Abstract
The purpose of the current review is to highlight the structure-function relationship of tendons and related structures to provide an overview for readers whose interest in tendons needs to be underpinned by anatomy. Because of the availability of several recent reviews on tendon development and entheses, the focus of the current work is primarily directed towards what can best be described as the ‘tendon proper’ or the ‘mid-substance’ of tendons. The review covers all levels of tendon structure from the molecular to the gross and deals both with the extracellular matrix and with tendon cells. The latter are often called ‘tenocytes’ and are increasingly recognized as a defined cell population that is functionally and phenotypically distinct from other fibroblast-like cells. This is illustrated by their response to different types of mechanical stress. However, it is not only tendon cells, but tendons as a whole that exhibit distinct structure-function relationships geared to the changing mechanical stresses to which they are subject. This aspect of tendon biology is considered in some detail. Attention is briefly directed to the blood and nerve supply of tendons, for this is an important issue that relates to the intrinsic healing capacity of tendons. Structures closely related to tendons (joint capsules, tendon sheaths, pulleys, retinacula, fat pads and bursae) are also covered and the concept of a ‘supertendon’ is introduced to describe a collection of tendons in which the function of the whole complex exceeds that of its individual members. Finally, attention is drawn to the important relationship between tendons and fascia, highlighted by Wood Jones in his concept of an ‘ectoskeleton’ over half a century ago – work that is often forgotten today.
Keywords: aponeuroses, bursae, fascia, retinacula, tendon sheaths, tenocytes
Introduction
Tendons generally connect muscles to bone, though occasional ‘intermediate tendons’ connect one muscle belly to another. They can also extend into muscles as ‘intramuscular tendons’ (Fig. 1) and this allows muscle fibres to have a pennate arrangement (Standring, 2004). Pennation depends upon a connection between the perimysium and the intramuscular parts of the tendon, rather than on a direct connection between the tendon and the muscle fibre itself. Thus, it is the collagen network of the perimysium that forms the basis for the mechanical link between tendon and muscle fibres and this is promoted by specialized ‘perimysial junctional plates’ (Passerieux et al. 2006, 2007). Although tendons are fundamentally concerned with transmitting tensile forces generated by muscle cells, they may also be subject to compression and shear as they pass around bony or fibrous pulleys. Like other mechanically loaded tissues, they are dominated by extracellular matrix (ECM) and in tendons, the ECM is largely that of a dense fibrous connective tissue (Fig. 2).
Fig. 1.
A sagittal section through the knee joint showing the presence of intramuscular tendons (arrows) within the muscle belly of the gastrocnemius (G) and hamstring (H) muscles. F, femur; QT, quadriceps tendon; P, patella; PT, patellar tendon; T, tibia.
Fig. 2.
(a) A low power, longitudinal section through the limb tendon of a young calf in a section stained with Haematoxylin and Eosin (H & E). The tenocytes (TC) are typically arranged in longitudinal rows between parallel bundles of collagen fibres (CF) and are only recognizable in such routine sections by their darkly staining nuclei (i.e. the cytoplasm is not visible). Note the waviness (crimp) of the collagen. (b) A low power transverse section through the limb tendon of a young calf stained with H & E. Note that the collagen fibres are grouped into fascicles (FA) separated by endotenon (E). The tenocytes are recognizable within the fascicles by their nuclei (arrows).
Flattened tendons of any type are called aponeuroses. They may be present as separate structures emerging from muscle bellies (e.g. the tendons of latissimus dorsi and pectoralis major) or form fibrous sheets on the surface of a muscle or within it (e.g. soleus, vastus intermedius and gluteus minimus; Fig. 3a). It should be noted that some tendons, which may be rounded or oval proximally, might become more flattened, aponeurotic and ‘fascial’ as they approach their attachment sites (Fig. 3b). As Wood Jones (1944b) points out, where a muscle belly has an aponeurotic covering, this suggests that some more superficial muscle moves over it (e.g. gastrocnemius moving over soleus). Interestingly, Finni et al. (2003) have shown that the strain within the tendon of soleus differs in the aponeurotic part of the tendon compared with the rest. They suggest that this is associated with the pattern of force transmission via intramuscular connective tissue. Indeed, non-homogeneous strains can occur within the aponeurosis of soleus and this could reflect a compartmentalized recruitment of muscle fibres for a sub-maximal contraction (Finni et al. 2003).
Fig. 3.
(a) The aponeurotic tendon (arrows) of gluteus minimus emerging from the surface of the muscle and attaching to the greater trochanter (GT) of the femur. I, ilium. (b) The pes anserinus tendon complex attaching to the tibia (T). Note the aponeurotic character of the distal part of the tendons (arrows). G, gastrocnemius; S, sartorius.
Tendons are not usually calcified – though calcification is common pathologically. They may, however, contain bony nodules in healthy individuals and such sesamoid bones are particularly common in the foot (Dennis & McKinney, 1990). Exceptions to the non-calcified character of tendons include avian tendons (Landis & Silver, 2002) and the deep part of fibrocartilaginous tendon attachment sites in man (Benjamin et al. 2002, 2006). Tendon and ligament mineralization is normally prevented because their cells produce a transcription factor (Msx2) that is down regulated when ossification occurs (Yoshizawa et al. 2004).
Although considerable attention has been directed towards structure-function relationships in cartilage and bone, tendons have not attracted a comparable level of interest. However, the increasing importance of tissue engineering and stem cell biology in biomedical science has raised interest in creating artificial tendons or in using mesenchymal stem cells to promote repair (Zhang & Chang, 2003; Smith & Webbon, 2005). Such work needs to be set against a sound understanding of the normal functional morphology of tendons. Thus, the purpose of the current review is to provide an anatomical foundation for those engaged in tendon research, but whose focus and/or expertise is more molecular than morphological. Because of the availability of several recent reviews on tendon entheses (i.e. attachment sites) and tendon development (Benjamin & Ralphs, 2000; Benjamin & McGonagle, 2001; Benjamin et al. 2002, 2006; Tozer & Duprez, 2005; Hoffmann & Gross, 2006; Shaw & Benjamin, 2007), the present article is principally directed towards what can best be described as the ‘tendon proper’ (the ‘mid-substance’ of tendons) together with structures such as bursae, retinacula and fat pads that are associated with tendons. To keep the size of the review within manageable bounds, myotendinous junctions are excluded and only tendons in the limbs are considered.
Tendon structure
Tendons come in various shapes and sizes. Some have shallow groves on their surface and others are divided into slips (e.g. the tendon of obturator internus). The largest tendon is the Achilles and its shape varies from proximal to distal as it approaches its calcaneal attachment site. As a general rule, extensor tendons are more flattened than flexor tendons – which tend to be round or oval (Fig. 4a,b). The flattened, aponeurotic character of extensor tendons in the hand, relates to the convex joint surfaces that are created at the metacarpophalangeal and interphalangeal joints when flexing the fingers. Flattening reduces the risk of subluxation – along with other adaptations such as fibrous interconnections between adjacent tendons and the formation of an extensor hood (Fig. 4b, inset). The longest tendons are those of the hands and feet. Here, the tendons serve not only to transmit muscle contraction to the skeleton, but also to modulate the speed at which the distal elements can move. They do this by a strategic location of their attachment sites nearer or farther away from the axis of movement (the point through which the axis passes is referred to in the language of biomechanics as the ‘centre of rotation’). That bulky muscles always give rise to tendons before the end of a limb is reached, ensures that the most distal segment (i.e. the hand or foot) is not handicapped in its function by its ponderous weight.
Fig. 4.
The gross anatomy of tendons in the hand. (a) The flexor digitorum superficialis tendons (FT) emerging from beneath the flexor retinaculum (FR) to enter the palm of the hand. Note their rounded character and the shallow grooves that are occasionally evident on their surface (arrows). L, lumbricals. (b) The web of extensor tendons (ET) on the dorsum of the hand collectively form a ‘supertendon’ complex in which the individual components are interconnected by films of connective tissue (CT) and obliquely-orientated juncturae tendinum (JT). Note the extensor hood (EH) over the metacarpophalangeal joints.
It is typical of the attachment of many tendons in the limbs that adjacent bony surfaces are used as pulleys (Fig. 5a,b). This was recognized by Kapandji (1982a,b) who provided excellent illustrations to show how the bone adjacent to the attachment site makes the moment arm of the Achilles and triceps brachii tendons more effective at different positions of the ankle or elbow joint. Thus, he shows how the triceps tendon ‘uncoils’ from the olecranon as the elbow is moved from flexion to extension and how the Achilles tendon uses the superior tuberosity of the calcaneus as a pulley to minimize the change in the tendon moment arm as the foot moves between dorsi- and plantar flexion.
Fig. 5.
(a) A sagittal section of the attachment of the Achilles tendon (AT) to the calcaneus (C), showing the relation of it to the superior tuberosity (ST) that acts as a tendon pulley during dorsiflexion. Note also the presence of Kager's fat pad (KP) filling the space between the Achilles tendon and flexor hallucis longus (FHL). It contains numerous blood vessels (arrows), some of which enter the deep surface of the Achilles tendon to supply it. (b) A sagittal section of a toe that is hyperextended at the metatarsophalangeal joint (MTJ) and flexed at both interphalangeal joints (IPJ). Note how the head of the metatarsal acts as a pulley not only for the plantar fascia in maintaining the medial longitudinal arch of the foot, but also for the flexor tendons (FT) when the phalanges are dorsiflexed at the MTJ.
Extracellular matrix
The principal molecules acting as structural components of tendons are well documented and the reader is referred elsewhere for a more detailed account (Kjaer, 2004). Briefly, tendons largely consist of collagens and proteoglycans and are dominated by the fibril-forming, type I collagen. However, other collagens (e.g. II, III, V, VI, IX, XI) are also present (Fukuta et al. 1998; Ottani et al. 2002; Kjaer, 2004). Proteoglycans are primarily responsible for the viscoelastic behaviour of tendons, but do not make any major contribution to their tensile strength (Puxkandl et al. 2002; Robinson et al. 2004). The principal role of the collagen fibres is to resist tension, although they still allow for a certain degree of compliance (i.e. reversible longitudinal deformation). Such apparently conflicting demands are probably resolved because of the hierarchical architecture of tendons. Thus, collagen molecules consist of polypeptide chains and three such chains combine together to form a densely packed, helical tropocollagen molecule. In turn, five tropocollagens constitute a microfibril, and microfibrils aggregate together to form fibrils. Fibrils are then grouped into fibres, fibres into fibre bundles and fibre bundles into fascicles. Some of the larger collections of fascicles are visible in gross dissections (Fig. 4a). During early development, the fibrils are small and of uniform diameter, but from adolescence onwards they become progressively larger and more variable in size (Strocchi et al. 1991). Aging causes a decrease in mean collagen fibril diameter – possibly regulated by type V collagen. The size shift may be related to the reduced mechanical strength of older tendons (Dressler et al. 2002). The greatest mean fibril diameters of tendons in man are reported to occur between 20 and 29 years of age and average diameters then decrease with increasing age (Sargon et al. 2005). It is of interest to note that the size of collagen fibrils can be reduced as a result of injury – e.g. in the tissue adjacent to ruptured human Achilles tendons (Magnusson et al. 2002).
