Abstract
A new, easily applicable technique providing en face preparations for light microscopy observations of the rat aorta and human thin wall arteries is described here. The major steps of the technique include attachment of the fixed and flattened vessel with the endothelium face down on a glass slide, covered with a water-soluble adhesive medium; drying and softening the vessel wall with another water-soluble medium; removal of the adventitia and most of the media; detaching the layer by placing the glass slide in water; and final attachment of the layer with the endothelium upwards. On such ‘thinned-wall’ preparations, 40–50 µm in thickness, the stained endothelial cells are clearly visible. Because of the preparation thickness and the use of water-soluble media during the preparation, some subendothelial lipid accumulations, characteristic of the early stages of atherosclerosis process, are well preserved.
Keywords: endothelium, en face technique, human arteries, rat aorta
Introduction
To study the surface area of the endothelial cells on large areas or even the entire endothelium of a vessel, a number of special techniques for en face light-microscopy observations have been developed. The endothelium can be observed directly on the intact and flattened vessel wall (O’Neill, 1947; Poole et al. 1958; Joris et al. 1982; Zand et al. 1991), or on the manually peeled vessel wall (Lautsch et al. 1953; Massmann, 1979; Fornas & Fortea, 1987). Alternatively, monolayers of endothelial cells can be produced by the so-called ‘Häutchen’ preparation technique existing in many modifications (Poole et al. 1958; Warren, 1965; Er Obase & Payling Wright, 1968; Pugatch & Saunders, 1968; Tsutsumi & Gore, 1969; Hirsch et al. 1980; Jones et al. 1992).
In the literature, a considerable number of studies have investigated the en face morphology of spontaneous and experimentally induced arterio- and atherosclerotic changes in the vessel wall (Efskind, 1941; Lautsch et al. 1953; Poole & Florey, 1958; Cotton & Wartman, 1961; Silkworth et al. 1975; Massmann, 1979; Joris et al. 1983; Zand et al. 1991; Babál & Pecháňová, 1992; Pecháňová & Babál, 1993; Haraoka et al. 1995; Jones et al. 1996; Zand et al. 1999). As these alterations include changes in the endothelial cells, and also the presence of mononuclear cell infiltration and lipid accumulations, they can be observed on the en face preparations. Using the Häutchen preparation, the nuclei and cell borders of the endothelial cells and also the mononuclear cells adhering to the endothelium are clearly visible (Cotton & Wartman, 1961; Silkworth et al. 1975; Jones et al. 1996). But in these preparations the cells are in a single layer, and usually the alcohol dehydration is a part of the preparation process; as a result, the subendothelial lipid accumulations cannot be detected. On the thicker en face preparations, on the other hand, the subendothelial lipid accumulations are clearly visible (Lautsch et al. 1953; Massmann, 1979), but the haematoxylin staining of all the cell layers makes the endothelial cell observations difficult. Satisfactory results can be obtained with the methods of Joris et al. (1982) and Zand et al. (1991), where the silver impregnated borders of the endothelial cells and the lipid accumulations are clearly visible. But the haematoxylin staining shows only the nuclei of the mononuclear cells adhering to the endothelium, as the nuclei of the endothelial cells cannot be observed. So up till now there has been no satisfactory method for presenting the complete endothelial cell morphology and the lipid accumulations simultaneously.
The technique described here represents a new way of producing preparations for en face observations from both the rat aorta and human vessels with thin walls, allowing clear visualization of the endothelium cell composition and subintimal lipid accumulations in the early stages of the atherosclerosis process.
Materials and methods
The aortas of adult male Wistar rats (300–350 g), and human internal thoracic arteries, were used for our en face preparations.
