Abstract
Cleft lip and palate (CL/P), as is true of many craniofacial malformations in humans, is etiologically complex and highly variable in expression. A/WySn mice are an intriguing model for human CL/P because they develop this dysmorphology with a variable expression pattern, incomplete penetrance and frequent unilateral expression on a homogeneous genetic background. The developmental basis for this variation in expression is unknown, but of great significance for understanding such expression patterns in humans. As a step towards this goal, this study used three-dimensional geometric morphometric and novel high throughput morphometric techniques based on three-dimensional computed microtomography of mouse embryos to analyze craniofacial shape variation during primary palate formation. Our analysis confirmed previous findings based on two-dimensional analyses that the midface in A/WySn embryos, and the maxillary prominence in particular, is relatively reduced in size and appears to be developmentally delayed. In addition, we find that shape variance is increased in A/WySn embryos during primary palate formation compared to both C57BL/6J mice and the F1 crosses between these strains. If the reduction in midfacial growth caused by the Wnt9b hypomorphic mutation pushes A/WySn mice closer on average to the threshold for cleft lip formation, the elevated shape variance may explain why some, but not all, embryos develop the dysmorphology in a genetically homogeneous inbred line of mice.
Keywords: cleft lip and palate, computed microtomography, craniofacial development, mouse embryos, palate formation, three-dimensional morphometrics
Introduction
Mouse models with craniofacial dysmorphologies have proved to be useful in advancing the identification of human genetic mutations that cause various congenital malformations (Thyagarajan et al. 2003; Murray & Schutte, 2004). Mouse models are particularly appropriate as models for human palate malformation because early facial development and morphology of mice is similar to that of humans at the time of lip formation (Trasler, 1968; Diewert & Wang, 1992). This study uses A/WySn mice, which develop cleft lip (CL) with an incidence of 20–30% (Juriloff, 1982), to investigate the relationship between skull shape and variation and risk of abnormal lip formation.
Juriloff et al. (2004) identified two epistatically interacting loci [clf1(Chr 11) andclf2(Chr 13)] that produce CL in A/WySn mice. The first mutation, clf1, maps to mouse chromosome 11 (Juriloff et al. 2004) which is homologous to human chromosome 17q21. One of the unidentified genetic factors for human cleft lip also maps to this chromosomal region (Peanchitlertkajorn et al. 2003). Juriloff et al. (2006) discovered that clf1 is a hypomorphic mutation of the Wnt9b gene, possibly due to a IAP transposon insertion 3’ to the gene (Juriloff et al. 2005). Wnt9b is expressed in the ectoderm of the facial primordia and the epithelial seam during lip fusion in the mouse embryo (Lan et al. 2006). Additionally, Wnt9b has been shown to modify Shh expression in chick neural plate development (Robertson et al. 2004). Although the functions of Wnt9b are not well known, members of the Wnt gene family perform diverse functions including regulation of cell proliferation, cell polarity and determination of cell fate (Wodarz & Nusse, 1998; Francis-West et al. 2003; Qian et al. 2003). Best characterized is the canonical Wnt signaling pathway in which Wnt9b among others is implicated. The canonical Wntsignaling pathway is involved in facial prominence outgrowth as well as fusion (Lan et al. 2006). A complete loss-of-function allele of Wnt9b produces cleft lip with incomplete penetrance on a C57BL/6J background (Carroll et al. 2005). Lan et al. (2006) suggest that this pathway acts upstream of Bmp4. The clf2 mutation remains unknown, but Ptch, Mtrr and Gas1 are current candidates of interest (Juriloff et al. 2004).
Due to the composite nature of facial fusion, cleft palate has a complex etiology. During facial development, multiple paired three-dimensional (3D) structures come together in a coordinated manner to form an intricate, single craniofacial structure. When a malformation such as cleft lip occurs, the normal process is disrupted, either by an interference of facial process fusion (Jara et al. 1995; Pezzetti et al. 1998; Sözen et al. 2001) or by the timing of the fusion of these structures (Fraser, 1970). It has been hypothesized that the size of the facial prominences is causally associated with CL formation and that a reduction in facial growth during palate formation increases the risk that the prominences will not contact and therefore not fuse (Wang & Diewert, 1992; Diewert & Lozanoff, 1993; Diewert & Lozanoff, 2002). In addition, Wang et al. (1995) found that there is a developmental delay by somite stage in terms of mesenchymal bridge formation of the upper lip in A/WySn mice. Therefore there is a narrower window during development in which the bridge must form compared to non-clefting strains. Variability may also play a role in CL because many human syndromes that include CL also exhibit increased phenotypic variation (Khan et al. 1986; Lacombe et al. 1995).
Several studies have suggested that maternal effects may be responsible for CL formation (Reed, 1936; Davidson et al. 1969; Bornstein et al. 1970; Juriloff & Fraser, 1980; Juriloff, 1982). There is also evidence of a maternal effect in humans (Khoury et al. 1983; Chung et al. 1987). Cleft lip frequency differentials have been attributed to genetic maternal effects among the ‘A’ strain of mice, including A/WySn (Juriloff, 1982). Additional studies have suggested that maternal effects influence the forward growth of the maxillary prominence (Wang et al. 1995).
This is the first study that applies 3D geometric morphometric techniques to the study of embryonic development and it is the first application of a body of high-throughput morphometric and visualization techniques that relies on direct analyses of volumetric datasets (Kristensen et al. submitted). Geometric morphometric methods offer ways of rigorously analyzing facial shape that allow us to ask specific questions about the morphological nature of face and palate formation. Until now, studies of the morphology of cleft lip and palate development have been confined to the realm of two-dimensional (2D) geometric morphometrics (Young et al. 2007) or traditional morphometrics (Ciriani & Diewert, 1986; Wang & Diewert, 1992), whereas this study utilizes innovative methods in micro-computed tomography (µCT) scanning protocols to examine the 3D morphology of A/WySn embryos and adults with a focus on phenotypic variation.
