Abstract
To examine the nature of hemispheric lateralization for neural processes underlying verbal fluency and visuo-spatial attention, we investigated a single pair of handedness discordant monozygotic (MzHd) twins. Imaging of the brain was undertaken using magnetic resonance imaging (MRI) and functional magnetic resonance imaging (fMRI) in combination with manual performance tasks. The twins were discordant for MRI anatomical asymmetries of the pars triangularis and planum temporale, whose asymmetry was consistent with verbal laterality on fMRI. Thus, the right-handed twin had left lateralized verbal with right lateralized visuo-spatial attention, while the left-handed twin had right lateralized verbal with left lateralized visuo-spatial activation; these data lend further support for to the conclusions of Sommer et al.
Keywords: cerebral laterality, hemispheric specialization, landmark, left handedness, line bisection, verbal fluency
Introduction
In the great majority of people, core verbal functions are supported by networks in the left cerebral hemisphere. By contrast, many core visuo-spatial functions are supported by right hemisphere networks (Marshall & Fink, 2003; Stephan et al. 2007). There are, however, rare exceptions to this complementary hemispheric specialization in right handers (RH), with somewhat more frequent exceptions in left handers (LH) (Knecht et al. 2001; Jansen et al. 2005). Evidence of atypical verbal lateralization has derived, for example, from cases of aphasic language disorder after right hemisphere damage (i.e. crossed aphasia) (Annett & Alexander, 1996), while evidence for atypical spatial lateralization has come from atypical right visuo-spatial neglect persisting after left hemisphere damage (Beis et al. 2004). The development of functional magnetic resonance imaging (fMRI) has allowed such atypical lateralizations to be investigated in healthy brains (Knecht et al. 2001; Tzourio-Mazoyer et al. 2004). These studies have indicated an increased incidence of atypical language lateralization in healthy non-right-handed people (Pujol et al. 1999; Knecht et al. 2000; Szaflarski et al. 2002).
One approach to examining the nature and putative consequences of functional hemispheric lateralization is to study healthy monozygotic (Mz) twins who are discordant for writing hand (Hd) (Sommer et al. 2002). Discordant handedness occurs in some 20–25% of Mz twins (cf. Annett, 2002; Singh et al. 2002). Sommer et al. (1999) have described one pair of female MzHd twins (with Handedness Preference Inventory scores of +100 and –100, respectively), in whom the right-handed twin showed typical left cerebral lateralization on a verbal task, with some degree of right cerebral lateralization on a visuo-spatial mental rotation task. The left-handed twin displayed the reverse pattern: right hemisphere lateralization for verbal tasks, with some degree of left hemisphere lateralization for visuo-spatial tasks.
In a constructive replication of Sommer et al. (1999) we now describe a pair of healthy MzHd twins with strong handedness preferences for writing, whose fMRI shows double crossed cerebral laterality, but in the left-handed twin only. Going beyond the original study of Sommer et al. (1999), we provide quantitative structural MRI analyses – focusing on the frontal, temporal and parietal cortices, including the frontal [pars opercularis (POP) and pars triangularis (PTR)] and the temporal [planum temporale (PT)] (cf. Vernooij et al. 2007) – in addition to fMRI and manual performance results. A further difference pertains to the fMRI verbal and visuo-spatial attention tasks employed: verbal fluency (Amunts et al. 2004), and line bisection judgment (Harvey et al. 1995; Fink et al. 2000, 2001), with regions of interest in the frontal and parietal cortices, respectively. (The verbal task ordinarily lateralizes to the left hemisphere, while the visuo-spatial attention task is expected to lateralize to the right, in right-handed singletons.) Using the uni- and bi-manual motor results combined with the visuo-spatial fMRI indices enables us to discuss whether the left hander's atypical lateralization is associated with a performance decrement relative to the dextral twin: evidence that helps to delineate the limits of normality.
Although models concerning genetic links between lateralization of (1) manual preference, (2) manual performance, (3) cognitive functions and (4) cerebral function have been developed, they lack the evidence to link them (cf. Provins, 1997). Population-based studies aimed at rigorous data collection on all four levels (1–4) are required. To date: (1) One notably large study, a meta-analysis of 20 000 twin pairs, has addressed the statistical relation between hand preference and genetics. Namely, Medland et al. (2006) reported a 25% genetic effect on handedness preference (leaving 75% unaccounted for (1 and 2). Pujol et al. (1999) linked handedness and cerebral laterality using functional imaging studies in singletons, reporting 4% of RH (n = 50) and 24% of LH (n = 50) showing atypical cerebral laterality on verbal fluency (although visuo-spatial tasks were not employed). Other studies employing smaller subject numbers and differing techniques (e.g. tasks and scanning methods) have obtained larger or smaller percentages of LH cerebral atypicality (e.g. Hund-Georgiadis et al. 2002) (3 and 4). Evidence linking these two levels has been lacking. As Annett (2002), Klar (2003) and McManus (1983, 1995, 2002) have articulated, the factors coded for genetically have ranged from typical versus atypical cerebral laterality (level 4) to random laterality at all four levels (i.e. 1, manual preference; 2, manual performance; 3, cognitive; and 4, cerebral function); a complex picture indeed. Moreover, Singh et al. (2002) describe the potential epigenetic explanations for within-twin pair differences. There is little doubt that explanation of cerebral laterality and its specific reference to twins will not be resolved by evidence from a single twin pair (or two).
Case description
The twins were 61-year-old females listed on the St Thomas’ UK Adult Twin Registry (Spector & MacGregor, 2002). Zygosity had been established according to a questionnaire method with 91% reliability (Jackson et al. 2001). The subject selection criteria for twins described here were: writing hands strongly discordant, healthy, adult, female, monozygotic, with normal medical and developmental histories. (This was part of a larger ongoing study, cf. Gurd et al. 2006.) The twins were of similarly normal birth-weight; the right-hander weighed 2.47 kg, and the left-hander (first born by 2.3 h), weighed 2.35 kg at birth, and there was no apparent indication of abnormal foetal development (such as twin–twin transfusion syndrome). As far as can be determined, their birth histories were normal, except that the LH twin was delivered with a fractured left arm (no further birth details were available). Both twins had a single hair whorl, in the clockwise direction. None of the immediate family members (i.e. siblings, parents, grand-parents or children) was left-handed, neither twin had switched their writing hand in childhood, and neither was a ‘hooked’ writer. It therefore appeared that their writing hand profiles were consistent with natural right- and left-handedness, respectively (cf. Dellatolas et al. 1993; Dellatolas, 1994). As adults, they had chosen the same profession. At testing, both were fit and well; the LH twin had a slight left-hand tremor (dating from birth, probably related to the neonatal arm fracture), and she wore a brace on her right leg (due to an orthopaedic injury sustained in her twenties).
Methods
Neuroimaging: MRI of the brain
Image acquisition
All images (structural and functional) were acquired on the same 1.5-T Siemens Magnetom SONATA (Erlangen, FRG) MRI scanner. fMRI was carried out using echo planar imaging (EPI) with whole brain coverage, using the standard head coil for radio frequency (RF) transmission and signal reception. Sequences with the following parameters were employed: repetition time (TR) = 3000 ms, echo time (TE) = 50 ms, voxel size = 3 × 3 × 3 mm3. Using a mid-sagittal scout image, 36 axial slices were positioned to cover the whole brain. Anatomical whole brain images were obtained using a T1-weighted, three-dimensional (3D) gradient-echo pulse sequence (FLASH, fast low-angle shot) with the following parameters: TR = 1200 ms, TE = 5.6 ms, TI 19° flip angle, matrix size = 160 × 256 × 208, voxel size = 1 mm isotropic, acquisition = coronal, averages = 3.
Quantitative MRI
Quantitative (structural) MRI volume estimation of cortical language areas [i.e. the pars opercularis (POP), pars triangularis (PTR) and planum temporale (PT)] was carried out as per Keller et al. (2007). The pars opercularis and pars triangularis of the inferior frontal gyrus have been referred to as Broca's area (e.g. Broca, 1861; Foundas et al. 1995, 1996), which is associated with language production. The planum temporale, located on the dorsal aspect of the temporal lobe, is associated with auditory language comprehension (Shapleske et al. 1999), although cerebral networks participating in language are not limited to these cortical regions (Pulvermueller, 2002). The areas of grey matter within selected frontal and temporal language regions were quantified using the Cavalieri method in conjunction with point counting on MR images that had been: (1) realigned perpendicular to the bicommissural plane, and (2) demarcated on orthogonal sagittal, coronal and axial sections to provide markers for subsequent point counting on coronal sections. Realignment and demarcation was performed using BrainVoyager software (http://www.Brainvoyager.com). The anatomical definitions of the POP and PTR were consistent with those of Petrides & Pandya (2004), Petrides (2006) and Tomaiuolo et al. (1999). Definition of the PT was consistent with those of Anderson et al. (1999) and Kim et al. (2000). The POP was bordered caudally by the inferior precentral gyrus, dorsally by the inferior frontal sulcus, ventrally by the Sylvian fissure and rostrally by the ascending ramus of the Sylvian fissure. The PTR was bordered caudally by the anterior ascending ramus of the Sylvian fissure, dorsally by the inferior frontal sulcus and rostro-ventrally by the horizontal ramus of the Sylvian fissure. All grey matter between the deepest points (i.e. the fundus) of the inferior frontal sulcus and circular insular sulcus was quantified. The posterior boundary of the planum temporale was delineated as the plane where the Sylvian fissure turns to ascend in a more vertical plane (or if it remains horizontal, at its termination). The medial boundary was the deepest extent of the Sylvian fissure, and the anterior boundary was Heschl's sulcus. (In cases where there was more than one Heschl's gyrus, or it bifurcated at any point along its length, the posterior gyrus was included in the planum temporale.)