At various levels of tendon organization, including the whole tendon, fascicles and fibrils, a helical architecture (often with superimposed ‘crimp’, i.e. a zig-zag undulation of collagen fibrils) occurs in certain tendons (Fig. 2a; Yahia & Drouin, 1989; van Gils et al. 1996; Roukis et al. 1996). This helical organization of tendon components makes them comparable to man-made ropes (Bozec et al. 2007) and the presence of crimp contributes to their inherent flexibility (Ker, 2002). Roukis et al. (1996) have suggested that the twisting that characterizes the tendon of tibialis posterior reduces the need for longitudinal slippage between fascicles during triplanar movements of the foot. The angle of torsion of the inner fibrils in a helical tendon fascicle may be less oblique than that of the outer fibrils and this may give the tendon regionally distinct compliance (Yahia & Drouin, 1989). It is of interest that some tendons are reported to show fascicular convergence towards their bony attachments (Fallon et al. 2002). This allows numerous muscle fibres to concentrate their action at a relatively small attachment site.
Sliding between and within fascicles
One of the important features in tendons is the ability of their fascicles to slide independently against each other. This allows them to transmit tension despite the changing angles of a joint as it moves (Fallon et al. 2002) and allows tendons to change shape as their muscles contract. To facilitate the sliding movement and to create a conduit for blood vessels, a thin film of loose connective tissue (‘endotenon’) is present between fascicles and/or fibre bundles (Fig. 2b; Kastelic et al. 1978; Fallon et al. 2002). This role of the endotenon is in line with a general function of loose (areolar) connective tissue elsewhere in the body, promoting movement between adjacent structures, as for example between the skin on the dorsum of the hand and the underlying tissues. The endotenon is continuous with a further sheet of connective tissue (epitenon) that surrounds the tendon as a whole. In addition, some tendons have a ‘paratenon’ that is separate from the tendon itself, but nevertheless surrounds it. It is also known as a false tendon sheath and the best example is that around the Achilles tendon. The reader should note, however, that there is great variation and/or confusion in the use of several of these terms by different authors and thus one cannot always be certain of the particular structure to which an author is referring. For example, some authors may describe a structure outside, but still related to a tendon, as being a ‘peritendon’ (or ‘peritenon’), rather than a ‘paratenon’. In our view, a ‘paratenon’ is a sheath that is quite distinct from the tendon itself. However, occasionally a peritenon is viewed as part of a paratenon or vice versa. Equally, there is confusion associated with the hierarchical character of tendons (see above) – in particular with the co-existence of the terms ‘fibre bundle’ (primary, secondary or tertiary) and ‘fascicle’. A fascicle is a bundle of fibres!
Sliding within tendons is not limited to sliding between fascicles, but also occurs between fibrils and this may account for up to 50% of the longitudinal deformation (i.e. strain) of a tendon (Screen et al. 2004). Any sliding of fibrils or fascicles relative to each other must occur within the proteoglycan-rich matrix surrounding them (Puxkandl et al. 2002). It is thus intriguing that lubricin, a molecule often associated with joint lubrication, is also present between the fascicles of certain tendons (Sun et al. 2006a). Sliding is most pronounced in the non fibrocartilaginous parts of a tendon, and the extent to which it may also occur within the basketweave complex of collagen fibres characteristic of wrap-around tendons (Benjamin & Ralphs, 1998) is unknown.
Tendon cells
The characteristic cell in tendons responsible for the secretion of the ECM, and thus collagen assembly and turnover, is the tenocyte. These cells are a specialized set of fibroblasts that are typically arranged in longitudinal rows, in close proximity to the collagen fibrils (Fig. 2a). During development, they form a hierarchy of extracellular compartments that are associated with fibrils and fibre bundles (Birk & Zycband, 1994). With increasing age, the cells flatten and become less numerous and their long, thin cytoplasmic projections shorten and diminish in number (Strocchi et al. 1991). Mature tendon cells thus have a complex system of sheet-like and finger-like processes that facilitate intercellular communication via gap junctions in a way that is comparable to the communication between osteocytes in bone (McNeilly et al. 1996). In addition, however, a further population of fibroblasts is found in the endotenon and epitenon, with cells in the former corresponding to the ‘internal fibroblasts’ of Banes et al. (1988).
Although there is no unique marker that selectively distinguishes tenocytes at all stages of development, a number of molecules have been considered as markers. Thus, the transcription factor scleraxis has been used to identify tendon or ligament cells at all stages of their development (Schweitzer et al. 2001), even though scleraxis is also necessary for the development of other mesodermal tissues (Brown et al. 1999). A second marker candidate is tenomodulin – a molecule whose expression is induced by scleraxis (Shukunami et al. 2006; Murchison et al. 2007). It regulates tenocyte proliferation and plays a role in the maturation of collagen fibrils (Docheva et al. 2005). Finally, there is tenascin-C. This is expressed by tenocytes in response to mechanical stress, but again is not specific for tendons alone, for it is also present in bone, smooth muscle and healing fibroblasts (Chiquet-Ehrismann & Tucker, 2004).
Response of tendon cells to mechanical load
There is now considerable evidence to suggest that tendons and tendon cells can respond to altered mechanical load and the reader is referred to Buchanan & Marsh (2002) and Kjaer (2004) for more exhaustive treatments. In man, collagen synthesis in the patellar tendon increases by nearly 100% as a result of just a single bout of acute exercise, and the effect is still evident 3 days later (Miller et al. 2005). It is particularly interesting to note that there may be an initial period in the training programme of an athlete where collagen turnover in tendons (i.e. the balance between synthesis and degradation) is actually increased and thus there is a net loss of collagen (Langberg et al. 1999, 2001). The authors suggest that this could enable a tendon to restructure and adapt to the increased loading pattern. They point out that it is not until training progresses that there is a net gain in collagen synthesis.
The mean fibril diameter of tendons, the diameter distribution, the fibril cross-sectional area and the number of fibrils all change in young mice exercised on a treadmill (Michna, 1984; Michna & Hartmann, 1989). Initially, the mean diameter of the fibrils increases (after 1 week of exercise), but later (between weeks 3–7) falls to a value less than the controls. As far as we are aware, a longer lasting increase in fibril diameter as a result of mechanical stimuli has only been shown thus far in skin collagen (Sanders & Goldstein, 2001) and this occurred in relation to an increased compressive or shear stress, rather than tensile stress. It should also be noted that stress shielding increases the number of small collagen fibrils in the patellar tendon (Majima et al. 2003).
At a cellular level, there seems to be no difference in the response of tenocytes to mechanical load between cells that have been extracted from different tendons, e.g. those associated with antagonistic muscles (Evans & Trail, 2001). However, in a given tendon, different stress patterns provoke different cellular reactions depending on the amount and duration of the tensional stress applied. Cell proliferation, for example, is stimulated by short periods of repetitive tension, but inhibited by more extended periods (Barkhausen et al. 2003).
One of the best lines of evidence that tenocytes can modulate their activity according to changing mechanical load comes from the observation that tendon cells in vitro can upregulate collagen synthesis when subjected to tensional forces. The response seems to depend on gap junctional communication between neighbouring cells, for when gap junctions are blocked, the cells no longer increase collagen synthesis in response to stretching forces applied in vitro (Waggett et al. 2006). The modulation of ECM synthesis involves two types of gap junctions – those characterized by the presence of connexin 32 and those containing connexin 43. The former junctions stimulate and the latter inhibit collagen synthesis (Waggett et al. 2006). It is important to note that junctions expressing both connexins link tenocytes within the same longitudinal row, but lateral connections between cells in adjacent rows only involve gap junctions containing connexin 43 (Waggett et al. 2006). In other words, stimulatory connexin 32-containing junctions are arranged along the line of principal tensile stress in tendons, whereas inhibitory connexin 43-containing junctions link cells in all directions (Waggett et al. 2006). Waggett et al. (2006) have also suggested that these two separate communication networks within tendons indicate independent functions. Tenocytes may have a basal level of synthesis maintained by systems involving connexin 32 signalling, which is enhanced by mechanical stress. The signalling of connexin 43 then becomes active, damps down the response to mechanical stress and maintains control. Since tendon cells can respond individually to mechanical stimuli, it must be important for their response to be coordinated along the tendon, so that local areas of weaker ECM do not develop.
In addition to its effects on collagen synthesis, the repetitive stretching of tenocytes in vitro upregulates pro-inflammatory cytokine production and the gene expression of mediators such as Cox-2, PGE2 and MMP-1 (Wang et al. 2004; Yang et al. 2005). The effect is more pronounced in the presence of interleukin (IL)-1β, at least at higher strain rates. Smaller levels of repetitive tensile stress have the opposite effect and reduce the production of proinflammatory agents (even when IL-1β is present). Thus, repetitive small-magnitude stretching seems to be anti-inflammatory, whereas large-magnitude stretching is pro-inflammatory. If the findings also prove to be applicable in vivo, then it follows that moderate exercise may be beneficial for reducing tendon inflammation (Yang et al. 2005).
It is interesting to note that tenocytes themselves may produce IL-1β, especially if they are located next to a site where the tendon is injured. Expression is highest 1 day after injury but can persist for several days (Koshima et al. 2007). The significance of IL-1β production in an injured tendon is that it can induce the expression of a wide range of pro-inflammatory agents such as Cox2, MMP1, MMP3, MMP13, ADAMTS-4 and IL-6. It also triggers the further expression of IL-1β mRNA (Tsuzaki et al. 2003) and this is presumably a mechanism for rapidly raising its local concentration. It should be noted, however, that in addition to such actions, IL-1β reduces the elastic modulus of tenocytes by disrupting actin filaments (Qi et al. 2006). The authors suggest that this acts as a protective mechanism against mechanical overuse of tendon cells during healing.
Suppression of proteoglycan and collagen synthesis in cultured tenocytes can be induced by glucocorticoids (Wong et al. 2004, 2005). These are among the substances commonly used by clinicians to suppress inflammation in patients with tendon injuries. Glucocorticoids can also suppress tenocyte proliferation and progenitor cell recruitment (Scutt et al. 2006). If such effects also occur in vivo, then this may explain why the integrity of the tendon as a whole may be affected by corticosteroid treatment. In contrast to corticosteroids, nitric oxide generally benefits tendon healing and enhances collagen synthesis (Xia et al. 2006). Nitric oxide synthetases are normally expressed at low levels and are upregulated by mechanical stimuli (Flick et al. 2006; Szomor et al. 2006). The absence of nitric oxide from tendons during wound healing is associated with prolonged inflammation (Darmani et al. 2004). In clinical practice, this has encouraged attempts to use pharmaceuticals that are intended to increase nitric oxide levels in the tissue in patients with tendinopathies (Murrell, 2007).
Neurovascular supply of tendons
Blood supply
An appreciation of the blood supply of tendons is of special interest to surgeons and thus our current understanding largely stems from studies of certain tendons in particular, viz. the Achilles tendon, digital tendons and numerous wrap-around tendons. A number of different approaches have been used to visualize the vessels – vascular injections of coloured dyes (with and without microdissections), routine histology or immunolabelling for laminin (a component of the basal lamina which surrounds all vessels), and Doppler ultrasonography. Unfortunately, results obtained by the use of one technique may be difficult to reconcile with those obtained by another.
As a general rule, tendons have a vascular supply that is considerably less than that of the more metabolically active muscles with which they are associated. This is why fresh tendons are white and muscles are red. Nevertheless, contrary to the view of early anatomists, tendons are still vascularized, and the presence of vessels is important for the normal functioning of tendon cells and the ability of tendons to repair. This is well illustrated by the pronounced effect that tenotomy has on the rat Achilles tendon (Jozsa et al. 1998). The blood flow within the tendon itself and in the muscle belly of gastrocnemius remains at a lower level for an extended period of time after tenotomy and this may inhibit repair. It is also commonly argued that reduced tendon blood supply can lead to tendon degeneration, particularly in association with certain tendons that have avascular or poorly vascularized regions, e.g. the Achilles tendon, tibialis posterior and supraspinatus (Rees et al. 2006). Nevertheless, this view is by no means universally accepted (Prado et al. 2006). Studies using Doppler ultrasonography suggest that the vascularity of tendons in a given individual can vary from day to day and according to exercise levels (Cook et al. 2005).