This study addresses three hypotheses about the facial architecture during A/WySn ontogeny and the development of CL. It has been previously shown that embryonic face shape affects the formation of the lip in A/J and C57BL/6J mice (Trasler, 1968) and the same has been suggested for humans (Fraser & Pashayan, 1970; Diewert & Shiota, 1990). We test the hypothesis that the 3D facial shape of A/WySn embryos is more variable than their C57BL/6J counterparts and that this increased variability leads to some individuals reaching the threshold for the phenotypic expression of CL. Secondly, we test the hypothesis that the A/WySn mice have a mean shape that is shifted towards this threshold. In this hypothesis, ‘typical’ amounts of variation around this shifted mean would be adequate to push some individuals over the threshold. Additionally, possible maternal effects are further investigated using two groups of F1 crosses of A/WySn and C57BL/6J, each with a different maternal strain, in the shape analysis.
These hypotheses were tested by using 3D geometric morphometric analyses on both embryonic and adult skull datasets of both A/WySn and C57BL/6J mice. The adults were included to investigate whether any mean shape or variance differences observed in the embryos extend to the adult phenotype. The use of C57BL/6J as a comparison strain here follows a precedent set by earlier work on the subject of CL (Trasler, 1968; Ciriani & Diewert, 1986; Wang & Diewert, 1992).
Methods
The embryonic dataset used in this study comprised 50 C57BJ/6J, 79 A/WySn and 72 of their F1 crosses: 36 with A/WySn mothers and C57BL/6J fathers (AC), and 36 with C57BL/6J mothers and A/WySn fathers (CA). A/WySn embryos with cleft lip were excluded from this study; at the early stages of lip formation, however, those that will develop clefts cannot be identified. As the two clf mutations unique to the A/WySn strain are recessive, the F1 specimens (AC and CA) are all heterozygous for those two mutations and do not develop the cleft lip malformation. The C57BL/6J embryos were generated at the University of Calgary (Hallgrímsson Lab) and the A/WySn and the heterozygote samples were generated at the University of British Columbia (Diewert Lab). All embryos were collected between 10.5 and 11.5 days post-fertilization and staged by tail somite number. They were immediately fixed in Bouin's solution and transferred to 70% ethanol after 24 h of fixation. Bouin's solution is effective for micro-computed tomography scanning because the embryos themselves cannot be suspended in solution during the scan, and Bouin's fixation gives embryos the necessary tissue rigidity to complete the scan without movement or drying artifacts. The adult dataset consisted of A/WySn skulls (n = 20) and C57BL/6J skulls (n = 20) over 90 days old and were also generated in the Hallgrímsson lab. All mice used in this study were housed and cared for at the University of Calgary or at the University of British Columbia in accordance with Canadian animal research laws and regulations and protocols were approved by the Animal Care Committees at each institution.
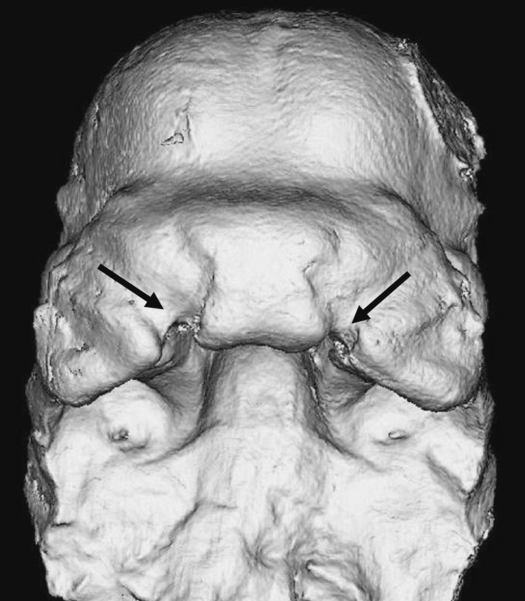
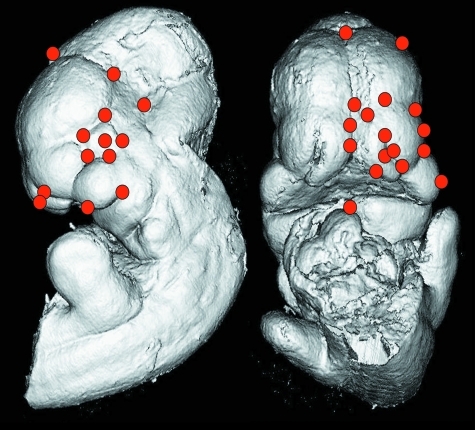
All embryos were imaged by a micro-computed tomography scanner (Skyscan 1072, Kontich, Belgium) at a 6.25 µm nominal resolution (100.0 kV, 98.4 µA, 0.90 rotation step, 2 frame averaging and a 3.8 s exposure time) corrected for both flat field and random movement errors. The scans were reconstructed (Cone_Rec, v1.4.4.0, Skyscan) and cropped (ImageJ, 1.37v, http://rsb.info.nih.gov/ij). An example image of a µCT-scanned A/WySn embryo with bilateral cleft lip is depicted in Fig. 1. Analyze 3D (http://www.mayo.edu/bir/) was used to digitize 52 3D landmarks directly from the volumetric reconstruction of the µCT scans (Fig. 2). The landmarks were chosen both to capture adequately the developing facial morphology and so that they could be precisely digitized on each individual. On many of the specimens, the mandibles had been previously removed to enhance visualization and staging of the developing face (Ciriani & Diewert, 1986). The landmarks were concentrated around the medial and lateral nasal prominences and the maxillary prominences. The landmarks are located at the juncture of identifiable structures, for example the juncture of the maxillary and lateral-nasal prominences, or at points of maximum curvature.