Under deep pentobarbital anesthesia, the thoracic cavity of each animal was opened, and a cannula was inserted into the ascending aorta. Pre-fixation silver impregnation of the cell borders was provided as described (Jones et al. 1992). The aorta was then perfusion-fixed in situ with 10% phosphate-buffered formalin for 10 min. After the perfusion, the abdominal cavity was opened and the internal organs were removed. The aorta from the arch to bifurcation was carefully cleaned from peri-adventitial connective tissue. The ventral branches were cut near to the main stem. Then the aorta was opened longitudinally by an incision along its ventral aspect. The side branches were cut and the aorta was removed. The thoracic and abdominal parts of the aorta were pinned out flat (using 10-mm tips of dental root-canal needles), luminal surface up, on polyethylene strips.
The human artery specimens were obtained from necropsies, within 4–12 h of death, carried out in the Department of Forensic Medicine of the Medical University of Sofia. The arteries were cleaned from the peri-adventitial connective tissue and then opened longitudinally. The side branches were cut, and the arteries were removed and also pinned out flat, endothelial surface upwards, on polyethylene strips. The rat aortas and human arteries were stored in 10% neutral buffered formalin for at least a week.
Description of the preparation technique
Immediately before making the en face preparations the rat aortas were stained with Carazzi's haematoxylin for 5 min. The parts from the human internal thoracic artery were also stained with Carazzi's haematoxylin or a combination of Sudan IV and Carazzi's haematoxylin.
Attaching the vessel on a glass slide with the endothelial surface downwards
The fixed and stained vessels were released from the polyethylene strips. They were then pressed carefully, with the endothelial side down, on a clean glass slide covered with a thin layer of water-soluble glue from a glue stick. We used Glue Stick (Stanger, Espelkamp, Germany), but other similar glue sticks, available in the trade markets, may also be used. This material takes 30 min to 1 h to dry, depending on the room temperature. At low magnification the adhesion of the vessel to the glass slide was inspected several times under a dissecting microscope. During the drying period, the small air bubbles under the vessels were eased out with a curved dental probe.
Removing of the media and adventitia of the vessel
Several drops of 65% glycerol-water solution were placed over the attached dried vessels. The drops were placed carefully over the vessel surface with the dental probe. The softening of the adventitia and media of the vessel occurs after 5–20 min, depending on the age and species differences in the wall thickness. After that, the rest of the solution was blotted. The next manipulation was provided under a dissecting microscope. In a corner, a small part of the softened adventitia and media was lifted up using the backside of a small lancet blade. Then, with a fine forceps, the lifted adventitia and media were peeled off.
Removal of the preparation from the glass slide
The final thin layer on the glass slide was detached by placing the slide in water. This procedure takes 2–3 min. Another 10–15 min in water was necessary for removing all of the small particles of the adhering material. A gentle flush with a syringe and small needle (27G) helped to remove the glue.
To measure the thickness of the layers produced, small parts of the preparations were cut, dehydrated in graded alcohols, and embedded in Durcupan. Thereafter, semi-thin sections were prepared and the thickness of the preparations was measured.
Attaching the preparation on a glass slide with the endothelial surface upwards
The detached layers were placed with the endothelial surface up on a glass slide, dried between glass slides and coverslipped with Entellan or Apathy's medium.
Results
Over the last 2 years, en face preparations from the aortas of over 150 rats have been prepared successfully using this technique. Specimens of over 30 internal thoracic arteries from humans of both sexes, 2–78 years old, have also been processed. In some of the human artery specimens, however, it was difficult to produce layers of satisfactory thickness for clear en face observations. Empirically, we discovered that the most suitable samples for our preparation process are the unaffected by the atherosclerotic process internal thoracic arteries of young individuals (up to 30 years of age). In older individuals, a thickened vessel wall with uneven endothelial surface and advanced atherosclerosis may be a problem. Vessels with these characteristics are difficult to attach properly with their endothelial surface down on the glass slides. This creates problems during the peeling of the adventitia-media layer and in the production of en face preparations. Therefore our technique can be used for en face studying of the internal thoracic artery in young individuals, and also in older ones, but only those in the initial stages of atherosclerosis.
