Abstract
The early-flowering habit of rapid-cycling accessions of Arabidopsis (Arabidopsis thaliana) is, in part, due to the genes of the autonomous floral-promotion pathway (AP). The AP promotes flowering by repressing expression of the floral inhibitor FLOWERING LOCUS C (FLC). AP mutants are therefore late flowering due to elevated levels of FLC, and this late-flowering phenotype is eliminated by loss-of-function mutations in FLC. To further investigate the role of the AP, we created a series of double mutants. In contrast to the phenotypes of single mutants, which are largely limited to delayed flowering, a subset of AP double mutants show a range of defects in growth and development. These phenotypes include reduced size, chlorophyll content, growth rate, and fertility. Unlike the effects of the AP on flowering time, these phenotypes are FLC independent. Recent work has also shown that two AP genes, FCA and FPA, are required for the repression and, in some cases, proper DNA methylation of two transposons. We show that similar effects are seen for all AP genes tested. Microarray analysis of gene expression in AP single and double mutants, however, suggests that the AP is not likely to play a broad role in the repression of gene expression through DNA methylation: very few of the genes that have been reported to be up-regulated in DNA methylation mutants are misexpressed in AP mutants. Together, these data indicate that the genes of the AP play important and sometimes functionally redundant roles in aspects of development in addition to flowering time.
A fundamental question in biology is how differentiating cells make decisions between alternative fates. The change from vegetative to reproductive development in Arabidopsis (Arabidopsis thaliana) is an attractive model for studying the factors that regulate such developmental transitions. Early in the life cycle, the undifferentiated stem cells of the shoot apical meristem give rise to organ primordia that will produce the vegetative portions of the plant (e.g. leaves). Later, the shoot apical meristem switches to producing primordia that will form the reproductive organs (e.g. flowers). Proper timing of this transition is critical for successful reproduction. Therefore, plants have evolved mechanisms to regulate flowering in response to both endogenous and environmental factors (Boss et al., 2004). These mechanisms help to ensure that plants flower at a suitable time in their development and a favorable time of year.
Vernalization is the promotion of flowering by prolonged exposure to cold temperature, such as would be experienced by plants in temperate climates during winter (Chouard, 1960). Flowering behavior in Arabidopsis can be divided into two types, winter annual and rapid cycling, based on their requirement for vernalization. Naturally occurring winter annual types are late flowering, but the late-flowering phenotype can be eliminated by vernalization. This late-flowering vernalization-responsive phenotype is created by the interaction of two genes: FLOWERING LOCUS C (FLC), a MADS domain-containing transcription factor that acts to repress flowering, and FRIGIDA (FRI), a gene of unknown biochemical function that activates the expression of FLC (Michaels and Amasino, 1999; Sheldon et al., 1999; Johanson et al., 2000). Therefore, prior to vernalization, FRI causes FLC to be expressed at high levels, which delays flowering. Vernalization eliminates the late-flowering phenotype of FRI and FLC through an epigenetic shutdown of FLC that is mediated by repressive histone modifications at the FLC locus (Bastow et al., 2004; Sung and Amasino, 2004). Thus, the interaction of FRI, FLC, and vernalization prevents flowering prior to winter and promotes flowering in the spring. Rapid-cycling accessions, in contrast, are early flowering in the absence of vernalization. This is most often due to naturally occurring mutations in FRI, in which case FLC expression is not activated, or to mutations in FLC itself (Johanson et al., 2000; Gazzani et al., 2003; Michaels et al., 2003). Therefore, rapid-cycling accessions appear to have evolved from winter annuals through loss- or reduction-of-function mutations in FRI or FLC. This loss of the vernalization requirement may have given rapid-cycling accessions an advantage as Arabidopsis spread into warmer climates that lack the prolonged cold temperatures required for vernalization.
Because rapid-cycling accessions, such as Columbia (Col), have lost FRI function, they contain low levels of FLC expression and thus do not require vernalization for early flowering. Mutagenesis in rapid-cycling backgrounds, however, has yielded mutations that restore the winter annual growth habit (Koornneef et al., 1991). The autonomous floral-promotion pathway (AP) is a group of genes that acts to promote flowering by suppressing FLC expression. Mutations in any one of these genes results in elevated FLC expression and delayed flowering. Similar to FRI-containing backgrounds, the elevated levels of FLC in AP mutants can be epigenetically silenced by vernalization (Michaels and Amasino, 1999; Sheldon et al., 1999). Thus, plants containing either dominant alleles of FRI or recessive AP mutations behave as winter annuals.
The AP contains seven genes, and although all of them have been cloned, relatively little is known about how these genes act to suppress FLC at the molecular level. Four contain domains that suggest roles in RNA binding or RNA metabolism. Three contain RNA-binding domains: FCA (Macknight et al., 1997) and FPA (Schomburg et al., 2001) contain two and three RRM-type RNA-binding domains, respectively, and FLOWERING LOCUS K (FLK; Lim et al., 2004; Mockler et al., 2004) contains three K homology domains. FY is related to a group of eukaryotic proteins that play a role in 3′ end processing of RNA transcripts (Simpson et al., 2003). FCA and FY proteins have been shown to physically interact and together regulate 3′ end selection of the FCA transcript (Simpson et al., 2003). The fact that four of the seven members of the AP are predicted to have RNA-related activities has led to the hypothesis that these proteins may regulate FLC posttranscriptionally; however, no direct evidence to support this model has been published. Interestingly, data have recently been published that indicate that FPA protein is localized to FLC chromatin and that FCA and FPA are required for RNA-mediated chromatin silencing of other loci (Baurle et al., 2007). This raises the possibility that FCA, FLK, FPA, and FY may repress FLC transcription though modulations in chromatin structure. This model is supported by the fact that two other AP proteins are thought to interact with a histone deacetylase complex. FVE and FLOWERING LOCUS D (FLD) have human homologs that coimmunoprecipitate with the histone deacetylase complex (He et al., 2003). FVE contains six WD repeats and is similar to mammalian retinoblastoma-associated proteins that are present in complexes involved in chromatin assembly and modification (Ausin et al., 2004; Kim et al., 2004). FLD encodes a protein that is homologous with the human protein LYSINE-SPECIFIC DEMETHYLASE1 (LSD1) that has been shown to act as a histone demethylase (Shi et al., 2004). The final member of the AP is LUMINIDEPENDENS (LD), which encodes a homeodomain-containing protein (Lee et al., 1994a). Work to date has focused primarily on the role of the AP in the regulation of flowering time. For most AP genes, loss-of-function mutations have few, if any, reported visible phenotypes aside from delayed flowering. A most notable exception is FY, for which weak alleles cause late flowering but null alleles cause embryo lethality (Henderson et al., 2005).
To gain further insight into the role of AP genes, we created a series of double mutants between fpa and other AP mutants. Interestingly, although the late-flowering phenotype of AP single mutants is eliminated by loss-of-function mutations in flc, we have found evidence that some AP genes act redundantly to promote flowering through FLC-independent mechanisms. In addition, a subset of double mutants show a similar set of pleiotropic phenotypes, indicating that some AP genes also function redundantly to control aspects of growth and development besides flowering time. This redundancy between AP mutants is supported by microarray analysis. We have also found evidence that most AP genes are required for the repression of a pair of transposons previously shown to be up-regulated in fca and fpa mutants (Baurle et al., 2007). Microarray analysis, however, suggests that the AP does not play a broad role in the repression of repetitive elements. Together, these data shed new light on the function of the AP in both the regulation of flowering time and other aspects of development.
RESULTS
FPA Plays Functionally Redundant Roles with FLD, FVE, and LD in Growth and Development
To investigate the relationships between AP genes, double mutant analysis was performed between fpa and other AP mutants. A previous study has examined genetic interactions among Arabidopsis flowering-time mutants (Koornneef et al., 1998). This work, however, was performed in a Landsberg erecta (Ler) background that contains an atypical weak allele of FLC (Koornneef et al., 1994; Lee et al., 1994b; Gazzani et al., 2003; Michaels et al., 2003) and did not include fld, flk, or ld mutants. Therefore, double mutants were created between fpa and fca, fld, flk, fve, and ld in a Col background, which contains a strong allele of FLC.
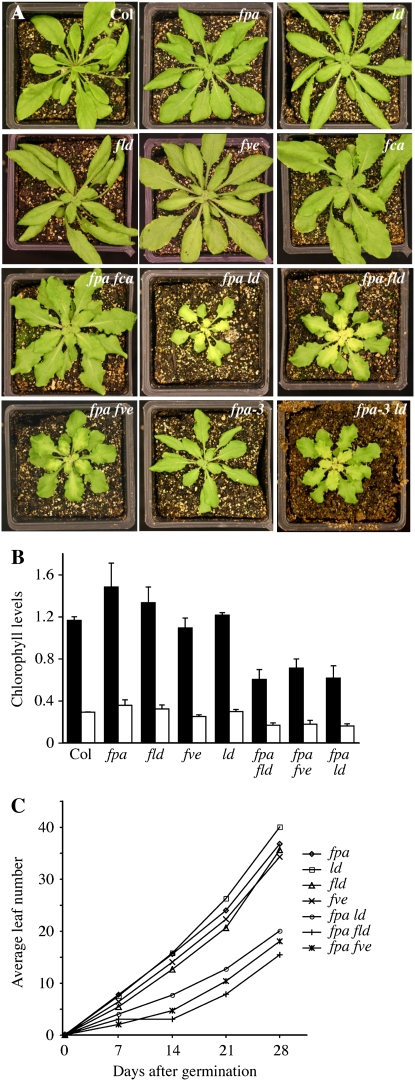
The visible phenotypes that have been reported for AP single mutants are primarily limited to delayed flowering (although it should be noted that fve mutants show altered responses to cold [Kim et al., 2004] and the expression of some GA-regulated genes is altered in fpa mutants [Meier et al., 2001]). In double mutants between fpa and other flowering-time mutants, the phenotypes of most lines were restricted to flowering time (see below). Surprisingly, however, three double mutants, fpa ld, fpa fld, and fpa fve, showed a similar set of pleiotropic phenotypes (Fig. 1A). Because these double mutants were all created with the same allele of fpa (fpa-7), we were concerned that a second mutation in the fpa-7 background might be responsible for the pleiotropic phenotypes. Therefore, we recreated the fpa ld double mutant using fpa-3. The resulting fpa-3 ld plants showed similar pleiotropic phenotypes to fpa-7 ld (Fig. 1A); thus, the phenotypes observed in the double mutants are indeed due to mutations in fpa.