Fig. 1.
Example of a 3D reconstruction from a µCT scan of an A/WySn embryo at tail somite stage 26 with bilateral cleft lip. This image is indicative of scan quality after fixation with Bouin's solution. The mandible was removed for a previous study.
Fig. 2.
Embryo landmarks shown on a 3D reconstruction of a µCT scan of a C57BL/6J embryo.
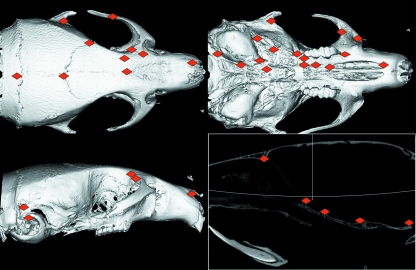
All adult crania 3D scans were obtained using a µCT system (vivaCT 40, Scanco Medical, Bassersdorf, Switzerland) providing a 35 µm nominal resolution (70 kV, 160 µA, 500 projections/180º). Subsequent to automatic reconstruction, the 3D data were digitized (Analyze 3D) at 68 landmarks on each individual (Fig. 3).
Fig. 3.
Adult landmarks shown on a 3D reconstruction of a µCT scan of a C57BL/6J skull.
A generalized least squares superimposition was used to obtain Procrustes coordinates for both embryos and adults using Morpheus et al. (Slice, 1994–1999). As the developing embryo changes shape considerably, yet predictably, as it grows, the Procrustes coordinates were standardized to the 16 tail somite stage by regression of the Procrustes coordinate data on tail somite stage for the pooled embryo sample (IMP program ThreeDStand6; Sheets, 2004b). This creates a new dataset in which most ontogenetic shape variation or all variation correlated with tail somite stage is removed from the samples. For the adult dataset, the residuals of Procrustes coordinates regressed on centroid size were calculated to standardize the data to the mean centroid size (ThreeDStand6). This removes allometric or size-related shape variation from the analysis.
Mean shapes were tested for significant differences between all sample groups using Goodall's F-tests of the Procrustes distances of the standardized data. These tests were performed in a second IMP module (Simple3D; Sheets, 2004a). This same module was used to calculate the variance of each group and a permutation function was run with 1600 repetitions to test for significant differences between the variances of each group.
A principal component analysis (PCA) was performed (MorphoJ, 0.09i, University of Manchester, UK; Klingenberg, 2006) on the symmetrical component of the standardized embryo dataset. Wireframes visualizing the shape changes along principal components (PC) were generated (Morphologika2, v2.4, Hull York Medical School; O’Higgins & Jones, 2006). PCA is a multivariate method that reduces the dimensionality of a given dataset so that all the variation in all samples combined is captured on the same number of axes as there are variables in that dataset. The first PC represents the greatest amount of variance and the following PCs represent subsequently smaller proportions of the total variance. Generally, the first PC represents the variation due to size; however, as here the PCA was performed using the standardized data, it allows us to explore the shape differences associated with the major axes of variation.
A canonical variate analysis (CVA) was also performed (MorphoJ) on the symmetrical component of the standardized embryo data. CVA maximizes between-group variance relative to within-group variance, as opposed to the variance across all individuals (PCA). Therefore, it maximally discriminates between groups, which in this study are defined as the four sets of embryos (C57BL/6J, A/WySn, AC and CA). The shape changes associated with each canonical variate (CV) describes the ways in which the groups are most differentiated.
A subset of the embryos at tail somite stage 16 (six C57BL/6J and six A/WySn) and all of the adult crania were used in a semi-automated high-throughput screening method of 3D shape analysis. This method allows us to explore mean shape differences between groups independent of landmarks. Only 12 embryos were chosen because this method cannot remove allometric effects and therefore all the embryos had to be at the exact same developmental stage. To examine only the craniofacial shape, the image reconstructions for each embryo were cropped after the first brachial arch to eliminate information on the shape of the rest of the embryo.
To calculate a mean shape, the µCT volumes were first transformed into the same orientation using intensity-based rigid image registration (Boyd et al. 2006). Following this, the grey scale value of each voxel in every image was divided by the sample size and then the corresponding voxel intensities of each superimposed image were added, resulting in an average image that contains information from the shape of each specimen. Surface-to-surface Euclidean distances were measured using a maximal sphere algorithm on the superimposed mean shapes. Further details of this method are outlined in Kristensen et al. (submitted). These distances were calculated between the A/WySn and C57BL/6J mean shapes for both the embryos and the skulls (the spheres were centered on the C57BL/6J mean and expanded outward toward the A/WySn mean) and then mapped onto the AWS mean image using a color-gradient scale.
Results
Adult
The Goodall's F-test revealed a statistically significant mean shape difference between A/WySn and C57BL/6J adult crania regressed data (between group Procrustes distance = 0.03038, F-score = 10.6955, P < 0.0001. The variances (A/WySn variance = 0.00103; C57BL/6J variance = 0.00104), however, were not statistically different.
