Abstract
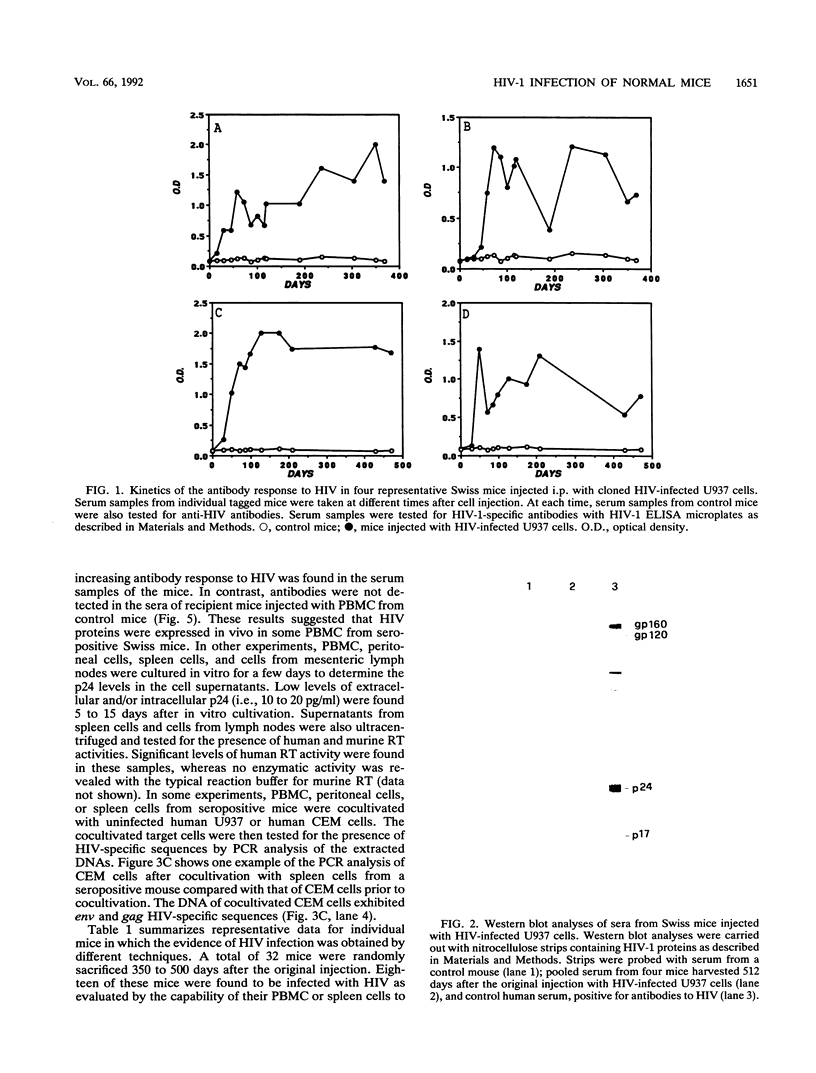
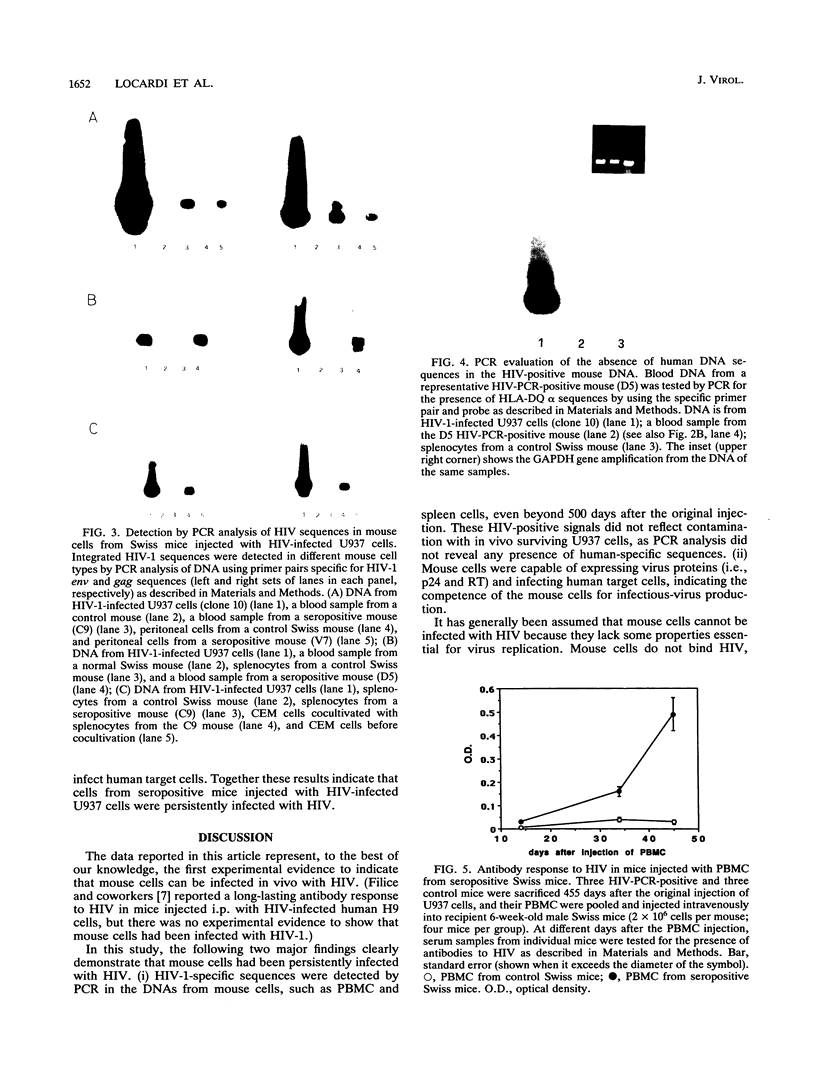
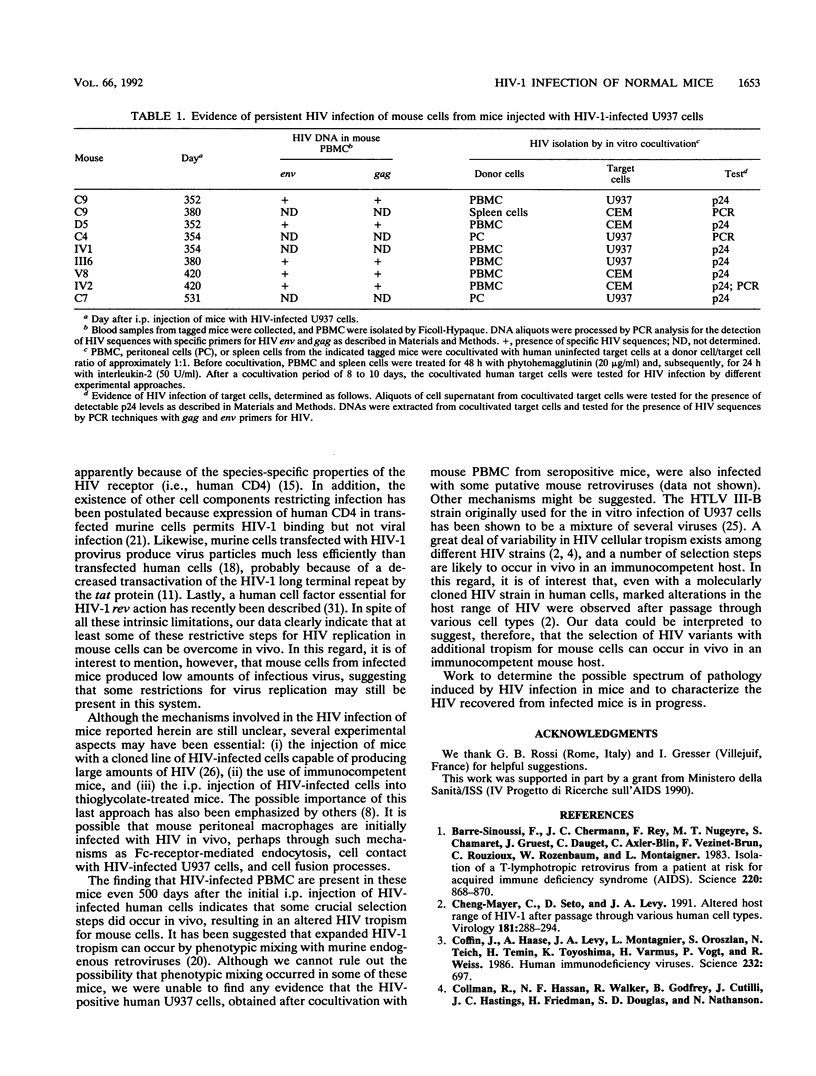
In this article, we report the establishment of persistent HIV type 1 infection of normal Swiss mice after a single intraperitoneal injection with high-producing HIV-infected U937 cells. Anti-HIV antibodies were found more than 500 days after the original injection, and p24 antigenemia was detected in approximately 50% of the mice. By polymerase chain reaction (PCR) techniques, HIV-specific gag and env sequences were detected in DNA samples from peripheral blood mononuclear cells (PBMC) and peritoneal cells of seropositive mice 300 to 500 days after inoculation with HIV-infected cells. These DNA samples did not contain human DNA sequences, as determined by PCR analysis using primers and the probe for the HLA-DQ alpha gene. Low levels of p24 and detectable human reverse transcriptase activity were found in cultures of PBMC and peritoneal macrophages. Cocultivation of PBMC, peritoneal cells, and spleen cells with human uninfected U937 or CEM (a T lymphoma cell line) cells resulted in HIV infection of the target cells, as determined by PCR analysis and/or p24 assays. The intravenous injection of untreated Swiss mice with the PBMC from PCR-positive mice resulted in the development of an increasing antibody response to HIV in the recipient animals. Together these results indicate that cells from seropositive Swiss mice were persistently infected with HIV and were capable of producing infectious virus. The development of persistent HIV infection in an immunocompetent mouse may represent the starting point for further studies aimed at defining the host mechanisms involved in the restriction of virus replication, defining the pathogenesis of HIV infection, and testing antiviral compounds and vaccines.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C., Seto D., Levy J. A. Altered host range of HIV-1 after passage through various human cell types. Virology. 1991 Mar;181(1):288–294. doi: 10.1016/0042-6822(91)90494-v. [DOI] [PubMed] [Google Scholar]
