Abstract
Chromogranins and secretogranins belong to the granin family of proteins, which are expressed in neuroendocrine and nervous tissue. In earlier publications we have described the development of region-specific antibodies against CgA and CgB. In this study we describe antibodies to SgII and SgIII and their usefulness for immunohistochemical staining. Peptides homologous to defined parts of secretogranins II and III were selected and synthesized. Antibodies were raised and immunostainings were performed on normal human pancreas. The SgII 154–165 (N-terminal secretoneurin), SgII 172–186 (C-terminal secretoneurin) and SgIII antibodies immunostained all insulin-immunoreactive cells, most of the glucagon cells and some of the pancreatic polypeptide cells. The SgII 225–242 antibody immunostained only the insulin-containing cells. None of the antibodies immunostained the somatostatin cells. This study is the first observation of the expression of SgIII in human tissues, where we show expression of SgIII in three of the four major islet cell types in human pancreas.
Keywords: antibodies, co-localization, immunostaining, peptides, secretogranin, secretoneurin
Introduction
Chromogranins (Cgs) and secretogranins (Sgs) are members of the granin family of proteins, which are expressed in neuroendocrine and nervous tissue (Taupenot et al. 2003; Helle, 2004). They share certain common physiochemical properties, such as calcium-binding, acidic isoelectric points due to an abundant content of acidic amino acid residues, and multiple pairs of basic amino acids, which are potential cleavage sites for enzymes such as the proconvertases (Taupenot et al. 2003). However, the Sgs do not have the N-terminally located disulfide-bonded loop, as do the Cgs (Taupenot et al. 2003; Helle, 2004). The members of the Sgs family tend to increase in number; we now recognize at least six members, designated SgII to SgVII (Helle, 2004).
Like the Cgs, the Sgs also exhibit several biological functions, some of them generated by cleavage products from the granins. The most important peptide generated from SgII is secretoneurin (SN; SgII 154–186) (Kirchmair et al. 1993; Marksteiner et al. 1993). SN has been found in both nervous and neuroendocrine tissue (Kirchmair et al. 1993; Marksteiner et al. 1993), and seems to be phylogenetically well conserved (Helle, 2004). Several biological functions are associated with SN, such as stimulation or inhibition of nervous transmitter release, chemotaxis of white blood cells and fibroblasts, proliferation of vascular smooth muscle cells and endothelial cells, and activation of cell adherence (Reinisch et al. 1993; You et al. 1996; Kahler et al. 1997; Dunzendorfer et al. 1998; Ischia et al. 2000). SgIII (1B1075) has been shown to interact with CgA in secretory granules (Hosaka et al. 2002).
The wide ranging biological functions of the granins merit further investigation. Earlier publications have reported immunohistochemical findings with newly developed region-specific antibodies to CgA and CgB (Portela-Gomes & Stridsberg, 2001; Portela-Gomes & Stridsberg, 2002). In this study three antibodies to SgII epitopes and one to SgIII epitopes are described, as is their expression in different human pancreatic islet cell types.
Materials and methods
Antibody production
Derived from the amino acid sequences of human SgII and rat SgIII, specific polypeptides were synthesized by a solid-phase system using Fmoc chemistry (Applied Biosystems model 430A, Foster City, CA, USA). The peptides were purified by reversed phase chromatography and analysed by plasma desorption mass spectrometry (PDMS, Bioion 20, Bioion Nordic AB, Uppsala, Sweden).
The sequences were selected to be specific for the desired protein, and homology was less than 50% to any other known protein sequence in the protein database SWISS-PROT (release 37.0, Dec 1998), except for the respective sequences from other species. The selected sequences for the respective peptides are shown in Table 1. To facilitate coupling to the carrier protein (see below), a cysteine residue was added to either the C-terminal or the N-terminal of the synthesized peptides. To enable labelling of the peptides with iodine, a tyrosine residue was interposed between the cysteine residue and the peptide if the peptide sequence lacked a tyrosine residue.
Table 1.
Co-localization of secretogranins II and III in human pancreatic endocrine cells. The amino acid sequences of the peptides used for production of antibodies are indicated
| Immunoreactivity/cell type | Insulin | Glucagon | Somatostatin | Pancreatic polypeptide |
|---|---|---|---|---|
| N-terminal secretoneurin (SgII 154–165) | ++++ | ++(+) | − | +(+) |
| C-terminal secretoneurin (SgII 172–186) | ++++ | +++ | − | ++ |
| Secretogranin II (SgII 225–242) | ++++ | − | − | − |
| Secretogranin III (SgIII 348–361) | ++++ | ++(+) | − | +(+) |
–, no immunoreactive cells; +, fewer than 10% positive cells; ++, 10–50%; +++, 50–90%; ++++, virtually all cells are immunoreactive.
+(+) and ++(+), an apparent variation in frequency of immunoreactive cells between ‘+ and ++’ and ‘++ and +++’, respectively.