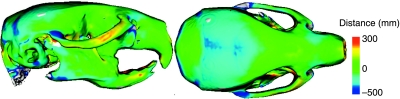
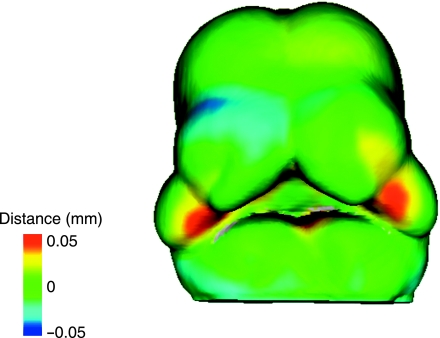
The result of the adult mean shape surface–surface map of the two strains is displayed in Fig. 4. The surface–surface map shows that the C57BL/6J mean shape has a higher cranium and is also larger at the posterior end of cranium. The A/WySn mean shape is wider at the anterior end of the zygomatic arch, but then narrows compared to C57BL/6J at the junction with the squamosal bone. A/WySn also has a longer nasal bone as well has higher area around the nasal–frontal suture.
Fig. 4.
Gradient map of Euclidean surface–surface distances taken from the C57BL/6J mean skull to the A/WySn mean skull and mapped onto the A/WySn mean skull. The position of the mandibles was not standardized in the individual adult scans and therefore the differences in the mandibular region of this figure are not biologically relevant.
Embryo
All but one pair-wise comparison of Procrustes distances between groups of the embryonic dataset proved to be significantly different according to the Goodall's F-test. Results of the significance tests are shown in Table 1. The only two groups that did not differ from one another were the two F1 crosses, AC and CA, although both groups proved to be different than C57BL/6J and A/WySn. The A/WySn embryos had the greatest shape variance when compared to the other three groups, and the F1 crosses had the least. The permutation test showed that the AC and CA groups did not differ from each other in this statistic (Table 2). The difference in variance between AC and C57BL/6J was deemed to be non-significant as well. The other comparisons, however, were significant at the P = 0.05 level after Bonferroni adjustment for multiple comparisons.
Table 1.
Procrustes distances between groups
P < 0.0000
P = 0.0326.
Table 2.
Differences in variance between groups
P < 0.001
P < 0.03.
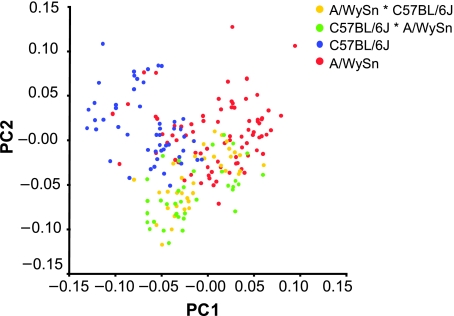
The PCA shows that the greatest axis of variation (PC1) separates the A/WySn, C57BL/6J and F1 crosses from each other. The separate F1 groups do not differ from one another on this axis (Fig. 5). Also, the A/WySn embryos seem to exhibit the most variability across PC1. This first PC represents 17.25% of the total variance in the sample. Along PC1, the maxillary and nasal process change in shape from more to less rotated towards the A/WySn end of the PC. The nasal processes also become less fused in this same direction. These shape changes are shown in Fig. 6.
Fig. 5.
Plot of PC1 and PC2 based on the Procrustes analysis of all four embryo groups.
Fig. 6.
Wireframes depicting the shape variation associated with the C57BL/6J and A/WySn ends of PC1, respectively.
The second greatest axis of variation (PC2), which represents 14.61% of the total variance, is also plotted in Fig. 5. This second PC distinguishes both of the F1 crosses from the A/WySn and C57BL/6J, but again, they do not differ from each other. The shape changes associated with this PC include lesser rotation of the maxillary prominences, more rotated and smaller nasal prominences and smaller nasal pits at the F1 cross portion of the distribution. Individually, the rest of the PCs do not account for more than 8% of the total variance and do not significantly differentiate any of the groups.
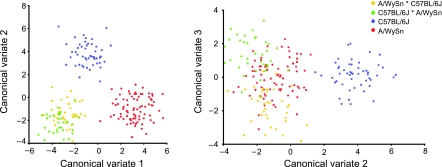
All three CVs make meaningful distinctions between the groups. A plot of CV1 and 2 shows a complete distinction between the C57BL/6J, A/WySn and both F1 groups (Fig. 7). CV1 differentiates C57BL/6J from A/WySn with both F1 crosses mixed together. Unlike PC1, however, the C57BL/6J strain plots in-between the F1 cross grouping and the A/WySn strain at either end. CV2 separates the C57BL/6J strain from both A/WySn and the F1 crosses which score similarly along this CV. The third and final CV is the only part of the entire analysis that made a distinction between the two different maternal groups of F1 crosses (Fig. 7).
Fig. 7.
Plots of both CV1 vs. CV2 and CV2 vs. CV3 based on the Procrustes analysis of the four embryo groups.
The shape change that separates the groups along CV1 largely involves the maxillary and nasal prominences, similar to the PCA results. At the F1 cross end of CV1, the maxillary prominences are less rotated and the head is larger and wider. At the A/WySn end, the nasal prominences are larger and the end points of the medial and lateral nasal processes are further apart.
The second CV shows how the shape of the C57BL/6J strain contrasts those of the A/WySn strain and the F1 crosses. The shape change at the C57BL/6J end of CV2 includes a more rotated maxillary prominence and the impression of a larger size overall. The nasal prominences not only seem larger, there is also less area between the medial nasal processes. The head of the C57BL/6J embryos also appears to be higher than the others and the nasal pits appear to be located more laterally.
The third CV illuminates a possible shape difference between the two maternal groups of F1 crosses. The AC group is characterized by smaller maxillary prominences and shorter head length. They also have larger nasal pits and their medial and lateral nasal processes are further apart. The CA group, in addition to larger overall nasal processes, has less area between the two medial nasal processes.