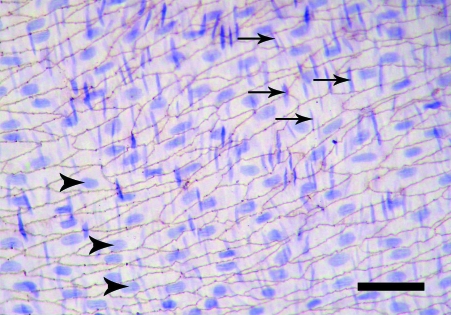
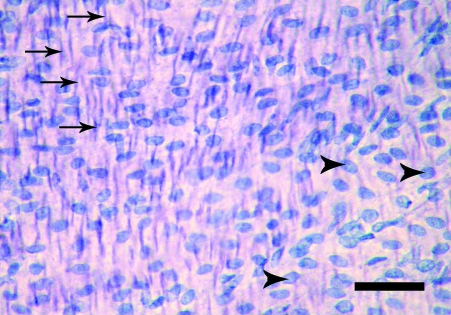
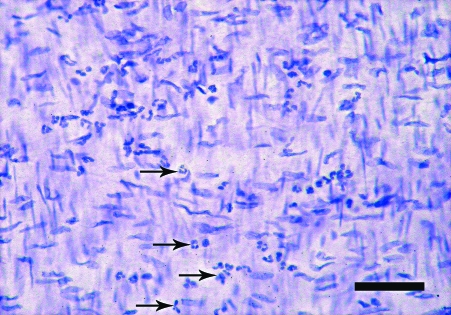
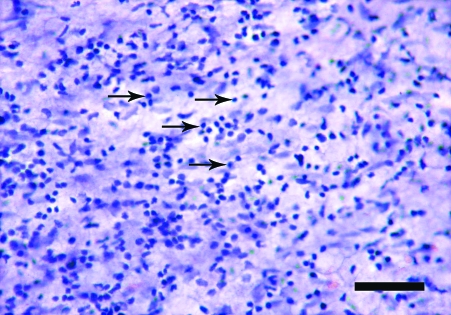
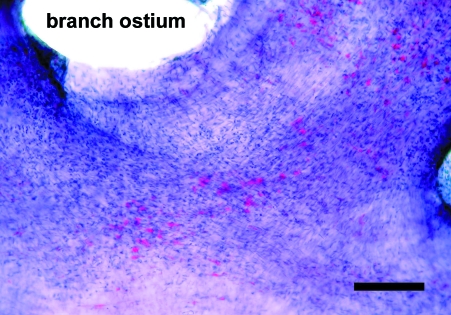
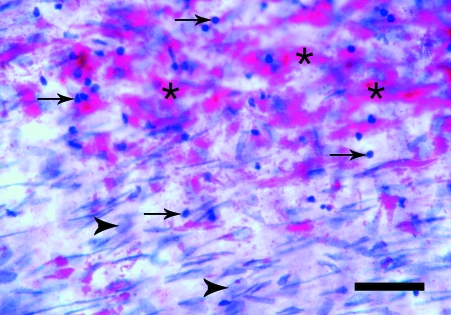
The en face preparations produced by the new technique described here, are composed of endothelium, subendothelial layer and few smooth muscle cells layers. On the semi-thin sections, a thickness of 40–50 µm was measured. In en face observations, the endothelium of the rat aorta is exposed clearly. The endothelial cells have clearly visible uninterrupted silver-stained borders and oval nuclei aligned parallel to the long axis of the vessel (Fig. 1). In the en face preparations from the human internal thoracic arteries, the nuclei of the endothelial cells, also oval in shape, are observed (Fig. 2). They have a primarily longitudinal orientation along the vessel. In the underlying smooth muscle cell layers of both the rat and human preparations the haematoxylin-stained nuclei are discernible (Figs 1 and 2). The latter, however, can be easily distinguished from the nuclei of the endothelial cells by their primarily perpendicular orientation to the vessel long axis and elongated spindle shape. In addition to the nuclei of the endothelial and smooth muscle cells, on some preparations from the human internal thoracic arteries, the nuclei of polymorphonuclear cells (Fig. 3) and mononuclear cells were detected (Fig. 4). Sudan IV staining of the human internal thoracic arteries reveals the presence of lipid accumulations in some cases (Figs 5 and 6). At low magnification (Fig. 5) they can be seen as small red spots located predominantly around the branch ostia. At higher magnification (Fig. 6), the relation of the lipid accumulations to the endothelial and mononuclear cells can be investigated.
