Limited available water is the single most important factor that reduces global crop yields, with far reaching socioeconomic implications. In North America alone, it is estimated that 40% of yearly maize (Zea mays) crop losses are due to suboptimal water availability (Boyer, 1982). Agriculture currently accounts for 70% of the fresh water used by humans. This rate of water use can exceed local regeneration rates, often relying on underground aquifers that are rapidly being depleted (Morison et al., 2008). The impending scarcity of water available for agriculture will surely increase overall costs of crop production and drive the need for crops that use water more efficiently. While tremendous progress has been made through breeding and through cultural practices that improve maize yields in water-limited environments, the potential for additional large improvements still exists, and positive impacts on yield and increased yield stability across a broad range of water availability is of great value to farmers, consumers, and the environment.
Maize plants are sensitive to water-deficit stress throughout the growing season. Stresses that occur during the flowering stage, either just before floral initiation or immediately after pollination, result in the most significant reductions in end-of-season grain yields (Claassen and Shaw, 1970; Boyer and Westgate, 2004). Water-deficit stress during the vegetative growth phases typically leads to reductions in overall productivity, resulting in grain loss through reductions in kernel numbers. Late-stage drought stress, during the grain-filling period, can frequently lead to reductions in yield by reducing kernel size as well as increasing rates of kernel abortion, depending upon the severity of the stress.
Improved plant performance under severe water-limited growth chamber and greenhouse conditions has been achieved through multiple transgenic approaches, including the use of osmotic protectants such as the sugar alcohols trehalose (Romero et al., 1997), mannitol (Tarczynski et al., 1993), galactinol (Taji et al., 2002), and ononitol (Sheveleva et al., 1997). Accumulation of zwitterionic compounds such as Pro (Kishor et al., 1995) and Gly-betaine (Rathinasabapathi et al., 1994), or of protein protectants such as HVA1 (Xu et al., 1996), have all demonstrated an ability to confer tolerance to a variety of abiotic stresses. To date, none of the above approaches has been shown to provide durable tolerance in an agriculture production setting. Expression of a maize CAAT box transcription factor, ZmNF-YB2, has been shown to confer drought tolerance and enhanced photosynthetic capacity under drought stress with improvements in grain yield observed across several growing seasons in maize (Nelson et al., 2007). These studies clearly demonstrate that plants are amenable to improved stress tolerance through multiple mechanisms of action.
Biotechnology approaches facilitate our ability to survey and capitalize on the extensive genetic diversity that exists in nature and to improve on conserved pathways important for adaptation to environmental stress. Adaptation requires rapid recovery in growth and maintenance of cellular function following stress. We have looked to model systems such as bacteria and plants for insights into adaptive stress response pathways and commonalities among stress response mechanisms broadly in search of candidates for crop improvement. In this article, we demonstrate that expression of related cold shock proteins (CSPs) from bacteria, CspA from Escherichia coli and CspB from Bacillus subtilis, promotes stress adaptation in multiple plant species. Interestingly, expression of CSP proteins in maize is not associated with negative pleiotropic effects, indicating that stress tolerance does not come at a cost to crop productivity under well-watered conditions.
RNA chaperones are ubiquitous and abundant proteins found in all living organisms and viruses. RNA tends to be kinetically trapped in misfolded forms, and RNA binding proteins, acting as chaperones, can resolve these structures, ensuring accessibility for its biological function. In bacteria, RNA chaperones are believed to play a general role in sustaining active growth by favoring active transcription, translation, and/or ribosome assembly. In E. coli, cell growth is arrested by cold shock, and the arrest is associated with a significant reduction in protein synthesis (Etchegaray et al., 1996; Etchegaray and Inouye, 1999). A small set of proteins rapidly accumulates during the cold shock (for review, see Gualerzi et al., 2003; Horn et al., 2007). These proteins have been designated as CSPs and can account for as much as 10% of the newly synthesized protein of cold-shocked cells (Goldstein et al., 1990). One such example of a CSP protein is the E. coli protein CspA, which contains a prototypical cold shock domain (CSD) that is composed of 65 to 70 amino acid residues and has been reported in bacteria, archaea, and eukaryotes, including plants (for review, see Karlson and Imai, 2003; Weber and Marahiel, 2003; Horn et al., 2007). In bacteria, the CSP proteins are 7 to 10 kD in size and contain the nucleic acid binding activity sufficient for their function as RNA chaperones. The CSD contains a polynucleotide binding function, and in E. coli CspA is reported to act as an RNA chaperone where it binds to and, when necessary, converts double-stranded RNA into single-stranded RNA with low sequence selectivity (Jiang et al., 1997). The chaperone function of CspA, as well as other CSP proteins, is thought to be important for stimulating growth following stress acclimation and during periods of high metabolic activity. A recently proposed model describes CSPs working in conjunction with a DEAD box helicase to rescue misfolded mRNA molecules and maintain proper initiation of translation (Hunger et al., 2006). This model is consistent with previously reported evidence for the colocalization of CSPs with ribosomes in transcriptionally active cells, where CSP proteins are proposed to be implicated in coupling transcription with translation (Mascarenhas et al., 2001; Weber et al., 2001; for review, see El-Sharoud and Graumann, 2007).
