Abstract
To clarify the anatomy of the pancreatic duct system and to investigate its embryology, we reviewed 256 pancreatograms with normal pancreatic head, 81 with pancreas divisum and 74 with pancreaticobiliary maljunction. Accessory pancreatograms were divided into two patterns. The long-type accessory pancreatic duct forms a straight line and joins the main pancreatic duct at the neck portion of the pancreas. The short-type accessory pancreatic duct joins the main pancreatic duct near its first inferior branch. The short-type accessory pancreatic duct is less likely to have a long inferior branch arising from the accessory pancreatic duct. The length of the accessory pancreatic duct from the orifice to the first long inferior branch was similar in the short- and long-type accessory pancreatic ducts. The first long inferior branch from the long-type accessory pancreatic duct passes though the main pancreatic duct near the origin of the inferior branch from the main pancreatic duct. Immunohistochemically, in the short-type accessory pancreatic duct, the main pancreatic duct between the junction with the short-type accessory pancreatic duct and the neck portion was located in the ventral pancreas. The long-type accessory pancreatic duct represents a continuation of the main duct of the dorsal pancreatic bud. The short-type accessory pancreatic duct is probably formed by the proximal main duct of the dorsal pancreatic bud and its long inferior branch.
Keywords: accessory pancreatic duct, endoscopic retrograde pancreatography, main pancreatic duct, pancreas divisum, pancreaticobiliary maljunction
Introduction
The human pancreas develops embryologically from the dorsal and ventral pancreatic buds, with fusion occurring at 78 weeks of gestation. The dorsal pancreatic bud gives rise to the anterior part of the head of the pancreas, in addition to the body and tail, while the ventral pancreatic bud develops into the posterior part of the head of the gland. Fusion of the pancreatic buds is accompanied by anastomosis of the ducts. The main drainage duct of the ventral pancreatic bud communicates with the main duct of the dorsal pancreatic bud, with the point of union lying between the isthmus and head of the pancreas; this becomes the dominant and more constant pancreatic duct (main pancreatic duct, MPD) (Adda et al. 1984; Skandalakis et al. 1993; Kamisawa et al. 1999, 2001; Kamisawa, 2004). Under normal conditions, the superior branch of the main ventral pancreatic duct fuses with the main dorsal pancreatic duct, while the inferior branch of the ventral pancreatic duct drains the posterior surface of the lower part of the head (Dawson & Langman, 1961; Kamisawa et al. 1999). The proximal part of the main dorsal pancreatic duct partially regresses to form the accessory pancreatic duct (APD, Santorini's duct), which opens into the minor duodenal papilla.
Pancreas divisum is a common congenital anomaly of the pancreas; it results from an abnormal fusion between the ventral and dorsal pancreatic ducts during fetal development (Lehman & Sherman, 1995; Kamisawa, 2004; Klein & Affronti, 2004). It is divided into complete and incomplete pancreas divisum. Complete pancreas divisum is defined as a completely separate pancreatic duct system in a grossly undivided gland. Incomplete pancreas divisum is a pancreatic anomaly with inadequate communication between the ventral and dorsal pancreatic duct (Tulassay et al. 1986; Sugawa et al. 1987). Based on the embryology and radiological findings, Hirooka et al. (1994) proposed the concept of branch fusion between the ventral and dorsal pancreatic ducts; this concept had previously been confirmed pathologically (Suda et al. 1991a,b).
Pancreaticobiliary maljunction is a congenital anomaly defined as the union of the pancreatic and biliary ducts outside of the duodenal wall. A common channel longer than 15 mm is usually present in pancreaticobiliary maljunction (Kimura et al. 1985; Kamisawa et al. 2005; Kamisawa & Okamoto, 2006). Several studies have reported on the pathogenesis of pancreaticobiliary maljunction during embryogenesis (Tanaka, 1995; Oi, 1996; Matsumoto et al. 2001), but the conclusions remain controversial.
Most embryological studies of the pancreatic duct system have been assessed by the direct injection of material into the pancreatic duct in resected or post-mortem specimens. We have prospectively examined the anatomy of the pancreatic duct of the head based on endoscopic retrograde pancreatography (ERP) and classified the accessory pancreatograms (Kamisawa et al. 1998a,b). We also examined the function of the APD using dye-injection ERP (Kamisawa et al. 1997, 1998a,b). Finally, we divided the accessory pancreatograms into two types (long and short type) based on their course and shape and the distance of the MPD from the orifice at the major duodenal papilla to the junction with the APD (Kamisawa et al. 1999). To clarify the anatomy of the pancreatic duct system of the head and to investigate the embryology of the normal pancreatic duct system and pancreaticobiliary malformation, all pancreatographic studies performed in our department up to the present time were reviewed.
Materials and methods
Materials
Between 1982 and 2005, we prospectively studied the anatomy of the pancreatic duct system using ERP. To achieve optimal visualization, several pancreatograms of the head of the pancreas were taken with the patient in the prone or slightly oblique position. When an adequate pancreatogram was not achieved via the major duodenal papilla, pancreatography via the minor duodenal papilla was tried. Among 3210 ERPs that were performed for evaluation of pancreatic diseases (n = 2130) such as pancreatic cancer, pancreatic cyst and chronic or acute pancreatitis, and biliary diseases (n = 1080) such as biliary cancer and biliary stones, there were 40 cases of complete pancreas divisum, 41 cases of incomplete pancreas divisum and 74 cases of pancreaticobiliary maljunction.