The gradient map representing the surface-to-surface Euclidean distances of the C57BL/6J and A/WySn whole volumes is illustrated in Fig. 8. This semi-automated method yielded comparable shape-change results to both PC1 and CV2, both of which separate the A/WySn and C57BL/6J strains. This analysis shows that the maxillary prominences of the C57BL/6J mean shape at this stage project further inward (are more rotated) than those of the A/WySn mean shape.
Fig. 8.
Colour scalar map of Euclidean surface–surface distances measured from the C57BL/6J mean embryo head to the A/WySn mean embryo head. The results are mapped onto the A/WySn mean embryo head image.
Discussion
Previous 2D analyses of A/WySn and C57BL/6J mice support our results of differences in craniofacial shape throughout ontogeny (Young et al. 2007). This 3D analysis strengthened that conclusion with new information concerning the possible involvement of morphological variation in the etiology of CL and, on a larger scale, the role developmental variation plays in dysmorphology.
The overall conclusion that A/WySn was less developed at the same tail-somite stage comes from three separate analyses: PCA, CVA and the semi-automated method. Specifically, the maxillary prominences were not as rotated and the medial nasal processes are closer to fusion in the C57BL/6J embryos.
The result that shape variation was highest in the A/WySn mice in the embryonic sample has implications for the ontogeny of such variation in both mutant and normal mice. In previous work on adult mice, A/WySn mice were found to have reduced integration of craniofacial shape variation but not increased shape variances (Hallgrímsson et al. 2004). The elevated shape variance in A/WySn embryos suggests incomplete penetrance or reduced canalization of craniofacial shape. Mutant phenotypes commonly have increased variation (Scharloo, 1964; Rendel, 1967; Hallgrímsson et al. 2002; Dworkin, 2005; Willmore et al. 2006). In A/WySnJ mice, this increased variance may not be retained at later ontogenetic stages because subsequent developmental events overwhelm this variation or simply because postnatal samples would not include those individuals who develop clefts as they die perinatally. However, we have suggested elsewhere that increased variance may play an etiologic role in some dysmorphologies, particularly those that are complex and show highly variable expressivity (Hallgrímsson et al. 2005). While the causes of increases or decreases in variance within a constant genotype are rarely known, nonlinear relationships between developmental determinants and phenotypic outcomes probably play a central role (Klingenberg & Nijhout, 1999; Hallgrímsson et al. 2006). A hypothesis in this case is that facial prominence outgrowth is reduced in A/WySn mice because of a perturbation to Wnt signaling caused by the hypomorphic mutation in Wnt9.At this reduced level, facial prominence outgrowth, probably via mesenchymal proliferation, is more sensitive to Wntsignaling than in the wild type where this pathway is sufficiently active to ensure a normal rate of growth. Such a result would be produced if facial prominence outgrowth has a nonlinear dependency on Wnt9b expression or canonical Wntsignalling, which could be tested experimentally.
Although the function of Wnt9b is not well understood, a role in regulating cell proliferation is plausible. Wnts are glycoproteins that act as signaling factors and are expressed in extracellular matrix. In mice, Wnts are expressed in the midfacial mesenchyme within the facial prominences (Lan et al. 2006; Brugmann et al. 2007). Canonical Wnt signaling is co-localized with Nmyc (Brugmann et al. 2007), which is a known target of Wnts and also a known regulator of cell proliferation, acting downstream of Sonic hedgehog (Kenney et al. 2003; Hatton et al. 2006).
The shape differences, which held through adulthood, are contrary to previous studies. The skulls of C57BL/6J are longer than A/WySn (Wang et al. 1995; Hallgrímsson et al. 2004). This finding is consistent with deficient growth of the maxillary prominence in A/WySn embryos. In contrast, our analysis showed that these mice have shorter faces and a higher cranium. However we found a wider anterior portion of the zygomatic arch in A/WySn mice, consistent with earlier conclusions in these same studies. A wider facial shape is also associated with increased risk of CL within human populations (Yoon et al. 2004).
Previous work found evidence for a maternal effect for cleft lip liability in these mice (Juriloff & Fraser, 1980; Juriloff, 1982). Ciriani & Diewert (1986) also found a significant difference between AC and CA and determined that the A/WySn maternal environment retards the growth and development of progeny. Based on this, we expected to find a maternal effect for craniofacial shape. However, in this study the only evidence for a maternal affect in the F1 crosses was CV3, which accounts for only a minor proportion of the variation in the sample. The mean shapes, after correction for tail-somite stage, did not differ between the maternal groups. Variance in shape also did not differ between these groups. This result indicates that although the mechanism by which maternal factors contribute to cleft lip liability is not known; it is certainly not reflected in craniofacial shape. It should be noted that the design of our study does not distinguish between maternal effects due to maternal inheritance and effects due to genomic imprinting.
A limitation of the methods used here for morphometrics of embryos was that fixation introduced unquantifiable alterations in size. Fixation artifact is unquantifiable in this case because all available methods that one could use to measure embryos involve distortion of some kind. Bouin's fixation, which is used here because it renders the tissue sufficiently rigid to stand up to scanning without deformation, is known to produce shape and size distortion but the degree of distortion is less than seen in many other methods (Denef et al. 1979). Morphometric analyses have also been performed on Bouin's fixed mouse embryos in previous studies (Wang & Diewert, 1992). Two factors mitigate this problem. Firstly, fixation artifact is extremely unlikely to vary systematically with genotype, although it is theoretically possible for differences in extracellular matrix properties to produce a genotype-specific fixation artifact. Secondly, our analysis was confined to a fairly limited developmental period during which the tissue composition of the structures involved does not change dramatically. We are fairly confident that any fixation artifact was extremely unlikely to have influenced the overall results.