Realigned and demarcated images were imported into Easymeasure software (Puddephat, 1999) for volume estimation of the grey matter within each area. Point counting intensities were optimized to achieve a coefficient of error of less than 5% (Roberts et al. 2000). The separation between the grid array test points used for point counting of the pars opercularis and pars triangularis was 0.3 cm (i.e. 3 pixels), and 0.4 cm (i.e. 4 pixels) for the planum temporale. The slice interval was every section for the POP, and every second section for the PTR and PT. To extract approximate lobar volumes (frontal, temporal, parietal and occipital), an automated MR image analysis technique, ‘LowD’ (developed to quantify cerebral asymmetries), was applied (Barrick et al. 2005). After a series of fully automated image processing procedures, a mask demarcating approximate lobes was produced by drawing lobar boundaries onto a mid-sagittal 2D array using a rendered 3D reference image for integrating column voxels into relevant lobar compartments.
Fronto-occipital torques were determined from the lobar volumes, by summation of the asymmetry coefficients of the frontal and occipital lobes (which are typically rightward frontal, and leftward occipital). Torque is derived from the summation of the asymmetry coefficients of frontal and occipital lobes for both grey and white matter. Cerebral torque is a shape change of the cerebral hemispheres which when clockwise is manifested as a rightward frontal and leftward occipital bias (Bear et al. 1986; Toga & Thompson, 2003). Negative (i.e. anticlockwise) asymmetry coefficients indicate leftward asymmetry, whereas positive (i.e. clockwise) ones indicate increased fronto-occipital torque (i.e. increased rightward frontal and leftward occipital) (cf. Barrick et al. 2005).
fMRI
Image processing
fMRI image analysis including realignment, normalization and statistical analysis was performed using Statistical Parametrical Mapping (SPM2, Wellcome Department of Imaging Neuroscience, London, UK) implemented in MATLAB (Mathworks Inc., Sherborn, MA, USA). The first two volumes of data acquisition, during which the MR signal reaches a steady state, were not reconstructed. The image time series was realigned to the first image of the remaining time series to correct for head movement between scans. The 3D anatomical data sets were then co-registered to the EPI image sets and spatially normalized to the stereotactic space of the EPI-MNI (Montreal Neurological Institute) brain using templates provided by SPM2. The voxel size after normalization was then 2 × 2 × 2 mm. Data were subsequently smoothed with an isotropic Gaussian kernel of 6 mm for single subject analysis at full-width-half-maximum.
Tasks
The first task administered was verbal fluency. In the MRI scanner, eight letters (F, A, S, C, T, N, P, L), and eight semantic categories (furniture, animals, fruit, clothing, countries, shops, vegetables, and vehicles) were each presented centrally in yellow Arial font on a dark blue screen. The subjects were requested to covertly list as many words as possible beginning with the given letter (letter-initial fluency), or belonging to the given category (semantic fluency) cued on the screen, and to do so as quickly as possible without repeating any words. They were requested to think the words in their heads (rather than speaking them out loud), to avoid movement artefacts during scanning. The individual task instructions were presented as printed text on the screen prior to commencement. The entire experiment was continuous, and lasted for a total of 7.2 min. It began with a baseline condition, in which a plus sign bordered by three asterisks on either side in yellow Arial font was centred on a dark blue screen (*** + ***). This baseline lasted for 9 s, and was followed by the stimulus presentation (i.e. a letter), which lasted for 18 s. The 9-s baseline condition was then repeated, followed by the semantic stimulus (i.e. a category name), which lasted for 18 s. The paradigm then iterated until each of the letter-initial and semantic fluency cues had been individually presented.
The second task administered was visuo-spatial line bisection judgment (i.e. landmark task). Two different visuo-spatial tasks, one experimental (line bisection judgment) and one high-level baseline (line crossing), were performed in the scanner. Individual black horizontal lines subtending a visual angle of 6.12*1.19° were back-projected onto a white screen subtending a visual angle of 8.24*6.21°. The viewing distance was 170 cm. In the baseline task, each line either did or did not have a vertical line that crossed the horizontal at right angles. In the experimental task, every line had a vertical crossing mark which either correctly bisected the horizontal line (50%), crossed it slightly to the left or right, or crossed it far to the left or right (of true centre). To avoid participants keeping a constant centre point in mind, stimuli were presented randomly above, below, to the left or to the right of the screen's centre. Subjects pressed one of two buttons using the index finger for a yes-response, or the middle finger for a no-response.
Stimuli were presented pseudo-randomly, avoiding runs exceeding three consecutive identical answers. The experiment was split into two experimental runs (using different hands), each lasting 8.8 min. Subjects were trained on a PC outside of the scanner on these tasks immediately before scanning to ensure that they were familiar with the tasks, the instructions and the speed of the stimulus presentations. Four blocks of trials were presented randomly per task, per experimental run. Each block lasted 45 s, and was interrupted by a 21-s low-level baseline. During each low-level baseline, a white screen was presented for 15 s, followed by 6 s of instructions for the following block, with 25 stimuli presented per block. Stimulus-onset time was 550 ms, with an inter-stimulus interval of 1250 ms. During the line bisection task, 40% of the stimuli were correctly bisected, and 60% were incorrectly bisected (15% each for slightly left, slightly right, far to the left and far to the right). Subjects responded by pressing one of the buttons to indicate whether or not the vertical mark was at the centre of the horizontal line. During the line crossing baseline task, 60% of the lines were crossed as per the first task, while 40% had no cross. Subjects were requested to indicate via button press whether there was a mark on the line or not. During both tasks, the horizontal lines were presented with equal frequency, and were distributed pseudo-randomly across the four positions.
Statistical analysis
Following stereotactic normalization and smoothing, statistical analysis was performed in similar manner, for both tasks. Data were analysed by modelling the different conditions as reference waveforms (i.e. box car functions convolved with the haemodynamic response function in the context of the general linear model in SPM2). Subject-specific low-frequency drifts in signal were removed with a high-pass filter of 128 s. Thus, a design matrix was defined for each subject, to model the alternating periods of experimental conditions (using a delayed boxcar reference vector to account for the delayed cerebral haemodynamic response function following stimulus presentation). Six parameters obtained from the realignment procedure were also included as additional regressors in the design matrix. After estimation of all the model parameters, specific effects were tested by applying appropriate linear contrasts to the parameter estimates for each condition, yielding a t-statistic for each and every voxel. The t-statistics (transformed to z-statistics) constitute subject-specific statistical parametric maps (SPM{z}). The SPM{z}maps were interpreted with reference to the probabilistic behaviour of Gaussian random fields. For whole brain analyses, areas of activation were identified as significant only if they exceeded a threshold of P < 0.05 (corrected for multiple comparisons at the cluster level) (Friston et al. 1995), with an underlying voxel level of P = 0.001 (uncorrected).
The anatomical localization of local maxima was assessed using standard neuroanatomy reference tools (Talairach & Tournoux, 1988; Duvernoy, 1999), and superposition of the respective SPM{z} maps onto the subjects’ anatomical (i.e. structural MRI) images. Separate laterality measures were calculated for the verbal and the visuo-spatial tasks as: percentage of activated voxels, per number of voxels in the region of interest (dominant – non-dominant/dominant + non-dominant), where the left hemisphere is considered dominant for the verbal task and the right hemisphere for the visuo-spatial task. The verbal laterality index was obtained from all conditions versus baseline, and the spatial laterality index was based on the activations during line-bisection judgment (versus line-crossing). The laterality indices varied between –1 and +1, with positive indices indicating the expected, typical laterality (i.e. verbal = left hemisphere; visuo-spatial = right hemisphere) and negative ones indicating the unexpected, atypical laterality (i.e. verbal = right hemisphere; visuo-spatial = left hemisphere).