Before immunization, the peptides were coupled to a carrier protein. Peptides (1 mg) were dissolved in 100 µL DMSO, after which 100 µL (1 mg) of Imject® Maleimide Activated Keyhole Limpet Hemocyanin (aKLH, Pierce, Boule Nordic AB, Huddinge, Sweden) was added. This mixture was allowed to react for 2 h at room temperature. The coupled peptides were then purified on a PD-10 column (Pharmacia Biotec, Uppsala, Sweden) with phosphate-buffered saline (PBS) as the moving phase. Aliquots of 200 µg (n = 5) coupled peptide were frozen and stored at –20 °C until immunization. The peptide complexes were injected into New Zealand white rabbits, using the intradermal injection technique to produce polyclonal antibodies (Stridsberg et al. 1995). The production of antibodies to SgII 225–242 (denoted CgC) has been described elsewhere (Stridsberg et al. 1995).
Tissue
Tissue specimens from six adult human pancreata were obtained from surgical samples removed at operation for pancreatic adenocarcinoma. The specimens were taken from both macro- and microscopically normal glandular regions at least at 3 cm distant from the neoplasm. Four of the specimens were taken from the body–tail region and two from the head (processus uncinatus). These specimens were fixed in 10% buffered neutral formalin for 18–20 h at room temperature, dehydrated and routinely processed via xylol to paraffin wax. Sections, 5 µm thick, were cut and attached to positively charged (Superfrost+, Menzel, Germany) glass slides. Haematoxylin–eosin was used for routine staining.
Immunostaining
Immunofluorescence methods were used for single or double staining with antibodies to Sgs and to various islet hormones. For double immunofluorescence staining, the sections were incubated overnight at room temperature with a cocktail of two antibodies, either one monoclonal (anti-mouse) and one polyclonal antibody, or two polyclonal antibodies raised in different animal species (anti-rabbit, anti-guinea-pig and/or anti-chicken). Thereafter the sections were incubated with biotinylated swine anti-rabbit IgG (Dako, Glostrup, Denmark) for 30 min and then transferred to a mixture of streptavidin–Texas Red (Vector Laboratories, Burlingame, CA, USA) and the appropriate fluorescein isothiocyanate (FITC)-conjugated goat secondary antibody (anti-mouse, anti-guinea-pig, or anti-chicken IgG; Sigma Chemical Co., St Louis, MO, USA). Before applying the respective primary antibodies, the sections were incubated with non-immune serum from the animal species producing the secondary antibodies, at a dilution of 1 : 10. The secondary antibody in question was pre-incubated overnight at 4 °C with 10 µL mL−1 normal serum, both from the animal species recognized by the other secondary antibody and from the species producing that antibody. Between each staining step, the sections were washed with PBS.
The primary antibodies were as follows: SgII 154–165 (N-terminal SN), SgII 172–186 (C-terminal SN), SgII 225–242 and SgIII 348–361, diluted at 1 : 160, 1 : 200, 1 : 160 and 1 : 20, respectively. The other primary antibodies were: mouse monoclonal antibodies to human somatostatin (Novo Nordisk S/A, Bagsvaerd, Denmark, clone Som-018), guinea-pig antibodies to human insulin (P. Westermark, Dept of Genetics and Pathology, Uppsala University; code Ma 47), and chicken antibodies to human glucagon and pancreatic polypeptide (A. Larsson, Dept. of Medical Sciences, Uppsala University). The working dilutions for immunofluorescence were 1 : 50, 1 : 200, 1 : 800 and 1 : 100, respectively.
The control stains entailed (1) omission of the primary antibodies, (2) replacement of the first layer of antibody by non-immune serum diluted 1 : 10 and by the diluent alone, (3) pre-incubation (24 h) of primary antibody with the relevant antigen (10 nmol mL−1 diluted antibody solution) before application to the sections. The secondary antibodies were tested in relation to the specificity of the species in which the primary antibodies had been raised, the secondary antibody in question being replaced by secondary antibodies from different animal species. These control tests were performed with streptavidin biotin complex (single staining) and immunofluorescence techniques (co-localization studies).
For co-localization studies, the sections were examined in a Vanox AHBS3 fluorescence microscope (Olympus, Tokyo, Japan) equipped with filters (Olympus) producing excitation at wavelengths 475–555 nm for Texas Red (filter no. 32821, dichroic mirror BH2-DMG), and 453–488 nm for FITC (no. 32822, BH2-DMIB). A double-band filter set (no. 39538, BH2-DFC5) for simultaneous visualization of Texas Red- and FITC-labelled cells was also used (excitation at 550–570 nm and 480–495 nm, respectively). Photographs were taken with Fujicolor 400 film.
Ethics
The study was approved by the local ethics committee at Uppsala University Hospital.