Typically, tissues adjacent to tendons, including tendon sheaths and tendon-associated adipose tissue (Fig. 5a), have a richer blood supply than do the tendons themselves and there is evidence that blood flow in the peritendinous tissues is increased as a result of enhanced physical activity (Langberg et al. 1998). The vessels within tendons are largely small and thin-walled. They are a feature both of the internum of the tendon and of its surface epitenon. Where longitudinal, inter-fascicular grooves are visible on the surface of tendons, vessels may lie within the grooves (Edwards, 1946). In the tendon itself, the vessels run longitudinally, parallel to the fascicles and within the endotenon. In digital tendons at least, most of the vessels are arterioles and venules, with the latter being more numerous (Brockis, 1953). Anastomoses between parallel vessels are common (Edwards, 1946). Numerous vessels enter tendons at their myotendinous junctions and some vascular injection studies suggest that this is also the case at entheses. However, Edwards (1946), who used such techniques extensively, was of the opinion that the enthesis is not an important region for the entry of blood vessels, which then supply the rest of the tendon. According to Edwards (1946) it is, however, a site where relatively large lymphatic vessels may be seen on the surface of the tendon. Equally, Scapinelli (1968), Alm & Stromberg (1974), and Ginsburg et al. (1980) all argue that the patellar tendon does not receive any vascular supply from its tibial attachment. It should be noted, however, that although histological studies show that healthy, normal enthesis fibrocartilage is avascular (Benjamin et al. 1986), where tissue damage occurs in older individuals, blood vessels can grow into fibrocartilaginous entheses (Benjamin et al. 2007). Consequently, such attachment sites can indeed be regarded as vascularized tissues. Furthermore, there is clear histological evidence of vascular continuity between bone and tendon at such sites. This general conclusion contrasts with the specific, regional findings of Zbrodowski et al. (1981) showing that there is little continuity between the vascular networks of bone and tendon at the entheses of digital flexor tendons. However, the latter work was based on macroscopical studies only.
Where tendons are surrounded by true synovial sheaths, their supplying vessels enter via a mesotenon. At the wrist and ankle, mesotenons are sheet-like folds, but in the digits they are reduced to isolated, cord-like vinculae (Fig. 6; Edwards, 1946). The blood supply of digital tendons is thus typically segmental (Kostopoulos et al. 2006) with well-vascularized regions alternating with hypovascular ones. The location of the blood vessels seems to be dictated by the relation of the tendons either to the phalanges or to the pulleys associated with the flexor sheaths. At the latter locations, vessels are typically inconspicuous, in line with the avascularity of wrap-around regions of tendons (see below).
Fig. 6.
Vinculae (V) associated with the tendons of flexor digitorum superficialis (FDS) and flexor digitorum profundus (FDP) in a finger. The vinculae are remnants of the mesotenon and convey blood vessels to the tendons.
Avascularity
Numerous studies have demonstrated the greatly diminished blood supply of tendons in regions where they wrap around bony pulleys (Petersen et al. 2000, 2002a, 2003). As certain tendons (e.g. fibularis longus and flexor hallucis longus) can press against bone at more than one location between the myotendinous junction and the enthesis, it follows that they can have a corresponding number of poorly vascularized regions. Such areas of diminished or absent blood supply are of particular clinical significance because they are commonly the sites of tendon degeneration and/or rupture. It follows that angiogenesis must be inhibited, either because inhibitory factors are expressed by tendon fibrocartilage cells or because of the inability of such cells to express stimulatory peptides. It is thus worth noting that VEGF (which promotes angiogenesis) is absent in adult wrap-around tendons (Petersen et al. 2002b), but that endostatin (an inhibitor of angiogenesis) levels are high (Pufe et al. 2003).
Nerve supply
The sensory innervation of tendons is of particular interest in relation to tendinopathies and the repair of ruptured tendons. There is now considerable evidence that nerves can grow into damaged or ruptured tendons in association with blood vessels and that the site where this happens correlates with the region of tendon pain (Messner et al. 1999; Alfredson & Lorentzon, 2007). Intriguingly, Bring et al. (2007) have shown that both the initial ingrowth of nerves into the site of a transected rat Achilles tendon and their subsequent disappearance as the tendon heals, can be modulated by physical activity. Such neuronal plasticity has led the authors to suggest that a pharmacological enhancement of the local release of sensory neuropeptides around damaged tendons could be considered as an adjunct to exercise-based rehabilitation programmes.
The neurovascular invasion of damaged tendon tissue has led to an interest in the use of sclerosing agents for treating painful tendons (Hoksrud et al. 2006; Zeisig et al. 2006; Alfredson & Lorentzon, 2007) and to the development of training programmes that can reduce tendon neovascularization. It seems that a prolonged programme of eccentric exercises can reverse the neovascularization that occurs in patients with Achilles tendinopathy (Ohberg & Alfredson, 2004), although a single training session does not alter Doppler activity within the Achilles tendon (Boesen et al. 2006).
The mid-substance of the rat Achilles tendon is poorly innervated and the majority of nerve fibres are located within the paratenon and not the tendon itself (Ackermann et al. 2001). Vessel-associated fibres are common. They are autonomic nerves that immunolabel for neuropeptide Y and noradrenaline (vasoconstrictive factors) and for vasoactive intestinal peptide (VIP) – a vasodilatory factor. It has been suggested that the nerve fibres regulate blood flow within the tendon (Ackermann et al. 2001). Further, free nerve fibres containing substance P and calcitonin gene-related peptide (CGRP) might be involved in collecting sensory information (including pain) and relaying this to the central nervous system (Ackermann et al. 2001). The human Achilles tendon is also primarily supplied by sensory nerves within the connective tissue sheaths of the tendon and between the tendon fascicles (Bjur et al. 2005). Several opioids have also been identified within the peritendinous tissue and it is therefore possible that the Achilles tendon has an intrinsic system that may be used to reduce pain within the surrounding tissue (Ackermann et al. 2001).
Zaffagnini et al. (2003) have reported the presence of Ruffini and Pacinian corpuscles within the pes anserinus tendons, particularly at their tibial attachment sites. Although Benjamin et al. (2004) confirm that Pacinian corpuscles can be found on the surface of subcutaneous entheses, the recent study of Shaw et al. (2007) on the rat Achilles tendon enthesis concluded that the attachment site itself is aneural. The authors speculate that the absence of nerve fibres is associated with the heavy loading to which the enthesis is subject. However, sensory fibres are conspicuous within the neighbouring adipose tissue and could play a proprioceptive role by monitoring changes in the angle that the tendon makes with the foot during dorsi- and plantar flexion.
Elastic recoil of tendons
Many tendons can recoil elastically when a stretching force is removed. Indeed, some tendons can return over 90% of the energy they store (Ker, 1981). The elastic recoil property seems to be structurally related to crimp and/or knots within fibrils in regions where fibrils are twisted or bent (Franchi et al. 2007). When a tendon is physiologically stretched in vivo, the crimp numbers within it may decrease by nearly 50% (Franchi et al. 2007) so that the degree of fibril undulation is markedly reduced. The elastic recoil of tendons has attracted considerable interest from those working in the fields of exercise physiology and biomechanics, and the reader is referred to the comprehensive reviews of Maganaris (2002) and Reeves (2006) for further details. Thus, only a brief consideration is given to the issue in the current article.
The ability of tendons to stretch and recoil enables them to save energy in running by allowing the limb to have shorter muscle fascicles or slower muscle fibres that can generate force more economically (Alexander, 1991). When an athlete is preparing for a jump, for example, the quadriceps tendon is first stretched and the energy is released at the time of the jump to make the jump more effective (Kurokawa et al. 2001). During jumping, the tendon is stretched by approximately 6%, 350–100 ms before toe off, and the shortening of the whole muscle–tendon unit only happens < 100 ms before toe off (Kurokawa et al. 2001). It is during this last time interval that all of the stored energy is released.
The stiffness of tendons varies with age, sex and physical activity. In vastus lateralis, tendon stiffness is greater in young men and older boys than it is in young boys (Kubo et al. 2001a). In adults, it decreases with training (Kubo et al. 2001b; Reeves, 2006). Kubo et al. (2001a) have made the interesting suggestion that the greater compliance of tendons in young boys may be important in reducing the risk of sporting injuries. The Achilles tendon of women can recoil elastically more than that of men, but in both genders, the tendon shows a relatively linear force–length relationship, particularly at high strains (Kubo et al. 2003). Intriguingly, both the stiffness and elasticity of the Achilles tendon vary between individuals – the stiffness ranging from 145–231 N mm−1, and the elastic modulus from 0.67 to 1.07 GPa (Lichtwark & Wilson, 2005). During hopping, an average of 38 J of energy is recovered from the elastic recoil of the Achilles tendon and this contributes 16% of the total average mechanical work performed during such an action. The high strains recorded in the study of Lichtwark & Wilson (2005) (the average peak strain was 8.3%) may reflect the complex architecture of the Achilles tendon.
It is evident that fatigue may change the elastic properties of the tendon of vastus lateralis. According to Kubo et al. (2001c), the peak moment of the muscle–tendon unit declined after 50 maximal isometric contractions by over 40% and the pennation angle of the vastus lateralis increased about 10%. Thus, the elasticity of a fatigued tendon and aponeurosis tends to be greater as evidenced by its ability to lengthen further with the same load.
Limb lengthening by distraction osteotomy has become a routine surgical procedure and studies in goats have shown that it is the muscle rather than the tendon that provides the extra length within the muscle–tendon unit necessary for proper limb function. While the muscle may elongate by almost 10% of its initial length, the tendon only does so by 3–4% (Elsalanty et al. 2007). It is important to note that length changes are more pronounced in younger (i.e. growing and skeletally immature) than in older (i.e. skeletally mature) animals (Szoke et al. 2005). Tendon lengthening also occurs in a nonuniform manner – being greater in regions that grow faster during normal development (Szoke et al. 2005).
Relationship of tendons to joint capsules
Many tendons attach immediately beyond the joint on which they principally act. This increases the speed with which they can move the joint, albeit at the expense of the most effective moment arm (Wood Jones, 1944a). Thus, they often ‘compete’ with the neighbouring joint capsule for bony anchorage – a conflict that may be resolved by the fusion of the two structures. This is well documented in the glenohumeral joint where the rotator cuff tendons blend imperceptibly with the joint capsule, but it is also a feature of the interphalangeal joints in both the fingers and toes, where the extensor tendon replaces the capsule dorsally (Fig. 7). It has been described more recently in relation to the tendon of gluteus minimus and the hip joint capsule (Walters et al. 2001). It should be recognized that the capsules of highly mobile joints need a degree of laxity to allow the joint with which they are associated to function throughout its whole range of movement. However, such laxity carries with it the risk that the capsule could get pinched within the joint. This was well recognized in the older literature, where the consensus was that the deeper fibres of certain muscles (e.g. the articularis genu component of vastus intermedius at the knee; Lanz & Wachsmuth (1938) retracted the joint capsule as their superficial fibres moved the joint. The common thread in all such examples is that tendon–capsule fusion reduces the risk of capsular entrapment and eliminates the need for an extra muscle purely concerned with tensing the capsule.
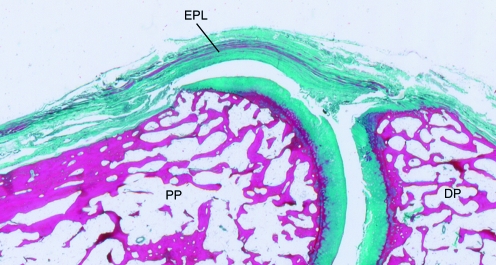
Fig. 7.
