Abstract
White light strongly promotes dormancy in freshly harvested cereal grains, whereas dark and after-ripening have the opposite effect. We have analyzed the interaction of light and after-ripening on abscisic acid (ABA) and gibberellin (GA) metabolism genes and dormancy in barley (Hordeum vulgare ‘Betzes’). Analysis of gene expression in imbibed barley grains shows that different ABA metabolism genes are targeted by white light and after-ripening. Of the genes examined, white light promotes the expression of an ABA biosynthetic gene, HvNCED1, in embryos. Consistent with this result, enzyme-linked immunosorbent assays show that dormant grains imbibed under white light have higher embryo ABA content than grains imbibed in the dark. After-ripening has no effect on expression of ABA biosynthesis genes, but promotes expression of an ABA catabolism gene (HvABA8′OH1), a GA biosynthetic gene (HvGA3ox2), and a GA catabolic gene (HvGA2ox3) following imbibition. Blue light mimics the effects of white light on germination, ABA levels, and expression of GA and ABA metabolism genes. Red and far-red light have no effect on germination, ABA levels, or HvNCED1. RNA interference experiments in transgenic barley plants support a role of HvABA8′OH1 in dormancy release. Reduced HvABA8′OH1 expression in transgenic HvABA8′OH1 RNAi grains results in higher levels of ABA and increased dormancy compared to nontransgenic grains.
Seed dormancy is a critical adaptive trait that is present in many plant species. It is imposed during the latter stages of embryo development and prevents germination prior to the completion of seed maturation (Baskin and Baskin, 1998). The persistence of dormancy after seed maturity is variable among species, but, in many species where it is retained, it offers adaptive advantages, such as avoidance of temporary conditions that do not support seedling establishment, and also the formation of seed banks in soils that remain viable for many years. In contrast to wild relatives, modern cereals, such as barley (Hordeum vulgare) and wheat (Triticum aestivum), have undergone strong selection by breeders against dormancy to promote quick and uniform germination in successive rounds of breeding (Simpson, 1990). As a consequence of the selective pressure, modern barley and wheat cultivars have low dormancy and are thus prone to preharvest sprouting.
In cereals and other seeds, it is well established through physiological and genetic studies that abscisic acid (ABA) plays an important role in the induction and maintenance of dormancy (Finkelstein, 2004; Gubler et al., 2005; Feurtado and Kermode, 2007). For example, many viviparous mutants in maize (Zea mays) have a defect in ABA biosynthesis or signaling, indicating a role for ABA in preventing precocious germination (McCarty, 1995). During seed development, ABA content is low during the early stages, increases rapidly, peaks around midmaturation, and thereafter declines gradually during seed desiccation (Bewley, 1997). In Arabidopsis (Arabidopsis thaliana) and tobacco (Nicotiana tabacum), it is clear that the early increase in ABA is derived from maternal tissue, but the increase during the midmaturation stage is due to ABA synthesized in the embryonic tissues (Karssen et al., 1983; Frey et al., 2004). Genetic studies using reciprocal crosses have ruled out the possibility that maternal ABA is responsible for the induction of dormancy. In Arabidopsis, analysis of mutants and expression patterns of ABA biosynthetic genes shows that expression of the 9-cis-epoxycarotenoid dioxygenase (NCED) genes plays a critical role in spatiotemporal regulation of ABA synthesis in the seed (Tan et al., 2003; Lefebvre et al., 2006). AtNCED6 and AtNCED9 are the most highly expressed NCEDs in developing seeds with AtNCED6 expressed specifically in the endosperm and AtNCED9 expressed both in the endosperm and embryo until the latter stages of maturation (Lefebvre et al., 2006). Functional analysis of AtNCED6 and AtNCED9 mutants reveals that ABA synthesized in the endosperm and possibly the embryo during the early to midstages of maturation contributes to dormancy (Lefebvre et al., 2006). The double mutant exhibits reduced ABA content and reduced dormancy. In contrast to NCEDs, many of the enzymes in ABA biosynthesis are encoded by single genes and these appear to be ubiquitously expressed during seed development. ABA catabolism has also been shown to play an important role in dormancy (Kushiro et al., 2004; Millar et al., 2006; Okamoto et al., 2006). Single and double mutants of the Arabidopsis ABA 8′-hydroxylase (ABA8′OH) gene family, CYP707A2 and CYP707A3, are more dormant and have increased ABA content, whereas overexpression results in decreased ABA content and reduced dormancy.
Cross talk between ABA and other hormones, such as GA and ethylene, is likely to be important in dormancy regulation (Feurtado and Kermode, 2007; Hilhorst, 2007). Application of GA can overcome seed dormancy in many species, including barley, suggesting that the ABA to GA ratio may be critical for dormancy maintenance (e.g. Jacobsen et al., 2002). Recent evidence indicates that ABA may act, at least in part, to inhibit germination by suppressing GA biosynthesis in Arabidopsis seeds (Seo et al., 2006). Expression levels of GA biosynthetic genes (AtGA3ox1 and AtGA3ox2) were elevated in seeds of an ABA-deficient mutant, aba2-2, compared to wild type. It is possible that the loss of dormancy in the aba2-2 mutant is due to loss of repression of GA biosynthesis. In contrast, ethylene appears to act at least in part by regulating ABA biosynthesis and signaling. Loss-of-function mutations in the ethylene signaling pathway, such as ein2 and etr1, result in higher ABA content and higher dormancy (Chiwocha et al., 2003).
Dormancy in barley and other cereals can be broken by changes in environmental conditions, such as temperature, light, oxygen, and nutrients, and also by after-ripening (Simpson, 1990; Jacobsen et al., 2002; Benech-Arnold et al., 2006). Evidence so far from hormone and gene expression studies of barley indicate that dormancy release is due at least in part to changes in ABA metabolism and possibly ABA signaling (Jacobsen et al., 2002; Benech-Arnold et al., 2006; Chono et al., 2006; Millar et al., 2006). Although embryos from dry dormant (D) and after-ripened (AR) barley grains contain similar levels of ABA, after 12-h imbibition ABA content in AR grains was 25% to 50% lower than that measured in embryos of imbibed D grains (Millar et al., 2006). The decline in ABA content in AR grains appeared to be due to conversion to phaseic acid, which is less active as a germination inhibitor (Jacobsen et al., 2002). The increase in ABA catabolism in embryos of imbibed AR grains correlated with increased gene expression of HvABA8′OH1 compared to embryos of D grains (Chono et al., 2006; Millar et al., 2006). Functional analyses in yeast (Saccharomyces cerevisiae) demonstrated that HvABA8′OH1 converted ABA to phaseic acid. Similar changes in ABA8′OH expression have been reported in AR Arabidopsis seeds compared to D seeds (Millar et al., 2006; Finch-Savage et al., 2007), indicating that ABA8′OH may function as a key regulator of dormancy release in seeds. Reported changes in embryo ABA sensitivity following dormancy loss in various cereals (Walker-Simmons, 1987; Wang et al., 1995; Benech-Arnold et al., 1999; Corbineau et al., 2000) can be explained at least in part by increased ABA catabolism (Benech-Arnold et al., 2006). This is further supported by the demonstration that ABA8′OH overexpression in Phaseolus seeds resulted in decreased sensitivity to ABA (Yang and Zeevaart, 2006).