Results
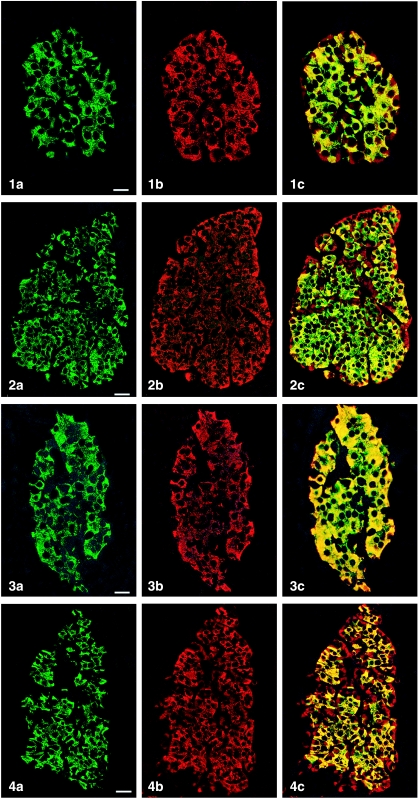
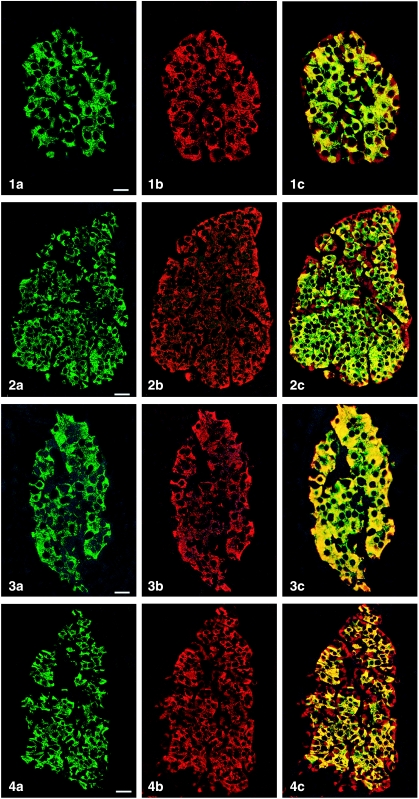
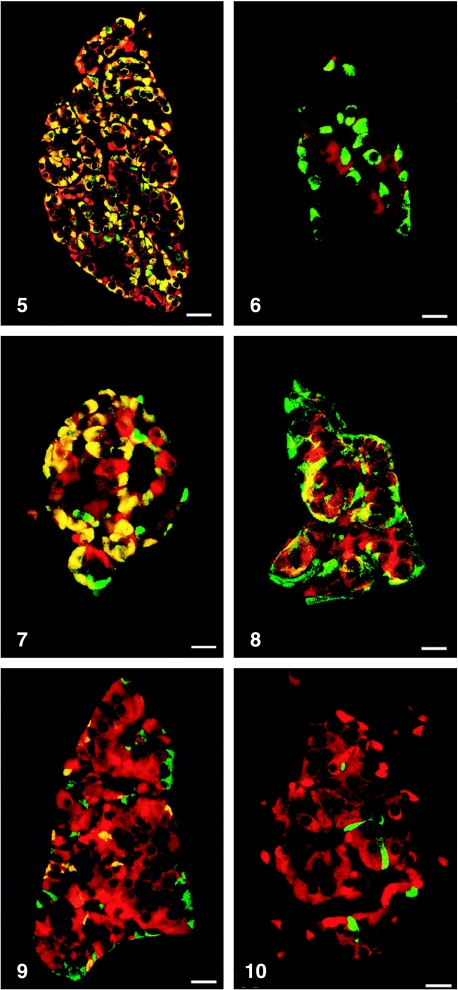
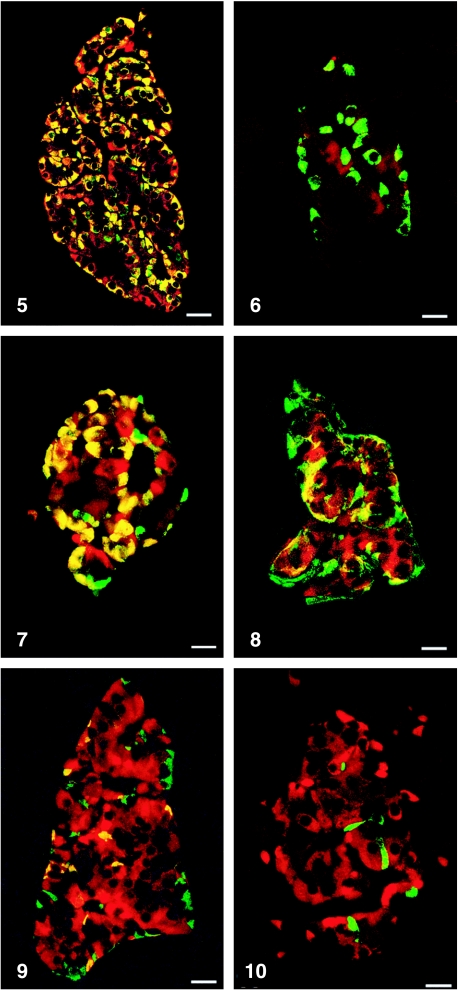
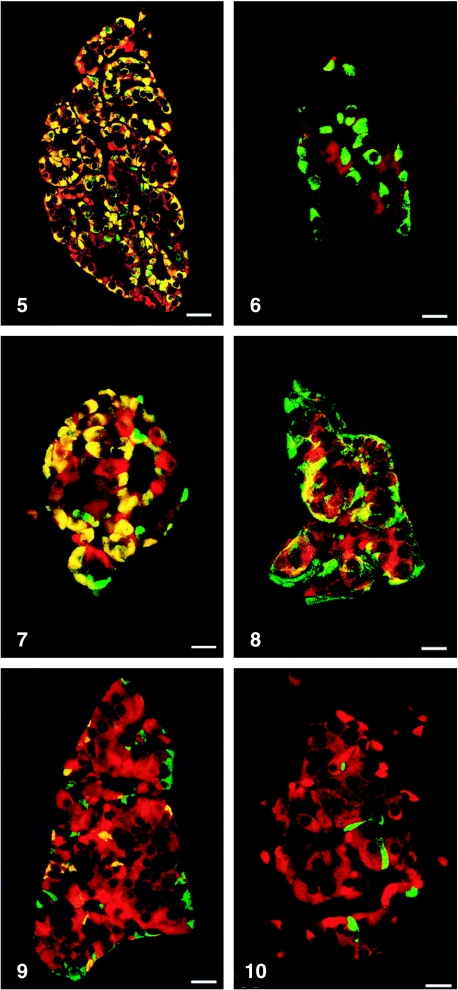
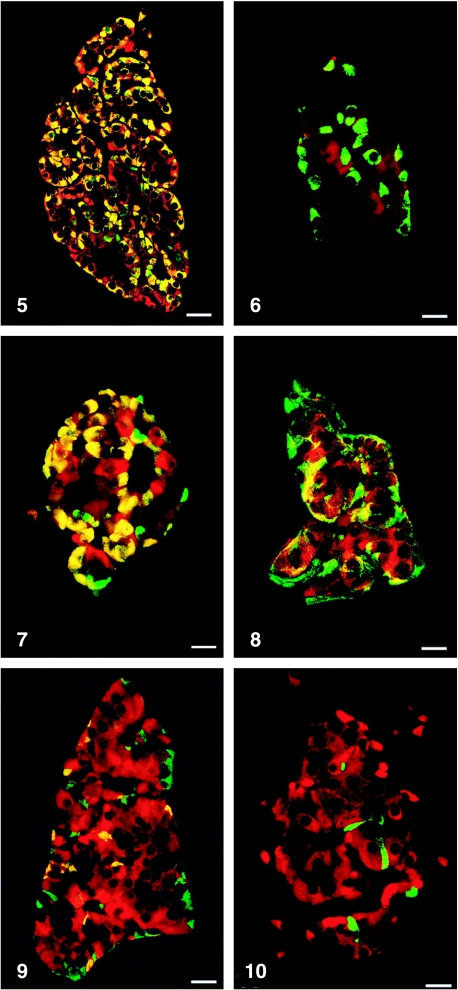
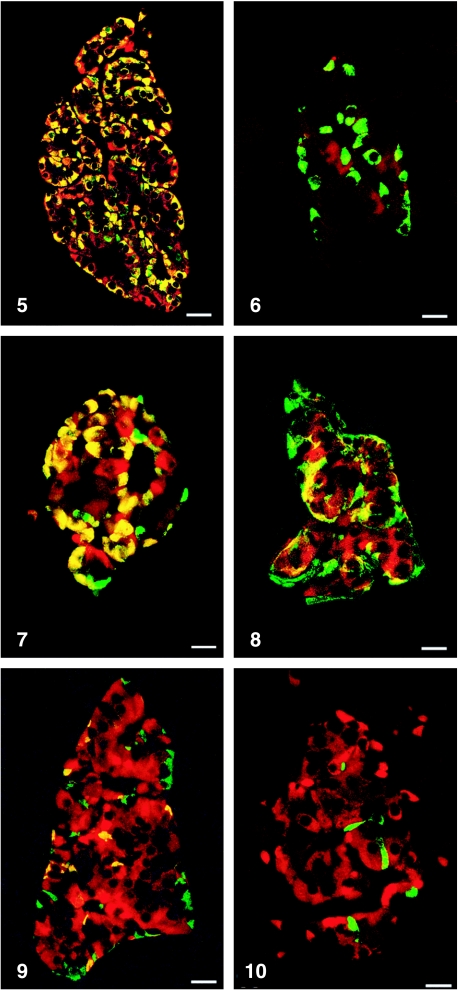
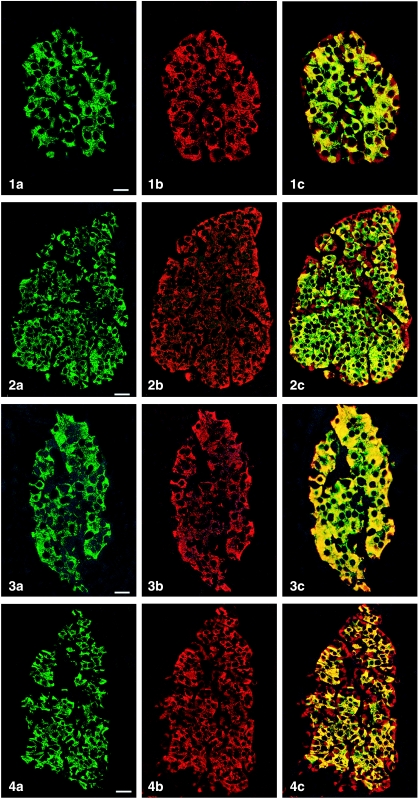
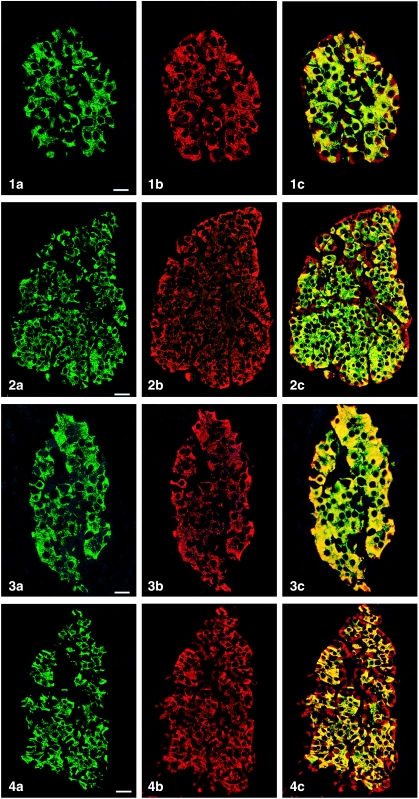