A sagittal section through the interphalangeal joint of the thumb stained with Masson's trichrome, showing how the tendon of extensor pollicis longus (EPL) replaces the joint capsule dorsally. DP, distal phalanx; PP, proximal phalanx.
It is worth recognizing that although tendons can pass over joints without fusing with the capsule, they can still press on the capsule, altering its shape and that of the joint cavity when their muscle contracts. This is exemplified by the peroneal tendons passing over the capsule of the ankle joint, in the region of the calcaneo-fibular ligament and by the tendon of iliopsoas passing over the hip joint capsule.
Tendons and fasciae
There is a close, but somewhat neglected, link between tendons and fasciae, for most tendons attach not only to bone, but also to adjacent dense fibrous connective tissues. This is a basic strategy for dissipating stress concentration at entheses and thus reducing the risk of failure or local wear and tear. Fascia-tendon connections are also important in linking muscles together to form ‘mechanical chains’– a concept of interest to manual therapists and considered in detail by Myers (1987).
One of the classic examples of a tendon that has both bony and fibrous attachments is the distal tendon of biceps brachii. This has a bony insertion on the radial tuberosity and a fascial connection to the deep fascia on the medial side of the forearm via the bicipital aponeurosis (Fig. 8). By tensing the deep fascia, the aponeurosis increases the effectiveness of the muscle as a supinator. Another example is the quadriceps tendon. This not only attaches to the superior pole of the patella, but also sends a sheet of fibres anterior to the patella that become continuous with the patellar tendon (Wood Jones, 1944b; Toumi et al. 2006).
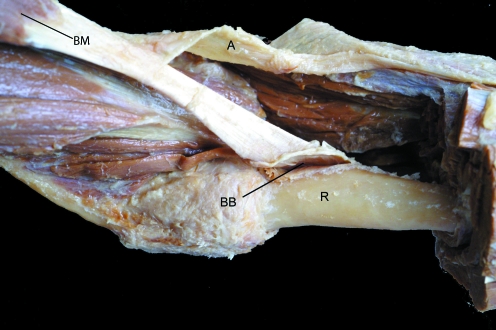
Fig. 8.
The biceps brachii muscle (BM) of the forearm has a tendon that attaches to the bicipital tuberosity of the radius (R) and an aponeurotic expansion (A) that merges with the deep fascia of the forearm. The bicipital bursa (BB) has been opened up at the tendon attachment site.
The ‘fascial ectoskeleton’ concept of Wood Jones
The importance of fascia and its functional relationship to muscles and tendons was well understood by Wood Jones (1944b) who considered fascia to form what he called an ectoskeleton within the limbs. An external skeleton (commonly referred to as an exoskeleton) is typical of arthropods. These animals are covered by a hard shell of chitin to the inner surface of which muscles are attached. Because the exoskeleton faces the outside world, the anchorage of its associated muscles is necessarily from the ‘inside-out’, in marked contrast to the ‘outside-in’ attachment of muscles to bones in man. The seminal work of Wood Jones (1944b) in which he compares the role of fascia in the limbs of man to that of an ectoskeleton is rarely cited today, but it is still highly relevant to modern biology and merits re-visiting. It relates closely to the current recognition (developed from animal studies) that muscles do not simply transmit their load to tendons and then to bone – and that muscles cannot be viewed as structures that are mechanically independent (Huijing, 2007). There is thus an increasing awareness that muscles can transmit forces beyond their confining epimysial envelope. Indeed Huijing (2007) considers two potential pathways – force transmission between adjacent muscles and force transmission to adjacent ‘non-muscular tissue’– a term which embraces the fascial ectoskeleton of Wood Jones (1944b).
Wood Jones (1944b) draws attention to the fact that many upper limb muscles have small, precise tendinous insertions on bones, but those in the lower limb often have larger and less discrete skeletal footprints – in line with their more powerful actions. The larger attachment area of the lower limb muscles is often promoted by an initial anchorage of the muscle bellies or their tendons to fascial sheaths. The fasciae envelop the limb musculature and extend between muscles or muscle groups so as to form septa and other fibrous partitions. Ultimately, of course, the fasciae also attach to bone. As Wood Jones (1944b) has highlighted, certain muscles in the gluteal region rely heavily on indirect attachments to bone via fasciae, rather than direct attachments via tendons. Furthermore, the relative contribution of tendon and fascia to the anchorage mechanism varies with age. Early in development, gluteus maximus is attached predominantly to the gluteal tuberosity, but it later develops a more extensive attachment to the fascia lata of the thigh (Wood Jones, 1944b). At the extreme end of the spectrum, tensor fasciae latae has completely abandoned its bony attachment to the gluteal tuberosity in man and instead attaches entirely to the iliotibial tract (Fig. 9a; Wood Jones, 1944b) – a thickening of the fascia lata. Thus effectively, tensor fasciae latae attaches to the whole stocking of dense connective tissue which ensheathes the thigh as a whole (Fairclough et al. 2006). In the upper limb, palmaris longus has also largely abandoned a direct bony attachment by attaching instead to the palmar fascia (Fig. 9b). A thickening of this ‘fascial tendon’ is characteristic of Dupuytren's contracture – a condition that produces an undesirable flexion of the fingers that can handicap patients considerably.
Fig. 9.
Two examples of tendons that have completely abandoned a bony enthesis and are attached to fascia instead. (a) Tensor fascia latae (TFL) attaching to the iliotibial tract (ITT). (b) The tendon of palmaris longus (PL) attaching to the palmar aponeurosis (PA).
Certain tendons in the lower limb, which are clearly tendinous and relatively distinct in their more proximal regions nearer to the muscle belly (e.g. semimembranosus, semitendinosus, gracilis and sartorius) end as flattened fascial expansions just below the knee, which give the parent muscles a wide grip on the ensheathing and partitioning fascias of the leg (Wood Jones, 1944b; Fig. 3b). Indeed, so widespread are the fascial connections of muscles in the lower limb that as Wood Jones (1944b) points out, it is difficult to perform clean dissections of muscles in the leg compared with the forearm. In the opinion of Wood Jones (1944b), it is the upright stance of man that largely accounts for the greater prominence of fascial connections of muscles and tendons in the lower compared with the upper limb. He suggests that it is a response to the demands for a stabilized limb that must not only provide for locomotion, but also support body weight in an upright position. In other words, the lower limb must act as a rigid column capable of providing passive support – and some muscles attach to the limb as a whole column, rather than to its moving parts. The valuable contribution of Wood Jones (1944b) has been to show that muscles and tendons that gain widespread insertions to fasciae, use these extensive sheets as a functional homologue of an invertebrate exoskeleton.
Functional networks of tendons – the ‘supertendon’ concept
Tendon networks are a particular feature of the hand and foot. On the dorsum of the hand, for example, there is a whole array of flattened extensor tendons that splay out from under the extensor retinaculum and head towards the fingers. The tendons are linked to each other by a highly variable collection of fibrous bands known as juncturae tendinum (Fig. 4b; von Schroeder & Botte, 1997). The bands are probably important in controlling the spacing of the extensor tendons, channelling forces between them and co-ordinating the extension of different fingers [see von Schroeder & Botte (2001) for further details and references]. However, the existence of the juncturae makes it difficult for different fingers to be extended independently. Along with the tendons themselves and their associated fascia, the bands contribute to the formation of a complex network (‘web’) of tendon tissue on the back of the hand – probably reflecting the development of this tendon tissue from a single blastema. Evidently, the key requirement on the extensor surface of the hand is for integrated functioning of the whole tendon web and any particular function of its individual elements is subservient to this primary role (von Schroeder & Botte, 2001). Consequently, subtle tendon variations on the back of the hand are common, for constant tendon anatomy is not essential here (von Schroeder & Botte, 2001). However, it is worth noting that variations in tendons and their interconnections are more frequent on the ulnar than on the radial side of the hand (von Schroeder & Botte, 2001). This is probably because the power grip (which gives maximum gripping force to the hand) is stronger on the radial side and because this part of the hand is so critical for a delicate precision grip (i.e. the grip characterized by opposition of the thumb to the fleshy pad of the terminal phalanx of a finger). It is also significant that the index finger (which is capable of a greater degree of independent movement than the others) has the least variable of the extensor tendons and the least prominent juncturae (von Schroeder & Botte, 2001). Despite the common reports of tendon variations on the dorsum of the hand, variation is not a particular feature of the muscles with which the tendons are associated (von Schroeder & Botte, 2001). Although at first sight this seems paradoxical, it is less surprising if a particular network of tendons is viewed as forming a single functional entity – a sort of ‘supertendon’. Although the morphology of the individual elements constituting the supertendon may vary, the function of the whole is constant and this is reflected in the character of the associated muscle.
A close cooperation between tendons associated with different muscles is a feature of the dorsal expansion that covers the posterior aspect of the fingers and toes. The expansions commence at the metacarpo(metatarso)phalangeal joints and continue to the base of the distal phalanges. They are aponeurotic sheets of tissue that represent the meeting point of tendons associated with different muscles, i.e. extensor tendons, lumbricals and interossei (Fig. 10a,b). In the case of the extensor digitorum tendon, it is worth noting that a single tendon splits up into different branches within the finger (Fig. 10b), which then not only distribute the mechanical forces across different joints (i.e. the proximal (PIP) and distal interphalangeal (DIP) joints), but also modulate joint function. The central slip of the extensor tendon does so by inserting at the base of the middle phalanx and is mainly under tension during flexion of the PIP joint. However, the two lateral branches of the extensor tendon pass beyond the PIP joint, to insert finally at the base of the terminal phalanx. During flexion of the PIP joint, their level of tension is low and thus they allow the DIP joint to be flexed further, even when the PIP joint is already flexed and the central slip is under tension. This modulation of tendon excursion is facilitated by the different radii of the pulleys over which the different slips of the tendon pass at the level of the PIP joint (Brand et al. 1987). Since the lateral slips pass closer to the axis of rotation of the PIP joints, their excursion is less limited than that of the central slip, which crosses the joint further away from its centre of rotation.
Fig. 10.
(a) A lateral view of the dorsal digital expansion (DE) of a finger on the proximal phalanx. Note the presence of interosseous (I) and lumbrical (L) muscles that attach to the expansion and the existence of a fibrous flexor sheath (FS) on the palmar aspect of the finger. (b) A dorsal view of the dorsal digital expansion over a metacarpophalangeal joint and its relationship to the more proximal extensor tendon (ET). The lateral slips of the extensor expansion have been displayed over the intermediate phalanx (arrows). (c) The three ‘wrap-around tendons’ in the region of the medial malleolus (MM) – tibialis posterior (TP), flexor digitorum longus (FDL) and flexor hallucis longus (FHL). The tendon of tibialis posterior has been displaced from its groove (arrow) to show the ‘articular character’ of the bone surface against which it presses. The left side of the photograph is distal. (d) The quadratus plantae muscle (QP) in the sole of the foot. It serves to adjust the oblique pull of flexor digitorum longus (FDL).
Recently, the complex interaction of the various digital tendons has been reviewed in the context of the co-evolution of the brain and body. Valero-Cuevas et al. (2007) suggest that the resulting information processing at a macroscopic level (i.e. the repetitive and predictable computation of tension levels in the associated tendons) is an example of an emerging principle of what they term ‘nonneural somatic logic’.
