Abstract
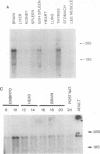
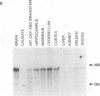
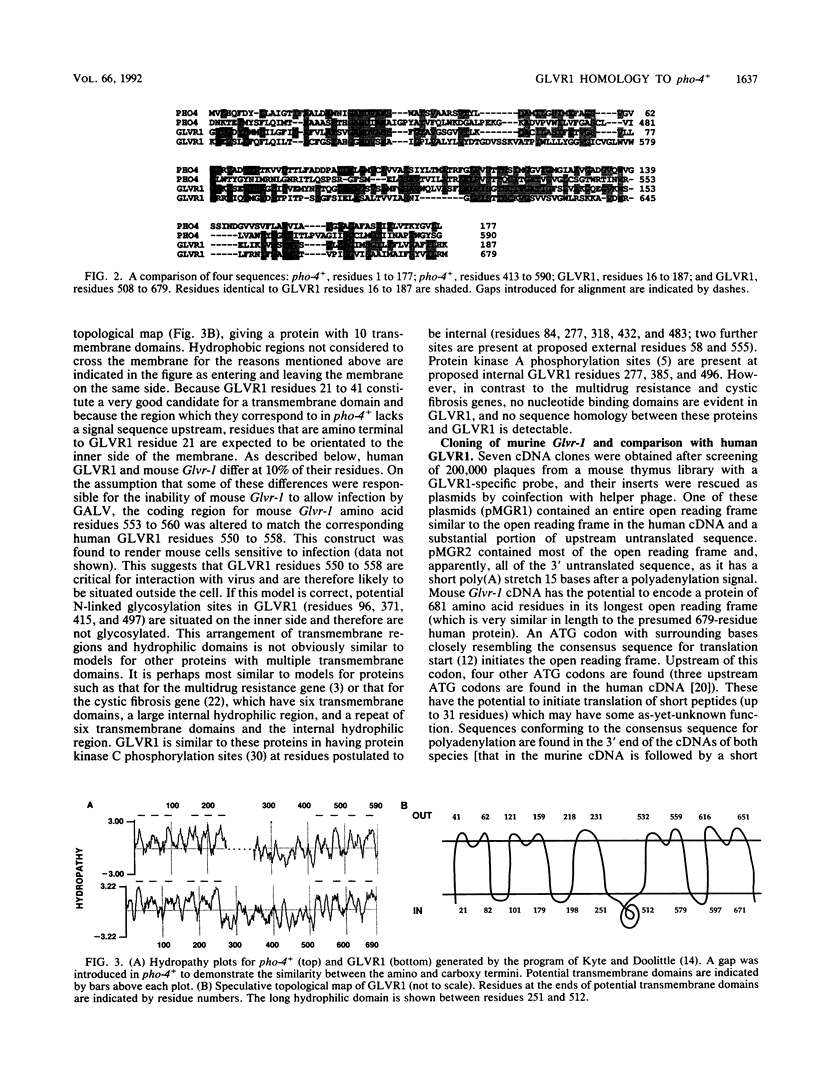
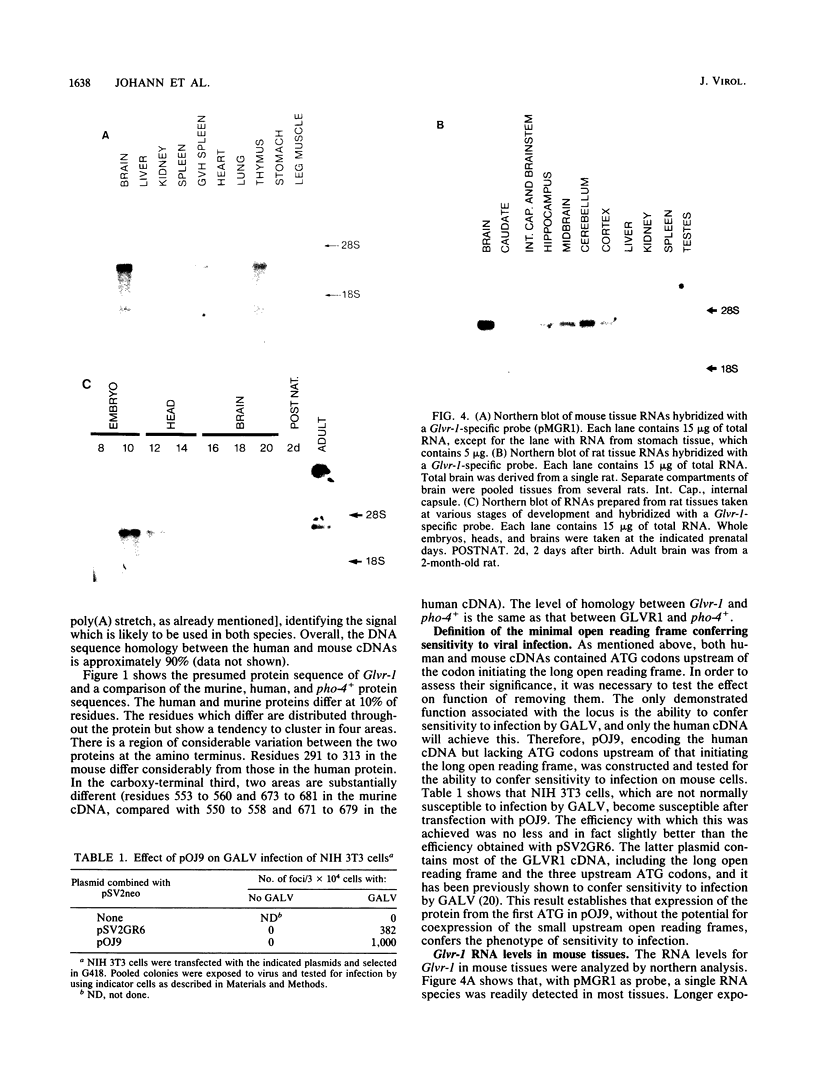
The human gene GLVR1 has been shown to render mouse cells sensitive to infection by gibbon ape leukemia virus. This indication that the GLVR1 protein acts as a virus receptor does not reveal the protein's normal physiological role. We now report that GLVR1 is homologous to pho-4+, a phosphate permease of Neurospora crassa, at a level sufficiently high to predict that GLVR1 is also a transport protein, although the substrate transported remains unknown. To characterize the gene further, we have cloned cDNA for the mouse homolog of the gene, Glvr-1. The sequence of the murine protein differs from that of the human protein in 10% of residues, and it may be presumed that some of these differences are responsible for the inability of gibbon ape leukemia virus to infect mouse fibroblasts. Glvr-1 RNA is most abundant in mouse brain and thymus, although it is present in all tissues examined. The pattern of RNA expression found in mouse tissues was also found in rat tissues, in which the RNA was expressed at high levels in all compartments of the brain except the caudate nucleus and was expressed most abundantly early in embryogenesis. Thus, high-level expression of Glvr-1 appears to be restricted to specific tissues and may have developmental consequences.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bova C. A., Olsen J. C., Swanstrom R. The avian retrovirus env gene family: molecular analysis of host range and antigenic variants. J Virol. 1988 Jan;62(1):75–83. doi: 10.1128/jvi.62.1.75-83.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Allen K. E., Slayman C. W. Vanadate-resistant mutants of Neurospora crassa are deficient in a high-affinity phosphate transport system. J Bacteriol. 1983 Jan;153(1):292–296. doi: 10.1128/jb.153.1.292-296.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Gibbons J. J., Jr, Lucas J. Immunomodulation by low-dose methotrexate. I. Methotrexate selectively inhibits Lyt-2+ cells in murine acute graft-versus-host reactions. J Immunol. 1989 Mar 15;142(6):1867–1873. [PubMed] [Google Scholar]
