Abstract
BACKGROUND
Although assessment for aspiration of small volumes of gastric contents in tube-fed patients receiving mechanical ventilation is important, available methods for this purpose are not wholly satisfactory. A potential method is immunoassay of tracheal secretions for the gastric enzyme pepsin.
OBJECTIVES
To determine the frequency with which pepsin in suctioned tracheal secretions from acutely ill, tube-fed patients receiving mechanical ventilation could be detected via an immunoassay.
METHODS
A convenience sample of 136 specimens of suctioned tracheal secretions was collected from 30 acutely ill, tube-fed adults receiving mechanical ventilation. Multiple samples were obtained from 26 of the 30 patients (range, 2−11 per subject). An immunoassay with rooster polyclonal antibodies to purified human pepsin was used to detect pepsin in the secretions.
RESULTS
Fourteen specimens tested positive for pepsin. Secretions from 5 patients accounted for the 14 pepsin-positive results. A significant relationship was found between the position of the head of the bed and the presence of pepsin in tracheal secretions (P< .001). Of the 14 pepsin-positive specimens, 13 (92.9%) were obtained from subjects in a flat position.
CONCLUSIONS
A pepsin immunoassay can be used to detect pepsin in human tracheal secretions. If pepsin in tracheal secretions is considered an indicator of aspiration of gastric contents, aspiration occurred in 5 of the 30 subjects. A flat position is strongly associated with the presence of pepsin in tracheal secretions.
Pulmonary aspiration of gastric contents is the most serious complication of tube feeding. However, little consensus exists on how frequently it occurs in patients receiving mechanical ventilation because researchers who studied this problem used widely varied definitions of aspiration and methods for its detection. A well-controlled study by Torres et al1 suggested that aspiration occurs in 32% of patients receiving mechanical ventilation when the patients are semirecumbent and in 68% when they are supine. Aspiration of gastric contents has numerous deleterious consequences, including transient hypoxemia, chemical pneumonitis, and potentially life-threatening nosocomial pneumonia.
Aspiration of gastric contents is one cause of ventilator-associated pneumonia, a condition that increases duration of mechanical ventilation, length of hospital stays, and use of medical resources. In a study2 of 120 critically ill patients receiving mechanical ventilation, patients with ventilator-associated pneumonia had a 16-day greater length of stay and almost a $30000 greater cost per case than did patients without pneumonia.
Although observed single aspirations of a large volume of gastric contents sometimes occur, most tube-fed patients experience repeated unobserved aspirations of small volumes of gastric contents that can ultimately lead to pneumonia.3 Clinicians try to detect aspiration early so that interventions can be implemented to prevent further aspiration events and poor outcomes. In clinical settings, methods used to detect aspiration of tube feedings include (1) adding food dye to the enteral formula and then observing for dye-stained tracheal secretions and (2) using glucose oxidase reagent strips to test tracheal secretions for the presence of glucose-rich formula. Unfortunately, neither method is wholly satisfactory.4 For example, the sensitivity and safety of the dye method5-7 and the specificity of the glucose method8,9 have been questioned. Therefore, the search for effective, yet harmless, clinical methods to detect aspiration of gastric contents should continue.
Because pepsin is plentiful in gastric juice, but not in tracheal secretions, several groups of investigators have suggested that pepsin would be a good marker or indicator for the aspiration of gastric juice.10,11 To test this hypothesis, Ufberg et al11 used unspecified methods to detect pepsin in specimens of gastric fluid and tracheal secretions obtained from 10 fasting preoperative patients. All 10 of the gastric specimens tested positive for pepsin, and all 10 of the tracheal specimens were negative. Ufberg et al concluded that a pepsin assay is a promising tool for the diagnosis of occult pulmonary aspiration of gastric contents.