A second caveat is that this study involves a comparison of two distantly related inbred strains. These strains have been compared extensively in the literature (Diewert & Lozanoff, 2002), but C57BL/6J mice differ from A/WySn mice in ways other than the clf mutations, which also involve craniofacial shape. In particular, C57BL/6J may have unusually robust primary palate formation (Wang & Diewert, 1992). Future studies will focus on comparisons of the clf loci with genetic background controlled. A related caveat for the comparisons of the embryo samples is that the C57BL/6J mice were entirely reared in Calgary whereas the majority of the A/WySn embryonic sample were reared in Vancouver. Although this is unlikely to have had major effects, this factor will also be eliminated in future studies.
The A/WySn strain as a model for CL is an illustrative example of how genetically similar individuals vary in the expression of a specific trait. Mutations and environmental effects have the potential to alter not only the mean phenotype, but the variance around that mean (Waddington, 1942, 1957; Scharloo, 1991; Gibson & Wagner, 2000; Dworkin et al. 2003; Milton et al. 2006). The increased facial shape variance provides evidence for this being true of the clf mutations in A/WySn. It is therefore misleading to refer to the genetic mutations clf1 and clf2 as causing CL. Rather, these mutations affect the physical interaction between developing components as size, maturity and position of one morphological element relative to others are necessary for proper development (Hall, 1992). The genetic factors that predispose A/WySn mice to CL/P create a developmental configuration in which the mean phenotype is shifted towards the CL/P threshold because the midface is hypoplastic and delayed and some individuals are pushed over the threshold for CL/P formation because growth of the face is also more variable during palate formation.
This study helps to elucidate the specific developmental processes that affect the morphological development of the primary palate and the possible perturbations that lead to dysmorphology. In evolutionary terms, the quantitative analysis of how genetic perturbations produce phenotypic variation in the growth of the facial prominences will help identify developmental processes of importance for the evolution of craniofacial morphology. For the study of cleft lip, a quantitative understanding of how developmental perturbations influence embryonic craniofacial growth and the formation of the CL/P dysmorphology is a prerequisite for early diagnosis of cleft lip in human embryos based on morphometric criteria. Such diagnoses may eventually become the basis for therapeutic strategies for in utero prevention of cleft lip in humans.
Acknowledgments
This work was supported by grants from the National Science and Engineering Research Council, Genome Canada and Genome Alberta (to BH), Canadian Institutes of Health Research (to SB and BH), Canadian Foundation for Innovation and Alberta Innovation and Science (to BH) and the Alberta Heritage Fund (to BH and SB).
References
- Bornstein S, Trasler DG, Fraser FC. Effect of the uterine environment on the frequency of spontaneous cleft lip in CL/FR mice. Teratology. 1970;3:295–298. doi: 10.1002/tera.1420030403. [DOI] [PubMed] [Google Scholar]
- Boyd S, Moser S, Kuhn M, Klinck J, Krauze PL, et al. Evaluation of three-dimensional registration methodologies for in-vivo microcomputed tomography. Ann Biomed Eng. 2006;34:1587–1599. doi: 10.1007/s10439-006-9168-7. [DOI] [PubMed] [Google Scholar]
- Brugmann SA, Goodnough LH, Gregorieff A, Leucht P, ten Berge D, Fuerer C, et al. Wnt signaling mediates regional specification in the vertebrate face. Development. 2007;134:3283–3295. doi: 10.1242/dev.005132. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–292. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Chung CS, Mi MP, Beechert AM. Genetic epidemiology of cleft lip with or without cleft palate in the population of Hawaii. Genet Epidemiol. 1987;4:415–423. doi: 10.1002/gepi.1370040603. [DOI] [PubMed] [Google Scholar]
- Ciriani D, Diewert VM. A comparative study of development during primary palate formation in A/WySn, C57BL/6, and their F1 crosses. J Craniofac Genet Dev Biol. 1986;6:369–377. [PubMed] [Google Scholar]
- Davidson JG, Fraser FC, Schlager G. A maternal effect on the frequency of spontaneous cleft lip in the A-J mouse. Teratology. 1969;2:371–376. doi: 10.1002/tera.1420020411. [DOI] [PubMed] [Google Scholar]
- Denef JF, Cordier AC, Mesquita M, Haumont S. The influence of fixation procedure, embedding medium and section thickness on morphometric data in thyroid gland. Histochemistry. 1979;63:163–171. doi: 10.1007/BF00644538. [DOI] [PubMed] [Google Scholar]
- Diewert VM, Lozanoff S. Growth and morphogenesis of the human embryonic midface during primary palate formation analyzed in frontal sections. J Craniofac Genet Dev Biol. 1993;13:162–183. [PubMed] [Google Scholar]
- Diewert VM, Lozanoff S. Animal models of facial clefting: experimental, congenital, and transgenic. In: Mooney MM, Siegel MI, editors. Understanding Craniofacial Anomalies: Etiopathogenesis of Craniosynostoses and Facial Clefting. New York: Wiley-Liss; 2002. pp. 251–272. [Google Scholar]