- Glass D. B., el-Maghrabi M. R., Pilkis S. J. Synthetic peptides corresponding to the site phosphorylated in 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase as substrates of cyclic nucleotide-dependent protein kinases. J Biol Chem. 1986 Feb 25;261(6):2987–2993. [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Haapala D. K., Robey W. G., Oroszlan S. D., Tsai W. P. Isolation from cats of an endogenous type C virus with a novel envelope glycoprotein. J Virol. 1985 Mar;53(3):827–833. doi: 10.1128/jvi.53.3.827-833.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Hino T., Kato H., Suzuki H., Saito K. Cytotoxic T lymphocyte response to minor H-42a alloantigen in H-42b mice: clonal inactivation of the precursor cytotoxic T lymphocytes by veto-like spleen cells that express the H-42a antigen. J Immunol. 1986 Oct 1;137(7):2080–2088. [PubMed] [Google Scholar]
- Kaelbling M., Eddy R., Shows T. B., Copeland N. G., Gilbert D. J., Jenkins N. A., Klinger H. P., O'Hara B. Localization of the human gene allowing infection by gibbon ape leukemia virus to human chromosome region 2q11-q14 and to the homologous region on mouse chromosome 2. J Virol. 1991 Apr;65(4):1743–1747. doi: 10.1128/jvi.65.4.1743-1747.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. W., Closs E. I., Albritton L. M., Cunningham J. M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991 Aug 22;352(6337):725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- Kozak C. A., Albritton L. M., Cunningham J. Genetic mapping of a cloned sequence responsible for susceptibility to ecotropic murine leukemia viruses. J Virol. 1990 Jun;64(6):3119–3121. doi: 10.1128/jvi.64.6.3119-3121.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kurtz M. E., Graff R. J., Adelman A., Martin-Morgan D., Click R. E. CTL and serologically defined antigens of B2m,H-3 region. J Immunol. 1985 Oct;135(4):2847–2852. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Mann B. J., Akins R. A., Lambowitz A. M., Metzenberg R. L. The structural gene for a phosphorus-repressible phosphate permease in Neurospora crassa can complement a mutation in positive regulatory gene nuc-1. Mol Cell Biol. 1988 Mar;8(3):1376–1379. doi: 10.1128/mcb.8.3.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann B. J., Bowman B. J., Grotelueschen J., Metzenberg R. L. Nucleotide sequence of pho-4+, encoding a phosphate-repressible phosphate permease of Neurospora crassa. Gene. 1989 Nov 30;83(2):281–289. doi: 10.1016/0378-1119(89)90114-5. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Sligh J. M., Cort S. P., Mawle A., Nicholson J. K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986 Jan 24;231(4736):382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- O'Hara B., Johann S. V., Klinger H. P., Blair D. G., Rubinson H., Dunn K. J., Sass P., Vitek S. M., Robins T. Characterization of a human gene conferring sensitivity to infection by gibbon ape leukemia virus. Cell Growth Differ. 1990 Mar;1(3):119–127. [PubMed] [Google Scholar]
- Praloran V., Raventos-Suarez C., Bartocci A., Lucas J., Stanley E. R., Gibbons J. J., Jr Alterations in the expression of colony-stimulating factor-1 and its receptor during an acute graft-vs-host reaction in mice. J Immunol. 1990 Nov 15;145(10):3256–3261. [PubMed] [Google Scholar]
- RUBIN H. The nature of a virus-induced cellular resistance to Rous sarcoma virus. Virology. 1961 Feb;13:200–206. doi: 10.1016/0042-6822(61)90054-x. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Wang H., Kavanaugh M. P., North R. A., Kabat D. Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nature. 1991 Aug 22;352(6337):729–731. doi: 10.1038/352729a0. [DOI] [PubMed] [Google Scholar]
- Woodgett J. R., Gould K. L., Hunter T. Substrate specificity of protein kinase C. Use of synthetic peptides corresponding to physiological sites as probes for substrate recognition requirements. Eur J Biochem. 1986 Nov 17;161(1):177–184. doi: 10.1111/j.1432-1033.1986.tb10139.x. [DOI] [PubMed] [Google Scholar]