Fig. 1.
En face preparation of the rat aorta. Normal endothelium. The nuclei of the endothelial cells (arrowheads) and of the smooth muscle cells (arrows) are clearly visible. Silver impregnation and haematoxylin. Bar, 50 µm.
Fig. 2.
En face preparation of the internal thoracic artery of a 20-year-old woman. Normal endothelium. The arrowheads indicate the nuclei of the endothelial cells; the arrows indicate the nuclei of the smooth muscle cells. Haematoxylin. Bar, 50 µm.
Fig. 3.
En face preparation of the internal thoracic artery of a 54-year-old man. Infiltration of the endothelium with polymorphonuclear cells (arrows). Haematoxylin. Bar, 50 µm.
Fig. 4.
En face preparation of the internal thoracic artery of a 73-year-old woman. Infiltration of the endothelium with mononuclear cells (arrows). Haematoxylin. Bar, 50 µm.
Fig. 5.
En face preparation of the internal thoracic artery of a 64-year-old man. Branch ostium. The areas of subintimal lipid accumulations are clearly visible stained in red. Sudan IV and haematoxylin. Bar, 200 µm.
Fig. 6.
En face preparation of the internal thoracic artery of a 64-year-old man. Together with the nuclei of the endothelial cells (arrowheads), the mononuclear cell infiltration (arrows) and the lipid accumulations (asterisks) are seen. Sudan IV and haematoxylin. Bar, 50 µm.
Discussion
In this paper, we present a new simple technique for en face observation of the endothelium. This technique can be used in studies of the aorta of the rat, frequently used in models of experimental atherosclerosis (Sano et al. 2004; Sakamoto et al. 2005; Almofti et al. 2006), and also in examining the en face characteristics of the human internal thoracic artery, which is a widely used arterial graft for the coronary system (Ojha et al. 2000; Marx et al. 2001). To evaluate the characteristics of our technique, we have briefly noted the other techniques for en face endothelial observations that have been previously described in the literature.
When the vessels examined have a thin wall, the endothelium can be observed en face on preparations containing the flattened whole vessel wall. In 1884, Zahn (1884) examined on such preparations the healing of lesions of the carotid or femoral arteries in rabbits. In 1947, O’Neill (1947) described a technique for examining the venous intima and the process of thrombosis in dogs. Segments from longitudinally opened canine veins were mounted flat on a metal frame, using coiled springs, and most of the adventitia was carefully removed. Then the silver-stained preparations were fixed, dehydrated, cleared and mounted in balsam. This method was used also by Samuels et al. (1952). Poole et al. (1958) presented a modification of the technique of O’Neill (1947) for examination of the regeneration of the rabbit aortic endothelium. Methods for whole thickness preparations from the aorta of the rat were later described by Joris et al. (1982) and Zand et al. (1991).
To observe the endothelium on thinner layers, some other techniques have been introduced. Lautsch et al. (1953) presented a modification of the technique of O’Neill (1947), which was applied to the arteries for the purpose of studying the early stages of the development of atherosclerosis. A corner of the inner layer of the human or rabbit aorta, mounted on a metal frame, was grasped manually with a forceps and separated from the major part of the media. Massmann (1979) described a technique for producing a ‘thick Häutchen’ by attaching pieces of pig aorta to a polystyrene foam plate and making cuts approximately 0.3 mm deep, enabling a 10–20 cell-thick layer to be peeled off. In a later technique, described by Fornas & Fortea (1987), the rat aorta was placed with the endothelium facing upwards; the adventitia was then fixed at both ends, and the media covered with the endothelium were stripped off with the help of fine forceps.