Region of interest (ROI) analyses
(1) Verbal: for verbal fluency, the summed contrast on both tasks (letter initial + semantic > baseline) was employed to represent the regions activated. Voxels surviving the threshold of P = 0.01 (corrected for peak height) were counted towards the mean letter-initial and semantic fluency (all conditions versus baseline). An ROI comprising the inferior and middle frontal gyri was then defined for each subject, for each hemisphere, using the Talairach Daemon (Lancaster et al. 1997) implemented in mri3dX (http://www.aston.ac.uk/lhs/staff/singhkd/mri3dX). (2) Visuo-spatial: to describe the regions activated during line bisection judgment, the contrast (line bisection > high-level baseline) was calculated for each subject. Voxels surviving the threshold of P = 0.01 (corrected for peak height) were counted towards the line-bisection (versus high-level baseline) values. One ROI for the entire parietal lobes was defined for each subject, for each hemisphere, using the Talairach Daemon (Lancaster et al. 1997) implemented in mri3dX.
Behavioural assessment
Handedness preference inventory (HPI)
The HPI questionnaire was returned by post, prior to scanning. It comprised 16 items from both the Briggs & Nebes (1975) and the Edinburgh Handedness (Oldfield, 1971) inventories (cf. Corey et al. 2001; Gurd et al. 2006). For each item on the questionnaire a self-reported hand preference for the particular motor task is recorded as ‘always right’ (+2), ‘usually right’ (+1), ‘no preference’ (0), ‘usually left’ (–1) and ‘always left’ (–2). The questions pertain to: handwriting, drawing, throwing a ball, using scissors for cutting paper, brushing your teeth with a toothbrush, using a knife for eating (later deleted from the analysis), using a spoon for eating, using a hammer, using a sports racket (e.g. for tennis), holding a broom for sweeping (upper hand), holding the top of a shovel, striking a match, unscrewing the lid of a jar, dealing playing cards (the card being dealt), holding thread to thread a needle, opening the lid of a box. The twins were originally recruited on the basis of strong laterality preference for writing; the RH scored +2, and the LH scored –2 on the HPI hand used for writing question (Q1). The values per item were summed, divided by 30, and multiplied by 100 to yield a handedness preference index (HPI) ranging from –100 to +100.
Manual performance laterality was assessed 1 day previous to scanning. The twins were tested in parallel, at the same time, on the same day, by different testers in different rooms of the same testing suite. Manual performance was measured on uni- and bi-manual tasks, which varied according to the degree of prior practice, and skill involved.
Finger tapping (WPS electronic tapping test, ETT)
Uni-manual performance was measured using an electronic finger tapping test (Nalcaci et al. 2001). This task is non-skilled and non-practised in everyday life. Subjects were required to tap on the button as rapidly as possible with their index finger, for 10 s. Ten trials were run, five with each hand. The order of administration was ABABABAB, where A indicates the writing hand. A laterality score was calculated using the formula (R – L/R + L) × 100.
Dot filling (Tapley & Bryden, 1985)
This uni-manual task is skilled as well as practised (in so far as it is similar to handwriting). Subjects are requested to mark as many dots as possible using a pencil, within 20 s. They are presented with circles, and asked to place a dot inside each one. The stimuli are given on a single sheet of A4 paper where the open circles are printed in four columns (linked at the top and bottom, to make a ‘W’ shape). A difference score (right hand average minus left hand average), and a laterality score (R – L/R + L) × 100 were calculated (where R and L refer to the right and left hand, irrespective of handedness for writing).
Peg moving (cf. Annett, 2002)
This uni-manual task is skilled but not practised. Following the procedures of Annett (2002), subjects were requested to move ten dowel pegs from one row of holes to another row on a purpose-built board, which was placed on a desk. Subjects were timed for their error-free (no dropped pegs) performance of trials for each hand (right versus left). They performed six trials in total, beginning with the writing hand (A), in the form ABABAB (where B refers to the non-writing hand). Results were scored as average time per hand, and the total was ‘left average minus right average’, such that the result is positive if the right hand is faster (L and R refer to hand used, irrespective of dominance). The method of calculating laterality scores was: (L – R/L + R) × 100 (Annett, 2002).
Bradykinesia laterality score (BLS) (Moore, 1987)
This is a motor assessment tool that assesses right and left hand usage on three separate timed tasks independently. It is a clinical tool designed for assessment of Parkinson's disease motor impairments. The assessment consists of the following three sub-tasks, which are each counted per hand, for a 20-s period. Testing begins with the dominant hand: (1) thumb tapping each ipsilateral finger in turn; (2) cycles alternating tapping the dorsum and the palm of the hand on the ipsilateral thigh; (3) number of taps of the index finger going between two marks placed 50 cm apart on the desk (at which the participant is seated). This is repeated with the non-dominant hand. To calculate the results, item (1) is divided by two, and then summed with items (2 and 3) to produce a sum (number of items/20 s) per hand. The BLS score is: score for dominant hand divided by score for non-dominant hand, all minus 1, then multiplied by 100; (score > 1 = dominant hand superiority).
Bi-manual co-ordination (Luria, 1969)
This bi-manual task was neither skilled nor practised. Subjects were requested to make simultaneous movements with the right and left hand, continuously tapping the lap while sitting. This alternated between fist and pat with right then left hand; with one hand a fist was tapped, while with the other a flat hand patted the lap. Subjects were requested to perform as quickly and accurately as possible for a total of 10 s. The total number of cycles and of errors made in sequential movements was counted by the experimenter. (Errors were reported as a percentage of the total number of cycles completed.)
Results
Neuroimaging: MRI of the brain
Quantitative MRI
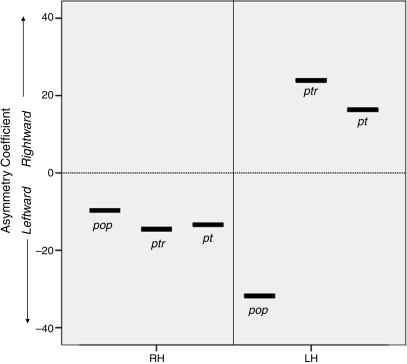
Volumes and volume asymmetries of the pars opercularis, pars triangularis, planum temporale and parietal lobes are presented in Table 1. Both twins had leftward asymmetry of the pars opercularis. The RH twin also had leftward asymmetry of the pars triangularis, planum temporale and parietal lobes. By contrast, the LH twin had rightward asymmetry of the pars triangularis, planum temporale and parietal lobes. Asymmetries of the language structures are illustrated in Fig. 1. Fronto-occipital torque measures showed positive (i.e. clockwise) values for both twins (RH = 5.52, LH = 12.46); hence there was no evidence of abnormal, absent or mirrored torque (within or between twins).
Table 1.
Quantitative MRI volume and volume asymmetry of the pars opercularis, pars triangularis and planum temporale (cm3). Proportional asymmetry coefficients of the pars opercularis, pars triangularis and planum temporal were determined using a standardized coefficient formula ((R – L)/(R + L) × 100), and asymmetry of the parietal lobes was automatically calculated using an absolute measure (R – L) within Low D software. Leftward asymmetries are negative and rightward asymmetries are positive. The right-handed twin (RH) had leftward asymmetry of all structures assessed while the left-handed twin (LH) only had leftward asymmetry of the pars opercularis
| Quantitative MRI volume | RH (cm3) | LH (cm3) |
|---|---|---|
| Left pars opercularis | 4.698 | 6.606 |
| Right pars opercularis | 3.807 | 3.420 |
| Pars opercularis asymmetry | –9.66 | –31.78 |
| Left pars triangularis | 5.454 | 6.516 |
| Right pars triangularis | 4.068 | 10.620 |
| Pars triangularis asymmetry | –14.56 | 23.95 |
| Left planum temporale | 2.304 | 2.464 |
| Right planum temporale | 1.760 | 3.424 |
| Planum temporale asymmetry | –13.39 | 16.30 |
| Left parietal lobe | 90.33 | 60.49 |
| Right parietal lobe | 86.70 | 60.92 |
| Parietal lobe asymmetry | –3.63 | 0.43 |
Fig. 1.