Using a pepsin assay developed by Anson12 (a method in which proteolytically active pepsin is allowed to digest a known quantity of hemoglobin), Badellino et al10 tested the same hypothesis in an animal model. Human gastric juice (2 mL/kg) was instilled intratracheally into 24 rabbits; similar volumes of isotonic sodium chloride solution were instilled intratracheally in 10 control rabbits. Bronchoalveolar lavage was performed 15 minutes, 30 minutes, or 60 minutes after the instillation of fluid. In the rabbits given human gastric juice, peptic activity was detected in the lavage fluid in 8 of 8 animals at 15 minutes, 6 of 8 at 30 minutes, and 5 of 8 at 60 minutes. Because the Anson method relies on the presence of proteolytically active pepsin to digest a hemoglobin substrate, it cannot be used to detect pepsin that has been degraded in the alkaline environment of the lung. This factor explains why fewer specimens tested positive for pepsin at the 30- and 60-minute times. No peptic activity was present in lavage fluid from control animals at any time.
In another study,13 the Anson method was used to assay for pepsin in 102 specimens of tracheal secretions collected from acutely ill human subjects. Only 2 of the 102 specimens contained sizeable amounts of pepsin (66.2 and 83.1 μg/mL). Because a variety of other proteins in addition to pepsin are detected with the Anson method, these values may be overestimations of the actual amount of pepsin in the 2 samples.
Purpose
The purpose of this exploratory study was to determine the frequency with which pepsin in suctioned tracheal secretions from acutely ill, tube-fed patients receiving mechanical ventilation could be detected via an immunoassay.
Methods
A convenience sample of 136 specimens of suctioned tracheal secretions was collected from 30 acutely ill, tube-fed adults receiving mechanical ventilation during a period of 2 1/2 months. Multiple samples were obtained from 26 of the 30 patients (range, 2−11 per patient). All 30 patients had enteral tubes for the administration of tube feedings and/or medications or for intermittent gastric decompression.
The institutional review board waived informed consent because no patient identifiers were used and specimens were discarded secretions. Visits were made to 4 adult intensive care units on weekdays to determine potential subjects. Inclusion criteria were treatment with mechanical ventilation, presence of a nasally inserted or endoscopically placed enteral tube, and no use of isolation precautions. The bedside nurses of selected patients were asked to collect the specimens in mucus traps during suctioning procedures and to give the specimens to the investigators. The bedside nurses decided whether or not isotonic sodium chloride solution was used during the suctioning procedure. The nurses placed the collected specimens in iced coolers provided by the investigators. Within a 2-hour period (usually <1 hour), specimens were transported to a research laboratory where they were assayed for pepsin. Information gathered at the time of data collection included whether or not tube feedings were in progress, the type of feeding tube in use, and the position of the head of the bed.
Pepsin Assay
An immunoassay with rooster polyclonal antibodies to purified human pepsin was used to detect pepsin in tracheal secretions. Because it uses an antibody to human pepsin, the assay is highly specific. The assay is also highly sensitive; a pepsin concentration as low as 1 μg/mL can be detected. All of the assays were performed by the same biochemist.
For the assay, 15 μL of each tracheal specimen was mixed with an equal volume of Laemmli Sample Buffer (Bio-Rad Laboratories, Hercules, Calif) containing 2-mercaptoethanol (Bio-Rad Laboratories) and boiled for 10 minutes. The treated sample was then resolved by using sodium dodecyl sulfate polyacrylamide gel electrophoresis and was transferred onto a nitrocellulose membrane (Bio-Rad Laboratories). After the membrane was blocked overnight in 50 mL of phosphate-buffered saline (pH 7.5; containing 0.5% Tween-20 [PBST] and 10% nonfat dry milk), it was incubated at room temperature for 2 hours with the rooster polyclonal antibodies to human pepsin diluted 1000-fold in PBST. The membrane was then washed 3 times in PBST and incubated at room temperature for 2 hours with the peroxidase-conjugated rabbit antichicken IgG (Sigma, St Louis, Mo) diluted 1:20 000 in PBST. Finally, after the membrane was rinsed 3 times in PBST, pepsin was visualized by using the chemiluminescent ICL kit (Amersham, Arlington, Ill) according to the manufacturer's procedures. Standard curves for the assay were created by assaying samples containing pepsin at concentrations of 2, 5, 10, 25, and 50 μg/mL.