There are a number of reports of light regulation of dormancy in cereals (Simpson, 1990); however, our understanding of the mechanisms involved remains poor. Typically, freshly harvested barley grains have little or no dormancy when imbibed in the dark, but germination is strongly inhibited by white light. The light promotion of dormancy is lost during after-ripening with AR grains germinating equally well in darkness or white light (Grahl and Thielebein, 1959; Burger, 1965; Grahl, 1965; Chaussat and Zoppolo, 1983; Jacobsen et al., 2002). Analysis of D grains imbibed for 24 h showed that embryo ABA content was 30% to 50% lower in grains imbibed in the dark compared to grains imbibed in white light (Jacobsen et al., 2002). Although dark imbibition and after-ripening both promote dormancy release and loss of ABA, it is not yet known whether they act independently or through a common molecular mechanism. In this study, we examine the effects of light and dark on expression of ABA and GA metabolism genes in D and AR barley grains during imbibition. We present evidence that white light promotion of dormancy is caused by blue light through induction of HvNCED1 expression. After-ripening appears to override the blue light promotion of HvNCED1 expression by activating ABA catabolism and GA biosynthesis genes in embryos of imbibing barley grains. In addition, we use transgenic barley lines to examine the role of HvABA8′OH1 in dormancy release. We show that reduced HvABA8′OH1 expression results in increased dormancy, but that it appears to have little effect on after-ripening.
RESULTS
Light Regulation of Dormancy and ABA Content in Barley
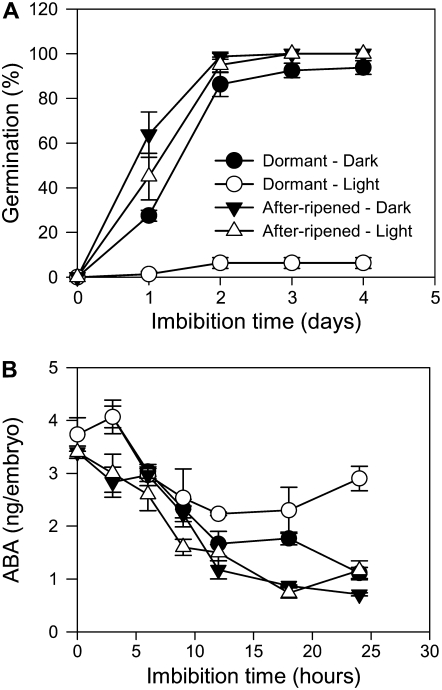
Barley plants grown under cool conditions produce grains that are highly D when imbibed under continuous white light and require extended periods of after-ripening for dormancy to decay (Jacobsen et al., 2002; Millar et al., 2006). Germination kinetics for D and AR grains of Betzes barley (a husked variety) from the same harvest showed that 100% of the AR grains germinated after 3-d imbibition in continuous white light compared with 5% germination for D grains imbibed under similar conditions (Fig. 1A). When the D and AR barley grains are imbibed in the dark, over 95% of grains germinated by 3 d, indicating that white light is a promoter of dormancy in barley and that imbibition in the dark breaks dormancy.
Figure 1.
Effect of white light and dark on germination and ABA content of D and AR barley grains during imbibition. A, Germination of D and AR barley grains irradiated with continuous white light or in dark: Measurements are averages of four replicates with error bars representing the se of the mean. B, Changes in embryo ABA content in D and AR grains imbibed under continuous white light or dark. Measurements are averages of three replicates with error bars representing the se of the mean.
To examine the effect of white light and dark on ABA content, ABA was extracted from embryos of D and AR grains imbibed in continuous light or dark and quantified by a competitive ELISA assay (Fig. 1B). ABA content in embryos of D grains imbibed under continuous white light declined from 3.7 ng per embryo to 2.2 ng per embryo during the first 12-h imbibition and then the ABA content stabilized before increasing over the next 12 h to 2.9 ng per embryo. In contrast, ABA content of D grains imbibed in the dark and AR grains imbibed in continuous white light or dark resulted in a steady decline from 3.7 ng ABA per embryo in dry embryos to 1.1 ng ABA per embryo after 24 h. These results are consistent with an earlier study that showed that release of dormancy in barley grains by after-ripening and dark imbibition was associated with a steady decline in embryo ABA content and a corresponding increase in phaseic acid during imbibition (Jacobsen et al., 2002).
White Light and After-Ripening Target Different ABA and GA Metabolism Genes
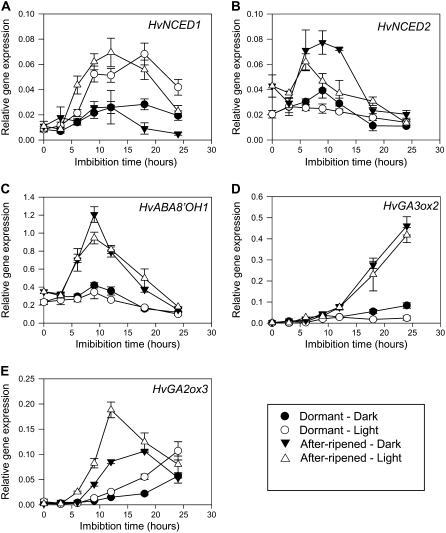
We have previously shown that the decline in ABA content in imbibing AR grains is likely to be due to increased expression of an ABA catabolic enzyme, HvABA8′OH1, and not related to any changes in the expression of HvNCEDs encoding for ABA biosynthetic enzymes (Millar et al., 2006). To determine whether the decrease in embryo ABA content associated with dark release of dormancy is also due to differential expression of HvABA8′OH1, we used quantitative reverse transcription (RT)-PCR to quantify expression levels of genes encoding HvABA8′OHs and HvNCEDs in embryos of D and AR grains imbibed under continuous white light and dark (Fig. 2, A–C). In barley, there are two ABA8′OH genes, HvABA8′OH1 and HvABA8′OH2, and two NCED genes, HvNCED1 and HvNCED2 (Millar et al., 2006).
Figure 2.
Effect of white light and dark on expression of ABA and GA metabolism genes in embryos of D and AR barley grains during imbibition. Measurements are averages of three replicates with error bars representing the se of the mean. A, HvNCED1. B, HvNCED2. C, HvABA8′OH1. D, HvGA3ox2. E, HvGA2ox3.
Both HvNCED genes were expressed in embryos of imbibed D and AR grain, but they showed different expression patterns in response to light and after-ripening. Continuous white light strongly induced HvNCED1 expression in embryos of D and AR grain by 6-h imbibition and remained high up to 24-h imbibition compared with grains that were imbibed in the dark (Fig. 2A). After-ripening had little effect on HvNCED1 expression in grains, whereas HvNCED2 expression was higher in embryos of AR grain after 6-h imbibition compared with D grain (Fig. 2B). White light had little effect on HvNCED2 expression in embryos of imbibing grains.
As previously shown, HvABA8′OH1 expression is over 10-fold higher than HvABA8′OH2 in imbibing grains (Millar et al., 2006). HvABA8′OH1 expression is strongly promoted by after-ripening in imbibing grains, but white light or dark had little effect on HvABA8′OH1 expression in embryos of D and AR grains. In imbibing grains, HvABA8′OH2 expression was very low in all treatments (data not shown).