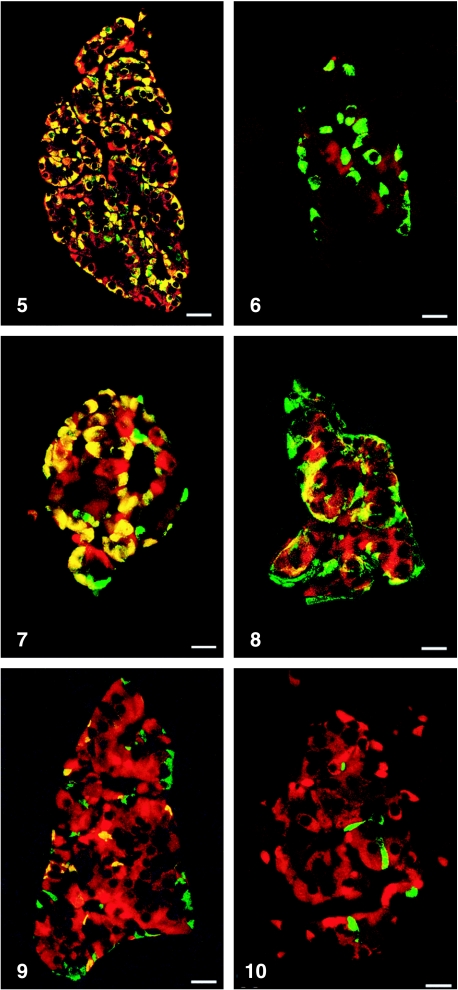
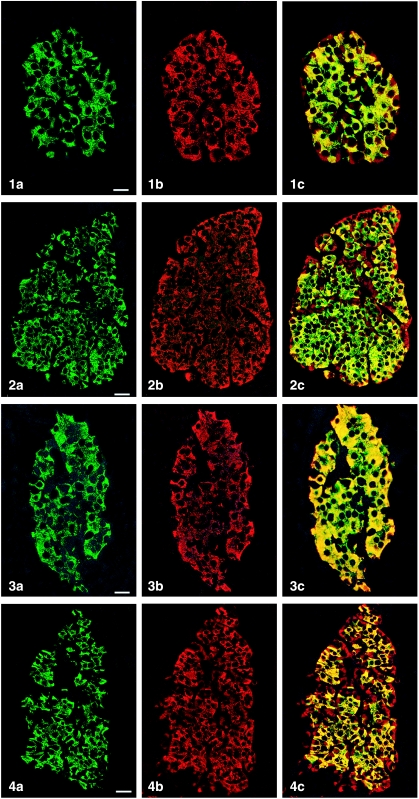
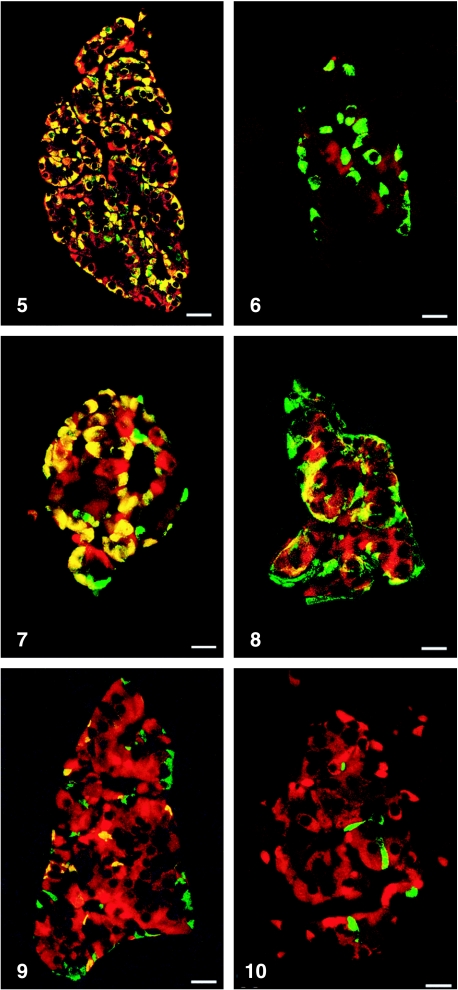
All peptides used were immunogenic and gave rise to useful antibodies. The expression of SgII and SgIII in the human pancreatic endocrine cell types is shown in Table 1. Virtually all insulin cells were immunostained by the three SgII and the SgIII antibodies (Figs 1, 2). Glucagon cells proved immunoreactive to two of the SgII antibodies (SgII 154–165 and 172–186) and the SgIII antibody (Figs 5–7). Some 10–50% of pancreatic polypeptide (PP) cells were immunoreactive to two of the SgII antibodies (SgII 154–165 and 172–186) and the SgIII antibody (Figs 8, 9). Somatostatin cells did not react with any of the antibodies used (Fig. 10).