- Diewert VM, Shiota K. Morphological observations in normal primary palate and cleft lip embryos in the Kyoto collection. Teratology. 1990;41:663–677. doi: 10.1002/tera.1420410603. [DOI] [PubMed] [Google Scholar]
- Diewert VM, Wang KY. Recent advances in primary palate and midface morphogenesis research. Crit Rev Oral Biol Med. 1992;4:111–130. doi: 10.1177/10454411920040010201. [DOI] [PubMed] [Google Scholar]
- Dworkin I. Evidence for canalization of Distal-less function in the leg of Drosophila melanogaster. Evol Dev. 2005;7:89–100. doi: 10.1111/j.1525-142X.2005.05010.x. [DOI] [PubMed] [Google Scholar]
- Dworkin I, Palsson A, Birdsall K, Gibson G. Evidence that Egfr contributes to cryptic genetic variation for photoreceptor determination in natural populations of Drosophila melanogaster. Curr Biol. 2003;13:1888–1893. doi: 10.1016/j.cub.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Francis-West PH, Robson L, Evans DJ. Craniofacial development: the tissue and molecular interactions that control development of the head. Adv Anat Embryol Cell Biol. 2003;169:III–VI. 1–138. doi: 10.1007/978-3-642-55570-1. [DOI] [PubMed] [Google Scholar]
- Fraser FC. The genetics of cleft lip and cleft palate. Am J Hum Genet. 1970;22:336–352. [PMC free article] [PubMed] [Google Scholar]
- Fraser FC, Pashayan H. Relation of face shape to susceptibility to congenital cleft lip. A preliminary report. J Med Genet. 1970;7:112–117. doi: 10.1136/jmg.7.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G, Wagner G. Canalization in evolutionary genetics: a stabilizing theory? Bioessays. 2000;22:372–380. doi: 10.1002/(SICI)1521-1878(200004)22:4<372::AID-BIES7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Hall B. Evolutionary and Developmental Biology. New York: Chapman & Hall; 1992. [Google Scholar]
- Hallgrímsson B, Willmore K, Hall BK. Canalization, developmental stability, and morphological integration in primate limbs. Yearbook Phys Anthropol. 2002;45:131–158. doi: 10.1002/ajpa.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgrímsson B, Dorval CJ, Zelditch ML, German RZ. Craniofacial variability and morphological integration in mice susceptible to cleft lip and palate. J Anat. 2004;205:501–517. doi: 10.1111/j.0021-8782.2004.00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgrímsson B, Brown J, Hall BK. The study of phenotypic variability: an emerging research agenda for understanding the developmental-genetic architecture underlying phenotypic variation. In: Hallgrimsson B, Hal BKL, editors. Variation: A Central Concept in Biology. New York: Elsevier Academic Press; 2005. pp. 525–551. [Google Scholar]
- Hallgrímsson B, Brown JJY, Ford-Hutchinson AF, Sheets HD, Zelditch ML, Jirik FR. The brachymorph mouse and the developmental-genetic basis for canalization and morphological integration. Evol Dev. 2006;8:61–73. doi: 10.1111/j.1525-142X.2006.05075.x. [DOI] [PubMed] [Google Scholar]
- Hatton BA, Knoepfler PS, Kenney AM, Rowitch DH, de Alboran IM, Olson JM, et al. N-myc is an essential downstream effector of Shh signaling during both normal and neoplastic cerebellar growth. Cancer Res. 2006;66:8655–8661. doi: 10.1158/0008-5472.CAN-06-1621. [DOI] [PubMed] [Google Scholar]
- Jara L, Blanco R, Chiffelle I, Palomino H, Carreno H. Association between alleles of the transforming growth factor alpha locus and cleft lip and palate in the Chilean population. Am J Med Genet. 1995;57:548–551. doi: 10.1002/ajmg.1320570406. [DOI] [PubMed] [Google Scholar]
- Juriloff DM. Differences in frequency of cleft lip among the A strains of mice. Teratology. 1982;25:361–368. doi: 10.1002/tera.1420250313. [DOI] [PubMed] [Google Scholar]
- Juriloff DM, Fraser FC. Genetic maternal effects on cleft lip frequency in A/J and CL/Fr mice. Teratology. 1980;21:167–175. doi: 10.1002/tera.1420210206. [DOI] [PubMed] [Google Scholar]
- Juriloff DM, Harris MJ, Dewell SL. A digenic cause of cleft lip in A-strain mice and definition of candidate genes for the two loci. Birth Defects Res Part A Clin Mol Teratol. 2004;70:509–518. doi: 10.1002/bdra.20041. [DOI] [PubMed] [Google Scholar]
- Juriloff DM, Harris MJ, Dewell SL, Brown CJ, Mager DL, Gagnier L, et al. Investigations of the genomic region that contains the clf1 mutation, a causal gene in multifactorial cleft lip and palate in mice. Birth Defects Res A Clin Mol Teratol. 2005;73:103–113. doi: 10.1002/bdra.20106. [DOI] [PubMed] [Google Scholar]
- Juriloff DM, Harris MJ, McMahon AP, Carroll TJ, Lidral AC. Wnt9b is the mutated gene involved in multifacial nonsyndromic cleft lip with or without cleft palate in A/WySn mice, as confirmed by a genetic complementation test. Birth Defects Res A Clin Mol Teratol. 2006;76:574–579. doi: 10.1002/bdra.20302. [DOI] [PubMed] [Google Scholar]
- Kenney AM, Cole MD, Rowitch DH. Nmyc upregulation by sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors. Development. 2003;130:15–28. doi: 10.1242/dev.00182. [DOI] [PubMed] [Google Scholar]
- Khan SN, Hufnagle KG, Pool R. Intrafamilial variability of popliteal pterygium syndrome: a family description. Cleft Palate J. 1986;23:233–236. [PubMed] [Google Scholar]