As stated earlier, with this assay, a pepsin concentration as low as 1 μg/mL can be detected. The sensitivity of the assay is not great enough to detect the small quantity of pepsinogen (0.1 μg/mL) known to be present in blood (presumably after direct absorption from gastric mucosal cells).14
Data Analysis
Simple descriptive statistics, independent sample t tests, and Pearson correlation coefficients were used to analyze the data. Results are expressed as mean ± SE.
Results
The mean volume of the 136 specimens of suctioned secretions was 2.4 ± 0.2 mL (range, 0.2−9.0 mL). Isotonic sodium chloride solution was used during the collection of 52 specimens. Of the 136 specimens, 23 were visibly bloody. The head of the bed was flat during the collection of 43 of the 136 specimens. Tube feedings were in progress when 85 of the specimens were collected. The remaining 51 specimens were collected before feedings were started or when the feedings were temporarily on hold for various reasons.
Of the 136 specimens, 14 tested positive for pepsin (see Figure for an example of positive vs negative results). Secretions from 5 subjects accounted for the 14 pepsin-positive results. Of the 14 positive results, 5 were for specimens collected from a single patient. In addition, 2 patients had positive results for 3 specimens each, 1 patient had positive results for 2 specimens, and 1 patient had positive results for 1 specimen (see the Table). No statistically significant relationships were found between the presence of pepsin in tracheal secretions and the use of tube feedings, presence of blood, or use of isotonic sodium chloride solution during suctioning. However, a statistically significant relationship was found between the position of the head of the bed and the presence of pepsin in tracheal secretions (P<.001). Of the 14 pepsin-positive specimens, 13 (92.9%) were obtained from patients in a flat-lying position.
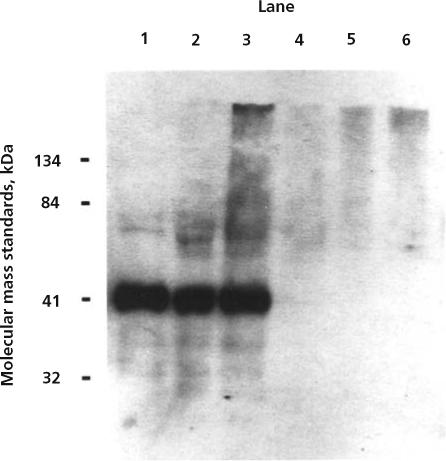
Immunoblot for human pepsin. Lane 1, a positive control (20 ng of human pepsin from gastric fluid); lanes 2 and 3, tracheal secretions positive for pepsin from subjects DD (specimen 1) and I (specimen 8), respectively; lanes 4, 5, and 6, tracheal secretions negative for pepsin from subjects C, E, and G, respectively. Molecular mass standards used were β-galactosidase (134 kDa), bovine serum albumin (84 kDa), carbonic anhydrase (41 kDa), and soybean trypsin inhibitor (32 kDa).