Fig. 1.
Islet double immunostaining for co-localization of (a) insulin and (b) SgII 154–165 (N-terminal SN) and (c) demonstrating co-localization of these substances in all insulin cells (yellow to yellow-green). A few insulin-negative cells at the periphery of the islet show immunoreactivity to SgII (red). Bar = 16 µm.
Fig. 2.
Islet double immunostained for co-localization of (a) insulin and (b) SgII 172–186 (C-terminal SN), and (c) demonstrating varying degrees of co-localization between these substances (yellow to yellow-green). Some insulin-negative cells, mainly at the periphery of the islet, show only SgII immunoreactivity. Bar = 27 µm.
Fig. 5.
Islet double immunostained for co-localization of glucagon and SgII 154–165 (N-terminal SN). C-terminal SN is present in most glucagon cells. Bar = 40 µm.
Fig. 7.
Islet double immunostained for co-localization of glucagon and SgIII. SgIII shows immunoreactivity in most glucagon cells, illustrated by the yellow colour. A small fraction of glucagon cells did not express SgIII (green). Bar = 20 µm.
Fig. 8.
Islet double immunostained for co-localization of pancreatic polypeptide (PP) and SgII 154–165 (N-terminal SN). Immunoreactivity to SgII was observed in a fraction of PP cells (yellow). Bar = 16 µm.
Fig. 9.
Islet double immunostained for co-localization of pancreatic polypeptide and SgIII, showing co-localization of these two substances in a fraction of pancreatic polypeptide cells (yellow). Bar = 22 µm.
Fig. 10.
Islet double immunostained for co-localization of for somatostatin and SgIII, showing that somatostatin and SgIII are present in different endocrine cells. Bar = 20 µm.
Fig. 3.
Islet double immunostained for co-localization of (a) insulin and (b) SgII 225–242, and (c) demonstrating that all insulin cells showed SgII immunoreactivity and vice versa. Bar = 16 µm.
Fig. 4.
Islet double immunostained for co-localization of (a) insulin and (b) SgIII, and (c) demonstrating that SgIII immunoreactivity occurs in all insulin cells. SgIII immunoreactivity is also present in other islet cells located at the islet periphery and often close to fibro-vascular stroma (red). Bar = 27 µm.
Fig. 6.
Islet double immunostained for co-localization of glucagon and SgII 225–242, illustrating that glucagon cells do not express this domain of SgII. Bar = 22 µm.
Figs 1a–10.
Human pancreatic islets double immunostained for co-localization of different hormones (FITC) and secretogranins (Sgs; Texas Red). A double-band filter set was used (1c–4c, 5–10).
Discussion
During recent years, several region-specific antibodies have been raised against various epitopes of the CgA and CgB molecules; these antibodies have been useful for radioimmunoassay analyses and for immunostaining in tissue sections (Portela-Gomes & Stridsberg, 2001; Portela-Gomes & Stridsberg, 2002; Stridsberg et al. 2004, 2005). The present findings focus on antibodies to SgII and SgIII, both being used for immunohistochemical analysis of human endocrine pancreas.
Granins are differently expressed in the various endocrine cells in the pancreas and the gastro-intestinal tract. Furthermore, it has been shown that specific regions of the CgA and CgB molecules are differently expressed in the endocrine cells of these organs (Portela-Gomes & Stridsberg, 2001; Portela-Gomes & Stridsberg, 2002). Thus, one specific region of CgA (CgA116–130) was expressed only in the islet PP cells. The most C-terminal region of CgA (CgA411–424) and two defined regions of CgB (CgB244–255, CgB647–657) were expressed in somatostatin cells. Insulin and glucagon cells expressed almost all regions of the CgA and CgB molecules (Portela-Gomes & Stridsberg, 2001; Portela-Gomes & Stridsberg, 2002). The present study obtained similar results regarding differential expression of SgII and SgIII. All antibodies immunostained insulin cells, while glucagon and PP cells did not express the SgII 225–242 epitope. Somatostatin cells were not immunostained with any of the Sg antibodies. Only insulin cells expressed the SgII 225–242 epitope, comparable to the expression of CgA 238–247 (Portela-Gomes & Stridsberg, 2001).