- Coffin J., Haase A., Levy J. A., Montagnier L., Oroszlan S., Teich N., Temin H., Toyoshima K., Varmus H., Vogt P. Human immunodeficiency viruses. Science. 1986 May 9;232(4751):697–697. doi: 10.1126/science.3008335. [DOI] [PubMed] [Google Scholar]
- Ferrantini M., Belardelli F., Locardi C. Role of endogenous alpha/beta interferon in the selection of virus nonproducer Friend leukemia cells after serial intraperitoneal passages in syngeneic mice. J Virol. 1988 Feb;62(2):600–605. doi: 10.1128/jvi.62.2.600-605.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filice G., Cereda P. M., Orsolini P., Soldini L., Romero E., Rondanelli E. G. Detection of IgG anti-HIV antibodies in mice experimentally infected with HIV-1. Microbiologica. 1989 Jan;12(1):75–80. [PubMed] [Google Scholar]
- Filice G., Cereda P. M., Varnier O. E. Infection of rabbits with human immunodeficiency virus. Nature. 1988 Sep 22;335(6188):366–369. doi: 10.1038/335366a0. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Powell D., Lightfoote M., Koenig S., Fauci A. S., Benn S., Rabson A., Daugherty D., Gendelman H. E., Hoggan M. D. Biological and biochemical characterization of a cloned Leu-3- cell surviving infection with the acquired immune deficiency syndrome retrovirus. J Exp Med. 1986 Jul 1;164(1):280–290. doi: 10.1084/jem.164.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Luciw P. A. Animal models of AIDS. FASEB J. 1989 Dec;3(14):2593–2606. doi: 10.1096/fasebj.3.14.2556312. [DOI] [PubMed] [Google Scholar]
- Hart C. E., Ou C. Y., Galphin J. C., Moore J., Bacheler L. T., Wasmuth J. J., Petteway S. R., Jr, Schochetman G. Human chromosome 12 is required for elevated HIV-1 expression in human-hamster hybrid cells. Science. 1989 Oct 27;246(4929):488–491. doi: 10.1126/science.2683071. [DOI] [PubMed] [Google Scholar]