Wrap-around tendons
The majority of long tendons in the limbs do not run a straight course from muscle to bone, but change direction by wrapping around bony or fibrous pulleys – at least in some positions of the joints that the tendons cross. Perhaps the clearest examples are the various tendons that change direction around the malleoli, as they pass between the leg to the foot (Fig. 10c). Such tendons have been called ‘wrap-around tendons’ by Vogel & Koob (1989), but they are commonly called ‘gliding tendons’ by German anatomists (e.g. Tillmann & Koch, 1995). The term ‘gliding tendons’ reminds the reader that such tendons exhibit a degree of longitudinal excursion over a bony or fibrous pulley as a result of muscle action. Both Benjamin & Ralphs (1998) and Pufe et al. (2005) cite numerous examples of gliding, or wrap-around, tendons. Curiously, while wrap-around tendons can alter the direction of pull of muscles, muscles can occasionally alter the pull of a tendon. This is a function classically ascribed to quadratus plantae (flexor accessorius), a small muscle in the sole of the foot that is widely believed to correct the line of pull of the obliquely orientated tendon of flexor digitorum longus (Fig. 10d; Standring, 2004).
A key factor to appreciate in relation to wrap-around tendons is that they are subject not only to tensional forces, but also to compression and shear. During an isometric contraction of a muscle, tensional forces are maximal towards the convex surface of the tendon, whereas compressive forces reach a peak on the concave side. However, when the tendon moves longitudinally as a result of muscle contraction, the concave surface of the tendon is also subject to shear. The gradient of forces acting across the width of a wrap-around tendon is reflected in its variable histological character at that location. Typically, the side of the tendon facing the source of compression is fibrocartilaginous (Fig. 11) and the tendon tissue nearer the convex surface is purely fibrous (Benjamin & Ralphs, 1998).
Fig. 11.
A histological view of a region of a tendon subject to compression (extensor pollicis longus as it crosses the interphalangeal joint of the thumb). Fibrocartilage cells (FC) rather than fibroblasts are typical of the compressed region of the tendon. Toluidine blue stained section.
Tendon sheaths, pulleys and retinacula
It is helpful in understanding why some tendons have synovial sheaths and others not, to compare the extensor and flexor tendons in the fingers, for only the latter have such sheaths. (Fig. 12a,b) According to Wood Jones (1944a) the reason relates to the range of movements promoted by the two sets of tendons. Flexion of the fingers can be carried further than extension, because the fingers can generally be flexed towards the palm at three neighbouring joints – the metacarpophalangeal and the two interphalangeal joints. Generally speaking, however, the fingers cannot be actively extended in the direction of the nails – flexed fingers are simply returned to a straight position. Consequently, as Wood Jones (1944a) points out, there are different requirements for keeping the extensor and flexor tendons in position. The extensor tendons just need steadying as they cross the finger joints, but the flexor tendons must be bound closely to the joints they cross to prevent bowstringing as their muscles contract. At the wrist, however, extension can proceed beyond the straight position to bend the hand upwards – so both the flexor and extensor tendons need to be bound down to prevent bowstringing. The structures holding tendons in position are known by a variety of names depending upon their location – retinacula, fibrous pulleys, annular ligaments or fibrous sheaths (Fig. 12a,c). It follows therefore that retaining structures are present on both the flexor and extensor sides of the wrist, but only on the flexor sides of the fingers (Wood Jones, 1944a). The flexor sheaths of the fingers form a tunnel with the bones, through which the flexor tendons thread (Fig. 12c) – like fishing line passing through a series of eyelets on a fishing rod (Semple, 1980). The sheaths are strengthened at intervals by a series of pulleys that are described as annular or cruciform according to differences in the direction of their fibres. It is important to recognize that although many anatomical texts depict them as discrete structures, they are really simply local thickenings of the fibrous sheath. How easy they are to recognize, depends on how abrupt their transitions are with the rest of the sheath. Their organization along the length of the finger minimizes the risk of the sheath buckling during finger movements, for this could impede the tendons that pass through it. The broader annular pulleys lie over the phalangeal shafts, and the narrower pulleys (both annular and cruciform) lie nearer to the joints (Doyle, 2001). Damage to the flexor sheaths of the fingers or their associated pulleys are common injuries in rock climbers and can result in prominent bowstringing (Klauser et al. 2002; Logan et al. 2004; Kubiak et al. 2006; Schoffl & Schoffl, 2006).
Fig. 12.
(a,b) Synovial sheaths associated with tendons in the wrist and hand as demonstrated in specimens injected with a blue dye. Note the presence of such sheaths around the digital flexor tendons (FT), but their absence in association with the digital extensor tendons (ET). On both the extensor (a) and flexor (b) sides, the synovial sheaths extend beyond the limits of the wrist retinacula (R) to allow for the longitudinal excursion of the tendons. The palmar bursa (PB) is evident in (b). (c) The digital flexor tendons (FT) entering the tunnel created by the fibrous flexor sheath (FS) and the phalangeal bones in a finger.
Any tendons passing beneath retinacula or threading through fibrous sheaths, are likely to be associated with shunt rather than spurt muscles. Such muscles are those that ‘shunt’ bones together at joints more effectively than they can produce angle changes at the joints (Standring, 2004). Because the insertional angle is kept constant, tendons associated with retaining structures such as retinacula, transmit equal force to the bones at all positions of the joints (MacConaill, 1948). Thus the multitude of tendons and their associated retinacula passing over the ankle and wrist joints, contribute greatly to the stability of these joints over a wide range of postures – a fact that MacConaill (1948) highlights as being of particular importance to an acrobat or a ballet dancer.
In addition to the fibrous sheath that binds down flexor tendons within the digits, the tendons themselves also contribute to holding each other in position. This is because the deep digital flexor tendon passes through a split in the superficial tendon (called ‘Camper's chiasma’) roughly half way down the finger, allowing one tendon to form a sling for the other (Fig. 12d). As Kapandji (1982b) has indicated, a mechanism which maintains the superficial flexor tendon in a superficial position right up to its attachment site, makes this tendon slightly more efficient at flexing the proximal interphalangeal joint, i.e. it gains a better moment arm than it would by ‘hugging’ the bone surface right up to its enthesis.
The need for retinacula in turn dictates the need for synovial tendon sheaths at the same location. These have traditionally been demonstrated by the use of coloured dyes in gross dissections (Fig. 12a,b) and are present wherever a tendon rubs against a bone or a fibrous tissue in order to reduce friction. They can thus be regarded as structures ancillary to retinacula. Typically, a synovial sheath has two layers that are continuous with each other – an outer parietal and an inner visceral layer; the sheath as a whole is often envisaged as an elongated bag invaginated from one side by the tendon (Semple, 1980; Standring, 2004). A point of continuity between the two layers of the tendon sheath (the mesotenon) carries blood vessels into the tendon. Where the invagination is not extensive, a mesotenon may not be recognizable, but in highly mobile tendons subject to considerable longitudinal excursion (e.g. in association with the flexor tendons of the digits), the invagination is so extensive that the mesotenon is reduced to a few strands, or ‘vinculae’ (Standring, 2004; Fig. 12d). These again serve for the conveyance of blood vessels. Even where the mesotenon is not reduced to vinculae, there is obviously still a need for slack so that the tendon can move within its sheath. According to Wood Jones (1944b), this is achieved by a reduplication of the synovium as a fold at one end of the tunnel – at the other end, he states that the sac wall tapers out in close adherence to the tendon. If adhesions develop between the two layers of a synovial sheath (because of inflammation), the tendon cannot glide within it and it becomes comparable to the cable of a rusty brake. Interestingly, synovial sheaths develop in the foetus before the onset of the muscular movements that demand their presence (Wood Jones, 1944a).
There are clear parallels between gliding surfaces involving tendons and those provided by articular cartilage in synovial joints (Amadio, 2005). The basic strategy to reduce friction is the same and, in both cases, lubricin (superficial zone protein) promotes boundary lubrication (Schumacher et al. 1994; Rees et al. 2002; Sun et al. 2006b). Although hyaluronan is present both in synovial fluid and in the fluid of tendon sheaths, Amadio (2005) raises the possibility that the chief role of hyaluronan is to provide a high viscosity nutrient delivery vehicle, rather than to act as a lubricant.
It should be noted that synovial sheaths associated with tendons in the wrist and elsewhere, extend beyond the limits of the retinacula to allow for a degree of longitudinal excursion (Fig. 12a,b). Because the range of movement at the wrist is greater on the flexor side, it follows that the sheaths here extend beyond the boundaries of the retinaculum to a greater extent than they do on the extensor side (Wood Jones, 1944a). Indeed, in the palm, the synovial sheaths extend out into the palmar bursa that reaches as far as the middle of the palm (Fig. 12b). Because the metacarpal bones of the thumb and the little finger are much more highly mobile than those of the other fingers, the synovial sheaths associated with the flexor tendons of these digits pass without interruption from wrist to fingers (Wood Jones, 1944a). This explains the dictum, well known to generations of medical students, that local infection in the thumb or little finger can spread more proximally than it can in the middle three fingers.
In addition to true synovial sheaths, a few tendons have ‘false sheaths’. The best known of these is that associated with the Achilles tendon. It is sometimes called a paratenon and is essentially a condensation of surrounding connective tissue. Although it is easy to distinguish between it and the deep fascia of the leg in the more proximal parts of the Achilles tendon, the two structures cannot readily be separated in the more distal region, nearer the calcaneal attachment of the tendon. The sheath is rich in blood vessels and nerves and together with the epitenon that adheres to the surface of the tendon itself, it is sometimes referred to as the peritenon. It can stretch 2–3 cm as the tendon moves (Myerson & McGarvey, 1999). Inflammatory changes in this sheath are a very common cause of Achilles tendon problems in runners, for the sheath is both vascularized and innervated.
Bursae
Bursae are structures closely related to tendon sheaths. However, whereas sheaths typically occur in the mid-substance of tendons in association with bony pulleys or fibrous retinacula, bursae are characteristic of tendon insertion sites (at which location they are sometimes called ‘subtendinous bursae’) or lie between a tendon and some overlying structure. Subtendinous bursae include the bicipital bursa at the insertion of the tendon of biceps brachii (Fig. 8) and the retrocalcaneal and deep infrapatellar bursae at the insertions of the Achilles and patellar tendons respectively (Standring, 2004). Subtendinous bursae may be no more than small fluid-filled spaces with a very local relationship to a tendon. However, some can be more elongated and it can become a matter of semantics to know whether to call such structures bursae or tendon sheaths. As well as subtendinous bursae (which, as their name suggests, lie deep to tendons), there is a further set of more superficial bursae facilitating movement between the skin and an adjacent tendon. Such a bursa is present on the superficial surface of the distal part of the Achilles tendon, but comparable bursae are present near other tendons as well – e.g. triceps brachii. All such bursae are again vulnerable to inflammation – commonly as an overuse injury stemming from excessive levels of shear and/or compression. It is thus intriguing to note that Oliva et al. (2005) have reported the case of a patient in whom there was clear evidence of cartilage metaplasia in the superficial bursa overlying the Achilles tendon. Such cartilage differentiation in the walls of the more deeply-placed retrocalcaneal bursa is well documented (Rufai et al. 1995; Canoso, 1998; Benjamin & McGonagle, 2001) and explains why such bursae are not lined by synovium in their deepest recesses.
Fat pads associated with tendons
There is a striking, though greatly neglected, association between fat and tendons. Large fat pads are particularly prominent immediately deep to the patellar and Achilles tendons in Man. They are associated with synovium, are richly innervated and vascularized (Shaw et al. 2007) and are likely to serve as mechanosensory organs for tendons and be implicated in tendinopathies in ways that we do not fully understand. It is intriguing to note that Hoffa's fat pad actually knits into the deep surface of the patellar tendon as finger-like extensions of fat – a feature that is clearly visible in axial MRIs (Toumi et al. 2006). The tip of Kager's fat pad (which is associated with the Achilles tendon) moves in and out of the retrocalcaneal bursa during plantar and dorsiflexion of the foot so as to minimize pressure changes in the bursa (Theobald et al. 2006). Canoso et al. (1988) have likened it to a freely moveable spacer and emphasized its importance in enabling the Achilles tendon to gain a more distal attachment to the calcaneus. This gives the tendon a biomechanical advantage. The tip of Kager's fat pad is probably also important in spreading synovial fluid within the bursa, reducing the risk of tendon adhesions to the superior tuberosity (Canoso et al. 1988; Theobald et al. 2006) and acting as an immune organ by virtue of its content of macrophages and lymphocytes (Shaw et al. 2007). More proximally, the fat cushions and protects blood vessels that enter the deep surface of the Achilles tendon to supply it (Theobald et al. 2006).