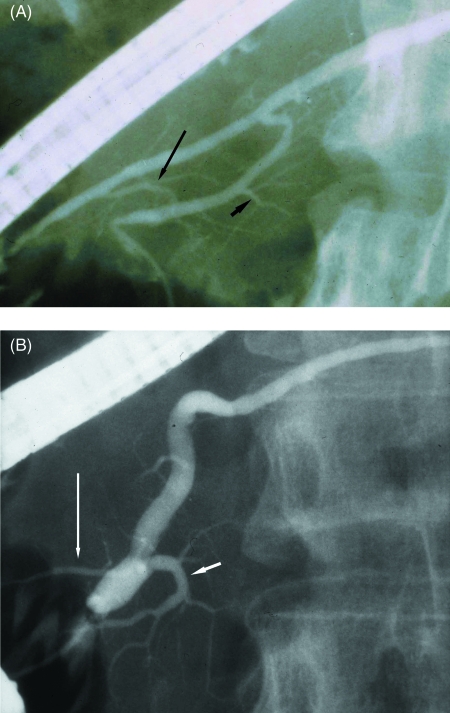
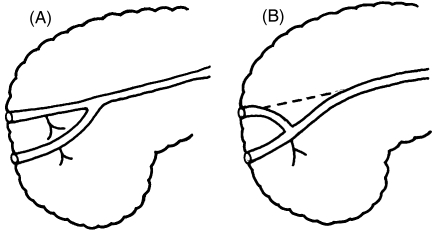
In total, 256 pancreatograms of normal pancreatic head with satisfactory imaging of the entire APD were reviewed, focusing on the long inferior branches arising from the APD and the MPD. The accessory pancreatograms are divided into two types [long type (171 cases) and short type (85 cases)] based on the distance of the MPD from the orifice to the junction with the APD. The long-type APD forms a straight line and joins the MPD at the neck portion of the pancreas (Fig. 1A). The short-type APD joins the MPD near its first inferior branch (Fig. 1B) (Kamisawa et al. 1999).
Fig. 1.
Retrograde pancreatography of normal cases (A) with the long-type accessory pancreatic duct which joined the main pancreatic duct in the neck portion and ran straight from the upper dorsal pancreatic duct (long arrow: the first long inferior branch from the accessory pancreatic duct; short arrow: inferior branch from the main pancreatic duct) and (B) with the short-type accessory pancreatic duct (long arrow) which joined the main pancreatic duct near the inferior branch (short arrow) and ran a descending course.
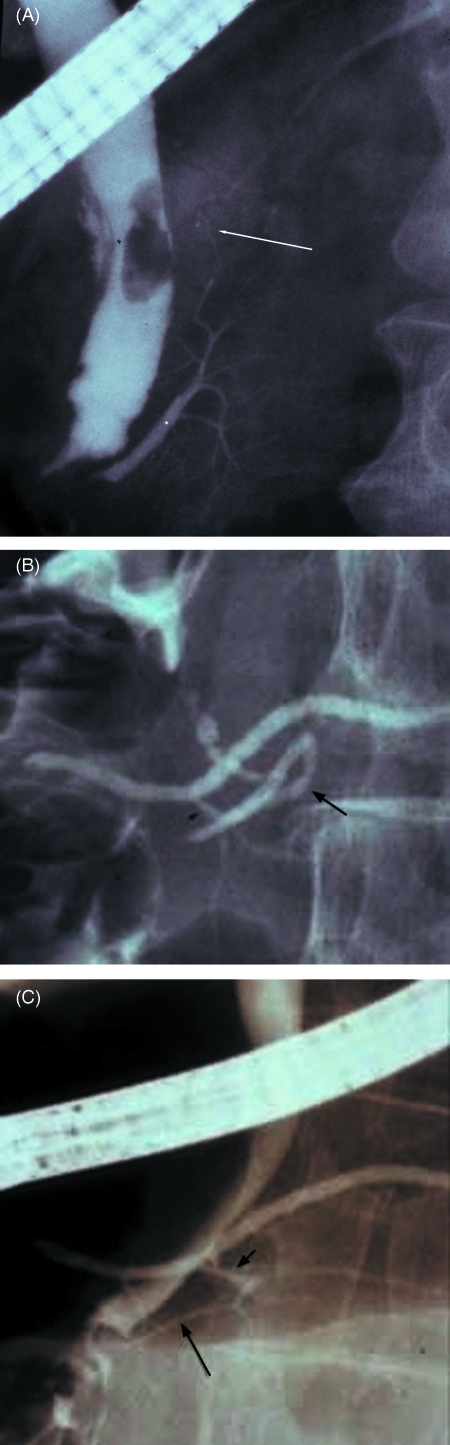
Complete pancreas divisum was diagnosed when filling of the entire dorsal pancreatic duct from the tail to the anterior part of the head was observed on pancreatography via the minor duodenal papilla, which was not connected to the ventral pancreatic duct. Incomplete pancreas divisum was diagnosed when the APD ran straight from the upstream dorsal pancreatic duct and one of the following was found: the presence of a long ventral pancreatic duct that ran toward the dorsal pancreatic duct, suggesting the involvement of the extreme end of the upper branch of the ventral pancreatic duct (Type 1, Fig. 2A); or the presence of a branch running toward the ventral pancreatic duct in a circuitous (Type 2, Fig. 2B) or a direct path (Type 3, Fig. 2C), suggesting the involvement of a branch from the dorsal pancreatic duct. The one characteristic common to all types was that the dorsal and ventral pancreatic ducts ran parallel to each other (Hirooka et al. 1994; Kamisawa et al. 2007).
Fig. 2.
Retrograde pancreatogram of (A) incomplete pancreas divisum (Type 1) showing fusion of the extreme end (arrow) of the upper branch of the ventral pancreatic duct to the dorsal pancreatic duct, (B) incomplete pancreas divisum (Type 2) showing fusion of the lower branch (short arrow) of the dorsal pancreatic duct with the lower branch (long arrow) of the ventral pancreatic duct, and (C) incomplete pancreas divisum (Type 3) showing fusion of the lower branch (short arrow) of the dorsal pancreatic duct with the slender ventral pancreatic duct (long arrow).