The endothelium may also be investigated on preparations one cell in thickness. These preparations, also called ‘Häutchen’ preparations, are principally produced after embedding the vessel inner surface and stripping off the other layers of the wall (Poole et al. 1958; Cotton & Wartman, 1961). Up to now, many modifications of the Häutchen technique have been developed to reliably produce more complete endothelial monolayers without retained smooth muscle elements (Hirsch et al. 1980). These modifications differ mainly in the method of attachment of the vessel intima to the slide. The monolayers of the endothelial cells have been produced by attaching the endothelium to slides covered with different concentrations of nitrocellulose solution or collodion (Sinapius, 1952; Poole et al. 1958; Schwartz & Benditt, 1973; Riese et al. 1978; Jones et al. 1992), or gelatin (Tsutsumi & Gore, 1969; Sade & Folkman, 1972), by freezing the vessel intima to a glass slide (Warren, 1965; Er Obase & Payling Wright, 1968; Riese et al. 1978; Babál & Pecháňová, 1992), or by pressing the vessel inner surface to cellulose acetate paper (Pugatch & Saunders, 1968) or sticky tape (Hirsch et al. 1980).
Compared with the other techniques for en face observations, our technique has various advantages. First, similarly to the Häutchen techniques, the vessel intima is embedded in a medium. However, the medium used in our technique is water-soluble – safe and easily removable by water – and this ensures that the endothelium is not damaged. In addition, such embedding medium can be obtained easily; therefore the cost of the preparation process is very low. Furthermore, the preparations produced by our technique are 40–50 µm thick and this ensures a good view on the endothelium. In thickness, the preparations resemble very thick microtome sections. We named the preparations produced by our technique ‘thinned-wall’ preparations. The preparations presented here contain not only the endothelium, but also the subendothelium and a few layers of smooth muscle cells. In these preparations, therefore, the underlying layers support the endothelium, and any artificial loss of the endothelial cells is impossible, so long as the procedure is performed carefully.
Another advantage is that, unlike in other en face techniques (Poole et al. 1958; Hirsch et al. 1980; Jones et al. 1992), during the preparation process only water-soluble media are used, rather than lipid solvents (alcohols, acetone). Therefore the subendothelial lipid accumulations in the early stages of the atherosclerotic process are well preserved and can be easily demonstrated. One possible disadvantage of the technique, however, is the difficulty of producing preparations from vessels thicker than the rat aorta or the human internal thoracic artery. It is also difficult to produce en face preparations where there is an advanced atherosclerotic process affecting the vessel wall.
In conclusion, and in view of the above-mentioned advantages, we believe that the present technique can be added to the arsenal of special techniques used in endothelial studies.
Acknowledgments
The authors wish to thank the Pathological Society of Great Britain and Ireland, Prof. Emerita Isabelle Joris and Dr Guido Majno (Department of Pathology, University of Massachusetts Medical School, USA), Dr Gregory Jones (Department of Surgery, Dunedin School of Medicine, New Zealand), Dr Guy M. Chisolm III (Cleveland Clinic, USA) and Prof. Nikolaus Freudenberg (Institute of Pathology, University Clinic, Freiburg, Germany) for their kind assistance in locating some of the references. The authors are very indebted to Prof. Karl Zilles (C. and O. Vogt Brain Research Institute, University of Düsseldorf, Germany) for his critical reading of earlier versions of the manuscript and for many helpful suggestions.