Earlier studies have reported the expression of SgII and SgIII in neuroendocrine cells in different organs. However, most of these studies were performed in experimental animals. In earlier studies on human endocrine pancreas, the cellular distribution of SgII was only partly described. Others have used antibodies raised against rat SN (amino acid sequences SgII 154–186) and reported immunoreactivity in insulin and glucagon cells of human pancreas, but in neither PP nor somatostatin cells (Schmid et al. 1994, 1995). Our results, using human SN sequences, were consistent to theirs, but we were also able to demonstrate immunoreactivity in a sub-population of PP cells.
A previous report showed that the use of region-specific antibodies raised against epitopes in the C-terminal region of SN can facilitate the diagnosis of malignant pheochromocytoma; these antibodies visualized distinctly spindle-shaped cells with long processes, cells that had been described in pheochromocytomas as being linked to malignant activity (Portela-Gomes et al. 2004). It has also been shown that gastrin cells and a few enterochromaffin cells can display SgII immunoreactivity (SgII 225–242; in that publication denoted CgC) (Portela-Gomes et al. 1997).
It has been reported that Sg III can bind to CgA and the binding site was shown to be localized between SgIII 214–373 and CgA 48–111 (Hosaka et al. 2002). Since the amino acid sequences to which some of our SgIII antibodies were raised (348–361) are included in the described binding site to CgA, it is interesting to note that the immunostaining pattern of SgIII was comparable to that reported earlier in islet cells for the CgA epitopes between amino acids 48 and 111 (Portela-Gomes & Stridsberg, 2001; Hosaka et al. 2002).
SgIII mRNA and protein expression have previously been described in rat brain and pituitary, co-localized with CgA in the same secretory granules (Ottiger et al. 1990; Hosaka et al. 2002; Sakai et al. 2004). Similarly, expression of SgIII has been reported in the pituitary of the frog Xenopus laevis (Kingsley et al. 1990; Holthuis & Martens, 1996). Upon stimulation, the mRNA for SgIII increased in parallel to that of proopiomelanocortin, and thus a functional role for SgII has been suggested in frog (Holthuis & Martens, 1996). However, ablation of the gene encoding for SgIII did not result in any major defects in mice, suggesting that the protein is of minor importance in mammals (Kingsley et al. 1990).
To conclude, this study is the first observation of expression of SgIII in human tissues, where we show expression of SgIII in three of the four major islet cell types in human pancreas. The functional significance of this observation remains to be established. However, it can be hypothesized that the binding of SgIII to CgA may be of importance.
Acknowledgments
This work was accorded grants from the Swedish Cancer Foundation, Lions Cancer Fund and Selanders Fund.
References
- Dunzendorfer S, Schratzberger P, Reinisch N, Kähler CM, Wiedermann CJ. Secretoneurin, a novel neuropeptide, is a potent chemoattractant for human eosinophils. Blood. 1998;91:1527–1532. [PubMed] [Google Scholar]
- Helle KB. The granin family of uniquely acidic proteins of the diffuse neuroendocrine system: comparative and functional aspects. Biol Rev Camb Philos Soc. 2004;79:769–794. doi: 10.1017/s146479310400644x. [DOI] [PubMed] [Google Scholar]
- Holthuis JCM, Martens GJM. The neuroendocrine proteins secretogranin II and III are regionally conserved and coordinately expressed with proopiomelanocortin in Xenopus intermediate pituitary. Journal of Neurochemistry. 1996;66:2248–2256. doi: 10.1046/j.1471-4159.1996.66062248.x. [DOI] [PubMed] [Google Scholar]
- Hosaka M, Watanabe T, Sakai Y, et al. Identification of a chromogranin A domain that mediates binding to secretogranin III and targeting to secretory granules in pituitary cells and pancreatic beta-cells. Mol Biol Cell. 2002;13:3388–3399. doi: 10.1091/mbc.02-03-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischia R, Gasser RW, Fischer-Colbrie R, Uchiyama Y, Takeuchi T. Levels and molecular properties of secretoneurin-immunoreactivity in the serum and urine of control and neuroendocrine tumor patients. J Clin Endocrinol Metab. 2000;85:355–360. doi: 10.1210/jcem.85.1.6314. [DOI] [PubMed] [Google Scholar]
- Kahler CM, Schratzberger P, Wiedermann CJ. Response of vascular smooth muscle cells to the neuropeptide secretoneurin. A functional role for migration and proliferation in vitro. Arterioscler Thromb Vasc Biol. 1997;17:2029–2035. doi: 10.1161/01.atv.17.10.2029. [DOI] [PubMed] [Google Scholar]