It is clear from studies of Arabidopsis that light plays a major role in coordinating ABA and GA metabolism in imbibed seeds. In imbibed seeds, red light promotes germination via phytochrome B by inhibiting NCED expression and promoting ABA catabolism and GA synthesis (Seo et al., 2006). To determine whether light/dark regulation of dormancy in barley also acts through coordinated changes in ABA and GA metabolism, we investigated whether GA 3-oxidases, which encode enzymes that catalyze the conversion GA20 to active GAs, and GA 2-oxidases, which encode GA deactivation enzymes, are regulated by white light in D and AR grains. Two HvGA3ox and four HvGA2ox genes have been identified in barley (Spielmeyer et al., 2004; Dewi, 2006), but only HvGA3ox2 and HvGA2ox3 were expressed at high levels in embryos during grain imbibition (Fig. 2, D–E). HvGA3ox2 expression increased rapidly and continued to increase up to 24-h imbibition in AR grains in both light and dark compared to D grains, where expression remained low over the 24-h imbibition period. White light had little effect on HvGA3ox1 expression. Expression of HvGA2ox3 increased in all treatments, but the increase was more rapid in AR grains and preceded the increase in HvGA3ox2 expression.
Taken together, these results suggest that white light stimulation of HvNCED1 expression plays a major role in maintenance of dormancy and high ABA content in embryos of D grains. In contrast, after-ripening counteracts the white light effect by promotion of ABA catabolism via increased HvABA8′OH1 expression and promotion of GA synthesis through increased HvGA3ox2 expression.
Blue Light Regulates HvNCED1 Expression and ABA Content in D Embryos
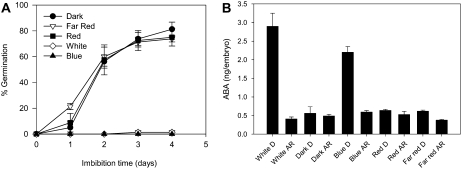
To investigate the relationship between the light spectrum and barley grain dormancy, D grains were imbibed under continuous blue, red, far-red, or white light or dark (Fig. 3A). The results show that blue light was as effective as white light in maintaining dormancy in imbibing D grains with <10% of the grains germinating by 3-d imbibition (compare Fig. 3A with Fig. 1A). Blue light did not have any effect on the germination of AR grains with over 90% of the AR grains germinating after 3-d imbibition under constant blue light. In contrast to blue light, germination of D grains imbibed under continuous red or far-red light was similar to dark-imbibed grains with over 80% D grains germinating after 3-d imbibition. To test whether higher fluences of far-red had any effect on germination of D grains, huskless D grains were imbibed under continuous 273 μm m−2 s−1 far-red light. The germination results for the high-intensity far-red light treatment were similar to those from dark-imbibed grains with germination over 95% after 4-d imbibition (data not shown). It is clear from these results that blue light promotes dormancy in freshly harvested barley grains and that red and far-red light have no effect.
Figure 3.
Effect of light quality on dormancy and embryo ABA content in barley grains during imbibition. A, Germination of D barley grains in dark and irradiated with continuous far-red, red, and blue light. Measurements are averages of four replicates with error bars representing the se of the mean. B, ABA content in embryos from D and AR grains imbibed for 24 h in dark and under continuous blue, red, far-red, and white light. Measurements are averages of three replicates with error bars representing the se of the mean.
We investigated whether blue light promotion of dormancy correlated also with increases in ABA content similar to that found in white light treatments. We measured ABA levels in embryos of D and AR grains imbibed under continuous blue light, red, far-red, and white light, and dark after 24-h imbibition. As shown in Figure 3B, embryo ABA content was 4-fold higher in blue light-treated D grains compared to dark, red, and far-red light-treated grains. The blue promotion of ABA content was similar to white light treatments. In AR grains, ABA content remained low regardless of the light treatment.
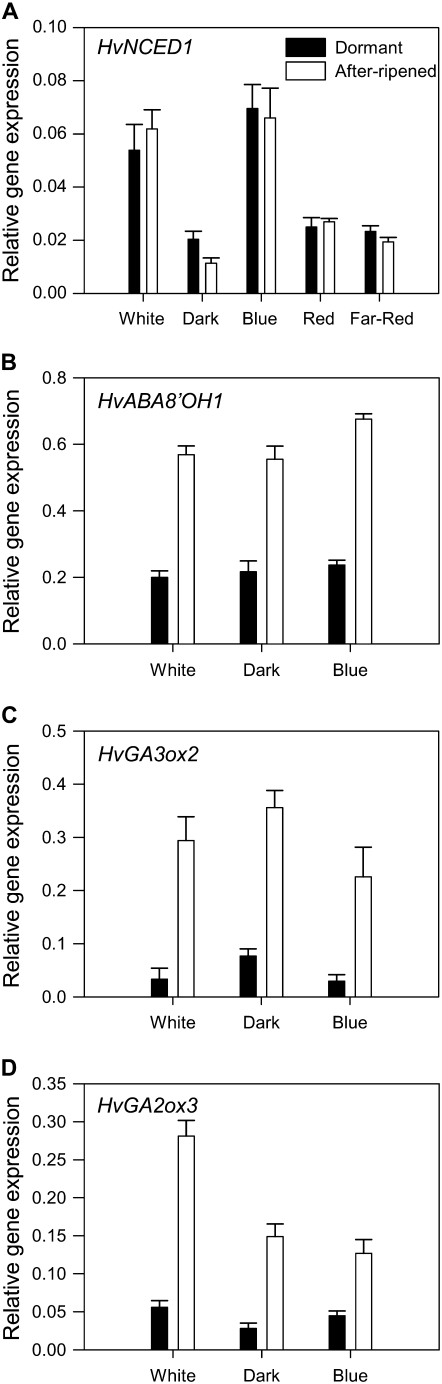
To test whether blue light regulation of dormancy and ABA content is associated with increased HvNCED1 expression, we monitored expression of genes encoding enzymes of ABA and GA metabolism in response to various light treatments after 12-h imbibition (HvNCED1, HvABA8′OH1, HvGA2ox3) and 24-h imbibition (HvGA3ox2). As shown in Figure 4A, HvNCED1 expression was induced 2- to 3-fold in D and AR grains imbibed under blue or white light compared with other light treatments. The response to blue light was not detected in ABA and GA metabolism genes (Fig. 4, B–D) that had been shown to be regulated by after-ripening (see Fig. 2).
Figure 4.
Effect of light quality on expression of ABA and GA metabolism genes in embryos of D and AR grains during imbibition. Gene expression was measured in embryos from grains that had been imbibed for 24 h under continuous blue, red, far-red, and white light and dark. Measurements are averages of three replicates with error bars representing the SE of the mean. A, HvNCED1. B, HvABA89OH1. C, HvGA3ox2. D, HvGA2ox3.
Reduced HvABA8′OH1 Expression in Barley Grains Results in Increased Dormancy
It has been previously shown that reduction in ABA8′OH1 expression in Arabidopsis is associated with increased ABA content in seeds and increased dormancy (Kushiro et al., 2004; Millar et al., 2006; Okamoto et al., 2006). Analysis of ABA metabolism and gene expression in barley grains indicates that after-ripening alleviates dormancy by increasing the expression of a small group of genes, including HvABA8′OH1. To study the role of HvABA8′OH1 in dormancy in barley, we constructed a hairpin RNA interference (RNAi) construct under the control of the maize ubiquitin promoter designed to cleave HvABA8′OH1 transcripts (designated binary vector pWBVec8-Ubi:HvABA8′OH1RNAi). The vector was transformed in Golden Promise barley and, from more than 30 transgenic lines, three single-locus lines were selected for further analysis. Grains from homozygous transgenic and null segregant plants from each of the three lines, together with grains from wild-type plants, were analyzed for HvABA8′OH1 expression, ABA content, and dormancy.