- Khoury MJ, Erickson JD, James LM. Maternal factors in cleft lip with or without palate: evidence from interracial crosses in the United States. Teratology. 1983;27:351–357. doi: 10.1002/tera.1420270309. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP, Nijhout HF. Genetics of fluctuating asymmetry: a developmental model of developmental instability. Evolution. 1999;53:358–375. doi: 10.1111/j.1558-5646.1999.tb03772.x. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP. MorphoJ. Manchester, UK: 2006. [Google Scholar]
- Lacombe D, Pedespan JM, Fontan D, Chateil JF, Verloes A. Phenotypic variability in van der Woude syndrome. Genet Couns. 1995;6:221–226. [PubMed] [Google Scholar]
- Lan Y, Ryan RC, Zhang Z, Bullard SA, Bush JO, Maltby KM, et al. Expression of Wnt9b and activation of canonical Wnt signaling during midfacial morphogenesis in mice. Dev Dyn. 2006;235:1448–1454. doi: 10.1002/dvdy.20723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton CC, Ulane CM, Rutherford S. Control of canalization and evolvability by hsp90. PLoS ONE. 2006;1:e75. doi: 10.1371/journal.pone.0000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JC, Schutte BC. Cleft palate: players, pathways, and pursuits. J Clin Invest. 2004;113:1676–1678. doi: 10.1172/JCI22154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Higgins P, Jones N. Tools for statistical analysis. Hull York Medical School, UK; 2006. [Google Scholar]
- Peanchitlertkajorn S, Cooper ME, Liu YE, Field LL, Marazita ML. Chromosome 17: gene mapping studies of cleft lip with or without cleft palate in Chinese families. Cleft Palate Craniofac J. 2003;40:71–79. doi: 10.1597/1545-1569_2003_040_0071_cgmsoc_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Pezzetti F, Scapoli L, Martinelli M, Carinci F, Bodo M, Carinci P, et al. A locus in 2p13-p14 (OFC2), in addition to that mapped in 6p23, is involved in nonsyndromic familial orofacial cleft malformation. Genomics. 1998;50:299–305. doi: 10.1006/geno.1998.5273. [DOI] [PubMed] [Google Scholar]
- Qian J, Jiang Z, Li M, Heaphy P, Liu YH, Shackleford GM. Mouse Wnt9b transforming activity, tissue-specific expression, and evolution. Genomics. 2003;81:34–46. doi: 10.1016/s0888-7543(02)00012-5. [DOI] [PubMed] [Google Scholar]
- Reed SC. Harelip in the house mouse. I. Effects of the external and internal environments. Genetics. 1936;21:339–360. doi: 10.1093/genetics/21.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendel JM. Canalization and Gene Control. London: Logos Press; 1967. [Google Scholar]
- Robertson CP, Braun MM, Roelink H. Sonic hedgehog patterning in chick neural plate is antagonized by a Wnt3-like signal. Dev Dyn. 2004;229:510–519. doi: 10.1002/dvdy.10501. [DOI] [PubMed] [Google Scholar]
- Scharloo W. Mutant expression and canalization. Nature. 1964;203:1095–1096. doi: 10.1038/2031095b0. [DOI] [PubMed] [Google Scholar]
- Scharloo W. Canalization: genetic and developmental aspects. Annu Rev Ecol Syst. 1991;22:65–93. [Google Scholar]
- Sheets D. IMP Suite (Integrated Morphometrics Package) Buffalo, NY: 2004a. Simple3D. [Google Scholar]
- Sheets D. IMP Suite (Integrated Morphometrics Package) Buffalo, NY: 2004b. ThreeDStand6. [Google Scholar]
- Slice D. Morpheus. Stony Brook, NY: 1994–1999. [Google Scholar]
- Sözen MA, Suzuki K, Tolarova MM, Bustos T, Fernandez Iglesias JE, Spritz RA. Mutation of PVRL1 is associated with sporadic, non-syndromic cleft lip/palate in northern Venezuela. Nat Genet. 2001;29:141–142. doi: 10.1038/ng740. [DOI] [PubMed] [Google Scholar]
- Thyagarajan T, Totey S, Danton MJ, Kulkarni AB. Genetically altered mouse models: the good, the bad, and the ugly. Crit Rev Oral Biol Med. 2003;14:154–174. doi: 10.1177/154411130301400302. [DOI] [PubMed] [Google Scholar]
- Trasler DG. Pathogenesis of cleft lip and its relation to embryonic face shape in A-J and C57BL mice. Teratology. 1968;1:33–49. doi: 10.1002/tera.1420010106. [DOI] [PubMed] [Google Scholar]
- Waddington CH. The canalisation of development and the inheritance of acquired characters. Nature. 1942;150:563. [Google Scholar]
- Waddington CH. The Strategy of the Genes. New York: MacMillan Company; 1957. [Google Scholar]
- Wang K-Y, Diewert VM. A morphometric analysis of craniofacial growth in cleft lip and noncleft mice. J Craniofac Genet Dev Biol. 1992;12:141–154. [PubMed] [Google Scholar]
- Wang KY, Juriloff DM, Diewert VM. Deficient and delayed primary palatal fusion and mesenchymal bridge formation in cleft lip-liable strains of mice. J Craniofac Genet Dev Biol. 1995;15:99–116. [PubMed] [Google Scholar]
- Willmore KE, Zelditch ML, Young N, Ah-Seng A, Lozanoff S, Hallgrimsson B. Canalization and developmental stability in the brachyrrhine mouse. J Anat. 2006;208:361–372. doi: 10.1111/j.1469-7580.2006.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Yoon YJ, Perkiomaki MR, Tallents RH, Barillas I, Herrera-Guido R, Fong CT, et al. Transverse craniofacial features and their genetic predisposition in families with nonsyndromic unilateral cleft lip and palate. Cleft Palate Craniofac J. 2004;41:256–261. doi: 10.1597/02-134.1. [DOI] [PubMed] [Google Scholar]
- Young NM, Wat S, Diewert VM, Browder LW, Hallgrimsson B. Comparative morphometrics of embryonic facial morphogenesis: implications for cleft-lip etiology. Anat Rec (Hoboken) 2007;290:123–139. doi: 10.1002/ar.20415. [DOI] [PubMed] [Google Scholar]