- Kingsley DM, Rinchik EM, Russell LB, Ottiger HP, Sutcliffe JG, Copeland NG, et al. Genetic ablation of a mouse gene expressed specifically in brain. EMBO J. 1990;9:395–400. doi: 10.1002/j.1460-2075.1990.tb08123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchmair R, Hogue Angeletti R, Gutierrez J, Fischer Colbrie R, Winkler H. Secretoneurin – a neuropeptide generated in brain, adrenal medulla and other endocrine tissues by proteolytic processing of secretogranin II (chromogranin C) Neuroscience. 1993;53:359–365. doi: 10.1016/0306-4522(93)90200-y. [DOI] [PubMed] [Google Scholar]
- Marksteiner J, Kirchmair R, Mahata SK, Mahata M, Fischer-Colbrie R, Hogue-Angeletti R, et al. Distribution of secretoneurin, a peptide derived from secretogranin II, in rat brain: an immunocytochemical and radioimmunological study. Neuroscience. 1993;54:923–944. doi: 10.1016/0306-4522(93)90585-4. [DOI] [PubMed] [Google Scholar]
- Ottiger HP, Battenberg EF, Tsou AP, Bloom FE, Sutcliffe JG. 1B1075: A brain- and pituitary-specific messenger RNA that encodes a novel chromogranin-secretogranin-like component of intracellular vesicles. J Neuroscience. 1990;10:3135–3147. doi: 10.1523/JNEUROSCI.10-09-03135.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portela-Gomes GM, Stridsberg M. Selective processing of chromogranin A in the different islet cells in human pancreas. J Histochem Cytochem. 2001;49:483–490. doi: 10.1177/002215540104900408. [DOI] [PubMed] [Google Scholar]
- Portela-Gomes GM, Stridsberg M. Region-specific antibodies against chromogranin B display different immunoreactivity in the human pancreatic islet cell types. Ann NY Acad Sci. 2002;971:341–344. doi: 10.1111/j.1749-6632.2002.tb04491.x. [DOI] [PubMed] [Google Scholar]
- Portela-Gomes GM, Stridsberg M, Johansson H, Grimelius L. Complex co-localization of chromogranins and neurohormones in the human gastrointestinal tract. J Histochem Cytochem. 1997;45:815–822. doi: 10.1177/002215549704500606. [DOI] [PubMed] [Google Scholar]
- Portela-Gomes GM, Stridsberg M, Grimelius L, Falkmer UG, Falkmer S. Expression of chromogranins A, B, and C (secretogranin II) in human adrenal medulla and in benign and malignant pheochromocytomas An immunohistochemical study with region-specific antibodies. APMIS. 2004;112:663–673. doi: 10.1111/j.1600-0463.2004.t01-1-apm1121003.x. [DOI] [PubMed] [Google Scholar]
- Reinisch N, Kirchmair R, Kähler CM, Hogure-Angeletti R, Fischer-Colbrie R, Wnkler H, et al. Attraction of human monocytes by the neuropeptide secretoneurin. FEBS Lett. 1993;334:41–44. doi: 10.1016/0014-5793(93)81676-q. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Hosaka M, Yoshinaga A, Hira Y, Harumi T, Watanabe T. Immunocytochemical localization of secretogranin III in the endocrine pancreas of male rats. Arch Histol Cytol. 2004;67:57–64. doi: 10.1679/aohc.67.57. [DOI] [PubMed] [Google Scholar]
- Schmid KW, Brink M, Freytag G, Kirchmair R, Böcker W, Fischer-Colbrie R, et al. Expression of chromogranin A and B and secretoneurin immunoreactivity in neoplastic and nonneoplastic pancreatic alpha cells. Virchows Arch. 1994;425:127–132. doi: 10.1007/BF00230348. [DOI] [PubMed] [Google Scholar]
- Schmid KW, Kunk B, Kirchmair R, Tötsch M, Böcker W, Fischer-Colbrie R. Immunohistochemical detection of secretoneurin, a novel neuropeptide endoproteolytically processed from secretogranin II, in normal human endocrine and neuronal tissues. Histochem J. 1995;27:473–481. [PubMed] [Google Scholar]
- Stridsberg M, Öberg K, Li Q, Engström U, Lundqvist G. Measurements of chromogranin A, chromogranin B (secretogranin I), chromogranin C (secretogranin II) and pancreastatin in plasma and urine from patients with carcinoid tumours and endocrine pancreatic tumours. J Endocrinol. 1995;144:49–59. doi: 10.1677/joe.0.1440049. [DOI] [PubMed] [Google Scholar]
- Stridsberg M, Eriksson B, Oberg K, Janson ET. A panel of 11 region-specific radioimmunoassays for measurements of human chromogranin A. Regul Pept. 2004;117:219–227. doi: 10.1016/j.regpep.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Stridsberg M, Eriksson B, Oberg K, Janson ET. A panel of 13 region-specific radioimmunoassays for measurements of human chromogranin B. Regul Pept. 2005;125:193–199. doi: 10.1016/j.regpep.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Taupenot L, Harper KL, O’Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- You ZB, Saria A, Fischer-Colbrie R, Terenius L, Goiny M, Herrera-Marschitz M. Effects of secretogranin II-derived peptides on the release of neurotransmitters monitored in the basal ganglia of the rat with in vivo microdialysis. Naunyn-Schmiedebergs Arch Pharmacol. 1996;354:717–724. doi: 10.1007/BF00166897. [DOI] [PubMed] [Google Scholar]