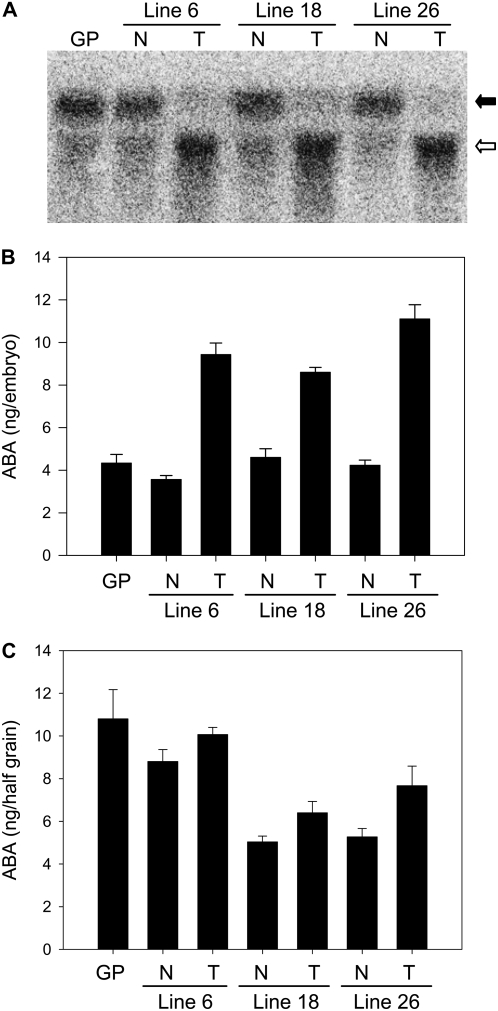
HvABA8′OH1 gene expression was monitored by RNA-blot analysis of embryos from RNAi and null grains imbibed for 18 h in the dark (Fig. 5A). In all three lines, HvABA8′OH1 transcript levels were down-regulated in embryos of RNAi grain compared to embryos from null and wild-type grains. Quantitative RT-PCR analysis failed to detect any effect of the RNAi construct on HvABA8′OH2 expression, demonstrating the specificity of the RNAi construct (data not shown). Quantitation of ABA content in dry D grains demonstrated that reduction of HvABA8′OH1 transcript level in RNAi grains correlated with increased ABA content in embryo and embryoless half-grains compared to null and wild-type grains (Fig. 5B). Embryos from RNAi grains from the three lines had ABA levels at least twice as high as the corresponding null segregant embryos. The effect of the RNAi was also detected on ABA content of endosperm from dry grains, but the effect was small (Fig. 5C). Endosperm tissue from RNAi grains had higher ABA content compared to endosperm from null segregant grains.
Figure 5.
Effect of RNAi-directed silencing of HvABA8′OH1 on gene expression and ABA content of barley grains. A, RNA-blot analysis of HvABA8′OH1 expression in embryos of wild-type Golden Promise (GP), and null (N) and transgenic (T) grains from HvABA8′OH1 RNAi lines 6, 18, and 26 imbibed for 18 h. The HvABA8′OH1 transcripts have a slower mobility (black arrow) than the RNAi transcripts (white arrow). B, ABA content in embryos from dry wild-type (GP), and null (N) and transgenic (T) grains from HvABA8′OH1 RNAi lines 6, 18, and 26. Measurements are averages of three biological replicates with error bars representing the se of the mean. C, ABA content in endosperm half-grains from dry wild-type (GP), and null (N) and transgenic (T) grains from HvABA8′OH1 RNAi lines 6, 18, and 26. Measurements are averages of three biological replicates with error bars representing the se of the mean.
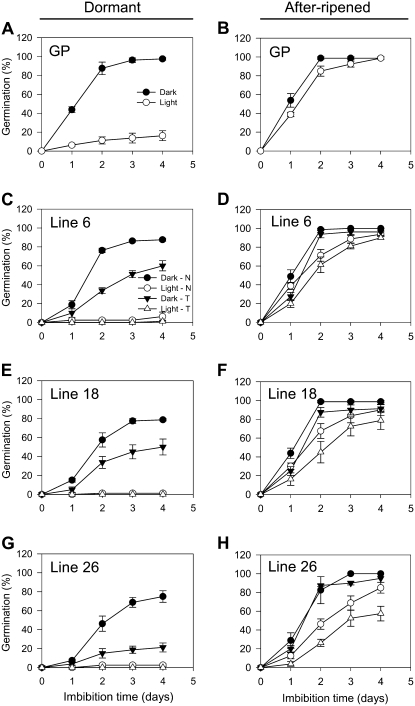
To determine the effect of reduced HvABA8′OH1 expression on grain dormancy, freshly harvested and AR grains from wild-type, RNAi, and null plants, together with grains from wild-type plants, were imbibed for 3 d under continuous white light or darkness. As shown in Figure 6, freshly harvested RNAi grains in the three lines studied were more D when dark imbibed. Line 26 had the highest level of ABA in dry embryos and had the highest dormancy with only 21% of the RNAi grains germinated compared with over 75% of the null segregant grains germinated. To test whether RNAi grains had a longer after-ripening period compared to null segregants, grains were AR for 1 month at 37°C and assayed for dormancy (Fig. 6, B, D, F, and H). AR grains from all the lines showed almost no dormancy in the dark, with germination levels of 95% and higher. When imbibed under continuous white light, AR grains from RNAi and null plants were partially D with lower germination compared to the dark-imbibed grains.
Figure 6.
Effect of after-ripening on dormancy release of wild-type and HvABA8′OH1 RNAi transgenic grains. D (A, C, E, and G) and AR (B, D, F, and H) grains from wild-type (A and B) and RNAi lines (C–H) were imbibed for 4 d under continuous white light or dark. The AR grains had been AR for 1 month at 37°C. Measurements are averages of four replicates with error bars representing the se of the mean. A and B, Wild-type Golden Promise grains (GP). C and D, Null (N) and transgenic (T) grains from line 6. E and F, Null (N) and transgenic (T) grains from line 18. G and H, Null (N) and transgenic (T) grains from line 26.