Descriptive information on 5 subjects with pepsin present in 1 or more tracheal secretions
| Subject/specimen No. | Date and time | Amount of pepsin, μg/mL | Tube feeding status | Isotonic sodium chloride solution used during suctioning | Position of head of bed, type of feeding tube |
|---|---|---|---|---|---|
| W-1 | September 12, 10 AM | 1.9 | Present | Yes | Head of bed flat for spinal cord injury, nasally placed small-bore tube |
| W-2 | September 13, 12 PM | 2.4 | Present | Yes | |
| W-3 | September 18, 8 AM | 3.7 | Present | No | |
| W-4 | September 18, 10 AM | 2.6 | Present | No | |
| W-6 | September 19, 12 PM | 2.2 | On hold | Yes | |
| DD-1 | October 2, 10 AM | 17.8 | Absent | Yes | Head of bed flat for spinal cord injury, nasally placed large-bore tube |
| DD-2 | October 2, 12 PM | 2.7 | Absent | Yes | |
| DD-3 | October 2, 2 PM | 6.3 | Absent | Yes | |
| I-8 | August 10, 2 PM | 9.5 | Present | No | Head of bed flat for spinal cord injury, percutaneous gastrostomy tube |
| I-9 | August 11, 12 PM | 5.1 | Present | No | |
| I-10 | August 11, 2 PM | 7.8 | Present | No | |
| X-1 | September 14, 10 AM | 4.2 | Present | Yes | Head of bed flat for spinal cord injury, nasally placed small-bore tube |
| X-2 | September 19, 10 AM | 1.9 | On hold | No | |
| V-3 | September 13, 2 PM | 3.1 | On hold | Yes | Head of bed elevated, nasally placed small-bore tube |
Discussion
One patient (W) had 5 specimens positive for pepsin during a 7-day period, another patient (X) had 2 specimens positive for pepsin during a 5-day period, and another (I) had 3 specimens positive for pepsin during a 24-hour period (see Table). These findings suggest that multiple episodes of aspiration occurred in these patients. In contrast, the 3 specimens that were positive for pepsin within a 4-hour period for patient DD possibly represent a single episode of aspiration. We do not know the length of time after a single aspiration of gastric juice that pepsin can be detected with this immunoassay.
The only variable significantly related to detection of pepsin in specimens was the position of the head of the bed at the time the specimens were collected. That 13 of the 14 pepsin-positive specimens were obtained from subjects in a flat-lying position is not surprising; several well-controlled studies1,15,16 indicated that aspiration is far more likely to occur when the head of the bed is not elevated. The primary reason for the supine position in subjects included in this study was the presence of actual or possible spinal cord injury.
Conclusions
The 14 specimens positive for pepsin strongly suggest that aspiration of gastric juice occurred in 5 of the 30 high-risk patients in the study. What remains to be learned is the significance of these findings in relation to clinical outcomes. As Bartlett and Gorbach17 pointed out, aspiration of small volumes of gastric contents is common even in healthy persons and may be unrecognized. A number of factors determine who will have pulmonary complications after aspiration, including the frequency of aspiration and the character and volume of the aspirate. Also important is the patient's ability to defend against pneumonia after aspiration. Especially significant risk factors for pneumonia are advanced age, high severity of illness, and immunosuppression.18,19
Our findings indicate that an immunoassay for pepsin in tracheobronchial secretions may be a better method for detecting pulmonary aspiration of gastric contents than are other commonly used methods. Additional work is needed to compare clinical outcomes with the presence or absence of pepsin in tracheal secretions obtained from critically ill, tube-fed patients receiving mechanical ventilation.
ACKNOWLEDGMENTS
This project was funded in part by the National Institute of Nursing Research grant R01 NR0 5007. The research was done in accordance with the St Louis University Health Sciences Center institutional review board and the ethical standards set forth in the Helsinki Declaration of 1975.