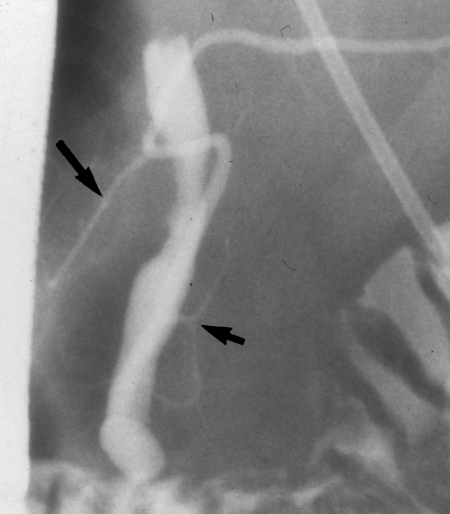
Patients with a long common channel (usually ≥ 15 mm) in which the sphincter muscle of Oddi lacked effect on the pancreaticobiliary junction, even during sphincter contraction, were diagnosed as having pancreaticobiliary maljunction (Kimura et al. 1985; Kamisawa et al. 2005; Kamisawa & Okamoto, 2006). The accessory pancreatograms of pancreaticobiliary maljunction cases were also classified into long-type (22 cases, Fig. 3), short-type (25 cases), ansa-type (ten cases) (Dawson & Langman 1961), incomplete pancreas divisum (ten cases) and halfway (seven cases).
Fig. 3.
Retrograde cholangiopancreatogram of pancreaticobiliary maljunction with the long-type accessory pancreatic duct (long arrow) (short arrow: inferior branch from the main pancreatic duct).
Pancreatograms of cases of pancreas divisum and pancreaticobiliary maljunction were also reviewed.
Measurement of lengths and diameters on pancreatograms
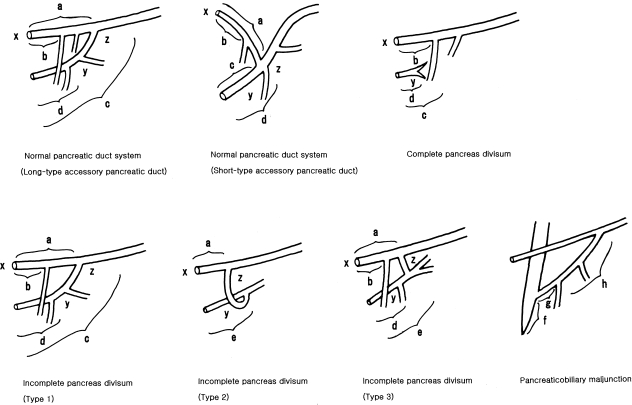
The number of long inferior branches (≥ 20 mm) arising from the APD and passing through the MPD to the caudal portion, and the presence of a long inferior branch (≥ 10 mm) arising from the MPD were determined.
In cases with a normal pancreatic duct system and incomplete pancreas divisum, the entire length of the APD (Fig. 4, a) was measured using a manual goniometer (Digital Map-meter, Comcurve-9, Koizumi Sokki Mfg Co. Ltd, Tokyo, Japan). In cases with long inferior branches arising from the APD, the length of the APD from the orifice at the minor duodenal papilla to the first long inferior branch (Fig. 4, b) was measured. In Type 1 incomplete pancreas divisum, the entire length of the MPD from the orifice at the major duodenal papilla to the junction with the APD (Fig. 4, c) was measured. In cases with a long inferior branch from the MPD, the length of the MPD from the orifice to the long inferior branch (Fig. 4, d) was measured. In Type 2 and 3 incomplete pancreas divisum, the length of the MPD from the orifice to the junction with the communicating branch (Fig. 4, e) was measured. In all cases, the maximum diameters of the APD (Fig. 4, x) and the MPD (Fig. 4, y) were measured. The maximum diameters of the MPD at the junction with the APD (Fig. 4, z) were measured in Type 1 incomplete pancreas divisum, along with the communicating branch in Types 2 and 3 (Fig. 4, z).
Fig. 4.
Schematic illustration of the pancreatic duct of normal cases with the long-type and short-type accessory pancreatic duct, complete pancreas divisum, incomplete pancreas divisum (Type 1–3), and pancreaticobiliary maljunction showing the portions whose length and diameter were measured. a, the entire length of the accessory pancreatic duct; b, length of the accessory pancreatic duct from orifice to the first long inferior branch; c, the entire length of the main (ventral) pancreatic duct from orifice to junction with the accessory pancreatic duct; d, length of the main (ventral) pancreatic duct from orifice to the long inferior branch; e, length of the main pancreatic duct from orifice to junction with the communicating branch in Type 2 and 3; x, maximum diameter of the accessory (dorsal) pancreatic duct; y, maximum diameter of the main (ventral) pancreatic duct; z, maximum diameter of the main pancreatic duct at junction with the accessory pancreatic duct in Type 1, and maximum diameter of the communicating branch in Types 2 and 3; f, length of the common channel in pancreaticobiliary maljunction; g, length of the main pancreatic duct from the proximal portion of the common channel to the inferior branch in pancreaticobiliary maljunction; h, length of the main pancreatic duct from the inferior branch to the junction with the accessory pancreatic duct.
In complete pancreas divisum cases, the length of the dorsal pancreatic duct from the orifice to the first long inferior branch (Fig. 4, b), the length of the entire ventral pancreatic duct (Fig. 4, c), the length of the ventral pancreatic duct from the orifice to the first long inferior branch (Fig. 4, d), and the maximum diameters of the dorsal pancreatic duct (Fig. 4, x) and the ventral pancreatic duct (Fig. 4, y) were measured.
In cases of pancreaticobiliary maljunction with the long-type or short-type APD, the length of the common channel (Fig. 4, f), the MPD from the proximal portion of the common channel to the first inferior branch (Fig. 4, g) and the MPD from the first inferior branch to the junction with the APD (Fig. 4, h) were measured.
Data were corrected for radiological magnification (O'Dwyer et al. 1981). Data were analysed statistically using the Mann–Whitney U-test. Values of P < 0.05 were considered statistically significant. Results are expressed as mean ± SD.