- Jacobsen H., Mestan J., Mittnacht S., Dieffenbach C. W. Beta interferon subtype 1 induction by tumor necrosis factor. Mol Cell Biol. 1989 Jul;9(7):3037–3042. doi: 10.1128/mcb.9.7.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulaga H., Folks T., Rutledge R., Truckenmiller M. E., Gugel E., Kindt T. J. Infection of rabbits with human immunodeficiency virus 1. A small animal model for acquired immunodeficiency syndrome. J Exp Med. 1989 Jan 1;169(1):321–326. doi: 10.1084/jem.169.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau N. R., Warton M., Littman D. R. The envelope glycoprotein of the human immunodeficiency virus binds to the immunoglobulin-like domain of CD4. Nature. 1988 Jul 14;334(6178):159–162. doi: 10.1038/334159a0. [DOI] [PubMed] [Google Scholar]
- Leonard J. M., Abramczuk J. W., Pezen D. S., Rutledge R., Belcher J. H., Hakim F., Shearer G., Lamperth L., Travis W., Fredrickson T. Development of disease and virus recovery in transgenic mice containing HIV proviral DNA. Science. 1988 Dec 23;242(4886):1665–1670. doi: 10.1126/science.3201255. [DOI] [PubMed] [Google Scholar]
- Leonard J., Khillan J. S., Gendelman H. E., Adachi A., Lorenzo S., Westphal H., Martin M. A., Meltzer M. S. The human immunodeficiency virus long terminal repeat is preferentially expressed in Langerhans cells in transgenic mice. AIDS Res Hum Retroviruses. 1989 Aug;5(4):421–430. doi: 10.1089/aid.1989.5.421. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Cheng-Mayer C., Dina D., Luciw P. A. AIDS retrovirus (ARV-2) clone replicates in transfected human and animal fibroblasts. Science. 1986 May 23;232(4753):998–1001. doi: 10.1126/science.3010461. [DOI] [PubMed] [Google Scholar]
- Locardi C., Petrini C., Boccoli G., Testa U., Dieffenbach C., Buttò S., Belardelli F. Increased human immunodeficiency virus (HIV) expression in chronically infected U937 cells upon in vitro differentiation by hydroxyvitamin D3: roles of interferon and tumor necrosis factor in regulation of HIV production. J Virol. 1990 Dec;64(12):5874–5882. doi: 10.1128/jvi.64.12.5874-5882.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusso P., di Marzo Veronese F., Ensoli B., Franchini G., Jemma C., DeRocco S. E., Kalyanaraman V. S., Gallo R. C. Expanded HIV-1 cellular tropism by phenotypic mixing with murine endogenous retroviruses. Science. 