Figure 1.
Phenotypes of AP mutants and fpa double mutants. A, Vegetative phenotypes of plants of the indicated genotypes. B, Analysis of chlorophyll levels in single and pleiotropic double mutants. Chlorophyll a and b levels are indicated by black and white bars, respectively. Error bars indicate 1 sd. C, Growth rate analysis of single and pleiotropic double mutants.
One of the most striking phenotypes of fpa ld, fpa fld, and fpa fve mutants is their pale green color, a phenotype that was strongest in the center of the rosette (Fig. 1A). Analysis of chlorophyll content showed that fpa ld, fpa fld, and fpa fve mutants contained approximately 50% less chlorophyll a and b than Col (Fig. 1B). A reduction in chlorophyll was not observed in fpa, fld, fve, or ld single mutants; thus, this phenotype was specific to the double mutants. The double mutants also showed slower growth. After 28 d of growth, fpa, fld, fve, or ld single mutants formed between 35 and 40 rosette leaves, whereas the double mutants formed only 15 to 20 leaves in the same period (Fig. 1C). A possible explanation for the reduction in growth rate is reduced photosynthetic capacity stemming from the lower levels of chlorophyll observed in the double mutants.
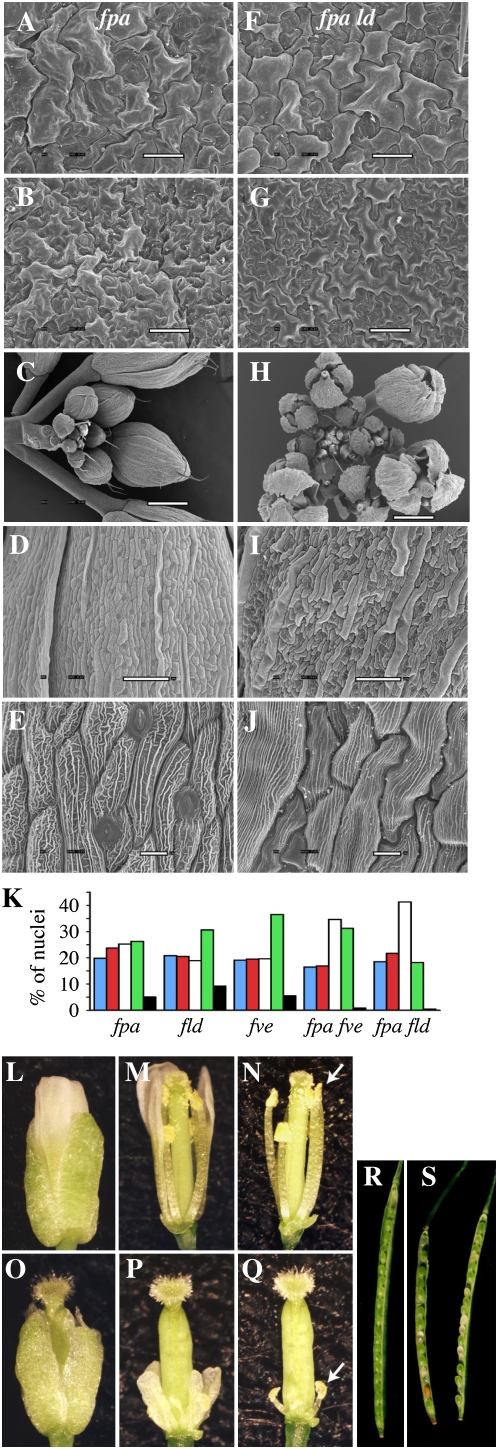
In addition to reduced growth rate and chlorophyll levels, fpa ld, fpa fld, and fpa fve double mutants showed additional defects, including wavy leaf margins and floral abnormalities (Figs. 1A and 2, C and H). Waving or crinkling of leaves can be caused by differential cell division or expansion on the top and bottom leaf surfaces. Scanning electron microscopy (SEM) was used to investigate cell size in the margins of wild-type and fpa ld leaves. If leaf waving in the double mutant is the result of differential cell expansion, a difference in cell size should be observed. Micrographs showed, however, that the cell size and organization on the adaxial and abaxial surfaces of the leaf were similar between fpa ld and the wild type (Fig. 2, A, B, F, and G). Because cell size is also correlated with DNA content, the degree of endoreduplication in leaves was also determined by fluorescence-activated cell sorter (FACS) analysis. The DNA content profiles of the single and double mutants were relatively similar (Fig. 2K). The double mutants did contain fewer 16n and 32n nuclei than the single mutants; however, this may be the result of the slower growth rate of the double mutants, as the level of endoreduplication in leaves increases with age (Galbraith et al., 1991). Taken together, these results suggest that the cause of the leaf waving may be alterations in cell number rather than cell size. This would be consistent with previous work that demonstrated that the crinkled leaves in cincinnata mutants of Antirrhinum are the result of excess cell division in leaf margins (Nath et al., 2003).
Figure 2.
Microscopic and FACS analyses of the pleiotropic phenotype of the fpa ld double mutant. A to J, Scanning electron micrographs of the adaxial (A and F) and abaxial (B and G) leaf surfaces, inflorescences (C and H), and sepals (D, E, I, and J) of fpa (A–E) and fpa ld (F–J) mutants. Bars = 50 μm (A–C, F, and G), 100 μm (D and I), and 10 μm (E and J). K, Nuclear DNA content as determined by FACS analysis. Bars represent the percentage of nuclei with DNA contents of 2n (blue), 4n (red), 8n (white), 16n (green), and 32n (black). Nuclei were isolated from leaf 5 from 30-d-old long-day-grown plants. More than 1,000 nuclei were analyzed for each genotype. L to Q, Flowers of flc (L–N) and fpa ld flc (O–Q) plants, whole (L and O), with sepals removed (M and P), and with sepals and petals removed (N and Q). White arrows (N and Q) indicate the positions of the anthers. R and S, Dissected siliques of Col (R) and fpa fpa; LD ld (S) plants.
Although SEM analysis did not reveal cellular abnormalities in leaves, clear differences between the wild type and fpa ld mutants were observed in flowers. In developing wild-type flowers, the sepals overlap to tightly enclose the flower (Fig. 2C). In fpa ld flowers, however, the sepals do not overlap one another in developing flowers, giving rise to gaps between the sepals (Fig. 2H). This phenotype was also observed in fpa fve and fpa fld mutants (data not shown) but was not seen in fpa fca or fpa flk double mutants. The sepals of fpa ld plants also show alterations in cell size and organization. The long axis of wild-type cells is primarily oriented along the length of the sepal (Fig. 2D); in fpa ld mutants, however, the orientation of cells appears more random (Fig. 2I). In addition, the average cell size appears to be larger in fpa ld sepals (Fig. 2, D and I). Another interesting phenotype of fpa ld double mutants was observed in the appearance of the ridges (possibly localized thickenings of the cell wall) on the surface of the sepal cells. In the wild type, the ridges are oriented both parallel and perpendicular to the long axis of the cell (Fig. 2E). In ld fpa cells, however, the perpendicular ridges are almost entirely absent (Fig. 2J).
Pleiotropic AP Double Mutants Exhibit Reduced Fertility
fpa fld, fpa fve, and fpa ld mutants all show strong reductions in fertility. Among these mutants, fpa ld mutants show the lowest fertility, typically setting fewer than 50 viable seeds per plant. SEM analysis showed that the flowers of fpa ld mutants have abnormal sepal development (Fig. 2, H–J). To further investigate the cause of the reduced fertility, flowers from fpa and fpa ld were dissected. Flowers were chosen from a position in the inflorescence where self-pollination typically occurs in wild-type flowers. At this stage, the stamens of fpa flowers have elongated such that the anthers are positioned near the stigmatic surface (Fig. 2, L–N). Also, the petals are longer than the sepals and are approximately the same length as the carpel. In contrast, fpa ld flowers show a strong reduction in petal and stamen size (Fig. 2, O–Q). Of particular note with regard to fertility, the stamens are much shorter than the carpel; thus, the anthers are not positioned to facilitate self-pollination (Fig. 2, O–Q, arrow).