Asymmetry coefficients for the pars opercularis (POP), pars triangularis (PTR) and planum temporale (PT) in the right-handed (RH) and left-handed (LH) twins. The pars opercularis is leftward asymmetrical in both twins. However, the pars triangularis and planum temporale are leftward asymmetrical in the right-handed twin, and rightward asymmetrical in the left-handed twin.
fMRI
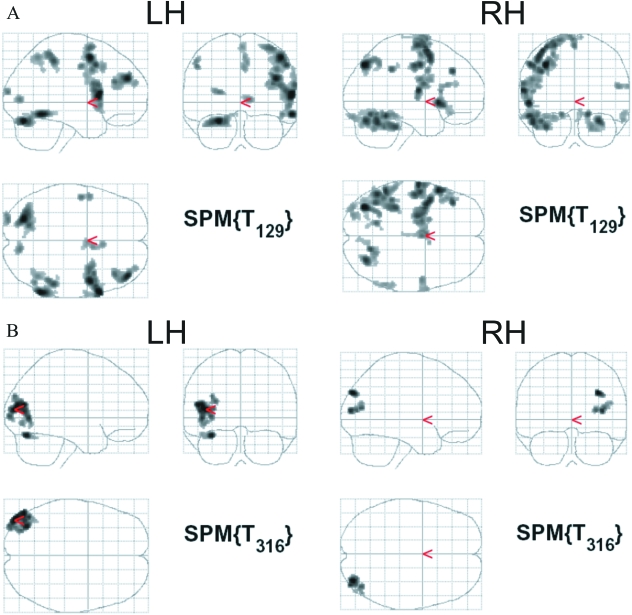
There were significant and extensive activations for both twins on the verbal fluency results (summed: letter initial and semantic versus baseline). The three largest clusters of activation for the RH twin were in the left precentral gyrus, the left medial inferior temporal gyrus and the right cerebellum, and for the LH twin in the right inferior frontal gyrus, the right posterior middle frontal gyrus and the left cerebellum. With respect to the ROI analyses on the inferior and middle frontal gyri, the RH twin obtained a verbal laterality score of +0.91, indicating typical left hemisphere lateralization. The left-handed twin obtained an fMRI verbal laterality score of –0.92 for verbal fluency, indicating a right hemisphere lateralization for the inferior and middle frontal gyri (see Table 2 and Fig. 2 for whole brain analysis).
Table 2.
fMRI verbal activations (whole brain) during the verbal fluency task (all conditions summed vs baseline) for the left- (LH) and right- (RH) handed twins
| Region | Side | x | y | z | Cluster size | T (voxel level) |
|---|---|---|---|---|---|---|
| LH: verbal task | ||||||
| Inferior frontal gyrus | R | 60 | 14 | 10 | 685** | 9.17 |
| Posterior middle frontal gyrus | R | 48 | 4 | 54 | 559** | 8.85 |
| Anterior middle frontal gyrus | R | 42 | 48 | 28 | 420** | 8.33 |
| Postcentral gyrus | L | –52 | –6 | 44 | 80* | 5.59 |
| Cingulate sulcus | R | 8 | 8 | 48 | 232** | 4.90 |
| Supramarginal gyrus | R | 52 | –40 | 50 | 351** | 6.70 |
| Middle occipital gyrus | L | –26 | –94 | 12 | 73* | 5.56 |
| Inferior temoral gyrus | R | 62 | –54 | –14 | 248** | 8.88 |
| Calcarine sulcus | R | 10 | –80 | 4 | 85* | 5.44 |
| Cerebellum | L | –26 | –76 | –22 | 523** | 9.13 |
| RH: verbal task | ||||||
| Inferior frontal gyrus | L | –56 | 16 | 2 | 302** | 6.65 |
| Middle frontal gyrus | L | –40 | 34 | 36 | 72* | 5.81 |
| Precentral gyrus | L | –64 | –8 | 14 | 1348** | 6.45 |
| Postcentral gyrus | L | –54 | –6 | 44 | 174** | 6.08 |
| R | 54 | –4 | 18 | 100** | 4.63 | |
| Medial inferior temporal gyrus | L | –50 | –58 | –26 | 980** | 6.28 |
| Lateral inferior temporal gyrus | R | 60 | –48 | –22 | 108** | 4.14 |
| L | –54 | –48 | –18 | |||
| Angular gyrus | L | –36 | –74 | 44 | 308** | 7.35 |
| Intraparietal sulcus | L | –44 | –40 | 40 | 73* | 5.34 |
| Inferior lingual gyrus | L | –16 | –60 | –16 | 70* | 5.11 |
| Inferior occipital gyrus | L | –34 | –88 | –20 | 120** | 4.45 |
| Cerebellum | R | 30 | –72 | –24 | 469** | 6.22 |
MNI coordinates refer to significantly activated clusters within an area of activation. x is the distance in millimetres to the right (+) or left (–) of the midsagittal (interhemispheric) line; y is the distance anterior (+) or posterior (–) to the vertical plane through the anterior commissure; and z is the distance above (+) or below (–) the intercommissural line. R = right, L = left, M = medial.
P < 0.05
P < 0.01, both corrected at the cluster level.
Fig. 2.
fMRI activations for the right-handed (RH) and left-handed (LH) twins during (A) verbal fluency: all conditions versus baseline, and (B) visuo-spatial: line bisection judgment versus high-level baseline (line crossing).
On the line bisection judgment task, there were significant and extensive activations for each twin, as per Fink et al. (2001, fig 1c) – a similar but grouped study (n = 11) of line bisection judgment (i.e. landmark) – in which parietal and occipital cortices showed significant effects. From Table 3 it can be seen that the local regions of maximum activation (height and extent) were slightly different in the left- and right-handed twins; the RH's maxima were in the right middle occipital gyrus and the right posterior intraparietal sulcus, whereas the LH's maxima were in the left occipito-temporal junction and the left dorsal cerebellar hemisphere. All cluster sizes were large, and therefore extended well beyond the named regions of maximal activation. It would appear that the LH had more extensive activation than her twin. The ROI was derived from Fink et al. (2001), a hypothesis-driven analysis limited to the parietal lobes. (The ROI analysis procedure in SPM adopts a lower threshold for significance in the ROI, as opposed to in whole brain analyses.) Specifically, the RH twin scored +0.50, indicating the expected right hemisphere lateralization, whereas the LH twin obtained an fMRI laterality score of –0.72, indicating left parietal lateralization on this visuo-spatial task (see Table 3, Fig. 2). Percentage errors on the line bisection judgment task were low and fairly well matched between twins, and between hands: 12%, 12% (RH dominant, non-dominant), 2%, 7% (LH dominant, non-dominant), and on the line crossing task: 4%, 2% (RH dominant, non-dominant), 4%, 3% (LH dominant, non-dominant).
Table 3.
fMRI visuo-spatial activations during the visuo-spatial line bisection judgment task (line bisection vs. line crossing) for the left- (LH) and right- (RH) handed twins
| Region | Side | x | y | z | Cluster size | T (voxel level) |
|---|---|---|---|---|---|---|
| LH: visuo-spatial task | ||||||
| Occipito-temporal junction | L | –42 | –88 | 10 | 628** | 5.00 |
| Dorsal cerebellar hemisphere | L | –34 | –74 | –20 | 104** | 4.71 |
| RH: visuo-spatial task | ||||||
| Middle occipital gyrus | R | 38 | –88 | 10 | 170** | 4.77 |
| Posterior intraparietal sulcus | R | 32 | –84 | 32 | 81* | 5.36 |
MNI coordinates refer to significantly activated clusters within an area of activation. x is the distance in millimetres to the right (+) or left (–) of the midsagittal (interhemispheric) line; y is the distance anterior (+) or posterior (–) to the vertical plane through the anterior commissure; and z is the distance above (+) or below (–) the intercommissural line. R = right, L = left, M = medial.
P < 0.05
P < 0.01, both corrected at the cluster level.
The region names and coordinates which are listed per cluster in Tables 2 and 3 refer only to the fMRI whole brain activations; they do not refer to the selected ROIs employed for the ROI analyses. (For the line bisection judgment task, for example, the parietal lobe activation is contained within the region entitled occipito-temporal junction, an extensive region consisting of 628 voxels.)
Behavioural: hand preference and performance
On the HPI, the RH twin scored +90 and the LH twin scored –50. The uni-manual performance results are presented in Table 4, which shows raw, laterality and z-score values for the tasks, relative to a comparable group of female MzHd twins with normal birth weights and birth histories. These are based on the means and standard deviations for 19/20 twin pairs reported in Gurd et al. (2006) (z-score = group mean – individual mean, divided by group standard deviation). (The current twin pair completed that group of 20 pairs. The means and standard deviations for the other 19 twin pairs were: RH: dot filling mean = 20.89, SD = 7.07; peg moving mean = 4.19, SD = 3.97; finger tapping mean = 5.27, SD = 6.57; LH: dot filling mean = –16.57, SD = 8.40; peg moving mean = –2.71, SD = 5.66; finger tapping mean = –3.03, SD = 8.28.) Table 4 also shows raw speed and accuracy measures for the bi-manual task, which was not performed on the larger group.
Table 4.