Studies in autopsied pancreases
Eight pancreases that were not included in the 3210 ERP cases were obtained from routine autopsies of cases without pancreatic diseases. Barium sulphate (approximately 50%) was injected into the MPD in the body of the pancreas, and pancreatograms of the head were taken in several directions. The pancreases were fixed in 10% neutral buffered formalin. Each tissue section crossed the MPD, the APD and the common bile duct in the same plane. Blocks of the head and body of the pancreas (width, 0.5 cm) parallel to this plane were examined. Serial sections were stained with haematoxylin and eosin; they were immunostained with anti-pancreatic polypeptide antibody (Milab, Malmo, Sweden) using the ABC method. The pancreatic head tissue was mapped for pancreatic polypeptide-immunoreactive cells (Fiocca et al. 1987; Suda et al. 1991b).
Results
Results of pancreatographic analysis of the normal pancreatic duct system, complete and incomplete pancreas divisum, and pancreaticobiliary maljunction, and immunohistochemical studies of autopsied pancreas are given in Table 1.
Table 1.
List of results of this study
| Pancreatographic analysis |
| Normal pancreatic duct system |
| Long-type accessory pancreatic duct |
| Short-type accessory pancreatic duct |
| Complete pancreas divisum |
| Incomplete pancreas divisum |
| Type 1 |
| Type 2 |
| Type 3 |
| Pancreaticobiliary maljunction |
| Immunohistochemical study of autopsied pancreases |
Pancreatographic analysis of the normal pancreatic duct system and complete pancreas divisum
Both the long-type and the short-type APDs had consistent findings on pancreatography. Long inferior branches arising from the long-type APD were observed in 133 of 171 cases (78%; one branch, n = 102; two branches, n = 30; three branches, n = 1). The length of the long-type APD from the orifice to the first inferior branch was 19.4 ± 4.0 mm. Long inferior branches from the dorsal pancreatic duct of complete pancreas divisum cases were observed in 29 of 40 cases (73%; one branch, n = 23; two branches, n = 6). The length of the dorsal pancreatic duct from the orifice to the first inferior branch was 18.1 ± 3.9 mm, similar to the length in the long-type APD.
A long inferior branch from the short-type APD was observed in 19 of 85 cases (22.4%; one branch, n = 19). The length of the short-type APD from the orifice to the long inferior branch (18.8 ± 4.2 mm) was similar to the length of the long-type APD from the orifice to the first long inferior branch.
A long inferior branch arising from the MPD was observed in 118 (69%) of 171 cases with the long-type APD, and the length of the MPD from the orifice to the long inferior branch was 17.4 ± 2.8 mm. A long inferior branch arising from the MPD was observed in 55 (65%) of 85 cases with the short-type APD, and the length of the MPD from the orifice to the long inferior branch was quite similar in the long-type and short-type APD cases (17.8 ± 4.5 mm) (Table 2).
Table 2.
Length and diameter of each factor in normal control, incomplete pancreas divisum (Type 1–3), and complete pancreas divisum (mm)
| a | b | c | d | e | x | y | z | |
|---|---|---|---|---|---|---|---|---|
| Normal control | ||||||||
| Long-type APD | 28.1 ± 8.9 | 19.4 ± 4.0 | 29.2 ± 9.9 | 17.4 ± 2.8 | 1.4 ± 0.4 | 3.1 ± 0.7 | 2.4 ± 0.8 | |
| (n = 171) | (n = 133) | (n = 118) | ||||||
| Short-type APD | 26.5 ± 5.6 | 18.8 ± 4.2 | 15.6 ± 5.4 | 17.8 ± 4.5 | 1.1 ± 0.6 | 3.1 ± 0.9 | 3.1 ± 0.9 | |
| (n = 85) | (n = 19) | (n = 55) | ||||||
| Incomplete pancreas divisum | ||||||||
| Type 1 | 31.1 ± 10.1 | 17.5 ± 1.5 | 41.1 ± 17.4* | 17.8 ± 4.2 | 1.8 ± 0.9 | 2.1 ± 0.9** | 1.4 ± 1.2** | |
| (n = 16) | (n = 14) | (n = 6) | (n = 13) | |||||
| Type 2 | 17.2 ± 2.7** | 18.3 ± 4.2 | 2.0 ± 1.4 | 2.5 ± 0.8 | 2.2 ± 0.8 | |||
| (n = 10) | (n = 10) | |||||||
| Type 3 | 23.1 ± 4.2* | 18.0 ± 1.6 | 19.6 ± 3.3 | 23.9 ± 6.8 | 2.7 ± 1.1** | 1.8 ± 0.9** | 1.7 ± 0.6 | |
| (n = 15) | (n = 15) | (n = 4) | (n = 15) | |||||
| Complete pancreas divisum | 18.1 ± 3.9 | 29.8 ± 21.0 | 19.1 ± 4.4 | 2.3 ± 0.9 | 1.7 ± 0.8** | |||
| (n = 40) | (n = 29) | (n = 15) | ||||||
P < 0.05
P < 0.01 compared with normal control. APD, accessory pancreatic duct.
a, the entire length of the accessory pancreatic duct; b, length of the accessory pancreatic duct from orifice to the first long inferior branch; c, the entire length of the main (ventral) pancreatic duct from orifice to junction with the accessory pancreatic duct; d, length of the main (ventral) pancreatic duct from orifice to junction with the inferior branch; e, length of the main pancreatic duct from orifice to junction with the communicating branch; x, maximum diameter of the accessory pancreatic duct; y, maximum diameter of the main (ventral) pancreatic duct; z, maximum diameter of the main pancreatic duct at junction with the accessory pancreatic duct in Type 1, and maximum diameter of the communicating branch in Type 2 and 3.