To determine whether the relative positions of the anthers and stigma are the only barrier to fertilization and seed set, fpa ld mutant flowers were manually pollinated with fpa ld pollen. Manually pollinated flowers did show increased seed set compared with self-pollinated flowers, but the quantity of seed produced was still low, approximately 10% of the seed set of the wild type. The low fertility seen in manually pollinated fpa ld flowers could be the result of defects in fpa ld pollen and/or defects in the ability of fpa ld flowers to receive pollen (or both). To test the effectiveness of fpa ld plants as pollen donors and recipients, reciprocal crosses were performed between fpa ld and Col. fpa ld plants fertilized with an excess of wild-type pollen formed approximately half the number of seeds as control crosses using the wild type as both the pollen donor and recipient. This indicates that, in addition to physical obstacles to pollination, fpa ld plants are less effective than wild-type plants as pollen recipients. Likewise, reduced seed set was also observed in crosses in which Col plants were fertilized with fpa ld pollen, indicating that fpa ld is less effective as a pollen donor as well. It should be noted, however, that fpa ld anthers (Fig. 2Q) shed very little visible pollen. As a result, even with manual pollination, fpa ld pollen may be limiting in crosses. Thus, it is not possible to conclude whether the observed reduction in seed set in crosses using fpa ld pollen is due to a reduction in the number or the viability of pollen grains.
The fpa ld Genotype Shows Reduced Transmission through the Female Gametophyte
In the course of creating the fpa AP double mutants described in this study, it was noticed that fpa fld, fpa fve, and fpa ld double mutants were identified in F2 populations at frequencies lower than the expected 1:16. These low frequencies suggested that the double mutants might have reduced gametophytic or zygotic viability. To investigate this possibility, lines were created that were either homozygous for fpa and heterozygous for ld (fpa fpa; LD ld) or heterozygous for fpa and homozygous for ld (FPA fpa; ld ld) and the genotype of selfed progeny was determined using molecular markers. Both lines were created in an flc mutant background and therefore were early flowering. Because only one mutation is segregating in each population, double mutants are predicted to constitute one-fourth of the selfed offspring. Double mutants were observed, however, at a much lower rate (Table I). In the selfed progeny of fpa fpa; LD ld plants, only one homozygous double mutant was identified from 82 plants, and from FPA fpa; ld ld plants, no double mutants were identified in 92 selfed progeny. An examination of developing siliques from fpa fpa; LD ld plants revealed the presence of aborted seeds (Fig. 2, R and S). Together, these data suggest that fpa ld double mutants have a high rate of embryo lethality.
Table I.
Fertility in fpa ld double mutants
| Parent Genotype(s) | No. of Plants with the Indicated Genotypes Observed in the Next Generation | ||
|---|---|---|---|
| fpa fpa; LD ld, selfed | fpa fpa; LD LD | fpa fpa; LD ld | fpa fpa; ld ld |
| 35 | 46 | 1 | |
| P = 5.3e-6 | |||
| FPA fpa; ld ld, selfed | FPA FPA; ld ld | FPA fpa; ld ld | fpa fpa; ld ld |
| 39 | 53 | 0 | |
| P = 3.7e-7 | |||
| FPA fpa; ld ld ♂ × Col ♀ (FPA FPA; LD LD) | FPA fpa; LD ld | FPA FPA; LD ld | |
| 48 | 47 | ||
| P = 0.92 | |||
| FPA fpa; ld ld ♀ × Col ♂ (FPA FPA; LD LD) | FPA fpa; LD ld | FPA FPA; LD ld | |
| 49 | 90 | ||
| P = 0.00051 | |||
In addition to obtaining fewer homozygous fpa ld plants than expected, the number of heterozygous plants obtained in the selfed progeny was also lower than anticipated. The progeny are predicted to contain a wild-type homozygote (fpa fpa; LD LD or FPA FPA; ld ld) to heterozygote (fpa fpa; LD ld or FPA fpa; ld ld) ratio of 1:2; however, the ratio for the progeny of fpa fpa; LD ld was 1:1.31 and that for FPA fpa; ld ld was 1:1.36 (Table I). This skewed ratio could be due to reduced embryo viability for heterozygotes. Another, nonmutually exclusive, possibility is that the fpa ld genotype shows reduced transmission through the male and/or female gametes. To investigate the latter possibility, reciprocal crosses were performed between FPA fpa; ld ld and Col and the genotype of the resulting F1 plants was determined. When FPA fpa; ld ld pollen was used to fertilize Col flowers, approximately equal numbers of FPA fpa and FPA FPA F1 plants were obtained, suggesting that there is no difference in the virility of FPA ld and fpa ld pollen (Table I). When FPA fpa; ld ld flowers were fertilized with Col pollen, however, 1.8-fold more FPA FPA F1 plants were obtained than FPA fpa plants, indicating that the FPA ld genotype is inherited more frequently than the fpa ld genotype. Thus, in addition to the increased embryo lethality of fpa fpa; ld ld homozygotes, the fpa ld genotype exhibits reduced transmission through the maternal parent.
fpa fve Double Mutants Do Not Flower in the Absence of Vernalization
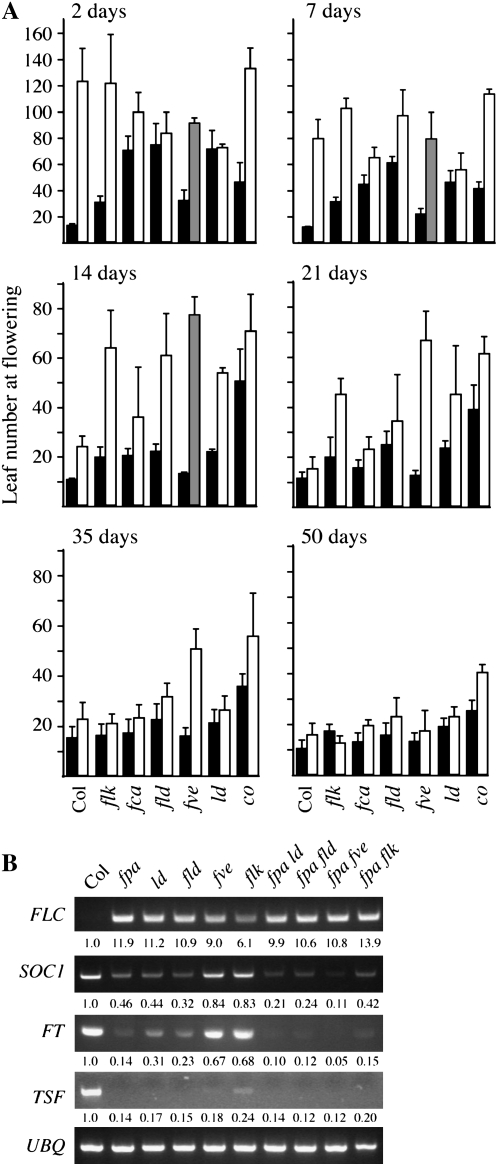
To examine the interactions between FPA and other members of the AP in the regulation of flowering time, we measured the flowering time of AP single mutants and double mutants with fpa under long days (Fig. 3A). Prior to planting, seeds were cold treated for 2 d; such short periods of cold exposure help to promote synchronous germination but do not significantly promote flowering (Schmitz et al., 2008). The AP single mutants could be divided into three classes based on flowering time: fpa mutants were the latest flowering, flowering after forming an average of 122 total leaves (rosette + cauline); these were followed by fca, fld, and ld mutants (averages of 70, 74, and 71 leaves, respectively); flk and fve mutants were the least late flowering (average of 30 and 32 leaves; respectively). Double mutants between fpa and other AP mutants exhibited a range of flowering behaviors. The flowering time of fpa fca and fpa fld was intermediate to that of either parent, whereas the flowering time of fpa ld double mutants was similar to that of the ld parent. fpa flk plants were the latest flowering of the double mutants and flowered at approximately the same time as fpa. Surprisingly, fpa fve plants did not flower under these conditions; these plants eventually underwent senescence after forming an average of 91 leaves. A constans (co) mutant was also included in this analysis. Because CO belongs to the photoperiod pathway, which acts independently of the AP, fpa and co mutants are predicted to show an additive effect on flowering time. Indeed, fpa co double mutants flower later than either parent (Fig. 3A). It is interesting that, with 2 d of cold treatment, none of the fpa AP double mutants flowered later than the fpa single mutant.
Figure 3.
Flowering time and gene expression in AP mutants and double mutants with fpa. A, Total number of leaves formed (rosette + cauline) by the indicated genotypes. Plants were grown under long days following the indicated period of cold treatment. Bars represent the number of leaves formed by the indicated genotypes without (black bars) or with (white bars) the fpa mutation. fpa fve mutants did not flower after 2, 7, or 14 d of cold treatment; the gray bars indicate the number of leaves formed at the time of senescence. Error bars indicate 1 sd. B, Semiquantitative RT-PCR analysis of gene expression in the indicated genotypes; numbers below the gels indicate fold changes relative to Col. UBQ10 was used as a constitutively expressed control.