Concluding remarks
The present review covers a comprehensive collection of works on a wide range of topics related to the functional morphology of tendons. We have tried to evaluate the novel contribution of many recent studies published within the last few years, but have also drawn attention to older works, particularly those of Wood Jones (1944a,b), that we think are still highly relevant today, but which are in danger of being forgotten. Among the key points we would emphasize that are rarely considered in other general reviews on tendons, are the interrelationships between tendons and fascia, and the existence of ‘supertendons’, i.e. the formation of tendon networks in which the function of the whole is greater than that of its individual parts. We have used our background as anatomists to ensure that a wide variety of different tendons have been evaluated when highlighting general principles of tendon design.
References
- Ackermann PW, Li J, Finn A, Ahmed M, Kreicbergs A. Autonomic innervation of tendons, ligaments and joint capsules. A morphologic and quantitative study in the rat. J Orthop Res. 2001;19:372–378. doi: 10.1016/S0736-0266(00)90029-9. [DOI] [PubMed] [Google Scholar]
- Alexander RM. Elastic mechanisms in primate locomotion. Z Morphol Anthropol. 1991;78:315–320. [PubMed] [Google Scholar]
- Alfredson H, Lorentzon R. Sclerosing polidocanol injections of small vessels to treat the chronic painful tendon. Cardiovasc Hematol Agents Med Chem. 2007;5:97–100. doi: 10.2174/187152507780363232. [DOI] [PubMed] [Google Scholar]
- Alm A, Stromberg B. Vascular anatomy of the patellar and cruciate ligaments. A microangiographic and histologic investigation in the dog. Acta Chir Scand Suppl. 1974;445:25–35. [PubMed] [Google Scholar]
- Amadio PC. Friction of the gliding surface. Implications for tendon surgery and rehabilitation. J Hand Ther. 2005;18:112–119. doi: 10.1197/j.jht.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banes AJ, Donlon K, Link GW, et al. Cell populations of tendon: a simplified method for isolation of synovial cells and internal fibroblasts: confirmation of origin and biologic properties. J Orthop Res. 1988;6:83–94. doi: 10.1002/jor.1100060111. [DOI] [PubMed] [Google Scholar]
- Barkhausen T, van Griensven M, Zeichen J, Bosch U. Modulation of cell functions of human tendon fibroblasts by different repetitive cyclic mechanical stress patterns. Exp Toxicol Pathol. 2003;55:153–158. doi: 10.1078/0940-2993-00302. [DOI] [PubMed] [Google Scholar]
- Benjamin M, McGonagle D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J Anat. 2001;199:503–526. doi: 10.1046/j.1469-7580.2001.19950503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Ralphs JR. Fibrocartilage in tendons and ligaments – an adaptation to compressive load. J Anat. 1998;193:481–494. doi: 10.1046/j.1469-7580.1998.19340481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Ralphs JR. The cell and developmental biology of tendons and ligaments. Int Rev Cytol. 2000;196:85–130. doi: 10.1016/s0074-7696(00)96003-0. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Evans EJ, Copp L. The histology of tendon attachments to bone in man. J Anat. 1986;149:89–100. [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Kumai T, Milz S, Boszczyk BM, Boszczyk AA, Ralphs JR. The skeletal attachment of tendons – tendon ‘entheses’. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:931–945. doi: 10.1016/s1095-6433(02)00138-1. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Redman S, Milz S, et al. Adipose tissue at entheses: the rheumatological implications of its distribution. A potential site of pain and stress dissipation? Ann Rheum Dis. 2004;63:1549–1555. doi: 10.1136/ard.2003.019182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Toumi H, Ralphs JR, Bydder G, Best TM, Milz S. Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J Anat. 2006;208:471–490. doi: 10.1111/j.1469-7580.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Toumi H, Suzuki D, Redman S, Emery P, McGonagle D. Microdamage and altered vascularity at the enthesis-bone interface provides an anatomic explanation for bone involvement in the HLA-B27-associated spondylarthritides and allied disorders. Arthritis Rheum. 2007;56:224–233. doi: 10.1002/art.22290. [DOI] [PubMed] [Google Scholar]
- Birk DE, Zycband E. Assembly of the tendon extracellular matrix during development. J Anat. 1994;184:457–463. [PMC free article] [PubMed] [Google Scholar]
- Bjur D, Alfredson H, Forsgren S. The innervation pattern of the human Achilles tendon: studies of the normal and tendinosis tendon with markers for general and sensory innervation. Cell Tissue Res. 2005;320:201–206. doi: 10.1007/s00441-004-1014-3. [DOI] [PubMed] [Google Scholar]
- Boesen MI, Koenig MJ, Torp-Pedersen S, Bliddal H, Langberg H. Tendinopathy and Doppler activity: the vascular response of the Achilles tendon to exercise. Scand J Med Sci Sports. 2006;16:463–469. doi: 10.1111/j.1600-0838.2005.00512.x. [DOI] [PubMed] [Google Scholar]
- Bozec L, van der Heijden G, Horton M. Collagen fibrils: nanoscale ropes. Biophys J. 2007;92:70–75. doi: 10.1529/biophysj.106.085704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand PW, Thompson DE, Micks JE. The biomechanics of the interphalangeal joints. In: Bowers WH, editor. The Interphalangeal Joints. Edinburgh: Churchill Livingstone; 1987. pp. 21–54. [Google Scholar]
- Bring DK, Kreicbergs A, Renstrom PA, Ackermann PW. Physical activity modulates nerve plasticity and stimulates repair after Achilles tendon rupture. J Orthop Res. 2007;25:164–172. doi: 10.1002/jor.20257. [DOI] [PubMed] [Google Scholar]
- Brockis JG. The blood supply of the flexor and extensor tendons of the fingers in man. J Bone Joint Surg. 1953;35B:131–138. doi: 10.1302/0301-620X.35B1.131. [DOI] [PubMed] [Google Scholar]
- Brown D, Wagner D, Li X, Richardson JA, Olson EN. Dual role of the basic helix-loop-helix transcription factor scleraxis in mesoderm formation and chondrogenesis during mouse embryogenesis. Development. 1999;126:4317–4329. doi: 10.1242/dev.126.19.4317. [DOI] [PubMed] [Google Scholar]
- Buchanan CI, Marsh RL. Effects of exercise on the biomechanical, biochemical and structural properties of tendons. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:1101–1107. doi: 10.1016/s1095-6433(02)00139-3. [DOI] [PubMed] [Google Scholar]
- Canoso JJ. The première enthesis. J Rheumatol. 1998;25:1254–1256. [PubMed] [Google Scholar]
- Canoso JJ, Liu N, Traill MR, Runge VM. Physiology of the retrocalcaneal bursa. Ann Rheum Dis. 1988;47:910–912. doi: 10.1136/ard.47.11.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R, Tucker RP. Connective tissues: signalling by tenascins. Int J Biochem Cell Biol. 2004;36:1085–1089. doi: 10.1016/j.biocel.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Cook JL, Kiss ZS, Ptasznik R, Malliaras P. Is vascularity more evident after exercise? Implications for tendon imaging. AJR Am J Roentgenol. 2005;185:1138–1140. doi: 10.2214/AJR.04.1205. [DOI] [PubMed] [Google Scholar]
- Darmani H, Crossan JC, Curtis A. Single dose of inducible nitric oxide synthase inhibitor induces prolonged inflammatory cell accumulation and fibrosis around injured tendon and synovium. Mediators Inflamm. 2004;13:157–164. doi: 10.1080/09511920410001713556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis KJ, McKinney S. Sesamoids and accessory bones of the foot. Clin Podiatr Med Surg. 1990;7:717–723. [PubMed] [Google Scholar]
- Docheva D, Hunziker EB, Fassler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. 2005;25:699–705. doi: 10.1128/MCB.25.2.699-705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JR. Palmar and digital flexor tendon pulleys. Clin Orthop Relat Res. 2001;383:84–96. doi: 10.1097/00003086-200102000-00011. [DOI] [PubMed] [Google Scholar]
- Dressler MR, Butler DL, Wenstrup R, Awad HA, Smith F, Boivin GP. A potential mechanism for age-related declines in patellar tendon biomechanics. J Orthop Res. 2002;20:1315–1322. doi: 10.1016/S0736-0266(02)00052-9. [DOI] [PubMed] [Google Scholar]
- Edwards DA. The blood supply and lymphatic drainage of tendons. J Anat. 1946;80:147–152. 142. [PubMed] [Google Scholar]
- Elsalanty M, Makarov M, Cherkashin A, Birch J, Samchukov M. Changes in pennate muscle architecture after gradual tibial lengthening in goats. Anat Rec. 2007;290:461–467. doi: 10.1002/ar.20513. [DOI] [PubMed] [Google Scholar]
- Evans CE, Trail IA. An in vitro comparison of human flexor and extensor tendon cells. J Hand Surg [Br] 2001;26:307–313. doi: 10.1054/jhsb.2001.0593. [DOI] [PubMed] [Google Scholar]
- Fairclough J, Hayashi K, Toumi H, et al. The functional anatomy of the iliotibial band during flexion and extension of the knee: implications for understanding iliotibial band syndrome. J Anat. 2006;208:309–316. doi: 10.1111/j.1469-7580.2006.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon J, Blevins FT, Vogel K, Trotter J. Functional morphology of the supraspinatus tendon. J Orthop Res. 2002;20:920–926. doi: 10.1016/S0736-0266(02)00023-2. [DOI] [PubMed] [Google Scholar]
- Finni T, Hodgson JA, Lai AM, Edgerton VR, Sinha S. Nonuniform strain of human soleus aponeurosis-tendon complex during submaximal voluntary contractions in vivo. J Appl Physiol. 2003;95:829–837. doi: 10.1152/japplphysiol.00775.2002. [DOI] [PubMed] [Google Scholar]
- Flick J, Devkota A, Tsuzaki M, Almekinders L, Weinhold P. Cyclic loading alters biomechanical properties and secretion of PGE2 and NO from tendon explants. Clin Biomech. 2006;21:99–106. doi: 10.1016/j.clinbiomech.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Franchi M, Fini M, Quaranta M, et al. Crimp morphology in relaxed and stretched rat Achilles tendon. J Anat. 2007;210:1–7. doi: 10.1111/j.1469-7580.2006.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuta S, Oyama M, Kavalkovich K, Fu FH, Niyibizi C. Identification of types II, IX and X collagens at the insertion site of the bovine achilles tendon. Matrix Biol. 1998;17:65–73. doi: 10.1016/s0945-053x(98)90125-1. [DOI] [PubMed] [Google Scholar]
- van Gils CC, Steed RH, Page JC. Torsion of the human Achilles tendon. J Foot Ankle Surg. 1996;35:41–48. doi: 10.1016/s1067-2516(96)80011-1. [DOI] [PubMed] [Google Scholar]