The short-type APD is less likely than the long-type APD to possess a long inferior branch arising from the APD. Although two long inferior branches arising from the long-type APD were detected in 18% of cases, only one long inferior branch was detected arising from the short-type APD. Furthermore, the length of the short-type APD from the orifice to the long inferior branch was similar to the length of the long-type APD from the orifice to the first long inferior branch. The length of the MPD from the orifice to the long inferior branch was similar in long-type APD and the short-type APD cases. The first long inferior branch from the long-type APD passes though the MPD near the origin of the inferior branch from the MPD, and the short-type APD joins the MPD near its inferior branch.
Pancreatographic analysis of incomplete pancreas divisum
The entire length of the MPD in Type 1 incomplete pancreas divisum cases was 41.1 ± 17.4 mm, which was significantly longer than that of normal cases with the long-type APD (P < 0.05). This implies that, embryologically, the extreme end of the upper branch of the ventral duct fuses with the dorsal pancreatic duct.
The length of the APD from the orifice to the first long inferior branch in normal cases with the long-type APD was similar to the length in Type 1 (17.5 ± 1.5 mm) and Type 3 (18.0 ± 1.6 mm) incomplete pancreas divisum cases, as well as the entire length (17.2 ± 2.7 mm) of the APD in Type 2 incomplete pancreas divisum cases. The entire length of the APD was significantly shorter in Type 2 (17.2 ± 2.7 mm, P < 0.01) and Type 3 (23.1 ± 4.2 mm, P < 0.05) incomplete pancreas divisum cases than in normal cases with the long-type APD (28.1 ± 8.9 mm). The length of the MPD from the orifice to the inferior branch in normal cases with the long-type APD was similar to the length in Type 1 (17.8 ± 4.2 mm) and Type 3 (19.6 ± 3.3 mm) incomplete pancreas divisum cases, as well as to the length of the MPD from the orifice to the junction with the communicating branch in Type 2 incomplete pancreas divisum cases (18.3 ± 4.2 mm). No long inferior branch from the MPD was seen in Type 2 incomplete pancreas divisum cases. These data suggest that the long inferior branch from the dorsal pancreatic duct and the inferior branch from the ventral pancreatic duct fuse embryologically in Type 2 incomplete pancreas divisum cases.
The length of the APD in Type 3 incomplete pancreas divisum cases was intermediate between the length of the APD from the orifice to the first inferior branch and entire length of the APD in normal cases with the long-type APD. Furthermore, four Type 3 incomplete pancreas divisum cases had a long inferior branch from the APD. These findings suggest that a second long inferior branch might have fused with the ventral pancreatic duct in some Type 3 incomplete pancreas divisum cases.
APD diameters were significantly grater in Type 3 incomplete pancreas divisum cases (2.7 ± 1.1 mm) than in normal cases with the long-type APD (1.4 ± 0.4 mm; P < 0.01). MPD diameters were significantly smaller in Type 1 (2.1 ± 0.9 mm) and Type 3 (1.8 ± 0.9 mm) incomplete pancreas divisum cases, as well as in complete pancreas divisum cases (1.7 ± 0.6 mm), than in normal cases with the long-type APD (3.1 ± 0.7 mm; P < 0.01). MPD diameters at the junction with the APD were significantly smaller in Type 1 incomplete pancreas divisum cases (1.4 ± 1.2 mm) than in normal cases with the long-type APD (2.4 ± 0.8 mm; P < 0.01) (Table 1). The diameter of the communicating branch was greater than that of the MPD in six Type 3 incomplete pancreas divisum cases. This suggests that this branch is derived from the dorsal pancreatic duct. A dominant APD, with diameter exceeding that of the MPD, was observed in seven Type 1 incomplete pancreas divisum cases, four Type 2 cases and 12 Type 3 cases. Marked hypoplasia of the MPD (maximum diameter less than 1 mm) was observed in three Type 1 incomplete pancreas divisum cases, one Type 2 case and six Type 3 cases (Table 2).
Pancreatographic analysis of pancreaticobiliary maljunction
In pancreaticobiliary maljunction cases, a long inferior branch from the MPD was detected in 15 (68.2%) of the 22 long-type APD cases and in 11 (44.0%) of the 25 short-type APD cases. In cases of pancreaticobiliary maljunction with the long-type APD, the length of the MPD from the proximal portion of the common channel to the inferior branch was 9.2 ± 5.1 mm, which was significantly shorter than the length of the MPD from the orifice to the inferior branch in normal cases with the long-type APD (P < 0.01). Furthermore, the length from the orifice to the inferior branch was 31.2 ± 9.9 mm, which was significantly longer than the length of the MPD from the orifice to the inferior branch in normal cases with the long-type APD (P < 0.01). In cases of pancreaticobiliary maljunction with the short-type APD, the length of the MPD from the proximal portion of the common channel to the inferior branch was 10.9 ± 3.8 mm, which was significantly shorter than the length of the MPD from the orifice to the inferior branch of normal cases with the long-type APD (P < 0.01); the length from the orifice to the inferior branch was 31.1 ± 7.8 mm, which was significantly longer than the length of the MPD from the orifice to the inferior branch in normal cases with the long-type APD (P < 0.01).
In cases of pancreaticobiliary maljunction with the long-type APD, the length of the MPD from the orifice to the junction with the APD was 44.5 ± 8.8 mm, which was significantly longer than the length of the MPD from the orifice to the junction with the APD in normal cases with the long-type APD (P < 0.01). In cases of pancreaticobiliary maljunction with the short-type APD, the length of the MPD from the orifice to the junction with the APD was 31.9 ± 6.8 mm, which was significantly longer than the length of the MPD from the orifice to the junction with the APD in normal cases with the short-type APD (P < 0.01).
In cases of pancreaticobiliary maljunction with the long-type APD, the length of the MPD from the inferior branch to the junction with the APD was 13.3 ± 6.8 mm, which was similar to the length of the MPD from the inferior branch to the junction with the APD in normal cases with the long-type APD (11.8 ± 3.4 mm). In cases of pancreaticobiliary maljunction with the short-type APD, the length of the MPD from the inferior branch to the junction with the APD was 2.1 ± 2.7 mm, which was similar to the length of the MPD from the inferior branch to the junction with the APD in normal cases with the short-type APD (1.8 ± 3.0 mm) (Table 3).