Because the late-flowering phenotype of AP mutants is due to an up-regulation in FLC expression, we investigated the mRNA levels of FLC in several AP mutant backgrounds. Overall, the level of FLC expression was well correlated with the delay in flowering (Fig. 3B), with fpa and fpa flk showing the highest levels of FLC transcript. Interestingly, in fpa fve mutants, which did not flower in the absence of vernalization, FLC levels were similar to those of fpa ld and fpa fld. Thus, the failure of fpa fve mutants to flower cannot be explained by FLC levels alone. Therefore, we also examined the expression of SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1), FT, and TWIN SISTER OF FT. These genes act as floral promoters and are repressed by FLC (Kardailsky et al., 1999; Kobayashi et al., 1999; Samach et al., 2000; Michaels et al., 2005; Yamaguchi et al., 2005; Searle et al., 2006). As expected, the expression of these genes was inversely correlated with flowering time. Unlike FLC, whose expression failed to differentiate between nonflowering fpa fve mutants and fpa ld and fpa fld mutants, SOC1 and FT levels were significantly lower in fpa fve mutants than in any of the other backgrounds examined. The observation that SOC1 and FT levels are lower in fpa fve suggests that the repression of SOC1 and FT in the fpa fve background may have an FLC-independent component. This possibility was examined further by determining the flowering time of an fpa fve flc triple mutant (see below).
fpa fve Double Mutants Require Longer Periods of Vernalization for Early Flowering
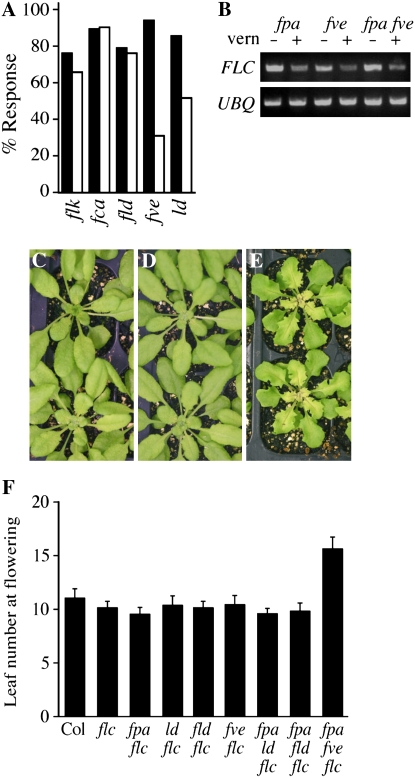
The late-flowering phenotype of AP mutants can be eliminated by vernalization, which causes an epigenetic repression of FLC. Because fpa fve mutants did not flower in the absence of vernalization, we were curious to determine the vernalization responsiveness of the fpa double mutants. The flowering times of AP single mutants and fpa double mutants were determined after cold treatments of various lengths. All lines showed a strong acceleration of flowering after 50 d of cold treatment (Fig. 3A). Following shorter periods of cold treatment, however, differences in vernalization response were apparent between the lines. After 21 d of cold, for example, most lines showed a reduction in leaf number equal to 70% to 90% of that seen after 50 d of cold treatment (Fig. 4A). fpa fve double mutants, however, showed only 33% of the maximal response after 21 d of cold. fpa ld plants also showed a significant, although less pronounced, reduction in vernalization response after 21 d (55%).
Figure 4.
Effects of vernalization and flc mutations in AP double mutants. A, Percentage vernalization response observed after 21 d of cold treatment. Percentage response was calculated by dividing the reduction in leaf number observed after the indicated periods of cold treatment by the reduction observed after 50 d of cold treatment. Bars represent the response of the indicated genotypes without (black bars) or with (white bars) the fpa mutation. B, Semiquantitative RT-PCR analysis of FLC expression after 21 d of cold treatment plus 10 d of recovery at warm temperatures. UBQ10 was used as a constitutively expressed control. C to E, fpa flc (C), ld flc (D), and fpa ld flc (E) plants grown under short days (plants grown under long days have similar phenotypes; data not shown). F, Flowering time measured as the total number of leaves formed prior to flowering under long days. Error bars indicate 1 sd. [See online article for color version of this figure.]
A complication with the analysis of the vernalization response of fpa fve plants is that they do not flower in the absence of vernalization (percentage response calculations were performed using the average leaf number at senescence). In fact, fpa fve plants did not consistently flower unless given at least 21 d of cold pretreatment (Fig. 3A). Therefore, it is difficult to determine whether the relatively late-flowering phenotype of fpa fve after 21 d of cold treatment is due to slower vernalization kinetics or an exceptionally strong initial block to flowering (i.e. if fpa fve plants flower in the absence of vernalization, they could be much later flowering than the other mutants, in which case the leaf number observed after 21 d of cold treatment would represent a response proportional to the other lines). In an attempt to distinguish between these possibilities, we examined FLC levels in fpa, fve, and fpa fve mutants after 21 d of cold treatment. Interestingly, FLC levels were similar in fpa and fpa fve mutants (Fig. 4B). Thus, the vernalization response appears normal in fpa fve double mutants and suggests that the delayed flowering observed in fpa fve after intermediate periods of vernalization may be independent of FLC levels (see below).
FLC Dependence of AP Double Mutant Phenotypes
The late-flowering phenotype of AP single mutants is due to elevated levels of FLC expression and is eliminated by loss-of-function mutations in flc (Michaels and Amasino, 2001). To determine whether FLC also plays a role in the pleiotropic phenotypes of AP double mutants, fpa fld flc, fpa ld flc, and fpa fve flc triple mutants were created. The pleiotropic phenotypes of these triple mutants were identical to those seen in the FLC-containing double mutants (Fig. 4, C–E); thus, FLC is not required for these phenotypes. We also evaluated the flowering time of the triple mutants. As reported previously, the late-flowering phenotype of fpa, fld, fve, and ld is eliminated in an flc-null background (Michaels and Amasino, 2001; He et al., 2003; Fig. 4F). The late-flowering phenotype of fpa ld and fpa fld was also eliminated in the absence of FLC. In contrast, fpa fve flc flowered significantly later than fpa flc or fve flc (Fig. 4F). These data indicate that the late-flowering phenotype of fpa ld and fpa fld is entirely dependent on FLC activity, whereas late flowering in fpa fve has an FLC-independent component. This FLC-independent block to flowering may be responsible for the late-flowering phenotype exhibited by fpa fve double mutants after intermediate periods of vernalization (Figs. 3A and 4A).
The AP Plays a Role in the Repression of Targets of RNA-Mediated Chromatin Silencing
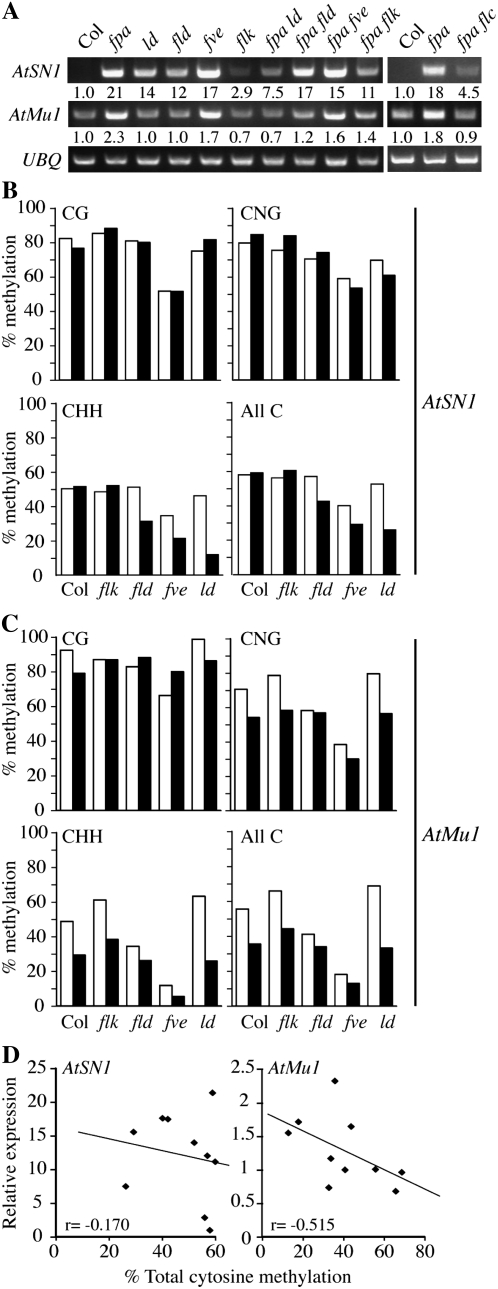
The retroelement AtSN1 (Xie et al., 2004) and the DNA transposon AtMu1 (Zilberman et al., 2003) are endogenous targets of RNA-mediated chromatin silencing. The silenced state of these loci is associated with high levels of DNA methylation, an increase in repressive histone modifications (e.g. histone H3K9 dimethylation), and a decrease in activating modifications (e.g. H3K4 dimethylation; Zilberman et al., 2003; Xie et al., 2004; Huettel et al., 2006). A recent study has identified mutations in fca and fpa as suppressors of RNA-mediated chromatin silencing (Baurle et al., 2007). AtSN1 and AtMu1 both show increased expression in fpa single and fpa fca double mutants, whereas only AtMu1 shows increased expression in fca mutants. The increased expression of AtMu1 in fpa fca double mutants was accompanied by a decrease in asymmetric cytosine methylation; however, no change in DNA methylation was observed at AtSN1 in fpa fca (Baurle et al., 2007).