- Ginsburg JH, Whiteside LA, Piper TL. Nutrient pathways in transferred patellar tendon used for anterior cruciate ligament reconstruction. Am J Sports Med. 1980;8:15–18. doi: 10.1177/036354658000800103. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Gross G. Tendon and ligament engineering: from cell biology to in vivo application. Regen Med. 2006;1:563–574. doi: 10.2217/17460751.1.4.563. [DOI] [PubMed] [Google Scholar]
- Hoksrud A, Ohberg L, Alfredson H, Bahr R. Ultrasound-guided sclerosis of neovessels in painful chronic patellar tendinopathy: a randomized controlled trial. Am J Sports Med. 2006;34:1738–1746. doi: 10.1177/0363546506289168. [DOI] [PubMed] [Google Scholar]
- Huijing PA. Epimuscular myofascial force transmission between antagonistic and synergistic muscles can explain movement limitation in spastic paresis. J Electromyography Kinesiology. 2007;17:708–724. doi: 10.1016/j.jelekin.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Jozsa L, Kannus P, Jarvinen TA, Balint J, Jarvinen M. Blood flow in rat gastrocnemius muscle and Achilles tendon after Achilles tenotomy. Eur Surg Res. 1998;30:125–129. doi: 10.1159/000008567. [DOI] [PubMed] [Google Scholar]
- Kapandji IA. The Physiology of the Joints. Lower Limb. Edinburgh: Churchill Livingstone; 1982a. [Google Scholar]
- Kapandji IA. The Physiology of the Joints. Upper Limb. Edinburgh: Churchill Livingstone; 1982b. [Google Scholar]
- Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connect Tissue Res. 1978;6:11–23. doi: 10.3109/03008207809152283. [DOI] [PubMed] [Google Scholar]
- Ker RF. Dynamic tensile properties of the plantaris tendon of sheep (Ovis aries. J Exp Biol. 1981;93:283–302. doi: 10.1242/jeb.93.1.283. [DOI] [PubMed] [Google Scholar]
- Ker RF. The implications of the adaptable fatigue quality of tendons for their construction, repair and function. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:987–1000. doi: 10.1016/s1095-6433(02)00171-x. [DOI] [PubMed] [Google Scholar]
- Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Klauser A, Frauscher F, Bodner G, et al. Finger pulley injuries in extreme rock climbers: depiction with dynamic US. Radiology. 2002;222:755–761. doi: 10.1148/radiol.2223010752. [DOI] [PubMed] [Google Scholar]
- Koshima H, Kondo S, Mishima S, et al. Expression of interleukin-1beta, cyclooxygenase-2, and prostaglandin E2 in a rotator cuff tear in rabbits. J Orthop Res. 2007;25:92–97. doi: 10.1002/jor.20241. [DOI] [PubMed] [Google Scholar]
- Kostopoulos E, Casoli V, Verolino P, Papadopoulos O. Arterial blood supply of the extensor apparatus of the long fingers. Plast Reconstr Surg. 2006;117:2310–2318. doi: 10.1097/01.prs.0000218799.33322.7f. [DOI] [PubMed] [Google Scholar]
- Kubiak EN, Klugman JA, Bosco JA. Hand injuries in rock climbers. Bull Hosp Jt Dis. 2006;64:172–177. [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Ito M, Fukunaga T. Effects of isometric training on the elasticity of human tendon structures in vivo. J Appl Physiol. 2001b;91:26–32. doi: 10.1152/jappl.2001.91.1.26. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Kawakami Y, Fukanaga T. Growth changes in the elastic properties of human tendon structures. Int J Sports Med. 2001a;22:138–143. doi: 10.1055/s-2001-11337. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Kawakami Y, Fukunaga T. Effects of repeated muscle contractions on the tendon structures in humans. Eur J Appl Physiol. 2001c;84:162–166. doi: 10.1007/s004210000337. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Fukunaga T. Gender differences in the viscoelastic properties of tendon structures. Eur J Appl Physiol. 2003;88:520–526. doi: 10.1007/s00421-002-0744-8. [DOI] [PubMed] [Google Scholar]
- Kurokawa S, Fukunaga T, Fukashiro S. Behavior of fascicles and tendinous structures of human gastrocnemius during vertical jumping. J Appl Physiol. 2001;90:1349–1358. doi: 10.1152/jappl.2001.90.4.1349. [DOI] [PubMed] [Google Scholar]
- Landis WJ, Silver FH. The structure and function of normally mineralizing avian tendons. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:1135–1157. doi: 10.1016/s1095-6433(02)00248-9. [DOI] [PubMed] [Google Scholar]
- Langberg H, Bulow J, Kjaer M. Blood flow in the peritendinous space of the human Achilles tendon during exercise. Acta Physiol Scand. 1998;163:149–153. doi: 10.1046/j.1365-201X.1998.00361.x. [DOI] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Petersen LJ, Bulow J, Kjaer M. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J Physiol. 1999;521 Pt 1:299–306. doi: 10.1111/j.1469-7793.1999.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H, Rosendal L, Kjaer M. Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J Physiol. 2001;534:297–302. doi: 10.1111/j.1469-7793.2001.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanz TV, Wachsmuth W. Praktische Anatomie. 4. Vol. 1. Berlin: Springer; 1938. [Google Scholar]
- Lichtwark GA, Wilson AM. In vivo mechanical properties of the human Achilles tendon during one-legged hopping. J Exp Biol. 2005;208:4715–4725. doi: 10.1242/jeb.01950. [DOI] [PubMed] [Google Scholar]
- Logan AJ, Makwana N, Mason G, Dias J. Acute hand and wrist injuries in experienced rock climbers. Br J Sports Med. 2004;38:545–548. doi: 10.1136/bjsm.2002.003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacConaill MA. The movement of bones and joints. J Bone Joint Surg. 1948;31B:100–104. [PubMed] [Google Scholar]
- Maganaris CN. Tensile properties of in vivo human tendinous tissue. J Biomech. 2002;35:1019–1027. doi: 10.1016/s0021-9290(02)00047-7. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Qvortrup K, Larsen JO, et al. Collagen fibril size and crimp morphology in ruptured and intact Achilles tendons. Matrix Biol. 2002;21:369–377. doi: 10.1016/s0945-053x(02)00011-2. [DOI] [PubMed] [Google Scholar]
- Majima T, Yasuda K, Tsuchida T, et al. Stress shielding of patellar tendon: effect on small-diameter collagen fibrils in a rabbit model. J Orthop Sci. 2003;8:836–841. doi: 10.1007/s00776-003-0707-x. [DOI] [PubMed] [Google Scholar]
- McNeilly CM, Banes AJ, Benjamin M, Ralphs JR. Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions. J Anat. 1996;189:593–600. [PMC free article] [PubMed] [Google Scholar]
- Messner K, Wei Y, Andersson B, Gillquist J, Rasanen T. Rat model of Achilles tendon disorder. A pilot study. Cells Tissues Organs. 1999;165:30–39. doi: 10.1159/000016671. [DOI] [PubMed] [Google Scholar]
- Michna H. Morphometric analysis of loading-induced changes in collagen-fibril populations in young tendons. Cell Tissue Res. 1984;236:465–470. doi: 10.1007/BF00214251. [DOI] [PubMed] [Google Scholar]
- Michna H, Hartmann G. Adaptation of tendon collagen to exercise. Int Orthop. 1989;13:161–165. doi: 10.1007/BF00268040. [DOI] [PubMed] [Google Scholar]
- Miller BF, Olesen JL, Hansen M, et al. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567:1021–1033. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison ND, Price BA, Conner DA, et al. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134:2697–2708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- Murrell GA. Using nitric oxide to treat tendinopathy. Br J Sports Med. 2007;41:227–231. doi: 10.1136/bjsm.2006.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers TW. Anatomy Trains: Myofascial Meridians for Manual and Movement Therapists. Edinburgh: Churchill Livingstone; 1987. [Google Scholar]
- Myerson MS, McGarvey W. Disorders of the Achilles tendon insertion and Achilles tendinitis. Instr Course Lect. 1999;48:211–218. [PubMed] [Google Scholar]
- Ohberg L, Alfredson H. Effects on neovascularisation behind the good results with eccentric training in chronic mid-portion Achilles tendinosis? Knee Surg Sports Traumatol Arthrosc. 2004;12:465–470. doi: 10.1007/s00167-004-0494-8. [DOI] [PubMed] [Google Scholar]
- Oliva F, Venanzi R, Fratoni S, Maffulli N. Chondroma of the subcutaneous bursa of the Achilles tendon. Bull Hosp Jt Dis. 2005;63:24–26. [PubMed] [Google Scholar]
- Ottani V, Martini D, Franchi M, Ruggeri A, Raspanti M. Hierarchical structures in fibrillar collagens. Micron. 2002;33:587–596. doi: 10.1016/s0968-4328(02)00033-1. [DOI] [PubMed] [Google Scholar]
- Passerieux E, Rossignol R, Chopard A, et al. Structural organization of the perimysium in bovine skeletal muscle: junctional plates and associated intracellular subdomains. J Struct Biol. 2006;154:206–216. doi: 10.1016/j.jsb.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Passerieux E, Rossignol R, Letellier T, Delage JP. Physical continuity of the perimysium from myofibers to tendons: involvement in lateral force transmission in skeletal muscle. J Struct Biol. 2007;159:19–28. doi: 10.1016/j.jsb.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Petersen W, Bobka T, Stein V, Tillmann B. Blood supply of the peroneal tendons: injection and immunohistochemical studies of cadaver tendons. Acta Orthop Scand. 2000;71:168–174. doi: 10.1080/000164700317413148. [DOI] [PubMed] [Google Scholar]
- Petersen W, Hohmann G, Stein V, Tillmann B. The blood supply of the posterior tibial tendon. J Bone Joint Surg [Br] 2002a;84:141–144. doi: 10.1302/0301-620x.84b1.11592. [DOI] [PubMed] [Google Scholar]
- Petersen W, Pufe T, Kurz B, Mentlein R, Tillmann B. Angiogenesis in fetal tendon development: spatial and temporal expression of the angiogenic peptide vascular endothelial cell growth factor. Anat Embryol (Berl) 2002b;205:263–270. doi: 10.1007/s00429-002-0241-1. [DOI] [PubMed] [Google Scholar]
- Petersen W, Pufe T, Zantop T, Paulsen F. Blood supply of the flexor hallucis longus tendon with regard to dancer's tendinitis: injection and immunohistochemical studies of cadaver tendons. Foot Ankle Int. 2003;24:591–596. doi: 10.1177/107110070302400804. [DOI] [PubMed] [Google Scholar]
- Prado MP, de Carvalho AE, Jr, Rodrigues CJ, Fernandes TD, Mendes AA, Salomao O. Vascular density of the posterior tibial tendon: a cadaver study. Foot Ankle Int. 2006;27:628–631. doi: 10.1177/107110070602700811. [DOI] [PubMed] [Google Scholar]
- Pufe T, Petersen W, Kurz B, Tsokos M, Tillmann B, Mentlein R. Mechanical factors influence the expression of endostatin – an inhibitor of angiogenesis – in tendons. J Orthop Res. 2003;21:610–616. doi: 10.1016/S0736-0266(02)00262-0. [DOI] [PubMed] [Google Scholar]
- Pufe T, Petersen WJ, Mentlein R, Tillmann BN. The role of vasculature and angiogenesis for the pathogenesis of degenerative tendons disease. Scand J Med Sci Sports. 2005;15:211–222. doi: 10.1111/j.1600-0838.2005.00465.x. [DOI] [PubMed] [Google Scholar]