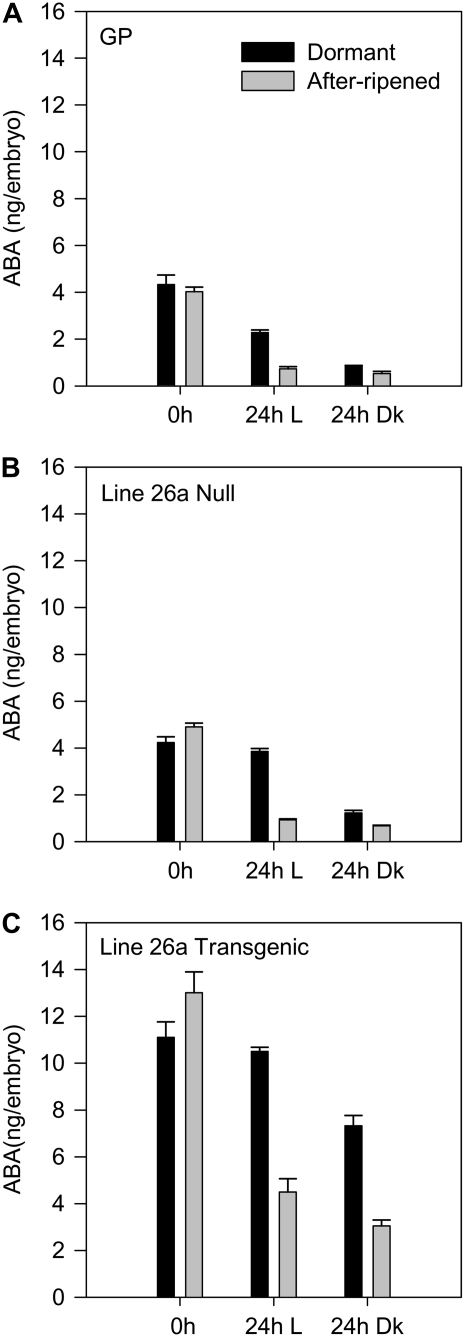
To investigate the effect of after-ripening and light on the ABA content in embryos of the RNAi grains, null and transgenic grain from line 26 and wild-type plants was AR for 1 month and ABA content measured in embryos of dry and imbibed grains (Fig. 7). After-ripening had no effect on ABA content of embryos from dry RNAi grains (Fig. 7C) similar to what had been shown in Betzes barley (Fig. 1B). Following 24-h imbibition, embryo ABA content remained high in the dormant RNAi grains imbibed in the light, but a small decrease was observed in dark-imbibed grains. After-ripening had a major effect on embryo ABA content in imbibed RNAi grains, with a decrease >50% after 24-h imbibition both in the light and dark. Although the ABA content in the null and wild-type grains (Fig. 7, A and B) was overall much lower, decreases in embryo ABA content were also detected in AR grains imbibed in the light and dark compared with D grains imbibed under similar conditions. We do not know whether this decrease in ABA content in the RNAi grains is catalyzed by any residual HvABA8′OH1 enzyme or due to alternative ABA catabolic or conjugation enzymes or by an alternative pathway.
Figure 7.
Effect of after-ripening and light on ABA content of D and 1-month AR grains from wild-type and HvABA8′OH1 RNAi plants. The embryos were isolated from dry grains (0 h) and grains imbibed for 24 h under white light (L) and dark (Dk). ABA measurements are averages of four biological replicates with error bars representing the se of the mean. A, Golden Promise (GP). B, Null grains from HvABA8′OH1 RNAi line 26. C, Transgenic grains from HvABA8′OH1 RNAi line 26.
DISCUSSION
We have shown that freshly harvested grains of Betzes barley grown under the conditions described here are highly D when imbibed under white light, but their dormancy can be rapidly alleviated by imbibing the grains in the dark. The white light-induced dormancy can also be broken by after-ripening the dry grains at 37°C for 4 months, by which time the grains germinated equally well in the light or dark. These results are consistent with earlier studies that showed that white light promoted dormancy in barley and a number of other cereals (Grahl and Thielebein, 1959; Burger, 1965; Grahl, 1965; Chaussat and Zoppolo, 1983). In cases where the grain dormancy is deep, dark imbibition may not be effective in releasing dormancy until the grains have been AR for a short time. Grahl (1965) showed that barley grains from cultivars with high dormancy were highly D after 1 week after-ripening when imbibed in the dark. After 5 to 13 weeks after-ripening at 20°C, the barley grains germinated in the dark, but remained D under continuous white light. After 38 weeks after-ripening, the grains germinated close to 100% in the dark or light. It is clear from these results that the light response varies with the depth of dormancy and that further work is required to understand the molecular changes associated with the after-ripening that accompany light repression of germination in barley and other cereals.
Analysis of ABA content and expression of ABA metabolism genes indicates that the white light effect on dormancy is correlated with increased ABA content and HvNCED1 expression in the embryos of imbibing grains compared to dark-imbibed grains. In contrast, no differences were observed in the expression of HvNCED2, HVABA8′OH1, and HvABA8′OH2 in embryos of D grains imbibed in white light or dark, indicating the white light effect on ABA content may be specific to HvNCED1. Embryo ABA content of D grains declined from 3.7 ng/embryo in dry seeds to 1.1 ng/embryo after 24-h imbibition in the light compared with 2.9 ng/embryo when imbibed in the dark in agreement with an earlier study (Jacobsen et al., 2002). Our results indicate that the maintenance of a high ABA content in embryos in light-imbibed grains may be due to increased ABA biosynthesis rather than a decrease in ABA catabolism. HvNCED1 expression increased in response to white light and it reached maximal expression after 18-h imbibition, which correlated with stabilization of ABA content in embryos compared to the further decline observed in dark-imbibed grains. The increase in ABA content as a result of increased HvNCED1 expression may facilitate white light-imposed dormancy, although it cannot be ruled out that the effect may also be due to a reduction in ABA sensitivity in response to white light.
Light stimulation of NCED mRNA expression has also been observed in tomato (Lycopersicon esculentum) leaves. Analysis of plants grown in a 12-h-light/12-h-dark cycle showed a diurnal pattern of LeNCED1 expression in leaves with the peak of expression at the end of the light period (Thompson et al., 2000). Switching from light/dark cycling to continuous dark resulted in LeNCED1 expression dropping to low levels, indicating that the diurnal cycling was due to positive regulation by light and not due to a circadian oscillator. Although there are no reports in plants that ABA content is regulated diurnally, diurnal redistribution between the chloroplast and cytosol has been reported (Slovik and Hartung, 1992). Interestingly, light stimulation of ABA biosynthesis has also been observed in the fungus Botrytis cinerea (Marumo et al., 1982) and hydroids, members of the animal phylum Eumetazoa (Puce et al., 2004). The light-stimulated increase in ABA content during hydroid regeneration can be blocked by fluridone, an ABA biosynthesis inhibitor.
Dormancy and germination in cereals has been shown to be dependent on light spectral quality (Chaussat and Zoppolo, 1983; Simpson, 1990). Our results show that the white light repression of germination is at least in part caused by the blue light component. By using narrow wavelength light-emitting diodes (LEDs), we showed that blue light was as effective as white light in promoting dormancy and that red light and far-red light had no effect compared to dark-imbibed grains. Our data are in agreement with an earlier study, which investigated the effect of the light spectrum from a 1,600-W xenon arc light on barley dormancy (Chaussat and Zoppolo, 1983). Only the blue region of the spectrum (435–455 nm) inhibited germination with longer wavelengths up to 700 nm having no effect. Our data extend these observations and indicate that blue light is also responsible for the white light-induced increase in HvNCED1 expression and ABA content in embryos of imbibed grains. Similarly, the component of white light that stimulated ABA content in mycelium of B. cinerea (see above; Marumo et al., 1982) has been shown to be blue light. Studies of wild oats (Avena fatua) have shown photoreversible germination by red and far-red light, indicating the presence of active phytochromes in the embryo (for review, see Simpson, 1990). The absence of any detectable effect of red and far-red light on barley germination and HvNCED1 expression indicates that phytochromes play no part in dormancy in barley. We note that the red/far-red reversibility of germination in wild oats was demonstrated using grains that were dehulled (Hou and Simpson, 1992), but we were unable to observe reversibility in huskless Betzes barley.