Motor performance results for dot filling (dot), peg moving (peg), finger tapping (tap) (mean and raw laterality scores, and z-scores based on n = 19 from Gurd et al. 2006), and co-ordination tasks for the right- (RH) and left- (LH) handed twins
| Uni-manual | Uni-manual | Uni-manual | Uni-manual | Bi-manual | ||
|---|---|---|---|---|---|---|
| Twin | Hand | Dot (no.) | Peg (s) | Tap (no.) | BLS (no.) | Co-ordination [cycles (% errors)] |
| RH | rh | 36.5 | 7.95 | 59.5 | 71.5 | n/a |
| lh | 24.5 | 19.30 | 48.4 | 67 | n/a | |
| laterality | +20 | +13 | +10.29 | +6.7 | 7.4 (18) | |
| (z-score) | –0.13 | +2.22 | +0.76 | n/a | n/a | |
| LH | rh | 29.0 | 8.82 | 54.0 | 71 | n/a |
| lh | 40.0 | 8.87 | 57.2 | 75 | n/a | |
| laterality | –16.00 | 0.00 | –2.88 | 5.6 | 7.0 (24) | |
| (z-score) | –0.07 | +0.48 | –0.02 | n/a | n/a |
(rh), performed with right hand; (lh), performed with left hand.
The RH twin was: (1) Right handed (versus mixed). She scored +2 for a strong preference to write with the right hand. She also preferred to perform most other manual tasks with her right hand; her HPI score was +90. (2) Consistently right handed with respect to performance superiority. All her uni-manual laterality values were positive, and all her z-scores were either close to zero (i.e. identical to the comparison group mean) or highly positive (i.e. strongly right hand superior). Her bradykinesia scores were right hand = 71.5, left hand = 67, laterality score = 6.7, all within normal limits and indicating right hand superiority, with no evidence of Parkinsonism (Moore, 1987). The outstanding value (a z-score greater than 2 SD above the comparison group mean) was on a task which was skilled but not practised, namely peg moving. This was the least sensitive manual performance task (Gurd et al. 2006). Closer analysis of the peg moving data revealed that on individual trials the timed results (seconds per trial) were: RH, 1 (first trial, right hand) 8 s; 2 (left) 11.15 s; 3 (right) 8.40 s; 4 (left) 9.87 s; 5 (right) 7.46 s; 6 (left) 9.81 s; and LH 1 (first trail, left hand) 9.84 s; 2 (right) 8.81 s; 3 (left) 8.18 s; 4 (right) 8.96 s; 5 (left) 8.59 s; 6 (right) 8.71 s. To further probe the high z-score in the RH twin, the data were trimmed (by removing a high value of 11.15 s on the first trial with the left hand; trial 2). For consistency, the first left and right hand were removed per twin; the trimmed values became: RH mean 7.93 s (right hand), 9.84 s (left hand). Compared with the raw mean (and SD) values from the group of 20 twin pairs reported in Gurd et al. (2006), the trimmed z-scores for the RH twin were +1.73 (right) and –0.65 (left). This brought the RH twin's right-hand performance within normal limits (i.e. ±2 standard deviations from the comparison group mean, or z-score range: –2 to +2). It indicates that the RH twin had superior performance with her right hand (not that she was particularly poor with her left hand).
The LH twin was: (1) Not fully left handed, but more aptly described as non-right handed. Although she strongly preferred to write with the left hand (i.e. she scored –2 for writing hand), she only preferred to perform three-quarters of the other manual tasks with her left hand; HPI = –50. (2) Not consistently left hand dominant with respect to uni-manual performance superiority, as two-thirds of her raw values were negative (indicating left hand superiority), and one was zero. On no uni-manual task was her performance superior with the right hand. Her bradykinesia scores, right hand = 71, left hand = 75, laterality score = 5.6, indicated superior left-hand performance, which was slightly better than her sister's (particularly with respect to the left-hand scores; the right-hand scores were identical), and there was no evidence of Parkinsonism (Moore, 1987). On the bi-manual co-ordination task, the LH twin was equally rapid (mean 7.4 cycles per 10 s), but slightly less accurate (24% errors, versus RH's 18%).
Discussion
There has been considerable controversy over the last 100 years concerning whether human left handedness is a normal biological variant, as opposed to being associated with cognitive, motor or neurological impairment (McManus, 1983, 2002; Sicotte et al. 1999; Beaton et al. 2000; Annett, 2002, 2003, 2004; Hund-Georgiadis et al. 2002; Knecht et al. 2002; Szaflarski et al. 2002; Dragovic et al. 2005). Not surprisingly, previous attempts to resolve this issue by testing groups of left- and right-handed singletons have led to conflicting conclusions (cf. Floel et al. 2005; Gurd et al. 2006; Hervé et al. 2006). By contrast, the present study employs a single case-control method, employing strongly handedness discordant Mz twins (cf. Steinmetz et al. 1995; Geschwind et al. 2002). According to this method, a left-handed twin is matched with her ‘ideal’ right-handed genetic control (but cf. Singh et al. 2002). Comparisons between fMRI cerebral lateralities are made in light of data on laterality of hand preference and performance, which show results within normal limits for both twins; this in the context of healthy brains as evidenced by quantitative structural MRIs. Previous studies may not have selected for strength of writing hand preference (cf. Sommer et al. 1999; Sommer et al. 2002), which may be a significant oversight (cf. Peters et al. 2006).
Quantitative MRI
Although the twins are discordant for structural asymmetries of two of the three cortical language areas measured, it is not possible to draw functional conclusions from this fact. Both twins had leftward volume asymmetry of the pars opercularis. The LH twin had atypical rightward asymmetry of the pars triangularis and planum temporale (Foundas et al. 1994, 1995, 1998; but cf. Keller et al. 2007), whilst the RH twin had leftward asymmetry in both. These anatomical regions were defined using gross morphological landmarks that are generally considered to demarcate the pars opercularis, pars triangularis and planum temporale from adjacent cortex. However, it should be noted that there are occasional discrepancies in definitions, particularly of the planum temporale, where posterior-most definitions might include landmarks other than the termination of the Sylvian fissure when horizontal, or when the Sylvian fissure turns to ascend vertically (Zetzsche et al. 2001).
With respect to the structural MRI of language areas, some (but not all) of the findings of Foundas and colleagues are consistent with the results presented here. Foundas et al. (1994) reported leftward asymmetry of the planum temporale in 10/10 epilepsy patients whose language was left lateralized, with rightward asymmetry of the same structure in the one patient whose language was right lateralized as assessed by the Wada test. They also reported a similar association of structural asymmetry of the pars triangularis with functional language lateralization in epilepsy patients using the Wada test (Foundas et al. 1996). Furthermore, Foundas et al. (1995, 1998) reported significant leftward asymmetry of the pars triangularis in right-handed singletons, and a loss of this leftward asymmetry in left handers. The earlier study reported that assessment of the combined asymmetry coefficients of the pars triangularis and planum temporale are most reliably associated with handedness (Foundas et al. 1995). Our results are consistent with those of Foundas et al. (1994, 1995, 1996, 1998), possibly supporting an association of language lateralization and handedness with asymmetry of the pars triangularis and planum temporale, but not with those of Knaus et al. (2007) (from the same group). Both fronto-occipital torque measures were positive for the RH and LH twins, indicating rightward asymmetry. This clockwise cerebral torque is considered to be the typical brain shape in healthy brains (LeMay, 1976; Keller et al. 2007).
Structure function issues remain necessarily contentious; most studies show some examples of dissociation between structural asymmetry of the pars triangularis and planum temporale (and pars opercularis) and handedness or language lateralization. Therefore, structural asymmetries of these regions do not necessarily have predictive value for the side of handedness or language lateralization, in so far as can be determined by currently available MRI technologies. There has also been some argument as to whether the dissociation between asymmetry of frontal language regions is of greater functional significance than that of planum temporale: ‘... the anatomical asymmetry of the supratemporal plane is neither necessary nor sufficient for the emergence of language function’ (Mesulam, 2000, p. 81). For example, Dorsaint-Pierre et al. (2006) have recently shown that asymmetry of the planum temporale has little predictive value with respect to the side of language lateralization. In particular, leftward planum temporale asymmetry was generally present regardless of the side of hemispheric language dominance. With respect to frontal regions, they reported an association between asymmetry of the posterior regions of the inferior frontal gyrus, particularly the pars opercularis, and language lateralization. This contrasts with our findings, as we show an association with pars triangularis, rather than with pars opercularis, a discrepancy that may be related to methodological differences between the two studies. In particular, Dorsaint-Pierre et al. (2006) automatically mapped cerebral asymmetry of the entire brain by determining grey matter concentration differences between homologous brain regions after spatial normalization and smoothing procedures. Conversely, we applied a manual stereological method on MR images in native space to estimate the volume of the convolutions of the inferior frontal gyrus by virtue of strict anatomical boundaries. We have previously shown that the voxel-based morphometric technique employed by Dorsaint-Pierre et al. (2006) may be less reliable than manual methods based on strict anatomical guidelines (Keller et al. 2004). It is also noteworthy that our subjects were neurologically healthy, in contrast to the brain-damaged subjects of Dorsaint-Pierre et al. (2006). Moreover, given the considerable variation in the morphology, volume and asymmetry of Broca's area between individuals, caution is urged in the interpretation of structure–function relationships described here (cf. Ono et al. 1990; Amunts et al. 1999, 2004; Tomaiuolo et al. 1999; Keller et al. 2007).