Table 3.
Length (mm) of the main pancreatic duct to the first inferior branch and the junction with the accessory pancreatic duct in controls and cases of pancreaticobiliary maljunction
| Length | Long type | Short type |
|---|---|---|
| MPD to the first inferior branch (controls) | 17.4 ± 2.8 | 17.8 ± 4.5 |
| MPD to the first inferior branch (PBM) | 31.2 ± 9.9* | 31.1 ± 7.8* |
| MPD from proximal portion of the common channel to the first inferior branch (PBM) | 9.2 ± 5.1** | 10.9 ± 3.8** |
| MPD to junction with APD (controls) | 29.2 ± 5.6 | 15.6 ± 5.4 |
| MPD to junction with APD (PBM) | 44.5 ± 8.8* | 31.9 ± 6.8* |
| MPD from proximal portion of the common channel to junction with APD (PBM) | 30.8 ± 11.7** | 11.8 ± 4.8** |
| MPD from the first inferior branch to junction with APD (controls) | 11.8 ± 3.4 | 1.8 ± 3.0 |
| MPD from the first inferior branch to junction with APD (PBM) | 13.3 ± 6.8 | 2.1 ± 2.7 |
| Common channel (PBM) | 20.3 ± 7.6 | 20.8 ± 6.5 |
MPD, main pancreatic duct; PBM, pancreaticobiliary maljunction; APD, accessory pancreatic duct.
P < 0.01, significantly longer than in controls.
P < 0.01, significantly shorter than in controls.
Immunohistochemical studies of autopsied pancreases
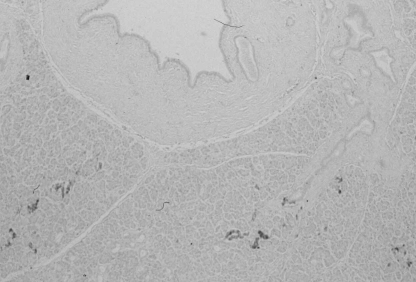
In five pancreases with the long-type APD, the APD and the MPD above the junction with the APD were always located in the dorsal pancreas with pancreatic polypeptide-poor islets, while the MPD from the orifice to the junction with the APD was located in the ventral pancreas with pancreatic polypeptide-rich islets. By contrast, in three pancreases with the short-type APD, the portion of the MPD located in the ventral pancreas was not confined to the area located from the orifice to the junction with the APD, but the neck portion was still located in the ventral pancreas, as indicated by the surrounding pancreatic polypeptide-rich islets (Figs 5 and 6).
Fig. 5.
Immunostaining of the autopsied pancreas with the short-type accessory pancreatic duct using anti-pancreatic polypeptide antibody. The main pancreatic duct in the neck portion, in addition to the portion from the orifice to the junction with the accessory pancreatic duct, was still located in the ventral pancreas as indicated by the surrounding pancreatic polypeptide-rich islets.
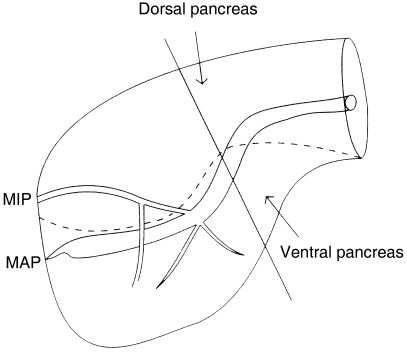
Fig. 6.
Schematic illustration of the pancreas head with the short-type accessory pancreatic duct, showing embryonic components by immunohistochemical study using anti-pancreatic polypeptide antibody. MIP, minor duodenal papilla; MAP, major duodenal papilla.
Discussion
We have prospectively examined the anatomy of the pancreatic duct of the head of the pancreas by ERP and classified the accessory pancreatograms into long-type and short-type according to the distance of the MPD from its orifice to the junction with the APD. The long-type APD forms a straight line and joins the MPD at the neck portion of the pancreas. The short-type APD joins the MPD near its first inferior branch (Kamisawa et al. 1999, 2004). We also examined the function of the APD using dye-injection ERP as follows: after injecting 23 mL of contrast medium containing indigocarmine through a catheter selectively cannulating the MPD, excretion of the dye from the minor duodenal papilla was observed endoscopically (Kamisawa et al. 1997, 1998a,b). Based on dye-injection ERP, the patency of the long-type APD was 75% (38/51), which was significantly greater than the patency of the short-type APD [36% (21/58); P < 0.01] (Kamisawa et al. 1999).
In this study, we clarified the anatomy of the pancreatic duct system of the head of the pancreas focusing on the long inferior branches arising from the APD and the MPD and investigated the embryology of the pancreatic duct system. The shape of the long-type APD was similar to that of the dorsal pancreatic duct of complete pancreas divisum. The long-type APD appears to represent an embryonic dorsal pancreatic duct, which is a persistent main duct of the dorsal pancreatic bud.
The short-type APD is less likely than the long-type APD to possess a long inferior branch arising from the APD. The first long inferior branch from the long-type APD passes through the MPD near the origin of the inferior branch from the MPD, and the short-type APD joins the MPD near its inferior branch. Based on these findings, we postulated a new embryological theory of pancreatic duct system development. It is likely that the short-type APD is formed by the most proximal part of the main duct of the dorsal pancreatic bud and its long inferior branch; the main duct of the dorsal pancreatic bud at the point of connection with the main duct of the ventral pancreatic bud is obliterated and replaced by this additional communication (Kamisawa et al. 1999, 2004) (Fig. 7). The connecting branch with the duct of the ventral pancreatic bud is the first inferior branch arising from the duct of the dorsal pancreatic bud in most short-type APDs, but the second inferior branch in cases with the short-type APD possessing a long inferior branch. On immunohistochemical study of autopsied pancreases with the short-type APD, the MPD from the orifice to the junction with the APD, as well as the MPD between the junction with the APD and the neck portion of the pancreas, was located in the ventral pancreas. This implies that the MPD between the junction with the APD and the neck portion originated from the ventral pancreatic duct, and that the duct of the dorsal pancreatic bud connected with the duct of the ventral pancreatic bud at two points. The immunohistochemical findings support our embryological hypothesis of the development of the short-type APD.