References
- Almofti MR, Huang Z, Yang P, Rui Y, Yang P. Proteomic analysis of rat aorta during atherosclerosis induced by high cholesterol diet and injection of vitamin D3. Clin Exp Pharmacol Physiol. 2006;33:305–309. doi: 10.1111/j.1440-1681.2006.04366.x. [DOI] [PubMed] [Google Scholar]
- Babál P, Pecháňová O. Activity of ATPase and 5′nucleotidase in endothelium of human atherosclerotic aortas. Cor Vasa. 1992;34:238–245. [PubMed] [Google Scholar]
- Cotton R, Wartman WB. Endothelial patterns in human arteries. Arch Pathol. 1961;71:3–12. [PubMed] [Google Scholar]
- Efskind L. Die Veränderungen im Gefässepithel bei Arteriosklerose. Acta Pathol Microbiol Scand. 1941;18:259–276. [Google Scholar]
- Er Obase D, Payling Wright H. A modified technique for producing ‘en face’ (Häutchen) preparations of endothelium for authoradiography. J Atheroscler Res. 1968;8:861–863. doi: 10.1016/s0368-1319(68)80050-x. [DOI] [PubMed] [Google Scholar]
- Fornas E, Fortea A. Autoradiography of endothelium in whole rat aorta by a new method. Atherosclerosis. 1987;66:95–98. doi: 10.1016/0021-9150(87)90183-3. [DOI] [PubMed] [Google Scholar]
- Haraoka S, Shimokama T, Watanabe T. Participation of T lymphocytes in atherogenesis: sequential and quantitative observation of aortic lesions of rats with diet-induced hypercholesterolaemia using en face double immunostaining. Virchows Archiv. 1995;426:307–315. doi: 10.1007/BF00191369. [DOI] [PubMed] [Google Scholar]
- Hirsch EZ, Martino W, Orr CH, White H, Chisolm GM., III A simple rapid method for the preparation of en faceendothelial (Häutchen) monolayers from rat and rabbit aortas. Atherosclerosis. 1980;37:539–548. doi: 10.1016/0021-9150(80)90061-1. [DOI] [PubMed] [Google Scholar]
- Jones GT, Martin BJ, Stehbens WE. Endothelium and elastic tears in the afferent arteries of experimental arteriovenous fistulae in rabbits. Int J Exp Path. 1992;73:405–416. [PMC free article] [PubMed] [Google Scholar]
- Jones GT, van Rij AM, Solomon C, Thomson IA, Packer SGK. Endothelin-1 is increased overlying atherosclerotic plaques in human arteries. Atherosclerosis. 1996;124:25–35. doi: 10.1016/0021-9150(95)05773-0. [DOI] [PubMed] [Google Scholar]
- Joris I, Zand T, Majno G. Hydrodynamic injury of the endothelium in acute aortic stenosis. Am J Pathol. 1982;106:394–408. [PMC free article] [PubMed] [Google Scholar]
- Joris I, Zand T, Nunnari JJ, Krolikowski FJ, Majno G. Studies on the pathogenesis of atherosclerosis. I. Adhesion and emigration of mononuclear cells in the aorta of the hypercholesterolemic rats. Am J Pathol. 1983;113:341–358. [PMC free article] [PubMed] [Google Scholar]
- Lautsch EV, McMillan GC, Duff GL. Technics for the study of the normal and atherosclerotic arterial intima from its endothelial surface. Lab Invest. 1953;2:397–407. [PubMed] [Google Scholar]
- Marx R, Clahsen H, Schneider R, Sons H, Klein RM, Gulker H. Histomorphological studies of the distal internal thoracic artery which support its use for coronary artery bypass grafting. Atherosclerosis. 2001;159:43–48. doi: 10.1016/s0021-9150(01)00483-x. [DOI] [PubMed] [Google Scholar]
- Massmann J. Mononuclear cell infiltration of the aortic intima in domestic swine. Exp Pathol. 1979;17:110–112. doi: 10.1016/s0014-4908(79)80035-6. [DOI] [PubMed] [Google Scholar]
- Ojha M, Leask RL, Johnston KW, David TE, Butany J. Histology and morphology of 59 internal thoracic artery grafts and their distal anastomoses. Ann Thorac Surg. 2000;70:1338–1344. doi: 10.1016/s0003-4975(00)01975-5. [DOI] [PubMed] [Google Scholar]