The pattern of functional lateralization versus structural asymmetry for the parietal lobe correlates differed again. While the RH was typically right lateralized on both fMRI and MRI measures, the LH only showed clear laterality on the fMRI, which was opposite in sign. Thus, the LH's structural MRI shows symmetry rather than asymmetry of the parietal lobes. We emphasize again that any ‘... relationship between structural asymmetry and functional lateralization … remains speculative’ (Vernooij et al. 2007, p. 1064). Structural asymmetries of cortical regions have long been conjectured to mediate handedness and lateralized human capabilities such as language (Geschwind & Levitsky, 1968; Galaburda & LeMay, 1978), but it is only relatively recently that such theories have been tested using neuroimaging techniques in healthy individuals, and these, unsurprisingly, have revealed substantial inter-individual variability.
fMRI
Differences in loci and extent of significant activations between twins can be accounted for by factors of: single subject data, inter-hemispheric variability in neuro-anatomy and overall activations between individuals, and the comparatively low magnet strength (i.e. 1.5 T). These differences are overcome by use of the fMRI laterality indices, in preference to raw comparisons (as per Sommer et al. 2002). Between-twin laterality discordances are found on both verbal and visuo-spatial tasks. Both twins are strongly lateralized on fMRI verbal laterality indices, and the LH twin also has strong laterality for visuo-spatial attention. Thus, the RH twin had the typical and the LH twin the atypical lateralities: the RH twin exhibited typical left hemisphere verbal dominance, whereas the LH twin exhibited atypical right hemisphere dominance. The RH exhibited typical right hemisphere visuo-spatial attention dominance, in contrast to the LH twin's atypical left hemisphere dominance. If there was a sense in which the twins could be described as mirrored (i.e. similar in magnitude but opposite in direction), that description would be better supported by the verbal than the visuo-spatial evidence: the verbal laterality indices as calculated here were strongly lateralized and opposite, whereas the visuo-spatial attention laterality indices were strongly lateralized for the LH twin only (VLI = –0.72), as compared with the RH twin's weaker value of (VLI = +0.50). It is important to consider the method of calculating laterality indices, in interpreting their significance: (1) the ROI per task is pre-specified anatomically (independently of an individual's maxima loci of activation at a whole brain analysis level); and (2) the algorithm per task is specifically adopted to account for differences in overall activation: difference (dominant – non-dominant), divided by summed (dominant + non-dominant), all multiplied by 100. This provides a percentage laterality indicator; qualitatively, a score of +50 indicates 50% dominant laterality (which is not substantial), as compared with a score of –72, which is close to three-quarters (although cut-offs for atypicality are in some sense arbitrary).
Behavioural hand preference and performance laterality
This twin pair is not mirrored with respect to hand preference (in contrast to the pair studied by Sommer et al. 1999) or to uni-manual performance laterality. With one exception, the uni-manual tasks are representative of a larger group of twin pairs reported by Gurd et al. (2006). The exception is in the RH's peg moving score, which shows particularly strong right-hand superiority. This is not a practised task. Because it involves skilled but unpractised fine motor control, it is considered to be less influenced by past cultural experience, and may therefore be a purer indicator of inherent laterality. (This result supports the description of our RH twin as indeed being right handed.) On the bi-manual task, the twins had virtually identical performance rates, although the LH twin was less efficient (higher error rate). The significance of laterality preference and performance results needs to be interpreted in the light of confounding factors: congenital hand tremor in the left hand of the LH twin, and braced right leg of the LH twin. However, it is important to note the similarity between twins with respect to the bradykinesia laterality scores, even if the RH had a slightly stronger dominant hand index.
Between-task comparisons, brain and behaviour
With respect to tasks, the HPI questionnaire did not appear to predict the degree of cerebral laterality on either verbal or spatial tasks. With respect to contrasts between twins, the RH displays a strong hand preference, whereas the LH is mixed handed, and the RH is not strongly lateralized on the fMRI spatial index, although the LH is (albeit in the opposite direction). Therefore, evidence from this twin pair case study raises several questions with respect to laterality of hand and brain (in MzHd twins), which pivot on definitions of handedness and purity of biological markers.
First, although the subject selection criteria were hand used for writing being equal to +2 (strongly right handed) and –2 (strongly left handed), other measures indicated that the LH twin was not as strongly lateralized for hand preference and performance as her RH twin. Is this related to the anomalous fMRI verbal and visuo-spatial laterality indices? (It would be difficult to argue that the LH twin simply lacked strong cerebral laterality, given the magnitude of her clearly lateralized verbal and visuo-spatial fMRI results although reversed in direction to those of her RH twin.) There is no evidence to suggest that the LH twin was a pathological left hander, given her slightly higher birth weight.
Secondly, that the hair whorls were both clockwise is important, given Klar's (2003) claim of a culture-independent relationship between hair whorl, handedness and language lateralization (cf. Weber et al. 2006; Beaton & Mellor, 2007; Jansen et al. 2007). Unfortunately, it is not clear how this measure interacts with the mechanisms responsible for twinning in monozygotic handedness discordant twins (cf. Singh et al. 2002). Several methodological points are apposite. (1) Subjects: gender and sample sizes have varied. In women, the use of hair whorl as a potential genetic indicator of handedness and its relationship to language lateralization has been less reported given the challenges of accurate measurement with longer hair (although female results may be more accurate in terms of completion, given the lower balding rates). Even the larger sample sizes lack adequate background data concerning developmental and medical histories (cf. Jansen et al. 2007). (2) Handedness assessment: this has tended to be varied and weak, typically limited to hand preference scales, and again employing widely varying cut-off points, even on the Edinburgh Handedness Inventory (Weber et al. 2006; Jansen et al. 2007). (3) Brain imaging laterality indices: these have been varied and limited. Techniques of assessing functional cerebral laterality (eg. fMRI versus PET, versus transcranial Doppler sonography) have varied, as have cut-off points and algorithms for calculating and delineating atypicality (Tzourio-Mazoyer et al. 2004; Weber et al. 2006; Jansen et al. 2007). Perhaps surprisingly, no previous mention of visuo-spatial lateralization and its relation to hair whorl direction has appeared in the literature (cf. Hatfield, 2006).
Summary and conclusions
This constructive replication augments the findings of Sommer et al. (1999); the existence of MzHd twins in whom the left hander has fully reversed cerebral lateralization for both language and visuo-spatial attention is now established. Our LH twin was more strongly lateralized on the spatial task than her twin, albeit in the opposite direction, and her atypical cerebral laterality was not just functional (cf. Galaburda & LeMay, 1978). This contrasts with the verbal indices, on which both twins were strongly lateralized, but in opposite directions. How common such atypical lateralization is remains to be determined (cf. Sommer et al. 2002, for a larger series of MzHd twins). On uni-manual performance, the LH showed no pronounced decrement relative to the RH; thus, demonstration of any necessary relationship between cerebral lateralization and hand performance remains elusive. This evidence constitutes somewhat of a challenge to genetic models of cerebral lateralization, as neither Annett (2002), Klar (cf. Hatfield, 2006) nor McManus (1995, 2002) accounted for the exclusivity of atypicality to the LH twin. And yet, there were no indications of pathological foetal, peri- or postnatal development in either of our twins, although it is possible that this was a late splitting twin pair with laterality under combined genetic and epigenetic influence. In future, it would be desirable to study complementary twin pairs: one, RH1, showing atypical verbal but typical spatial, contrasting with her twin LH1, showing typical verbal but atypical spatial, and a second, with RH2 showing typical verbal but atypical spatial, and LH2 showing atypical verbal but typical visuo-spatial laterality on functional imaging.
Acknowledgments
We are grateful to the two individual twins, to P. Dobson, H. Burnham, G. Cossu, J. Critchley, T. Crow, D. Luscombe, C. Furlong, H. Osborne, T. Spector and P. H. Weiss for their contributions to the study, and to A. Beaton and other referees for their comments on the manuscript. Data collection was funded by the British Academy (grant #ORG-35414, J.M.G.), and the MRC (J.C.M.). Further support, from the Critchley Charitable Trust, the Oxford Centre for Cognitive Neuroscience and the University of Hertfordshire (J.M.G.), is gratefully acknowledged.