Plants possess several classes of photoreceptors that absorb in the blue region of the spectrum. Phototropins, cryptochromes, and the ZTL/FKF/LPK2 receptors are classified as blue light receptors, but it is well known that the red/far-red light receptors, phytochromes, also absorb and respond to the blue region of the spectrum (Banerjee and Batschauer, 2005; Wang, 2005). Although we do not know which photoreceptor is involved in the blue light regulation of ABA content and HvNCED1 expression in barley embryos, recent progress made in the understanding of photoregulation of ABA metabolism in Arabidopsis seeds may provide useful insights (Oh et al., 2006; Seo et al., 2006). In Arabidopsis seeds, AtNCED6 expression is regulated in a red/far-red photoreversible manner in imbibed seeds and this response was absent in the phyB mutant (Seo et al., 2006). The change in ABA content correlated positively with the photoreversible red/far-red light regulation of AtNCED6. AtNCED9 may be regulated in a similar manner (Oh et al., 2006). In contrast, expression of other ABA biosynthetic genes did not show any response to red/far-red light. PIL5, a phytochrome-interacting protein, has been shown to mediate the phytochrome B regulation of AtNCED6, AtNCED9, and GA biosynthetic genes (Oh et al., 2006).
Our results indicate that after-ripening overrides the white light-induced dormancy by enhancing ABA catabolism and GA biosynthesis in imbibed grains. It has been shown previously that after-ripening increases the expression of HvABA8′OH1 in embryos by more than 2-fold compared to embryos from D grains imbibed in the light (Chono et al., 2006; Millar et al., 2006). The decrease in ABA content observed in embryos of AR grains imbibed in white light is consistent with the increase in HvABA8′OH1 expression and indicates that increases in the expression of HvNCED1 and HvNCED2 are not able to reverse the decline in ABA content. In addition to HvABA8′OH1, expressions of HvGA3ox2 and HvGA2ox3 were also higher in AR grains compared to D grains imbibed in the light or dark. Analysis of expression kinetics reveals that HvABA8′OH1 expression began to increase after 6-h imbibition, whereas HvGA3ox2 began to increase after 12-h imbibition. There is evidence to support the proposal that HvGA3ox2 expression in barley may be repressed by ABA in D grains in a similar way to that observed in Arabidopsis seeds (Seo et al., 2006). Also, imbibition of barley grains in the presence of 5 μm ABA reduced HvGA3ox2 expression by 45% compared to control treatments (Dewi, 2006). In addition, we found that expression of HvGA3ox2 was higher in D grains imbibed in the dark compared to grains imbibed in the light, correlating positively with changes in ABA content. Nevertheless, the decline in ABA content in the dark-imbibed D grains did not correlate with the large increase in HvGA3ox2 expression observed in AR grains. These results suggest that changes in ABA content in AR grains may only be partly responsible for the dramatic increase in HvGA3ox2 expression in AR grains compared to D grains.
We have used an RNAi approach to study the role of HvABA8′OH1 in barley dormancy. RNAi silencing of HvABA8′OH1 expression resulted in approximately 2-fold higher ABA content in embryos of transgenic grains compared to null segregant grains. The increase in ABA content correlated positively with increased depth of dormancy associated with dark-imbibed RNAi grains that had not been AR. Interestingly, decreased HvABA8′OH1 expression in the RNAi grains had only a small effect on after-ripening time compared to wild-type and null grains, indicating that increased ABA catabolism is not solely responsible for loss of dormancy by after-ripening. It has been reported that AR grains have reduced sensitivity to ABA compared to D grains, suggesting that after-ripening regulates ABA signaling components in addition to ABA metabolism (Walker-Simmons, 1987; Corbineau et al., 2000). A decrease in ABA sensitivity following after-ripening may explain in part the high germinability of AR HvABA8′OH1 RNAi grains even though the embryos have a high ABA content compared to wild-type grains. The expression of VP1, a member of the ABI3 family of transcription factors, has been positively correlated with the level of dormancy in wild oats (Jones et al., 1997) and sorghum (Sorghum bicolor; Carrari et al., 2001), but we could not detect any differences in HvVP1 expression in D and AR barley grains (F. Gubler, unpublished data). Transcriptome analysis of imbibed Arabidopsis seeds has shown that LIPID PHOSPHATE PHOSPHATASE2 (LPP2), which negatively regulates ABA signaling, is more highly expressed in AR seeds compared to D seeds, indicating support that loss of dormancy by after-ripening is associated with changes in ABA signaling and decreased ABA sensitivity (Carrera et al., 2008).
In conclusion, our results show that manipulation of HvABA8′OH1 provides an attractive opportunity to increase grain dormancy without unduly increasing after-ripening time. This is particularly attractive in cereal grains, which are prone to preharvest sprouting, such as wheat (Triticum aestivum). Alternative strategies that increase after-ripening time may result in lengthy delays before replanting and thus disadvantage breeders and farmers.
MATERIALS AND METHODS
Plant Material
Barley (Hordeum vulgare ‘Betzes’) plants were grown in naturally lit phytotron glasshouses with air temperature set at 17°C/9°C day/night cycle as previously described (Jacobsen et al., 2002; Millar et al., 2006). Heads were harvested at maturity, dried for 7 d, and threshed by hand to prevent damage to the husk and embryo. One-half of the threshed grains was stored at −20°C to preserve dormancy and the other half was incubated at 37°C for 4 months to after-ripen.
Germination Assays
For germination assays, quadruplicate sets of 20 grains were placed on 9-cm plastic petri dishes containing two 9-cm Whatman Number 1 filter papers and 6 mL of water. The plates were sealed with parafilm and incubated at 20°C under continuous white light at 130 μmol m−2 s−1 (Philips TLD 36W/865 fluorescent tubes) or wrapped in two layers of aluminum foil for darkness. Grains with emerged coleorhizae were scored as germinated.
Imbibitions under different light quality regimes were performed using monochromatic LEDs (for spectra, see Supplemental Fig. S1) in a light-tight box with temperature maintained at 20°C ± 0.5°C. Blue light was provided by NSPB510S-W/ST LEDs (Nichia Chemical Pty), far-red by L735-03AU LEDs, and red by 660-04U LEDs (both from EPITEX). White and blue light intensities were measured with an Apogee QMSS Quantum Meter, and red and far-red intensities with a Licor LI-1800 spectroradiometer. Intensities of blue, red, and far-red light were 26, 8, and 63 μmol m−2 s−1, respectively.
Gene Expression Analyses
RNA was prepared from embryos isolated from dry and imbibed grains using a method adapted from the hexadecyltrimethylammonium procedure described by Chang et al. (1993). Twenty embryos were ground in liquid nitrogen and the powder added to 1.8 mL of hot RNA lysis buffer containing 2% hexadecyltrimethylammonium (w/v). Following purification, the RNA was used for RNA-blot analysis or quantitative real-time PCR. For RNA-blot analysis, 20 μg of RNA was fractionated on 1.2% agarose gel containing formaldehyde and blotted onto a nylon membrane. The blot was hybridized with 32P-labeled dUTP HvABA8′OH1 riboprobes. The riboprobes were transcribed from PCR templates that spanned the 5′ and 3′ regions of the HvABA8′OH1 cDNA (578–859 bp and 1,144–1,311 bp; accession no. DQ145932).