Fig. 7.
Schematic illustration of embryology of a pancreatic duct system. (A) Long-type accessory pancreatic duct, continuation of the main duct of the dorsal primordium. (B) Short-type accessory pancreatic duct, formed by the proximal main duct of the dorsal primordium and its long inferior branch, with the main duct of the dorsal primordium at the point of connection with the main duct of the ventral primordium being obliterated and replaced by this additional communication.
Incomplete pancreas divisum is a pancreatic anomaly with inadequate communication between the ventral and dorsal pancreatic ducts (Tulassay et al. 1986; Sugawa et al. 1987). Under the concept of branch fusion (Hirooka et al. 1994), we evaluated the pancreatographic findings in incomplete pancreas divisum cases and confirmed branch fusion via detailed pancreatogram measurements. Interestingly, some incomplete pancreas divisum cases had a dominant APD (n = 23) or marked MPD hypoplasia (n = 10).
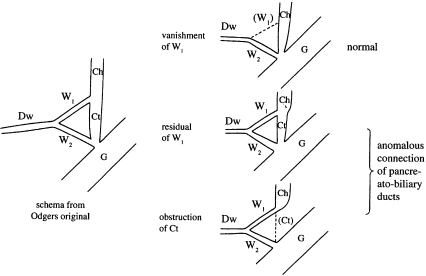
Pancreaticobiliary maljunction is a congenital anomaly characterized by a long common channel (Kimura et al. 1985; Kamisawa et al. 2005; Kamisawa & Okamoto 2006). As small radicles of the pancreas derived from a long common channel have been demonstrated in some patients with pancreaticobiliary maljunction (Matsumoto et al. 2001; Miyazaki &Ikeda, 2002), it has been suggested that the origin of a long common channel is the ventral pancreatic duct (Suda et al. 1991a,b; Matsumoto et al. 2001). According to Odgers (1930), the ventral pancreas, which fuses with a pair of ventral buds in the 5th week of fetal life, is connected by two ducts (W1 and W2) to the hepatic diverticulum; the left ventral duct (W1) usually regresses in the 6th week. One of the major hypotheses dealing with the pathogenesis of pancreaticobiliary maljunction suggests that pancreaticobiliary maljunction is related to the W1 remnant and regression of the terminal bile duct (Fig. 8) (Tanaka, 1995; Oi, 1996). Another hypothesis suggests that pancreaticobiliary maljunction results from a misarrangement, in which the terminal bile duct joins with one of the ducts of the ventral pancreas (Matsumoto et al. 2001). According to both of these hypotheses, the length of the MPD from the orifice to the inferior branch or the junction with the APD in pancreaticobiliary maljunction should be similar to that in normal cases. However, in the present study, cases of pancreaticobiliary maljunction with both the long-type and the short-type APD had a significantly shorter MPD length from the proximal portion of the common channel to the inferior branch, compared with the MPD length from the orifice to the inferior branch in normal cases. Likewise, the length from the orifice to the inferior branch was significantly greater in pancreaticobiliary maljunction cases than in normal cases. As the length of the MPD between the inferior branch and the junction with the APD was approximately equivalent in pancreaticobiliary maljunction and normal cases, these differences are probably due to a long common channel. If a long common channel in pancreaticobiliary maljunction is the result of embryological adhesion of the right ventral pancreatic duct and the terminal portion of the bile duct, the length of the MPD from the orifice to the inferior branch in pancreaticobiliary maljunction is longer than in normal cases due to the length of the terminal bile duct. Furthermore, the length of the MPD from the proximal portion of the common channel to the junction with the APD is shorter than in normal cases due to the length of the ventral pancreatic duct. Small radicles of the pancreas may be derived from the long common channel, which is composed of the pancreatic and bile ducts.
Fig. 8.
Schema of Oi's (1996) theory of the embryological development of pancreaticobiliary maljunction. Ch, choledochus; Ct, terminal portion of choledochus; W1, duct of left lobe; W2, duct of right lobe; Dw, the main pancreatic duct; G, gut.
In conclusion, there are two embryological types of APD. The long-type APD represents a continuation of the main duct of the dorsal pancreatic bud. The short-type APD is probably formed by the proximal main duct of the dorsal pancreatic bud and its long inferior branch. Branch fusion in incomplete pancreas divisum was confirmed by pancreatogram measurements. A long common channel in pancreaticobiliary maljunction cases might be due to embryological adhesion of the right ventral pancreatic duct and the terminal portion of the bile duct.