References
- Amunts K, Schleicher A, Burgel U, et al. Broca's region revisited: cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Amunts K, Weiss PH, Mohlberg H, et al. Analysis of the neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotactic space – The role of Brodmann's areas 44 and 45. NeuroImage. 2004;22:42–56. doi: 10.1016/j.neuroimage.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Anderson B, Southern B, Powers R. Anatomic asymmetries of the posterior superior temporal lobe: a post mortem study. Neuropsychiatry Neuropsychol Behav Neurol. 1999;12:247–254. [PubMed] [Google Scholar]
- Annett M, Alexander MP. Atypical cerebral dominance: predictions and tests of the right shift theory. Neuropsychologia. 1996;34:1215–1227. doi: 10.1016/0028-3932(96)00048-6. [DOI] [PubMed] [Google Scholar]
- Annett M. Handedness and Brain Asymmetry: The Right Shift Theory. East Sussex: Psychology Press; 2002. [Google Scholar]
- Annett M. Cerebral asymmetry in twins: predictions of the right shift theory. Neuropsychologia. 2003;41:469–479. doi: 10.1016/s0028-3932(02)00137-9. [DOI] [PubMed] [Google Scholar]
- Annett M. Hand preference observed in large healthy samples: classification, norms and interpretations of increased non-right-handedness by the right shift theory. British J Psych. 2004;95:339–353. doi: 10.1348/0007126041528130. [DOI] [PubMed] [Google Scholar]
- Barrick T, Mackay C, Prima S, et al. Automatic analysis of cerebral asymmetry: an exploratory study of the relationship between brain torque and planum temporale asymmetry. Neuroimage. 2005;24:678–691. doi: 10.1016/j.neuroimage.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Bear D, Schiff D, Saver J, et al. Quantitative analysis of cerebral asymmetries. Fronto-occipital correlation, sexual dimorphism and association with handedness. Arch Neurol. 1986;43:598–603. [Google Scholar]
- Beaton AA, Hughdahl K, Ray P. Lateral asymmetries and interhemispheric transfer in aging: A review and some new data. In: Mandal MK, Bulman-Fleming MB, Tiwari G, editors. Side Bias: A Neuropsychological Perspective. The Netherlands: Kluwer Publications; 2000. pp. 101–152. [Google Scholar]
- Beaton AA, Mellor G. Direction of hair whorl and handedness. Laterality. 2007;12:295–301. doi: 10.1080/13576500601112358. [DOI] [PubMed] [Google Scholar]
- Beis JM, Keller C, Morin N, et al. Right spatial neglect after left hemisphere stroke: qualitative and quantitative study. Neurology. 2004;63:1600–1605. doi: 10.1212/01.wnl.0000142967.60579.32. [DOI] [PubMed] [Google Scholar]
- Briggs GG, Nebes RD. Patterns of hand preference in a student population. Cortex. 1975;11:230–238. doi: 10.1016/s0010-9452(75)80005-0. [DOI] [PubMed] [Google Scholar]
- Broca P. Perte de la parole. Ramollisement chronique et destruction partielle du lobe anterieur gauche du cerveau. Bull Soc Anthro. 1861;2:235–238. [Google Scholar]
- Corey DM, Hurley M, Foundas A. Right and left handedness defined. Neuropsychiatr Neuropsych Behav Neurol. 2001;14:144–152. [PubMed] [Google Scholar]
- Dellatolas G, Luciani S, Castresana A, et al. Pathological left-handedness. Left-handedness correlatives in adult epileptics. Brain. 1993;116:1565–1574. doi: 10.1093/brain/116.6.1565. [DOI] [PubMed] [Google Scholar]
- Dellatolas G. Anomalous brain and anomalous model. Commentary. Brain Cogn. 1994;26:196–198. doi: 10.1006/brcg.1994.1051. [DOI] [PubMed] [Google Scholar]
- Dorsaint-Pierre R, Penhune VB, Watkins K, et al. Asymmetries of the planum temporale and Heschl's gyrus: relationship to language lateralization. Brain. 2006;29:1164–1176. doi: 10.1093/brain/awl055. [DOI] [PubMed] [Google Scholar]
- Dragovic M, Hammond G, Jablensky A. Schizotypy and mixed-handedness revisited. Psychiatry Res. 2005;136:143–152. doi: 10.1016/j.psychres.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Brain: Surface, Blood Supply, and Three-Dimensional Sectional Anatomy. Vienna: Springer; 1999. [Google Scholar]
- Fink GR, Marshall JC, Shah NJ, et al. Line bisection judgments implicate right parietal cortex and cerebellum as assessed by fMRI. Neurology. 2000;54:1324–1331. doi: 10.1212/wnl.54.6.1324. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Weiss PH, et al. The neural basis of vertical and horizontal line bisection judgments: an fMRI study of normal volunteers. Neuroimage. 2001;14:S59–67. doi: 10.1006/nimg.2001.0819. [DOI] [PubMed] [Google Scholar]
- Floel A, Buyx A, Breitenstein C, et al. Hemispheric lateralization of spatial attention in right-and left-hemispheric language dominance. Behav Brain Res. 2005;158:269–275. doi: 10.1016/j.bbr.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Leonard CM, Gilmore R, et al. Planum temporale asymmetry and language dominance. Neuropsychologia. 1994;32:1225–1231. doi: 10.1016/0028-3932(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Foundas A, Leonard C, Heilman K. Morphologic cerebral asymmetries and handedness. The pars triangularis and planum temporale. Arch Neurol. 1995;52:501–508. doi: 10.1001/archneur.1995.00540290091023. [DOI] [PubMed] [Google Scholar]
- Foundas A, Leonard C, Gilmore R, et al. Pars triangularis asymmetry and language dominance. Proc Natl Acad Sci USA. 1996;93:719–722. doi: 10.1073/pnas.93.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foundas A, Eure K, Luevano L, et al. MRI asymmetries of Broca's area: the pars triangularis and pars opercularis. Brain Lang. 1998;64:282–296. doi: 10.1006/brln.1998.1974. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Turner R, et al. Characterizing evoked hemodynamics with fMRI. Neuroimage. 1995;2:157–165. doi: 10.1006/nimg.1995.1018. [DOI] [PubMed] [Google Scholar]
- Galaburda A, LeMay M. Right-left asymmetries in the brain. Structural differences between the hemispheres may underlie cerebral dominance. Science. 1978;199:852–856. doi: 10.1126/science.341314. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Miller BL, DeCarli C, et al. Heritability of lobar brain volumes in twins supports genetic models of cerebral laterality and handedness. Proc Natl Acad Sci USA. 2002;99:3176–3181. doi: 10.1073/pnas.052494999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N, Levitsky W. Human brain left-right asymmetries in temporal speech region. Science. 1968;161:186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- Gurd J, Schulz J, Cherkas L, et al. Hand preference and performance in 20 pairs of monozygotic twins with discordant handedness. Cortex. 2006;42:934–945. doi: 10.1016/s0010-9452(08)70438-6. [DOI] [PubMed] [Google Scholar]
- Harvey M, Milner AD, Roberts RC. An investigation of hemispatial neglect using the landmark task. Brain Cogn. 1995;27:59–78. doi: 10.1006/brcg.1995.1004. [DOI] [PubMed] [Google Scholar]
- Hatfield JS. The genetic basis of hair whorl, handedness, and other phenotypes. Medical Hypotheses. 2006;66:708–714. doi: 10.1016/j.mehy.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Hervé P-Y, Crivello F, Perchey G, et al. Handedness and cerebral anatomical asymmetries in young adult males. NeuroImage. 2006;29:1066–1079. doi: 10.1016/j.neuroimage.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Hund-Georgiadis M, Lex U, Friederici AD, et al. Non-invasive regime for language lateralization in right-and left-handers by means of functional MRI and dichotic listening. Exp Brain Res. 2002;145:166–176. doi: 10.1007/s00221-002-1090-0. [DOI] [PubMed] [Google Scholar]
- Jackson R, Snieder H, Davis H, et al. Determination of twin zygosity: a comparison of DNA with various questionnaire indices. Twin Res. 2001;4:12–18. doi: 10.1375/1369052012092. [DOI] [PubMed] [Google Scholar]
- Jansen A, Floel A, Menke R, et al. Dominance for language and spatial processing: limited capacity of a single hemisphere. NeuroReport. 2005;16:1017–1021. doi: 10.1097/00001756-200506210-00027. [DOI] [PubMed] [Google Scholar]
- Jansen A, Lohmann H, Scharfe S, et al. The association between scalp hair-whorl direction, handedness and hemispheric language dominance: is there a common genetic basis of lateralization? NeuroImage. 2007;35:853–861. doi: 10.1016/j.neuroimage.2006.12.025. [DOI] [PubMed] [Google Scholar]