For quantitative real-time PCR analysis, 50 μg RNA was treated with RNAse-free DNAse (Promega) and further purified on a Qiagen RNeasy column (Qiagen). Two micrograms of DNAse-treated RNA were used to synthesize cDNA using SuperScript III (Invitrogen Life Sciences). The resulting cDNA was diluted 50-fold and 10 μL were used in 20-μL quantitative PCR reactions with Platinum Taq (Invitrogen Life Sciences) and SYBR Green (Invitrogen). Specific primers used were: HvABA8′OH1, 5′-GGACACTGACGGATGGAGAAC-3′, 5′-CCATGACCTTCACCCGCAAG-3′ (Millar et al., 2006); HvABA8′OH2, 5′-GAGATGCTGGTGCTCATC-3′, 5′-ACGTCGTCGCTCGATCCAAC-3′ (Millar et al., 2006); HvNCED1, 5′-CCAGCACTAATCGATTCC-3′, 5′-CCAGCACTAATCGATTCC-3′ (Millar et al., 2006); HvNCED2, 5′-CATGGAAAGAGGAAGTTGC-3′, 5′-GAAGCAAGTGTGAGCTAAC-3′ (Millar et al., 2006); HvGA3ox1 (Spielmeyer et al., 2004); HvGA3ox2 (Spielmeyer et al., 2004); HvGA2ox1 (EST sequence CB76549); HvGA2ox3 (EST sequence BU972476); HvGA2ox4, 5′-TCCTAGCCAGCCAGCAACT-3′, 5′-GGCATGGACAGGACACAGA-3′ (Dewi, 2006); HvGA2ox5, 5′-ACAAGAGCAGCACCCACAA-3′, 5′-AACCACAGGACCAGGACGA-3′ (Dewi 2006); and HvActin, 5′-GCCGTGCTTTCCCTCTATG-3′, 5′-GCTTCTCCTTGATGTCCCTTA-3′ (Trevaskis et al., 2006). Reactions were run on a Rotor-gene 3000A real-time PCR machine (Corbett Research) and data analyzed with Rotor-gene software. The expression of Actin (AY145451) was used as a control to normalize gene expression in the various treatments. Three replicates were carried out for each experiment. All experiments showed similar trends in separate biological repeats.
ABA Measurements
The content of isolated embryos and endosperm half-grains was measured using a Phytodetek Competitive ELISA kit (Agdia). Ten embryos were isolated from dry and imbibed barley grains and frozen on dry ice. The remaining half-grains, which included the starchy endosperm, aleurone, glumes, and seed coat, were cut into small pieces and frozen on dry ice. The frozen plant material was transferred to plastic tubes containing 80% methanol and two stainless steel ball bearings and homogenized in a Qiagen tissue lyser at 30 cycles s−1. The homogenate was mixed overnight at 4°C and centrifuged at 2,000 rpm to pellet the plant debris. The pellet was extracted five times with 80% methanol and the supernatants combined and concentrated in a SpeedyVac (Savant) until the methanol was removed. The aqueous extract (approximately 100 μL) was diluted to 1 mL by addition of Tris-buffered saline (25 mm Trizma base, 100 mm sodium chloride, 1 mm magnesium chloride, 3 mm sodium azide, pH 7.5) and ABA content was measured in the competitive ELISA assay as described by the Phytodetek protocol. Three biological replicates were carried out for each experiment.
Transformation of Barley with Hairpin RNAi Construct
A hairpin RNAi construct targeting the HvABA8′OH1 RNAi gene was made by inserting a PCR product spanning the region 578 to 859 bp of the HvABA8′OH1 cDNA (Millar et al., 2006) in both orientations into the hairpin RNAi vector pStarling. The region from 578 to 859 bp was chosen as the optimal target for RNAi because it is not conserved between HvABA8′OH1 and HvABA8′OH2. The hairpin RNAi HvABA8′OH-1 construct was subcloned into the NotI site of the binary vector, pWBVec8 (Wang et al., 1998) before being transferred into Agrobacterium tumefaciens strain AGL0 by triparental mating. Transformation of Golden Promise barley was performed using the Agrobacterium-mediated technique as described by Jacobsen et al. (2006).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Spectra of blue, red, and far-red LED lights.
Supplementary Material
Acknowledgments
We thank Ingrid Venables for assistance with transformation of barley; Professor Jim Reid and Ian Cummings, School of Plant Science, University of Tasmania, for introducing us to LED technology; Mike Hauptman for building the light cabinet; and Dr. John Evans, School of Biological Sciences, Australian National University, for assistance with light intensity measurements.
This work was supported by the Grains Research and Development Corporation and Commonwealth Scientific and Industrial Research Organisation.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Frank Gubler (frank.gubler@csiro.au).
The online version of this article contains Web-only data.
References
- Banerjee R, Batschauer A (2005) Plant blue-light receptors. Planta 220 498–502 [DOI] [PubMed] [Google Scholar]
- Baskin CC, Baskin JM (1998) Seeds; Ecology, Biogeography, and Evolution of Dormancy and Germination. Academic Press, San Diego
- Benech-Arnold RL, Giallorenzi MC, Frank J, Rodriguez V (1999) Termination of hull-imposed dormancy is correlated with changes in embryonic ABA content and sensitivity. Seed Sci Res 9 39–47 [Google Scholar]
- Benech-Arnold RL, Gualano N, Leymarie J, Côme D, Corbineau F (2006) Hypoxia interferes with ABA metabolism and increases ABA sensitivity in embryos of dormant barley grains. J Exp Bot 57 1423–1430 [DOI] [PubMed] [Google Scholar]
- Bewley JD (1997) Seed germination and dormancy. Plant Cell 9 1055–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger WC (1965) Effect of light on the germination of barley and its relation to dormancy. J Inst Brew 71 244–250 [Google Scholar]
- Carrari F, Perez-Flores L, Lijavetzky D, Enciso S, Sanchez R, Benech-Arnold R, Iusem N (2001) Cloning and expression of a sorghum gene with homology to maize vp1. Its potential involvement in pre-harvest sprouting resistance. Plant Mol Biol 45 631–640 [DOI] [PubMed] [Google Scholar]
- Carrera E, Holman T, Medhurst A, Dietrich D, Footitt S, Theodoulou FL, Holdsworth MJ (2008) Seed after-ripening is a discrete developmental pathway associated with specific gene networks in Arabidopsis. Plant J 53 214–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney K (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11 113–116 [Google Scholar]
- Chaussat R, Zoppolo J (1983) Lumière et germination de l'orge. Bios 14 30–32 [Google Scholar]
- Chiwocha SDS, Abrams SR, Ambrose SJ, Cutler AJ, Loewen M, Ross ARS, Kermode AR (2003) A method for profiling classes of plant hormones and their metabolites using liquid chromatography-electrospray ionization tandem mass spectrometry: an analysis of hormone regulation of thermodormancy of lettuce (Lactuca sativa L.) seeds. Plant J 35 405–417 [DOI] [PubMed] [Google Scholar]
- Chono M, Honda I, Shinoda S, Kushiro T, Kamiya Y, Nambara E, Kawakami N, Kaneko S, Watanabe Y (2006) Field studies on the regulation of abscisic acid content and germinability during grain development of barley: molecular and chemical analysis of pre-harvest sprouting. J Exp Bot 57 2421–2434 [DOI] [PubMed] [Google Scholar]
- Corbineau F, Benamar A, Come D (2000) Changes in sensitivity to ABA of the developing and maturing embryo of wheat cultivars with different sprouting susceptibility. Isr J Plant Sci 48 189–197 [Google Scholar]
- Dewi K (2006) The role of gibberellins in early growth regulation and dormancy breakage in barley (Hordeum vulgare L. ‘Himalaya’). PhD thesis. Australian National University, Canberra, Australia