A defect in RNA-mediated chromatin silencing could provide an explanation for the pleiotropic phenotypes observed in fpa fld, fpa fve, and fpa ld. Therefore, we examined the transcript levels and DNA methylation status of AtSN1 and AtMu1 in fpa, fld, fve, and ld single mutants as well as in fpa fld, fpa fve, and fpa ld double mutants. An fpa flk double mutant, along with flk, was also included as an example of a nonpleiotropic fpa double mutant. Interestingly, AtSN1 showed increased expression in all lines tested (Fig. 5A). Among single mutants, fpa and fve showed the highest expression of AtSN1, whereas flk showed a more modest up-regulation. The fact that all AP mutants tested showed elevated AtSN1 expression suggests that the up-regulation of AtSN1 might be due to increased FLC expression. Therefore, we examined the expression of AtSN1 in fpa and fpa flc (Fig. 5A) and found that AtSN1 transcript levels are indeed lower in fpa flc. It should be noted, however, that the levels of AtSN1 in fpa flc are higher than in Col. Thus, some, but not all, of the increase in AtSN1 expression observed in fpa mutants is FLC dependent. In contrast to AtSN1, which showed elevated expression in all AP mutants tested, AtMu1 showed increased expression only in fpa and fve (2.3- and 1.7-fold; Fig. 5A). Like AtSN1, the up-regulation of AtMu1 appears to require FLC (Fig. 5A). For both AtSN1 and AtMu1, none of the double mutants showed higher expression than fpa or fve single mutants. Thus, the pleiotropic phenotypes of the fpa double mutants do not correlate with the magnitude of AtSN1 or AtMu1 activation.
Figure 5.
Expression and DNA methylation analysis of AtSN1 and AtMu1. A, Semiquantitative RT-PCR analysis of AtSN1 and AtMu1 expression. UBQ10 was used as a constitutively expressed control. B and C, DNA methylation analysis of AtSN1 (B) and AtMu1 (C). White bars represent the indicated genotypes, and black bars indicate double mutants with fpa. D, Relationship between expression and the level of total cytosine methylation.
Bisulfite sequencing was used to investigate cytosine methylation at AtSN1 and AtMu1. fpa and fve mutants showed the greatest up-regulation of both AtSN1 and AtMu1; however, their effects on DNA methylation are quite different, especially at AtSN1. In fve mutants, CG, CNG (N = A, T, G, or C), and CHH (H = A, T, or C) methylation were all reduced at AtSN1 (Fig. 5B). The level of DNA methylation in fpa, flk, fld, and ld single mutants, however, was similar to that of the wild type. Interestingly, the pleiotropic double mutants, fpa fld, fpa fve, and fpa ld, all showed lower CHH methylation than either single mutant at AtSN1. CHH methylation in the nonpleiotropic fpa flk mutant, in contrast, was similar to that in the fpa and flk single mutants (Fig. 5B). Thus FLD, FVE, and LD have roles that are at least partially redundant with FPA in methylation at the AtSN1 locus. Like AtSN1, AtMu1 also showed significant changes in DNA methylation. Among the AP single mutants, fve again showed the largest reduction in each type of methylation (Fig. 5C). At AtMu1, however, fpa and fld showed reduced DNA methylation as well, particularly at CHH sites. fpa fve double mutants showed a greater reduction in methylation than the corresponding singles, again suggesting that AP proteins play redundant roles in DNA methylation. We also examined the correlation between DNA methylation and transcript levels for AtSN1 and AtMu1. At AtSN1, there is little correlation between the level of cytosine methylation and expression level (Fig. 5D). Notably, many lines (fpa, flk, fld, and fpa flk) show increased AtSN1 transcript levels with no significant change in DNA methylation compared with the wild type. AtMu1, in contrast, showed a much stronger correlation between total DNA methylation and expression (Fig. 5D). In summary, these results show that the AP has clear effects on both the expression and DNA methylation state of AtSN1 and AtMu1; however, there is no obvious link between the phenotypes of pleiotropic AP double mutants and the degree of DNA methylation or expression at AtSN1 or AtMu1.
Microarray Analysis Provides Additional Evidence for Functional Redundancy in the AP
In order to gain further insight into the function of the AP, we conducted microarray analysis. Col, fpa, fld, ld, fpa ld, and fpa fld plants were grown until the 10-leaf stage, at which point the aboveground portions were harvested and RNA was extracted. Because of differences in flowering time between Col and the AP mutants, plants were grown under short days to ensure that all plants remained in the vegetative stage of development at the time of harvest. For each genotype, RNA from four biological replicates was used to prepare labeled copy RNA, which was hybridized to GeneChip Arabidopsis ATH1 Genome Arrays (Affymetrix). For our initial analysis, comparisons were made between Col and each of the single and double mutants. Gene expression was considered to have changed significantly if transcript levels changed by at least 2-fold with an associated P value equal to or less than 0.01.
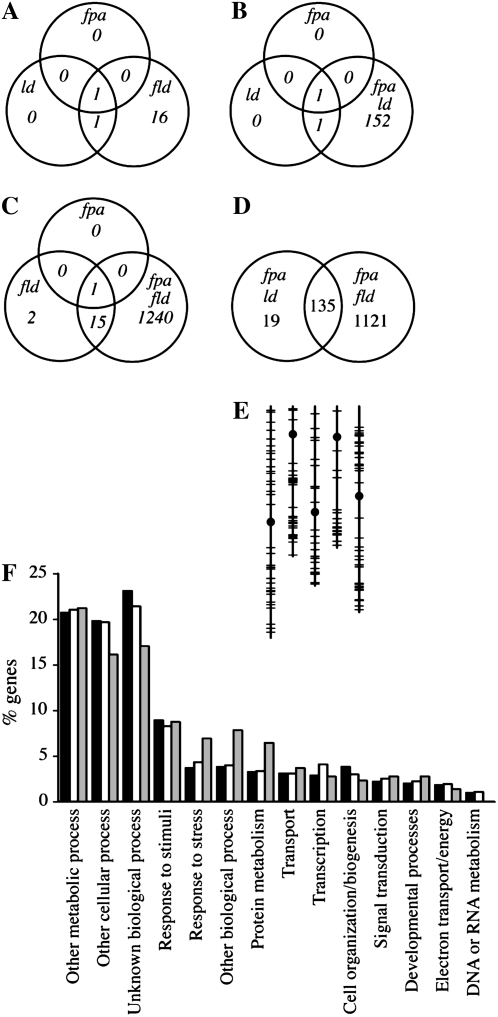
fpa, ld, and fld mutants all show a similar late-flowering vernalization-responsive phenotype, and as expected, all three mutants show highly elevated levels of FLC (Table II; Fig. 6A). As single mutants, fpa, ld, and fld affect the expression of a relatively small number of transcripts (one, two, and 18, respectively; Table II). This result is consistent with previous experiments using partial genome arrays (Wilson et al., 2005; Marquardt et al., 2006). Not surprisingly, given their pleiotropic phenotypes, a larger number of transcripts are altered in the fpa ld and fpa fld double mutants (Fig. 6, B and C). What was surprising, however, was the difference in the number of differentially expressed transcripts: 154 transcripts for fpa ld and 1,256 for fpa fld (Supplemental Table S1). Because these double mutants have similar phenotypes, one would predict that the genes responsible for these phenotypes should be misexpressed in both backgrounds. Therefore, we examined the overlap between the genes affected in fpa ld and fpa fld. Of the 154 genes misexpressed in fpa ld, 135 were also misexpressed in fpa fld (Fig. 6D). Moreover, if the criterion of significance is relaxed to any change supported by a P value of <0.05, 151 of the 154 genes showing differential expression in fpa ld are also differentially expressed in fpa fld. Thus, up to 98% of the genes affected by fpa ld are also affected by fpa fld. Another interesting discovery is that FPA and LD play redundant roles in the regulation of genes that require FLD for proper expression. Sixteen of 18 transcripts that were misexpressed in fld mutants were not affected in fpa or ld mutants (Table II); thus, FLD, but not FPA or LD, is required for the proper regulation of these 16 transcripts. Interestingly, however, in the fpa ld double mutant, 13 of those 16 transcripts are differentially expressed in the same direction and approximate magnitude as fld (Table II). Therefore, FPA and LD seem to function redundantly to regulate many of the same genes as FLD. In order to gain insight into the functions of the common targets of the double mutants, the genes misexpressed in fpa ld and fpa fld were grouped into functional categories using the Gene Ontology annotation search at The Arabidopsis Information Resource (http://www.arabidopsis.org/tools/bulk/go/index.jsp). The distribution of these genes into functional categories was compared with the distribution for the entire genome and the distribution for genes represented on the array (Fig. 6F). Genes predicted to be involved in stress, protein metabolism, and “other biological processes” were overrepresented in transcripts affected in the pleiotropic double mutants, whereas genes associated with unknown biological processes were underrepresented.
Table II.
Fold changes in gene expression relative to the wild type
Fold changes in boldface have an associated P value between 0.01 and 0.05; all other fold changes have P values of <0.01. n.c., No significant change in expression.
| Accession No. | fpa | ld | fld | fpa ld | Protein |
|---|---|---|---|---|---|
| AT5G10140 | 41.2 | 53.8 | 38.8 | 45.1 | FLC |
| AT5G42900 | n.c. | −4.3 | −11.7 | −11.3 | Unknown protein |
| AT2G33830 | n.c. | n.c. | −5.6 | −11.2 | Dormancy/auxin associated |
| AT1G22740 | n.c. | n.c. | −4.0 | −5.3 | Ras-related protein 7 |
| AT4G33985 | n.c. | n.c. | −3.9 | −3.9 | Unknown protein |
| AT3G50260 | n.c. | n.c. | −3.8 | −3.1 | DNA-binding/transcription factor |
| AT5G48250 | n.c. | n.c. | −3.6 | −7.5 | Zinc finger (B-box type) |
| AT3G58620 | n.c. | n.c. | −3.6 | −4.7 | TETRATRICOPETIDE-REPEAT THIOREDOXIN-LIKE4 |
| AT4G28085 | n.c. | n.c. | −3.5 | −2.7 | Unknown protein |
| AT3G16180 | n.c. | n.c. | −3.4 | −6.8 | Proton-dependent oligopeptide transport |
| AT5G58670 | n.c. | n.c. | −3.1 | −3.4 | PHOSPHOLIPASE C1 |
| AT5G05410 | n.c. | n.c. | −2.5 | −2.6 | DRE-BINDING PROTEIN2A |
| AT1G18390 | n.c. | n.c. | −2.4 | −2.6 | Protein kinase |
| AT1G62810 | n.c. | n.c. | −2.0 | −2.2 | Copper amine oxidase |
| AT2G18680 | n.c. | n.c. | 3.4 | 4.5 | Unknown protein |
| AT5G23870 | n.c. | n.c. | −2.4 | n.c. | Pectinacetylesterase family protein |
| AT3G04010 | n.c. | n.c. | −2.1 | n.c. | Glycosyl hydrolase |
| AT1G59218 | n.c. | n.c. | 2.6 | n.c. | Disease resistance protein |
Figure 6.