References
- Adda G, Hannoun L, Loygue J. Development of the human pancreas: variations and pathology. A tentative classification. Anat Clin. 1984;5:275–283. doi: 10.1007/BF01798752. [DOI] [PubMed] [Google Scholar]
- Dawson W, Langman J. An anatomical–radiological study on the pancreatic duct pattern in man. Anat Rec. 1961;139:59–68. doi: 10.1002/ar.1091390109. [DOI] [PubMed] [Google Scholar]
- Fiocca R, Sessa F, Tenti P, et al. Pancreatic polypeptide (PP) cells in the PP-rich lobe of the human pancreas are identified ultrastructurally and immunohistochemically as F cells. Histochemistry. 1987;77:511–523. doi: 10.1007/BF00495805. [DOI] [PubMed] [Google Scholar]
- Hirooka T, Kataoka S, Ohchi H, Maruo T, Toyonaga T, Dozaiku T. Branch fusion between the ventral and dorsal pancreatic duct. Dig Endosc. 1994;6:87–93. [Google Scholar]
- Kamisawa T, Tabata I, Tajima T, Tsushima T, Yoshida Y. Patency of the human accessory pancreatic duct as determined by dye-injection endoscopic retrograde pancreatography. Digestion. 1997;58:78–82. doi: 10.1159/000201427. [DOI] [PubMed] [Google Scholar]
- Kamisawa T, Tu Y, Egawa N, Sakaki N, Ishiwata J, Okamoto A. New classification for the accessory pancreatic duct by ERP. Dig Endosc. 1998a;10:308–311. doi: 10.1111/j.1443-1661.1998.tb00574.x. [DOI] [PubMed] [Google Scholar]
- Kamisawa T, Tu Y, Egawa N, Ishiwata J, Okamoto A. Patency of the accessory pancreatic duct in relation to its course and shape: a dye-injection endoscopic retrograde pancreatography study. Am J Gastroenterol. 1998b;93:2135–2140. doi: 10.1111/j.1572-0241.1998.00609.x. [DOI] [PubMed] [Google Scholar]
- Kamisawa T, Koike M, Okamoto A. Embryology of the pancreatic duct system. Digestion. 1999;60:161–165. doi: 10.1159/000007642. [DOI] [PubMed] [Google Scholar]
- Kamisawa T, Tu Y, Egawa N, Ishiwata J, Okamoto A. A new embryologic hypothesis of annular pancreas. Hepatogastroenterology. 2001;48:277–278. [PubMed] [Google Scholar]
- Kamisawa T. Clinical significance of the minor duodenal papilla and accessory pancreatic duct. J Gastroenterol. 2004;39:605–615. doi: 10.1007/s00535-004-1390-1. [DOI] [PubMed] [Google Scholar]
- Kamisawa T, Egawa N, Nakajima H, Tsuruta K, Okamoto A, Matsukawa M. Origin of the long common channel based on pancreatographic findings in pancreaticobiliary maljunction. Dig Liver Dis. 2005;37:363–367. doi: 10.1016/j.dld.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Kamisawa T, Okamoto A. Biliopancreatic and pancreatobiliary refluxes in cases with and without pancreaticobiliary maljunction: diagnosis and clinical implications. Digestion. 2006;73:228–236. doi: 10.1159/000095424. [DOI] [PubMed] [Google Scholar]
- Kamisawa T, Egawa N, Tu Y, Tsuruta K, Okamoto A. Pancreatographic investigation of embryology of complete and incomplete pancreas divisum. Pancreas. 2007;34:96–102. doi: 10.1097/01.mpa.0000240607.49183.7e. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ohto M, Saisho H, Unozawa T, Tsuchiya Y, Morita M, et al. Association of gall bladder carcinoma and anomalous pancreaticobiliary ductal union. Gastroenterology. 1985;89:1258–1265. doi: 10.1016/0016-5085(85)90641-9. [DOI] [PubMed] [Google Scholar]
- Klein SD, Affronti JP. Pancreas divisum, an evidence-based review: part I, pathophysiology. Gastrointest Endosc. 2004;60:419–425. doi: 10.1016/s0016-5107(04)01815-2. [DOI] [PubMed] [Google Scholar]
- Lehman GA, Sherman S. Pancreas divisum. Diagnosis, clinical significance, and management alternative. Gastrointest Endosc Clin N Am. 1995;5:145–170. [PubMed] [Google Scholar]
- Matsumoto Y, Fujii H, Itakura J, et al. Pancreaticobiliary maljunction: etiologic concepts based on radiologic aspects. Gastrointest Endosc. 2001;53:614–619. doi: 10.1067/mge.2001.113920. [DOI] [PubMed] [Google Scholar]
- Miyazaki R, Ikeda S. Diagnosis of pancreaticobiliary maljunction by ERCP. In: Koyanagi Y, Aoki T, editors. Pancreaticobiliary Maljunction. Tokyo: Igaku Tosho; 2002. pp. 47–50. [Google Scholar]
- O'Dwyer JA, Pemberton J, Thompson RPH. Measurement of the endoscopic retrograde cholangiogram. Dig Dis Sci. 1981;28:561–564. doi: 10.1007/BF01308107. [DOI] [PubMed] [Google Scholar]
- Odgers PNB. Some observation on the development of the ventral pancreas in man. J Anat. 1930;65:1–7. [PMC free article] [PubMed] [Google Scholar]
- Oi I. The development of the ventral pancreas and anomalous bilio-pancreatic junction. J Biliary Tract Pancreas. 1996;17:723–729. (in Japanese) [Google Scholar]
- Skandalakis LJ, Gray SW, Skandalakis JE. Surgical embryology and anatomy of the pancreas. Surg Clin North Am. 1993;73:661–697. doi: 10.1016/s0039-6109(16)46080-9. [DOI] [PubMed] [Google Scholar]
- Suda K, Matsumoto Y, Miyano T. Narrow segment distal to choledochal cyst. Am J Gastroenterol. 1991a;86:1259–1263. [PubMed] [Google Scholar]
- Suda K, Mogaki M, Matsumoto Y. Gross dissection and immunohistochemical studies on branch fusion type of ventral and dorsal pancreatic ducts: a case report. Surg Radiol Anat. 1991b;13:333–337. doi: 10.1007/BF01627768. [DOI] [PubMed] [Google Scholar]
- Sugawa CH, Walt AJ, Nunez DC, Masuyama H. Pancreas divisum: is it a normal anatomic variant? Am J Surg. 1987;153:62–67. doi: 10.1016/0002-9610(87)90202-9. [DOI] [PubMed] [Google Scholar]
- Tanaka T. Pathogenesis of choledochal cyst. Am J Gastroenterol. 1995;90:685. [PubMed] [Google Scholar]
- Tulassay Z, Papp J, Farkas IE. Diagnostic aspects of incomplete pancreas divisum. Gastrointest Endosc. 1986;32:428. doi: 10.1016/s0016-5107(86)71934-2. [DOI] [PubMed] [Google Scholar]