- Feurtado JA, Kermode AR (2007) A merging of paths: abscisic acid and hormonal cross-talk in the control of seed dormancy maintenance and alleviation. In K Bradford, H Nonogaki, eds, Seed Development, Dormancy and Germination. Annual Plant Reviews, Vol 27. Blackwell Publishing, Oxford, pp 176–223
- Finch-Savage WE, Cadman CSC, Toorop PE, Lynn JR, Hilhorst HWM (2007) Seed dormancy release in Arabidopsis Cvi by dry after-ripening, low temperature, nitrate and light shows common quantitative patterns of gene expression directed by environmentally specific sensing. Plant J 51 60–78 [DOI] [PubMed] [Google Scholar]
- Finkelstein RR (2004) The role of hormones during seed development and germination. In PJ Davies, ed, Plant Hormones: Biosynthesis, Signal Transduction, Action! Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 223–247
- Frey A, Godin B, Bonnet M, Marion-Poll A (2004) Maternal synthesis of abscisic acid controls seed development and yield in Nicotiana plumbaginifolia. Planta 218 958–964 [DOI] [PubMed] [Google Scholar]
- Grahl A (1965) Lichteinfluss auf die keimung des getreides in abhangigkeit von der keimruhe. Landbauforschung Volkenrode 2 97–106 [Google Scholar]
- Grahl A, Thielebein M (1959) Einfluss von licht auf die keimung der gerste. Naturwissenschaften 46 336–337 [Google Scholar]
- Gubler F, Millar AA, Jacobsen JV (2005) Dormancy release, ABA and pre-harvest sprouting. Curr Opin Plant Biol 8 183–187 [DOI] [PubMed] [Google Scholar]
- Hilhorst HWM (2007) Definitions and hypotheses of seed dormancy. In K Bradford, H Nonogaki, eds, Seed Development, Dormancy and Germination. Annual Plant Reviews, Vol 27. Blackwell Publishing, Oxford, pp 50–71
- Hou JQ, Simpson GM (1992) After-ripening and phytochrome action in seeds of dormant lines of wild oat (Avena fatua). Physiol Plant 86 427–432 [Google Scholar]
- Jacobsen JV, Pearce DW, Poole AT, Pharis RP, Mander LN (2002) Abscisic acid, phaseic acid and gibberellin contents associated with dormancy and germination in barley. Physiol Plant 115 428–441 [DOI] [PubMed] [Google Scholar]
- Jacobsen JV, Venables I, Wang MB, Matthews P, Ayliffe M, Gubler F (2006) Barley (Hordeum vulgare L.). In K Wang, ed, Agrobacterium Protocols, Vol 1, Ed 2. Humana Press, Totawa, NJ [DOI] [PubMed]
- Jones HD, Peters NCB, Holdsworth MJ (1997) Genotype and environment interact to control dormancy and differential expression of the VIVIPAROUS 1 homologue in embryos of Avena fatua. Plant J 12 911–920 [DOI] [PubMed] [Google Scholar]
- Karssen CM, Brinkhorst-van der Swan DLC, Breekland AE, Koornneef M (1983) Induction of dormancy during seed development by endogenous abscisic acid: studies on abscisic acid-deficient genotypes of Arabidopsis thaliana (L.) Heynh. Planta 157 158–165 [DOI] [PubMed] [Google Scholar]
- Kushiro T, Okamoto M, Nakabayashi K, Yamagishi K, Kitamura S, Asami T, Hirai N, Koshiba T, Kamiya Y, Nambara E (2004) The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: key enzymes in ABA catabolism. EMBO J 23 1647–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V, North H, Frey A, Sotta B, Seo M, Okamoto M, Nambara E, Marion-Poll A (2006) Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J 45 309–319 [DOI] [PubMed] [Google Scholar]
- Marumo S, Katayama M, Komori E, Ozaki Y, Natsume M, Kondo S (1982) Microbial production of abscisic acid by Botrytis cinerea. Agric Biol Chem 46 1967–1968 [Google Scholar]
- McCarty DR (1995) Genetic control and integration of maturation and germination pathways in seeds development. Annu Rev Plant Biol 46 71–93 [Google Scholar]
- Millar AA, Jacobsen JV, Ross JJ, Helliwell CA, Poole AT, Scofield G, Reid JB, Gubler FG (2006) Seed dormancy and ABA metabolism in Arabidopsis and barley: the role of ABA 8′-hydroxylase. Plant J 45 942–954 [DOI] [PubMed] [Google Scholar]
- Oh E, Yamaguchi S, Kamiya Y, Bae G, Chung W, Choi G (2006) Light activates the degradation of PIL5 protein to promote seed germination through gibberellin in Arabidopsis. Plant J 47 124–139 [DOI] [PubMed] [Google Scholar]
- Okamoto M, Kuwahara M, Seo M, Kushiro M, Asami T, Iria N, Kamiya Y, Koshiba T, Nambara E (2006) CYP707A1 and CYP707A2, which encode abscisic acid 8′-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol 141 97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce S, Basile G, Bavestrello G, Bruzzone S, Cerrano C, Giovine M, Arillo A, Zocchi E (2004) Abscisic acid signaling through cyclic ADP-ribose in hydroid regeneration. J Biol Chem 279 39783–39788 [DOI] [PubMed] [Google Scholar]
- Seo M, Hanada A, Kuwahara A, Endo A, Okamoto M, Yamauchi Y, North H, Marion-Poll A, Sun T, Koshiba T, et al (2006) Regulation of hormone metabolism in Arabidopsis seeds: phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J 48 354–366 [DOI] [PubMed] [Google Scholar]
- Simpson GM (1990) Seed Dormancy in Grasses. Cambridge University Press, Cambridge, UK
- Slovik S, Hartung W (1992) Compartmental distribution and redistribution of abscisic acid in intact leaves. II. Model analysis. Planta 187 26–36 [DOI] [PubMed] [Google Scholar]
- Spielmeyer W, Ellis M, Robertson M, Shahjahan A, Lenton JR, Chandler PM (2004) Isolation of gibberellin metabolic pathway genes from barley and comparative mapping in barley, wheat and rice. Theor Appl Genet 109 847–855 [DOI] [PubMed] [Google Scholar]
- Tan BC, Joseph LM, Deng WT, Liu L, Li QB, Cline K, McCarty DR (2003) Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J 35 44–56 [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Jackson AC, Parker RA, Morpeth DR, Burbridge A, Taylor IB (2000) Abscisic acid biosynthesis in tomato: regulation of zeaxanthin epoxidase and 9-cis-epoxycarotenoid dioxygenase mRNAs by light/dark cycles, water stress and abscisic acid. Plant Mol Biol 442 833–845 [DOI] [PubMed] [Google Scholar]
- Trevaskis B, Hemming MN, Peacock WJ, Dennis EJ (2006) HvVRN2 responds to daylength, whereas HvVRN1 is regulated by vernalization and developmental status. Plant Physiol 140 1397–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Simmons MK (1987) ABA levels and sensitivity in developing wheat embryos of sprouting resistant and susceptible cultivars. Plant Physiol 84 61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H (2005) Signaling mechanisms of higher plant photoreceptors: a structure-function perspective. Curr Top Dev Biol 68 227–261 [DOI] [PubMed] [Google Scholar]
- Wang M, Heimovaara-Dijkstra S, Van Duijn B (1995) Modulation of germination of embryos isolated from dormant and nondormant grains by manipulation of endogenous abscisic acid. Planta 195 586–592 [Google Scholar]
- Wang MB, Matthews PR, Upadhyaya NM, Waterhouse PM (1998) Improved vectors for Agrobacterium tumefaciens-mediated transformation of monocot plants. Acta Hortic 461 401–407 [Google Scholar]
- Yang SH, Zeevaart JAD (2006) Expression of ABA 8′-hydroxylases in relation to leaf water relations and seed development in bean. Plant J 47 675–686 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.