- Keller S, Wilke M, Wieshmann U, et al. Comparison of standard and optimized voxel based morphometry for analysis of brain changes associated with temporal lobe epilepsy. NeuroImage. 2004;23:860–868. doi: 10.1016/j.neuroimage.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Keller S, Highley JR, Garcia-Finana M, et al. Sulcal variability, stereological measurement and asymmetry of Broca's area on MR images. J Anat. 2007;211:534–555. doi: 10.1111/j.1469-7580.2007.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Crespo-Facorro B, Andreasen N, et al. An MRI-based parcellation method for the temporal lobe. Neuroimage. 2000;11:271–288. doi: 10.1006/nimg.2000.0543. [DOI] [PubMed] [Google Scholar]
- Klar AJ. Human handedness and scalp hair-whorl direction develop from a common genetic mechanism. Genetics. 2003;165:269–276. doi: 10.1093/genetics/165.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus TA, Corey DM, Bollich AM, et al. Anatomical asymmetries of anterior perisylvian speech-language regions. Cortex. 2007;43:499–510. doi: 10.1016/s0010-9452(08)70244-2. [DOI] [PubMed] [Google Scholar]
- Knecht S, Deppe M, Drager B, et al. Language lateralization in healthy right-handers. Brain. 2000;123:74–81. doi: 10.1093/brain/123.1.74. [DOI] [PubMed] [Google Scholar]
- Knecht S, Drager B, Floel A, et al. Behavioural relevance of atypical language lateralization in healthy subjects. Brain. 2001;124:1657–1665. doi: 10.1093/brain/124.8.1657. [DOI] [PubMed] [Google Scholar]
- Knecht S, Flöel A, Dräger B, et al. Degree of language lateralization determines susceptibility to unilateral brain lesions. Nature Neurosci. 2002;5:695–699. doi: 10.1038/nn868. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Summerln JL, Rainey L, et al. The Talairach Daemon, a database server for Talairach Atlas Labels. NeuroImage. 1997;54:633. [Google Scholar]
- LeMay M. Morphological cerebral asymmetries of modern man, fossil man, and nonhuman primate. Ann NY Acad Sci. 1976;280:349–366. doi: 10.1111/j.1749-6632.1976.tb25499.x. [DOI] [PubMed] [Google Scholar]
- Luria AR. Frontal lobe syndromes. In: Vinken PJ, Bruyn GW, editors. Handbook of Clinical Neurology. Vol. 2. Amsterdam: North-Holland; 1969. pp. 725–757. [Google Scholar]
- Marshall JC, Fink G. Cerebral localization, then and now. NeuroImage. 2003;20:S2–S7. doi: 10.1016/j.neuroimage.2003.09.001. [DOI] [PubMed] [Google Scholar]
- McManus I. The interpretation of laterality. Cortex. 1983;19:187–214. doi: 10.1016/s0010-9452(83)80014-8. [DOI] [PubMed] [Google Scholar]
- McManus IC. Familial sinistrality: the utility of calculating exact genotype probabilities for individuals. Cortex. 1995;31:3–24. doi: 10.1016/s0010-9452(13)80102-5. [DOI] [PubMed] [Google Scholar]
- McManus C. Right Hand, Left Hand: The Origins of Asymmetry in Brains, Bodies, Atoms and Cultures. London: Weidenfeld & Nicholson; 2002. [Google Scholar]
- Medland SE, Duffy DL, Wright MJ, et al. Handedness in twins: joint analysis of data from 35 samples. Twin Res Human Genetics. 2006;9:46–53. doi: 10.1375/183242706776402885. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M. Behavioural neuroanatomy: Large-scale networks, association cortex, frontal syndromes, the limbic system and hemispheric specializations. In: Mesulam M-M, editor. Principles of Behavioural and Cognitive Neurology. 2. Oxford: Oxford University Press; 2000. pp. 1–120. [Google Scholar]
- Moore AP. Impaired sensorimotor integration in parkinsonism and dyskinesia: a role for corollary discharges? J Neurol Neurosurg Psychiatr. 1987;50:544–552. doi: 10.1136/jnnp.50.5.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalcaci E, Kalaycioglu C, Cicek M, et al. The relationship between handedness and fine motor performance. Cortex. 2001;37:493–500. doi: 10.1016/s0010-9452(08)70589-6. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ono M, Kubik S, Abernathey CD. Atlas of the Cerebral Sulci. Stuttgart: Thieme; 1990. [Google Scholar]
- Peters M, Reimers S, Manning JT. Hand preference for writing and associations with seleted demographic and behavioural variables in 255,100 subjects: the BBC internet study. Brain Cogn. 2006;62:177–189. doi: 10.1016/j.bandc.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. The frontal cortex. In: Paxinos G, Mai JK, editors. The Human Nervous System. San Diego: Elsevier Academic Press; 2004. pp. 950–972. [Google Scholar]
- Petrides M. Broca's area in the human and nonhuman primate brain. In: Grodzinsky Y, Amunts K, editors. Broca's Region. New York: Oxford University Press; 2006. pp. 31–46. [Google Scholar]
- Provins KA. Handedness and speech: a critical reappraisal of the role of genetic and environmental factors in the cerebral lateralization of function. Psych Rev. 1997;104:554–571. doi: 10.1037/0033-295x.104.3.554. [DOI] [PubMed] [Google Scholar]
- Puddephat M. University of Liverpool; 1999. Computer interface for convenient application of stereological methods for unbiased estimation of volume and surface area: studies using MRI with particular reference to the human brain. PhD thesis. [Google Scholar]
- Pujol J, Deus J, Losilla JM, et al. Cerebral lateralization of language in normal left handed people studied by fMRI. Neurology. 1999;52:1038–1043. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- Pulvermueller F. The Neuroscience of Language. On Brain Circuits of Words and Serial Order. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Roberts N, Puddephat M, McNulty V. The benefit of stereology for quantitative radiology. Br J Radiol. 2000;73:679–697. doi: 10.1259/bjr.73.871.11089458. [DOI] [PubMed] [Google Scholar]
- Shapleske J, Rossell SL, Woodruff PW, et al. The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Res Rev. 1999;29:26–49. doi: 10.1016/s0165-0173(98)00047-2. [DOI] [PubMed] [Google Scholar]
- Sicotte N, Woods R, Mazziotta J. Handedness in twins: a meta-analysis. Laterality. 1999;4:265–286. doi: 10.1080/713754339. [DOI] [PubMed] [Google Scholar]
- Singh SM, Murphy B, O’Reilly R. Epigenetic contributors to the discordance of monozygotic twins. Clin Genet. 2002;62:97–103. doi: 10.1034/j.1399-0004.2002.620201.x. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Ramsey NF, Bouma A, et al. Cerebral mirror-imaging in a monozygotic twin. Lancet. 1999;354:1445–1446. doi: 10.1016/s0140-6736(99)04130-6. [DOI] [PubMed] [Google Scholar]
- Sommer I, Ramsey N, Mandl R, et al. Language lateralization in monozygotic twin pairs concordant and discordant for handedness. Brain. 2002;125:2710–2718. doi: 10.1093/brain/awf284. [DOI] [PubMed] [Google Scholar]
- Spector TD, MacGregor AJ. The St. Thomas’ UK Adult Twin Registry. Twin Res. 2002;5:440–443. doi: 10.1375/136905202320906246. [DOI] [PubMed] [Google Scholar]
- Steinmetz H, Herzog A, Schlaug G, et al. Brain (a)symmetry in monozygotic twins. Cerebral Cortex. 1995;5:296–300. doi: 10.1093/cercor/5.4.296. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Fink GR, Marshall JC. Mechanisms of hemispheric specialization: insights from analyses of connectivity. Neuropsychologia. 2007;45:209–228. doi: 10.1016/j.neuropsychologia.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Binder JR, Possing ET, et al. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59:238–244. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Tapley SM, Bryden MP. A group test for the assessment of performance between the hands. Neuropsychologia. 1985;23:289–304. doi: 10.1016/0028-3932(85)90105-8. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- Tomaiuolo F, MacDonald J, Caramanos Z, et al. Morphology, morphometry and probability mapping of the pars opercularis of the inferior frontal gyrus. Eur J Neurosci. 1999;11:3033–3046. doi: 10.1046/j.1460-9568.1999.00718.x. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Josse G, Crivello F, et al. Interindividual variability in the hemispheric organization for speech. NeuroImage. 2004;21:422–435. doi: 10.1016/j.neuroimage.2003.08.032. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Smits M, Wielopolski PA, et al. Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right- and left-handed healthy subjects: a combined fMRI and DTI study. NeuroImage. 2007;35:1064–1076. doi: 10.1016/j.neuroimage.2006.12.041. [DOI] [PubMed] [Google Scholar]
- Weber B, Hoppe C, Faber J, et al. Association between scalp hair-whorl direction and hemispheric language dominance. NeuroImage. 2006;30:539–543. doi: 10.1016/j.neuroimage.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Zetzsche T, Meisenzahl EM, Preuss UW, et al. In-vivo analysis of the human planum temporale (PT): does the definition of PT borders influence the results with regard to cerebral asymmetry and correlation with handedness? Psychiatry Res. 2001;107:99–115. doi: 10.1016/s0925-4927(01)00087-7. [DOI] [PubMed] [Google Scholar]