Microarray analysis of AP single and double mutants. A to D, Overlaps in differentially expressed (both up-regulated and down-regulated) transcripts. E, Chromosomal distribution of the 135 genes showing differential expression in both fpa ld and fpa fld. Vertical lines represent chromosomes I to V (left to right), circles indicate centromeres, and horizontal lines indicate differentially expressed genes. F, Gene Ontology analysis. Bars represent the percentage of genes in the Arabidopsis genome (black), on the ATH1 array (white), or in the 135 genes differentially expressed in both fpa ld and fpa fld (gray) that fall into the indicated Gene Ontology categories.
Given the effects of the AP on the expression and DNA methylation of AtSN1 and AtMu1, we examined the microarray data for evidence that the AP may play a widespread role in regulating gene expression via DNA methylation. Most cytosine methylation is located in paracentromeric heterochromatic regions of the genome (Zhang et al., 2006). In plants, CG methylation is thought to be maintained by METHYLTRANSFERASE1 (MET1), whereas DOMAINS REARRANGED METHYLTRANSFERASE1 (DRM1), DRM2, and CHROMOMETHYLASE3 (CMT3) perform non- CG methylation (Henderson and Jacobsen, 2007). Interestingly, the effects of met1 and drm1 drm2 cmt3 mutations on gene expression are quite different. The majority of the transcripts up-regulated in met1 mutants are pseudogenes (74%) located near the centromeres; in contrast, the majority of up-regulated transcripts in drm1 drm2 cmt3 triple mutants correspond to known genes (69%) and do not cluster near the centromeres (Zhang et al., 2006). Although it is not possible to distinguish between direct targets and indirect targets from the microarray data alone, it is noteworthy that the genes misexpressed in fpa ld and fpa fld show no evidence of a paracentromeric distribution (Fig. 6E) or an enrichment in pseudogenes. This suggests that the AP is not generally required for MET1-mediated DNA methylation. Moreover, a comparison of the genes up-regulated in met1 or drm1 drm2 cmt3 mutants (Zhang et al., 2006) with the genes up-regulated in AP mutants shows little overlap. For example, of the 493 genes up-regulated in fpa fld mutants (Supplemental Table S1), only three and six genes are reported as being up-regulated in met1 and drm1 drm2 cmt3 mutants, respectively (Zhang et al., 2006). Therefore, the AP does not appear to play a major role in gene regulation via DNA methylation.
DISCUSSION
Mutations affecting flowering time in Arabidopsis were first identified more than 45 years ago (Redei, 1962), and, especially in the last two decades, the regulation of flowering time has been a vigorous field of research. To date, the phenotypes ascribed to flowering-time mutants, including those of the AP, have largely been limited to flowering time. Therefore, it was surprising to find that double mutants between fpa and fld, fve, or ld had a number of strong pleiotropic phenotypes that had not been reported previously. One previous study has examined interactions between flowering-time genes through double mutant analysis (Koornneef et al., 1998). This study was performed in the Ler genetic background and included an fpa fve double mutant (fld and ld mutants were not used in that study); however, no pleiotropic phenotypes were reported (Koornneef et al., 1998). The reasons for phenotypic differences between our fpa fve double mutant and the previously reported double mutant are unclear, but two possible, nonmutually exclusive, explanations are that the differences are due to genetic background (our work was performed in the Col background) and/or to allele strength. Differences between the Ler and Col genetic backgrounds with regard to flowering time are well documented. The Ler allele of FLC contains a retrotransposon in intron I that leads to reduced levels of expression; therefore, FRI and AP mutations show a weaker late-flowering phenotype in Ler than in Col (Koornneef et al., 1994; Lee et al., 1994b; Gazzani et al., 2003; Michaels et al., 2004). In fact, fld and ld mutants show almost no effect on flowering time in the Ler background (Koornneef et al., 1994; Lee et al., 1994b; Sanda and Amasino, 1996). Because the pleiotropic phenotypes of fpa double mutants are FLC independent, however, it seems unlikely that the Ler allele of FLC is responsible for the lack of pleiotropic phenotypes for fpa fve in the Ler background. This, of course, does not rule out the possibility that other loci that differ between the Ler and Col backgrounds might be involved. Another cause for the differences in phenotypes are the alleles of fpa and fve used: fpa-7 and fve-4 (this study) and fpa-1 and fve-1 (Koornneef et al., 1998). fpa-1, fpa-7, and fve-4 likely represent null alleles; fpa-1 and fve-4 contain premature stop codons (Schomburg et al., 2001; Ausin et al., 2004); and fpa-7 contains a T-DNA insertion (Michaels and Amasino, 2001). fve-1, however, contains an Ala-to-Val missense mutation that may not represent a total loss of function. That fve-1 is not a null allele is supported by the fact that another missense mutation in Ler, fve-2, has a stronger late-flowering phenotype than fve-1 (Ausin et al., 2004). Thus, it is possible that if an fpa fve double mutant were constructed in the Ler genetic background using a null allele of fve, the double mutant may have pleiotropic phenotypes similar to those seen in the Col background. Unfortunately, a null allele of fve in the Ler background has not been described.
As single mutants, the phenotypes of fpa, fld, fve, and ld are primarily limited to delayed flowering as a result of FLC up-regulation. Our microarray data support this model: in fpa, ld, and fld single mutants, a relatively small number of genes show significant changes in expression. Moreover, FLC shows the largest change in expression between the wild type and the single mutants (Table II; Fig. 6A). As double mutants, however, fpa fld, fpa fve, and fpa ld double mutants show a range of pleiotropic phenotypes that are not observed in the single mutants or in other double mutants with fpa. These phenotypes are FLC independent and include reduced growth rate, chlorophyll content, and fertility and disruptions in cellular organization (e.g. sepals). As would be predicted from the pleiotropic phenotypes, a larger number of genes exhibit differential expression in fpa ld and fpa fld double mutants. Given that fpa ld and fpa fld have similar phenotypes, it was somewhat surprising that the number of genes showing differential expression in fpa fld was approximately 8-fold higher than in fpa ld (Fig. 6D). Although the numbers of genes showing differential expression was quite different between fpa ld and fpa fld, the overlap between the sets of genes is striking; of the genes affected in fpa ld, 98% show a significant change in expression in fpa fld (P > 0.05). Thus, it appears that the genes affected by fpa ld are largely a subset of the genes affected by fpa fld. Because FLC is expressed at higher levels in both fpa ld and fpa fld than in Col, it is possible that some of the genes differentially expressed in both backgrounds are, in fact, downstream targets of FLC. This seems unlikely, however, given that FLC levels are roughly similar in single and double mutants (Table II) and that the pleiotropic phenotypes of fpa fld, fpa fve, and fpa ld are FLC independent. This suggests that the genes differentially expressed in fpa ld and fpa fld are FLC-independent targets of the AP and that FPA acts redundantly with FLD and LD to regulate these genes (this regulation, of course, could be direct or indirect). Evidence of functional redundancy between AP genes was also observed elsewhere in the microarray data. fpa and ld mutants showed differential expression of only one and two transcripts, respectively, whereas 18 transcripts were differentially expressed in fld. Interestingly, the majority of the transcripts showing differential expression in fld were also differentially expressed in fpa ld double mutants. Overall, these data suggest that, although each of the genes of the AP is essential for the repression of FLC, AP proteins also act with varying degrees of redundancy to regulate the transcription of other genes in the genome.
For the most part, double mutants between fpa and other AP mutants did not show an additive delay in flowering time. In fact, several double mutants flowered earlier than the fpa single mutant. In general, the flowering time of the fpa double mutants is correlated with the severity of their pleiotropic phenotypes: no pleiotropic phenotypes were observed for fpa flk mutants, which flower at approximately the same time as fpa; fpa fca mutants have minor pleiotropic phenotypes (the leaves of fpa fca mutants are wider and more serrated than those of Col, fpa, or fca; Fig. 1A) and flower slightly earlier than fpa; finally, fpa ld and fpa fld have the most severe pleiotropic phenotypes and are the most early flowering. Although the exact reason for the earlier flowering in pleiotropic mutants is not clear, it is possible that stress may play a role. A number of different stresses, such as temperature, crowding, and water and nutrient availability, are known to accelerate flowering (Crone and McDaniel, 1997). At least in the case of fpa ld and fpa fld, this model is supported by their slower growth, pleiotropic phenotypes, and differential regulation of stress-associated genes observed in these double mutants. The one exception to this correlation is the fpa fve double mutant. In the absence of vernalization, fpa fve plants invariably died without flowering. It should be noted, however, that fpa fve plants died before forming as many leaves as fpa plants (Fig. 3A). Therefore, in addition to any effect on flowering time, fpa fve mutants also appear to have reduced longevity. fpa fve plants did not consistently flower unless vernalized for at least 21 d and, following 21 or 35 d of cold pretreatment, flowered later that any of the other AP single or double mutants. This delay in flowering after intermediate periods of vernalization does not appear to be due to slower vernalization kinetics; rather, it is likely due to an FLC-independent block to flowering. This is supported by the result that the fpa fve double mutant is late flowering in an flc-null background. Thus, in addition to the regulation of FLC, FPA and FVE function redundantly to promote flowering through an FLC-independent mechanism.