- Puxkandl R, Zizak I, Paris O, et al. Viscoelastic properties of collagen: synchrotron radiation investigations and structural model. Phil Trans R Soc Lond B Biol Sci. 2002;357:191–197. doi: 10.1098/rstb.2001.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Fox AM, Alexopoulos LG, et al. IL-1beta decreases the elastic modulus of human tenocytes. J Appl Physiol. 2006;101:189–195. doi: 10.1152/japplphysiol.01128.2005. [DOI] [PubMed] [Google Scholar]
- Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology (Oxford) 2006;45:508–521. doi: 10.1093/rheumatology/kel046. [DOI] [PubMed] [Google Scholar]
- Rees SG, Davies JR, Tudor D, et al. Immunolocalisation and expression of proteoglycan 4 (cartilage superficial zone proteoglycan) in tendon. Matrix Biol. 2002;21:593–602. doi: 10.1016/s0945-053x(02)00056-2. [DOI] [PubMed] [Google Scholar]
- Reeves ND. Adaptation of the tendon to mechanical usage. J Musculoskelet Neuronal Interact. 2006;6:174–180. [PubMed] [Google Scholar]
- Robinson PS, Lin TW, Reynolds PR, Derwin KA, Iozzo RV, Soslowsky LJ. Strain-rate sensitive mechanical properties of tendon fascicles from mice with genetically engineered alterations in collagen and decorin. J Biomech Eng. 2004;126:252–257. doi: 10.1115/1.1695570. [DOI] [PubMed] [Google Scholar]
- Roukis TS, Hurless JS, Page JC. Functional significance of torsion of the tendon of tibialis posterior. J Am Podiatr Med Assoc. 1996;86:156–163. doi: 10.7547/87507315-86-4-156. [DOI] [PubMed] [Google Scholar]
- Rufai A, Ralphs JR, Benjamin M. Structure and histopathology of the insertional region of the human Achilles tendon. J Orthop Res. 1995;13:585–593. doi: 10.1002/jor.1100130414. [DOI] [PubMed] [Google Scholar]
- Sanders JE, Goldstein BS. Collagen fibril diameters increase and fibril densities decrease in skin subjected to repetitive compressive and shear stresses. J Biomech. 2001;34:1581–1587. doi: 10.1016/s0021-9290(01)00145-2. [DOI] [PubMed] [Google Scholar]
- Sargon MF, Ozlu K, Oken F. Age-related changes in human tendo calcaneus collagen fibrils. Saudi Med J. 2005;26:425–428. [PubMed] [Google Scholar]
- Scapinelli R. Studies on the vasculature of the human knee joint. Acta Anat (Basel) 1968;70:305–331. doi: 10.1159/000143133. [DOI] [PubMed] [Google Scholar]
- Schoffl VR, Schoffl I. Injuries to the finger flexor pulley system in rock climbers: current concepts. J Hand Surg [Am] 2006;31:647–654. doi: 10.1016/j.jhsa.2006.02.011. [DOI] [PubMed] [Google Scholar]
- von Schroeder HP, Botte MJ. Functional anatomy of the extensor tendons of the digits. Hand Clin. 1997;13:51–62. [PubMed] [Google Scholar]
- von Schroeder HP, Botte MJ. Anatomy and functional significance of the long extensors to the fingers and thumb. Clin Orthop Relat Res. 2001;383:74–83. doi: 10.1097/00003086-200102000-00010. [DOI] [PubMed] [Google Scholar]
- Schumacher BL, Block JA, Schmid TM, Aydelotte MB, Kuettner KE. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys. 1994;311:144–152. doi: 10.1006/abbi.1994.1219. [DOI] [PubMed] [Google Scholar]
- Schweitzer R, Chyung JH, Murtaugh LC, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- Screen HR, Lee DA, Bader DL, Shelton JC. An investigation into the effects of the hierarchical structure of tendon fascicles on micromechanical properties. Proc Inst Mech Eng [H] 2004;218:109–119. doi: 10.1243/095441104322984004. [DOI] [PubMed] [Google Scholar]
- Scutt N, Rolf CG, Scutt A. Glucocorticoids inhibit tenocyte proliferation and Tendon progenitor cell recruitment. J Orthop Res. 2006;24:173–182. doi: 10.1002/jor.20030. [DOI] [PubMed] [Google Scholar]
- Semple C. The design of tendons and their sheaths. In: Owen R, Goodfellow J, Bullough P, editors. Scientific Foundations of Orthopaedics and Traumatology. London: Heinemann; 1980. pp. 74–78. [Google Scholar]
- Shaw HM, Benjamin M. Structure-function relationships of entheses in relation to mechanical load and exercise. Scand J Med Sci Sports. 2007;17:203–315. doi: 10.1111/j.1600-0838.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- Shaw HM, Santer RM, Watson AHD, Benjamin M. Adipose tissue at entheses: the innervation and cell composition of the retromalleolar fat pad associated with the rat Achilles tendon. J Anat. 2007;211:436–443. doi: 10.1111/j.1469-7580.2007.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukunami C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol. 2006;298:234–247. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Smith RK, Webbon PM. Harnessing the stem cell for the treatment of tendon injuries: heralding a new dawn? Br J Sports Med. 2005;39:582–584. doi: 10.1136/bjsm.2005.015834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standring S. Gray's Anatomy: The Anatomical Basis of Clinical Practice. Edinburgh: Elsevier Churchill Livingstone; 2004. [Google Scholar]
- Strocchi R, De Pasquale V, Guizzardi S, et al. Human Achilles tendon: morphological and morphometric variations as a function of age. Foot Ankle. 1991;12:100–104. doi: 10.1177/107110079101200207. [DOI] [PubMed] [Google Scholar]
- Sun Y, Berger EJ, Zhao C, An KN, Amadio PC, Jay G. Mapping lubricin in canine musculoskeletal tissues. Connect Tissue Res. 2006a;47:215–221. doi: 10.1080/03008200600846754. [DOI] [PubMed] [Google Scholar]
- Sun Y, Berger EJ, Zhao C, Jay GD, An KN, Amadio PC. Expression and mapping of lubricin in canine flexor tendon. J Orthop Res. 2006b;24:1861–1868. doi: 10.1002/jor.20239. [DOI] [PubMed] [Google Scholar]
- Szoke G, Lee SH, Simpson AH, Prescott J. Response of the tendon during limb lengthening. J Bone Joint Surg. 2005;87:583–587. doi: 10.1302/0301-620X.87B4.15393. [DOI] [PubMed] [Google Scholar]
- Szomor ZL, Appleyard RC, Murrell GA. Overexpression of nitric oxide synthases in tendon overuse. J Orthop Res. 2006;24:80–86. doi: 10.1002/jor.20009. [DOI] [PubMed] [Google Scholar]
- Theobald PS, Bydder G, Dent C, Nokes L, Pugh N, Benjamin M. The functional anatomy of Kager's fat pad in relation to retrocalcaneal problems and other hindfoot disorders. J Anat. 2006;208:91–97. doi: 10.1111/j.1469-7580.2006.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillmann B, Koch S. Functional adaptation processes of gliding tendons. Sportverletz Sportschaden. 1995;9:44–50. doi: 10.1055/s-2007-993421. [DOI] [PubMed] [Google Scholar]
- Toumi H, Higashiyama I, Suzuki D, et al. Regional variations in human patellar trabecular architecture and the structure of the proximal patellar tendon enthesis. J Anat. 2006;208:47–57. doi: 10.1111/j.1469-7580.2006.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozer S, Duprez D. Tendon and ligament: development, repair and disease. Birth Defects Res C Embryo Today. 2005;75:226–236. doi: 10.1002/bdrc.20049. [DOI] [PubMed] [Google Scholar]
- Tsuzaki M, Guyton G, Garrett W, et al. IL-1 beta induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J Orthop Res. 2003;21:256–264. doi: 10.1016/S0736-0266(02)00141-9. [DOI] [PubMed] [Google Scholar]
- Valero-Cuevas FJ, Yi JW, Brown D, McNamara RV, Paul C, Lipson H. The tendon network of the fingers performs anatomical computation at a macroscopic scale. IEEE Trans Biomed Eng. 2007;54:1161–1166. doi: 10.1109/TBME.2006.889200. [DOI] [PubMed] [Google Scholar]
- Vogel KG, Koob TJ. Structural specialization in tendons under compression. Int Rev Cytol. 1989;115:267–293. doi: 10.1016/s0074-7696(08)60632-4. [DOI] [PubMed] [Google Scholar]
- Waggett AD, Benjamin M, Ralphs JR. Connexin 32 and 43 gap junctions differentially modulate tenocyte response to cyclic mechanical load. Eur J Cell Biol. 2006;85:1145–1154. doi: 10.1016/j.ejcb.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Walters J, Solomons M, Davies J. Gluteus minimus: observations on its insertion. J Anat. 2001;198:239–242. doi: 10.1046/j.1469-7580.2001.19820239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Li Z, Yang G, Khan M. Repetitively stretched tendon fibroblasts produce inflammatory mediators. Clin Orthop Relat Res. 2004;422:243–250. doi: 10.1097/01.blo.0000126337.65685.e4. [DOI] [PubMed] [Google Scholar]
- Wong MW, Tang YN, Fu SC, Lee KM, Chan KM. Triamcinolone suppresses human tenocyte cellular activity and collagen synthesis. Clin Orthop Relat Res. 2004;421:277–281. doi: 10.1097/01.blo.0000118184.83983.65. [DOI] [PubMed] [Google Scholar]
- Wong MW, Tang YY, Lee SK, Fu BS. Glucocorticoids suppress proteoglycan production by human tenocytes. Acta Orthop. 2005;76:927–931. doi: 10.1080/17453670610046118. [DOI] [PubMed] [Google Scholar]
- Wood Jones F. The Principles of Anatomy as Seen in the Hand. London: Ballière, Tindall and Cox; 1944a. [Google Scholar]
- Wood Jones F. Structure and Function as Seen in the Foot. London: Bailliere, Tindall and Cox; 1944b. [Google Scholar]
- Xia W, Szomor Z, Wang Y, Murrell GA. Nitric oxide enhances collagen synthesis in cultured human tendon cells. J Orthop Res. 2006;24:159–172. doi: 10.1002/jor.20060. [DOI] [PubMed] [Google Scholar]
- Yahia LH, Drouin G. Microscopical investigation of canine anterior cruciate ligament and patellar tendon: collagen fascicle morphology and architecture. J Orthop Res. 1989;7:243–251. doi: 10.1002/jor.1100070212. [DOI] [PubMed] [Google Scholar]
- Yang G, Im HJ, Wang JH. Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene. 2005;363:166–172. doi: 10.1016/j.gene.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa T, Takizawa F, Iizawa F, et al. Homeobox protein MSX2 acts as a molecular defense mechanism for preventing ossification in ligament fibroblasts. Mol Cell Biol. 2004;24:3460–3472. doi: 10.1128/MCB.24.8.3460-3472.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaffagnini S, Golano P, Farinas O, et al. Vascularity and neuroreceptors of the pes anserinus: anatomic study. Clin Anat. 2003;16:19–24. doi: 10.1002/ca.10073. [DOI] [PubMed] [Google Scholar]
- Zbrodowski A, Gajisin S, Grodecki J. The anatomy of the digitopalmar arches. J Bone Joint Surg [Br] 1981;63:108–113. doi: 10.1302/0301-620X.63B1.7204462. [DOI] [PubMed] [Google Scholar]
- Zeisig E, Ohberg L, Alfredson H. Sclerosing polidocanol injections in chronic painful tennis elbow-promising results in a pilot study. Knee Surg Sports Traumatol Arthrosc. 2006;14:1218–1224. doi: 10.1007/s00167-006-0156-0. [DOI] [PubMed] [Google Scholar]
- Zhang AY, Chang J. Tissue engineering of flexor tendons. Clin Plast Surg. 2003;30:565–572. doi: 10.1016/s0094-1298(03)00074-9. [DOI] [PubMed] [Google Scholar]