REFERENCES
- 1.Torres A, Serra-Batlles J, Ros E, et al. Pulmonary aspiration of gastric contents in patients receiving mechanical ventilation: the effect of body position. Ann Intern Med. 1992;116:540–543. doi: 10.7326/0003-4819-116-7-540. [DOI] [PubMed] [Google Scholar]
- 2.Byers JF, Sole ML. Analysis of factors related to the development of ventilator-associated pneumonia: use of existing databases. Am J Crit Care. 2000;9:344–349. [PubMed] [Google Scholar]
- 3.Pingleton SK. Aspiration of enteral feeding in mechanically ventilated patients: how do we monitor? Crit Care Med. 1994;22:1524–1525. [PubMed] [Google Scholar]
- 4.Metheny NA, Clouse RE. Bedside methods for detecting aspiration in tube-fed patients. Chest. 1997;111:724–731. doi: 10.1378/chest.111.3.724. [DOI] [PubMed] [Google Scholar]
- 5.Potts RG, Zaroukian MH, Guerrero PA, Baker CD. Comparison of blue dye visualization and glucose oxidase test strip methods for detecting pulmonary aspiration of enteral feedings in intubated adults. Chest. 1993;103:117–121. doi: 10.1378/chest.103.1.117. [DOI] [PubMed] [Google Scholar]
- 6.Maloney JP, Halbower AC, Fouty BF, et al. Systemic absorption of food dye in patients with sepsis. N Engl J Med. 2000;343:1047–1048. doi: 10.1056/NEJM200010053431416. [DOI] [PubMed] [Google Scholar]
- 7.Maloney JP, Bhargava R, Ryan TA, Batchelder SL, Halbower AC, Moss M. FD&C Blue No. 1 food dye is a mitochondrial poison that can be absorbed from enteral tube feedings in sepsis: a report of 3 deaths linked to systemic absorption [abstract]. JPEN J Parenter Enteral Nutr. 2001;25:S25. [Google Scholar]
- 8.Elpern EH, Jacobs ER, Tangney CC, Bone RC. Nonspecificity of glucose reagent strips as a marker of formula feeding aspiration [abstract]. Am Rev Respir Dis. 1986;131:A288. [Google Scholar]
- 9.Kinsey GC, Murray MJ, Swensen SJ, Miles JM. Glucose content of tracheal aspirates. Crit Care Med. 1995;23:1451–1453. [PubMed] [Google Scholar]
- 10.Badellino MM, Buckman RF, Jr, Malaspina PJ, Eynon CA, O'Brien GM, Kueppers F. Detection of pulmonary aspiration of gastric contents in an animal model by assay of peptic activity in bronchoalveolar fluid. Crit Care Med. 1996;24:1881–1885. doi: 10.1097/00003246-199611000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Ufberg JW, Bushra JS, Patel D, et al. Human trial of a pepsin assay to detect pulmonary aspiration of gastric contents during endotracheal intubation. Acad Emerg Med. 2001;8:421–422. [Google Scholar]
- 12.Anson M. Estimation of pepsin, trypsin, papain and cathepsin with hemoglobin. J Gen Physiol. 1938;22:79–89. doi: 10.1085/jgp.22.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metheny NA, Stewart BJ, Smith L, Yan H, Diebold M, Clouse RE. pH and concentrations of pepsin and trypsin in feeding tube aspirates as predictors of tube placement. JPEN J Parenter Enteral Nutr. 1997;21:279–285. doi: 10.1177/0148607197021005279. [DOI] [PubMed] [Google Scholar]
- 14.Samloff IM, Liebman WM. Radioimmunoassay of group I pepsinogens in serum. Gastroenterology. 1974;66:494–502. [PubMed] [Google Scholar]
- 15.Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogue S, Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354:1851–1858. doi: 10.1016/S0140-6736(98)12251-1. [DOI] [PubMed] [Google Scholar]
- 16.Ibanez J, Penafiel A, Raurich JM, Marse P, Jorda R, Mata F. Gastroesophageal reflux in intubated patients receiving enteral nutrition: effect of supine and semirecumbent positions. JPEN J Parenter Enteral Nutr. 1992;16:419–422. doi: 10.1177/0148607192016005419. [DOI] [PubMed] [Google Scholar]
- 17.Bartlett JG, Gorbach SL. The triple threat of aspiration pneumonia. Chest. 1975;68:560–566. doi: 10.1378/chest.68.4.560. [DOI] [PubMed] [Google Scholar]
- 18.Cook DJ, Walter SD, Cook RJ, et al. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med. 1998;129:433–440. doi: 10.7326/0003-4819-129-6-199809150-00002. [DOI] [PubMed] [Google Scholar]
- 19.Zaloga GP. Pathophysiology of aspiration/aspiration pneumonia.. Paper presented at: 20th Clinical Congress, American Society of Parenteral and Enteral Nutrition; Washington, DC. January 14−17, 1996. [Google Scholar]