Modifications in chromatin structure play an important role in regulating transcription in eukaryotic genomes (Li et al., 2007). The relationship between various histone modifications and between histone modifications and DNA methylation, however, remains unclear. Repressive histone modifications have been implicated in the regulation of FLC by the AP and by vernalization; FLD and FVE are required for the deacetylation of histone tails at the FLC locus, whereas vernalization increases the methylation of H3K9 and H3K27 at FLC (He et al., 2003; Bastow et al., 2004; Sung and Amasino, 2004). Recent work has shown that FPA, FCA, and homologs of FLD are also required for the repression of loci that are normally associated with high levels of DNA methylation. In fpa fca double mutants, the expression of both AtSN1 and AtMu1 is up-regulated (Baurle et al., 2007) and mutations in the FLD homologs lsd1-like1 (ldl1) and ldl2 lead to ectopic expression of FWA (Jiang et al., 2007), a gene whose DNA is highly methylated and transcriptionally inactive in the sporophyte (FWA is normally expressed the central cell and in endosperm; Soppe et al., 2000; Kinoshita et al., 2004; Ikeda et al., 2007). In some cases, the activation of these normally silenced loci is accompanied by a decrease in DNA methylation, particularly at asymmetric sites (AtMu1 in fpa fca mutants and FWA in ldl1 and ldl2 mutants), whereas, in other cases (AtSN1 in fpa fca mutants), no change in DNA methylation is observed (Baurle et al., 2007; Jiang et al., 2007).
In our work, we found that all members of the AP tested showed elevated levels of AtSN1 expression and that FPA and FVE are required for AtMu1 suppression. Thus, the majority of the AP appears to play a role in the silencing of at least some loci associated with high levels of DNA methylation. Our microarray analysis, however, showed little overlap between the genes showing altered expression in AP mutants and DNA methylation mutants (Zhang et al., 2006). Therefore, is seems unlikely that the AP plays a genome-wide role in DNA methylation. The result that FPA has been shown to be physically associated with the chromatin of both AtSN1 and FLC (Baurle et al., 2007) suggests that the AP may function as a repressive complex at these loci. Given that DNA methylation has not been reported at the FLC locus, it appears that DNA methylation is not a requirement for the recruitment of FPA and possibly other AP components. It is worth noting that the activation of methylated loci without a reduction in DNA methylation is not unprecedented; morpheus' molecule1 mutations lead to the activation of methylated loci without affecting DNA methylation (Amedeo et al., 2000). Thus, processes downstream of DNA methylation are required for transcriptional silencing, and the AP may play a role in this process. Finally, it is interesting that, although the flowering habits of FRI-containing winter annuals are virtually indistinguishable from those of AP mutants, no naturally occurring AP mutants have yet been identified. The work presented here shows that the AP plays roles in aspects of growth and development independent of flowering time. Thus, it is possible that the effect of FRI is more flowering specific than that of AP mutations and that AP mutants confer a selective disadvantage that would make them less likely to become established in naturally occurring populations.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) fpa-7 (Michaels and Amasino, 2001), fpa-3 (Meier et al., 2001), fca-9 (Bezerra et al., 2004), ld-1 (Redei, 1962), fld-3 (He et al., 2003), fve-4 (Michaels and Amasino, 2001), and flc-3 (Michaels and Amasino, 1999) are in the Col genetic background and have been described previously. flk (SALK_112850) was obtained from the Arabidopsis Biological Resource Center. Plants were grown under cool-white fluorescent light (approximately 100 μmol m−2 s−1) at 22°C. Long days consisted of 16 h of light followed by 8 h of darkness. Short days consisted of 8 h of light followed by 16 h of darkness. For vernalization, seedlings were sown on agar-solidified medium (2 g L−1 Murashige and Skoog salts, 0.5 g L−1 15-16-17 Peat-lite special fertilizer [Scotts], and 0.8% agar) and were kept at 4°C under long days for the indicated periods of time.
Gene Expression Analysis
For semiquantitative reverse transcription (RT)-PCR analysis, RNA isolation, RT, and PCR were performed as described previously (Michaels et al., 2004). Primer sequences are shown in Supplemental Table S2. For all experiments, the data shown are representative of at least three independent experiments. For vernalized samples, seedlings were sown on agar-solidified medium, stratified for 2 d at 4°C, and grown for 10 d under long days at 22°C prior to cold treatment.
Microscopy
SEM was performed as described previously (Jacob et al., 2007).
Microarray Analysis
Total RNA was prepared from short-day-grown plants using the Spectrum Plant Total RNA kit (Sigma). Labeling, hybridization, and scanning were performed according to the manufacturer's instructions for the ATH1 Genome Arrays (Affymetrix). CEL files were uploaded into ArrayAssist software (Stratagene). The data were normalized using the GeneChip Robust Multi Array algorithm, and significance analysis was performed using P value correction for multiple testing (Benjamini-Hochberg false-discovery rate). P values corrected according to Benjamini-Hochberg are presented. Microarray data can be downloaded from Supplemental Data Set S1.
DNA Methylation Analysis
DNA was isolated from long-day-grown plants (10-leaf stage) using the DNeasy Plant Mini kit (Qiagen). Bisulfite conversion and methylation analyses for AtSN1 and AtMu1 were performed as described (Baurle et al., 2007). At least 15 individual clones were sequenced and analyzed per genotype.
Chlorophyll Content
Tissue discs (0.5 cm in diameter) were taken from recently fully expanded leaves. Chlorophyll content was determined spectroscopically as described (Ritchie, 2006).
FACS
Nuclei were isolated from nearly fully expanded leaves from plants at the 10-leaf stage. Plants were grown in long days. DNA content was determined essentially as described (Dolezel et al., 2007) using procedure A. Ribonuclease (10 mg mL−1) was added to the buffer just before chopping, and 1 mg mL−1 propidium iodide was used as a fluorochrome. The FACS analysis was carried out on the FACSCalibur system with CellQuest Pro version 4 software (BD Biosciences).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Microarray analysis of AP mutants.
Supplemental Table S2. PCR primers and amplification conditions.
Supplemental Data Set S1. Raw microarray data.
Supplementary Material
Acknowledgments
We thank Yannick Jacob, Lei Ding, and Xuhong Yu for useful discussions and critical evaluation of the manuscript.