1990 Feb 16;247(4944):848–852. doi: 10.1126/science.2305256. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- McCune J. M., Namikawa R., Kaneshima H., Shultz L. D., Lieberman M., Weissman I. L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988 Sep 23;241(4873):1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., Baird S. M., Wilson D. B., Spector D. H., Spector S. A. Human immunodeficiency virus infection of human-PBL-SCID mice. Science. 1991 Feb 15;251(4995):791–794. doi: 10.1126/science.1990441. [DOI] [PubMed] [Google Scholar]
- Namikawa R., Kaneshima H., Lieberman M., Weissman I. L., McCune J. M. Infection of the SCID-hu mouse by HIV-1. Science. 1988 Dec 23;242(4886):1684–1686. doi: 10.1126/science.3201256. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Puddu P., Locardi C., Sestili P., Varano F., Petrini C., Modesti A., Masuelli L., Gresser I., Belardelli F. Human immunodeficiency virus (HIV)-infected tumor xenografts as an in vivo model for antiviral therapy: role of alpha/beta interferon in restriction of tumor growth in nude mice injected with HIV-infected U937 tumor cells. J Virol. 1991 May;65(5):2245–2253. doi: 10.1128/jvi.65.5.2245-2253.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P., Balfe P., Peutherer J. F., Ludlam C. A., Bishop J. O., Brown A. J. Human immunodeficiency virus-infected individuals contain provirus in small numbers of peripheral mononuclear cells and at low copy numbers. J Virol. 1990 Feb;64(2):864–872. doi: 10.1128/jvi.64.2.864-872.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Trono D., Baltimore D. A human cell factor is essential for HIV-1 Rev action. EMBO J. 1990 Dec;9(12):4155–4160. doi: 10.1002/j.1460-2075.1990.tb07638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J., Hinrichs S. H., Reynolds R. K., Luciw P. A., Jay G. The HIV tat gene induces dermal lesions resembling Kaposi's sarcoma in transgenic mice. Nature. 1988 Oct 13;335(6191):606–611. doi: 10.1038/335606a0. [DOI] [PubMed] [Google Scholar]