- O’Neill JF. The effect on venous endothelium of alterations in blood flow through the vessels in vein wall and the possible relation to thrombosis. Ann Surg. 1947;126:270–288. [PubMed] [Google Scholar]
- Pecháňová O, Babál P. Activity of some adenine nucleotide degradation enzymes in human atherosclerotic aorta endothelium. Folia Biol (Praha) 1993;39:188–194. [PubMed] [Google Scholar]
- Poole JCF, Florey HW. Changes in the endothelium of the aorta and the behaviour of macrophages in experimental atheroma of rabbits. J Pathol Bacteriol. 1958;75:245–251. doi: 10.1002/path.1700750202. [DOI] [PubMed] [Google Scholar]
- Poole JCF, Sanders AG, Florey HW. The regeneration of aortic endothelium. J Pathol Bacteriol. 1958;75:133–143. doi: 10.1002/path.1700750116. [DOI] [PubMed] [Google Scholar]
- Pugatch EMJ, Saunders AM. A new technique for making Häutchen preparations of unfixed aortic endothelium. J Atheroscler Res. 1968;8:735–738. doi: 10.1016/s0368-1319(68)80032-8. [DOI] [PubMed] [Google Scholar]
- Riese K-H, Freudenberg N, Haas W. ‘En face’ preparation methods for investigation of endothelia and mesothelia. Pathol Res Pract. 1978;162:327–336. doi: 10.1016/S0344-0338(78)80047-8. [DOI] [PubMed] [Google Scholar]
- Sade RM, Folkman J. En face stripping of vascular endothelium. Microvasc Res. 1972;4:77–80. doi: 10.1016/0026-2862(72)90018-0. [DOI] [PubMed] [Google Scholar]
- Sakamoto W, Isomura H, Fujie K, Takahashi K, Nakao K, Izumi H. Relationship of coffee consumption with risk factors of atherosclerosis in rats. Ann Nutr Metab. 2005;49:149–154. doi: 10.1159/000086170. [DOI] [PubMed] [Google Scholar]
- Samuels PB, Samuels BM, Webster DR. New techniques in the study of venous endothelium. Lab Invest. 1952;1:50–60. [PubMed] [Google Scholar]
- Sano J, Shirakura S, Oda S, Hara T, Ishihara T. Foam cells generated by a combination of hyperglycemia and hyperlipemia in rats. Pathol Int. 2004;54:904–913. doi: 10.1111/j.1440-1827.2004.01778.x. [DOI] [PubMed] [Google Scholar]
- Schwartz SM, Benditt EP. Cell replication in the aortic endothelium: a new method for the study of the problem. Lab Invest. 1973;28:699–707. [PubMed] [Google Scholar]
- Silkworth JB, McLean B, Stehbens WE. The effects of hypercholesterolemia on aortic endothelium studied en face. Atherosclerosis. 1975;22:335–348. doi: 10.1016/0021-9150(75)90015-5. [DOI] [PubMed] [Google Scholar]
- Sinapius D. Über das Aortenendothel. Arch Pathol Anat. 1952;322:662–694. doi: 10.1007/BF00957503. [DOI] [PubMed] [Google Scholar]
- Tsutsumi H, Gore I. Isolation of living endothelial cells by gelatin-film stripping of vascular walls. Stain Technol. 1969;44:139–142. doi: 10.3109/10520296909063339. [DOI] [PubMed] [Google Scholar]
- Warren BA. A method for the production of ‘en face’ preparations one cell in thickness. J R Microsc Soc. 1965;84:407–413. doi: 10.1111/j.1365-2818.1965.tb02141.x. [DOI] [PubMed] [Google Scholar]
- Zahn FW. Untersuchungen über die Vernarbung von Querrissen der Arterienintima und Media nach vorheriger Umschnürung. Arch Pathol Anat Physiol Klin Med. 1884;96:1–15. [Google Scholar]
- Zand T, Hoffman AH, Savilonis BJ, Underwood JM, Nunnari JJ, Majno G, et al. Lipid depositions in rat aortas with intralumunal hemispherical plug stenosis. Am J Pathol. 1999;155:85–92. doi: 10.1016/S0002-9440(10)65103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zand T, Majno G, Nunnari JJ, Hoffman AH, Savilonis BJ, MacWilliams B, et al. Lipid deposition and intimal stress and strain. A study in rats with aortic stenosis. Am J Pathol. 1991;139:101–113. [PMC free article] [PubMed] [Google Scholar]