This work was supported by grants to S.D.M. from the National Science Foundation (grant no. IOB–0447583) and the National Institutes of Health (grant no. 1R01 GM–075060–01).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Scott D. Michaels (michaels@indiana.edu).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Amedeo P, Habu Y, Afsar K, Mittelsten Scheid O, Paszkowski J (2000) Disruption of the plant gene MOM releases transcriptional silencing of methylated genes. Nature 405 203–206 [DOI] [PubMed] [Google Scholar]
- Ausin I, Alonso-Blanco C, Jarillo JA, Ruiz-Garcia L, Martinez-Zapater JM (2004) Regulation of flowering time by FVE, a retinoblastoma-associated protein. Nat Genet 36 162–166 [DOI] [PubMed] [Google Scholar]
- Bastow R, Mylne JS, Lister C, Lippman Z, Martienssen RA, Dean C (2004) Vernalization requires epigenetic silencing of FLC by histone methylation. Nature 427 164–167 [DOI] [PubMed] [Google Scholar]
- Baurle I, Smith L, Baulcombe DC, Dean C (2007) Widespread role for the flowering-time regulators FCA and FPA in RNA-mediated chromatin silencing. Science 318 109–112 [DOI] [PubMed] [Google Scholar]
- Bezerra IC, Michaels SD, Schomburg FM, Amasino RM (2004) Lesions in the mRNA cap-binding gene ABA HYPERSENSITIVE 1 suppress FRIGIDA-mediated delayed flowering in Arabidopsis. Plant J 40 112–119 [DOI] [PubMed] [Google Scholar]
- Boss PK, Bastow RM, Mylne JS, Dean C (2004) Multiple pathways in the decision to flower: enabling, promoting, and resetting. Plant Cell (Suppl) 16 S18–S31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouard P (1960) Vernalization and its relations to dormancy. Annu Rev Plant Physiol 11 191–238 [Google Scholar]
- Crone W, McDaniel C (1997) Flowering response to transplanting and rooting manipulations in Arabidopsis thaliana (Brassicaceae). Int J Plant Sci 158 231–235 [Google Scholar]
- Dolezel J, Greilhuber J, Suda J (2007) Estimation of nuclear DNA content in plants using flow cytometry. Nat Protocols 2 2233–2244 [DOI] [PubMed] [Google Scholar]
- Galbraith DW, Harkins KR, Knapp S (1991) Systemic endopolyploidy in Arabidopsis thaliana. Plant Physiol 96 985–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzani S, Gendall AR, Lister C, Dean C (2003) Analysis of the molecular basis of flowering time variation in Arabidopsis accessions. Plant Physiol 132 1107–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Michaels SD, Amasino RM (2003) Regulation of flowering time by histone acetylation in Arabidopsis. Science 302 1751–1754 [DOI] [PubMed] [Google Scholar]
- Henderson IR, Jacobsen SE (2007) Epigenetic inheritance in plants. Nature 447 418–424 [DOI] [PubMed] [Google Scholar]
- Henderson IR, Liu F, Drea S, Simpson GG, Dean C (2005) An allelic series reveals essential roles for FY in plant development in addition to flowering-time control. Development 132 3597–3607 [DOI] [PubMed] [Google Scholar]
- Huettel B, Kanno T, Daxinger L, Aufsatz W, Matzke AJ, Matzke M (2006) Endogenous targets of RNA-directed DNA methylation and Pol IV in Arabidopsis. EMBO J 25 2828–2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Kobayashi Y, Yamaguchi A, Abe M, Araki T (2007) Molecular basis of late-flowering phenotype caused by dominant epi-alleles of the FWA locus in Arabidopsis. Plant Cell Physiol 48 205–220 [DOI] [PubMed] [Google Scholar]
- Jacob Y, Mongkolsiriwatana C, Veley KM, Kim SY, Michaels SD (2007) The nuclear pore protein AtTPR is required for RNA homeostasis, flowering time, and auxin signaling. Plant Physiol 144 1383–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Yang W, He Y, Amasino RM (2007) Arabidopsis relatives of the human lysine-specific demethylase1 repress the expression of FWA and FLOWERING LOCUS C and thus promote the floral transition. Plant Cell 19 2975–2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson U, West J, Lister C, Michaels S, Amasino R, Dean C (2000) Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science 290 344–347 [DOI] [PubMed] [Google Scholar]
- Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D (1999) Activation tagging of the floral inducer FT. Science 286 1962–1965 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Hyun Y, Park JY, Park MJ, Park MK, Kim MD, Lee MH, Moon J, Lee I, Kim J (2004) A genetic link between cold responses and flowering time through FVE in Arabidopsis thaliana. Nat Genet 36 167–171 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Miura A, Choi Y, Kinoshita Y, Cao X, Jacobsen SE, Fischer RL, Kakutani T (2004) One-way control of FWA imprinting in Arabidopsis endosperm by DNA methylation. Science 303 521–523 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T (1999) A pair of related genes with antagonistic roles in mediating flowering signals. Science 286 1960–1962 [DOI] [PubMed] [Google Scholar]
- Koornneef M, Alonso-Blanco C, Blankestijn-de Vries H, Hanhart CJ, Peeters AJ (1998) Genetic interactions among late-flowering mutants of Arabidopsis. Genetics 148 885–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Blankestijn-de Vries H, Hanhart C, Soppe W, Peeters T (1994) The phenotype of some late-flowering mutants is enhanced by a locus on chromosome 5 that is not effective in the Landsberg erecta wild-type. Plant J 6 911–919 [Google Scholar]
- Koornneef M, Hanhart CJ, van der Veen JH (1991) A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol Gen Genet 229 57–66 [DOI] [PubMed] [Google Scholar]
- Lee I, Aukerman MJ, Gore SL, Lohman KN, Michaels SD, Weaver LM, John MC, Feldmann KA, Amasino RM (1994. a) Isolation of LUMINIDEPENDENS: a gene involved in the control of flowering time in Arabidopsis. Plant Cell 6 75–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Michaels SD, Masshardt AS, Amasino RM (1994. b) The late-flowering phenotype of FRIGIDA and LUMINIDEPENDENS is suppressed in the Landsberg erecta strain of Arabidopsis. Plant J 6 903–909 [Google Scholar]
- Li B, Carey M, Workman JL (2007) The role of chromatin during transcription. Cell 128 707–719 [DOI] [PubMed] [Google Scholar]
- Lim MH, Kim J, Kim YS, Chung KS, Seo YH, Lee I, Hong CB, Kim HJ, Park CM (2004) A new Arabidopsis gene, FLK, encodes an RNA binding protein with K homology motifs and regulates flowering time via FLOWERING LOCUS C. Plant Cell 16 731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknight R, Bancroft I, Page T, Lister C, Schmidt R, Love L, Westphal L, Murphy G, Sherson S, Cobbett C, et al (1997) FCA, a gene controlling flowering time in Arabidopsis, encodes a protein containing RNA-binding domains. Cell 89 737–745 [DOI] [PubMed] [Google Scholar]
- Marquardt S, Boss PK, Hadfield J, Dean C (2006) Additional targets of the Arabidopsis autonomous pathway members, FCA and FY. J Exp Bot 57 3379–3386 [DOI] [PubMed] [Google Scholar]
- Meier C, Bouquin T, Nielsen ME, Raventos D, Mattsson O, Rocher A, Schomburg F, Amasino RM, Mundy J (2001) Gibberellin response mutants identified by luciferase imaging. Plant J 25 509–519 [DOI] [PubMed] [Google Scholar]
- Michaels S, Amasino R (1999) FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11 949–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM (2001) Loss of FLOWERING LOCUS C activity eliminates the late-flowering phenotype of FRIGIDA and autonomous pathway mutations but not responsiveness to vernalization. Plant Cell 13 935–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, Bezerra IC, Amasino RM (2004) FRIGIDA-related genes are required for the winter-annual habit in Arabidopsis. Proc Natl Acad Sci USA 101 3281–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, He Y, Scortecci KC, Amasino RM (2003) Attenuation of FLOWERING LOCUS C activity as a mechanism for the evolution of summer-annual flowering behavior in Arabidopsis. Proc Natl Acad Sci USA 100 10102–10107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, Himelblau E, Kim SY, Schomburg FM, Amasino RM (2005) Integration of flowering signals in winter-annual Arabidopsis. Plant Physiol 137 149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockler TC, Yu X, Shalitin D, Parikh D, Michael TP, Liou J, Huang J, Smith Z, Alonso JM, Ecker JR, et al (2004) Regulation of flowering time in Arabidopsis by K homology domain proteins. Proc Natl Acad Sci USA 101 12759–12764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath U, Crawford BC, Carpenter R, Coen E (2003) Genetic control of surface curvature. Science 299 1404–1407 [DOI] [PubMed] [Google Scholar]
- Redei GP (1962) Supervital mutants in Arabidopsis. Genetics 47 443–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie RJ (2006) Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth Res 89 27–41 [DOI] [PubMed] [Google Scholar]
- Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, Coupland G (2000) Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288 1613–1616 [DOI] [PubMed] [Google Scholar]
- Sanda SL, Amasino RM (1996) Ecotype-specific expression of a flowering mutant phenotype in Arabidopsis thaliana. Plant Physiol 111 641–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz RJ, Sung S, Amasino RM (2008) Histone arginine methylation is required for vernalization-induced epigenetic silencing of FLC in winter-annual Arabidopsis thaliana. Proc Natl Acad Sci USA 105 411–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomburg FM, Patton DA, Meinke DW, Amasino RM (2001) FPA, a gene involved in floral induction in Arabidopsis, encodes a protein containing RNA-recognition motifs. Plant Cell 13 1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle I, He Y, Turck F, Vincent C, Fornara F, Krober S, Amasino RA, Coupland G (2006) The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis. Genes Dev 20 898–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon CC, Burn JE, Perez PP, Metzger J, Edwards JA, Peacock WJ, Dennis ES (1999) The FLF MADS box gene: a repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell 11 445–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y (2004) Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119 941–953 [DOI] [PubMed] [Google Scholar]
- Simpson GG, Dijkwel PP, Quesada V, Henderson I, Dean C (2003) FY is an RNA 3′ end-processing factor that interacts with FCA to control the Arabidopsis floral transition. Cell 113 777–787 [DOI] [PubMed] [Google Scholar]
- Soppe WJ, Jacobsen SE, Alonso-Blanco C, Jackson JP, Kakutani T, Koornneef M, Peeters AJ (2000) The late flowering phenotype of fwa mutants is caused by gain-of-function epigenetic alleles of a homeodomain gene. Mol Cell 6 791–802 [DOI] [PubMed] [Google Scholar]
- Sung S, Amasino RM (2004) Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature 427 159–164 [DOI] [PubMed] [Google Scholar]
- Wilson IW, Kennedy GC, Peacock JW, Dennis ES (2005) Microarray analysis reveals vegetative molecular phenotypes of Arabidopsis flowering-time mutants. Plant Cell Physiol 46 1190–1201 [DOI] [PubMed] [Google Scholar]
- Xie Z, Johansen LK, Gustafson AM, Kasschau KD, Lellis AD, Zilberman D, Jacobsen SE, Carrington JC (2004) Genetic and functional diversification of small RNA pathways in plants. PLoS Biol 2: E104 [DOI] [PMC free article] [PubMed]
- Yamaguchi A, Kobayashi Y, Goto K, Abe M, Araki T (2005) TWIN SISTER OF FT (TSF) acts as a floral pathway integrator redundantly with FT. Plant Cell Physiol 46 1175–1189 [DOI] [PubMed] [Google Scholar]
- Zhang X, Yazaki J, Sundaresan A, Cokus S, Chan SW, Chen H, Henderson IR, Shinn P, Pellegrini M, Jacobsen SE, et al (2006) Genome-wide high-resolution mapping and functional analysis of DNA methylation in Arabidopsis. Cell 126 1189–1201 [DOI] [PubMed] [Google Scholar]
- Zilberman D, Cao X, Jacobsen SE (2003) ARGONAUTE4 control of locus-specific siRNA accumulation and DNA and histone methylation. Science 299 716–719 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.