Several reports described below support the hypothesis that the endogenous function of CSPs in plants relies on RNA binding/chaperone activity through the CSD and that these proteins, similarly to bacteria, regulate stress responses through a posttranscriptional mechanism. Mussgnug et al. (2005) describe the function of NAB1, a CSD-containing RNA chaperone in Chlamydomonas that links its RNA binding/chaperone activity with a target protein important for high light acclimation. NAB1 plays a role in high light acclimation by regulating the size of the light-harvesting antennae of PSII through posttranscriptional control of light-harvesting chlorophyll binding protein. Binding of NAB1 protein stabilizes light-harvesting chlorophyll binding protein mRNA at the preinitiation level via sequestration or masking and thereby suppresses translation. NAB1 activity accounts for approximately 50% of the translational repression of the protein, suggesting a mechanism that fine tunes expression at a posttranscriptional level and allows for very rapid response to occur under changing environmental conditions. The CSD domain of NAB1 was determined to be necessary and sufficient for specific high-affinity binding to this target. This RNA masking function was described previously in Xenopus oocytes for another CSD-containing protein, FRGY2 (Matsumoto et al., 1996; Manival et al., 2001).
In higher plants, Karlson et al. (2002) reported that wheat (Triticum aestivum) expresses a homolog of E. coli CSPA. The researchers found that this homolog, WCSP1, contains two RNA binding domains and increases in concentration during cold treatment. Kim et al. (2007) investigated a Gly-rich RNA binding protein from Arabidopsis (Arabidopsis thaliana), GRP2, and demonstrated that this protein plays a role in salt and cold stress adaptation. The researchers also found that GRP2 can rescue cold-sensitive E. coli CSP quadruple knockout and exhibits other properties consistent with an RNA binding/chaperone function. AtCSP2 (synonymous with GRP2) is also associated with floral transition and seed development (Fusaro et al., 2007; Sasaki et al., 2007). The possibility that CSPs functions in stress adaptation at least partially mediated by its role in the development and protection of reproductive structures should be explored. In maize, floral transition and reproductive development stages are the most sensitive to the water deficit stress conditions in terms of yield impact.
In this article, we demonstrate that bacterial CSPs can confer improved stress adaptation to multiple plant species. The action of CSPs in plants through a conserved stress adaptation mechanism common to plants and bacteria is supported by data showing that a functional RNA binding motif is required for the improved stress tolerance in both E. coli and maize. Stress tolerance at both vegetative and reproductive stages is reported with enhanced yield stability observed in maize under water-limiting conditions that were either imposed at various stages during plant development through controlled irrigation or occurred naturally in the western dryland region of the U.S. corn belt. Breadth of tolerance across environments and germplasms are key elements in establishing the value of transgenic strategies for crop stress tolerance improvement and require years of rigorous field testing to characterize the potential benefits.
ARABIDOPSIS COLD TOLERANCE
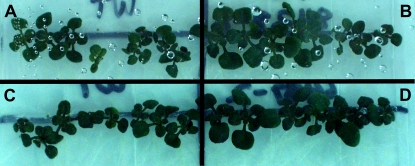
The expression of bacterial CSPs was shown to improve cold tolerance in transgenic Arabidopsis seedlings germinated and grown at low temperatures on standard agar media in petri dishes (Fig. 1). Transgenic Arabidopsis seedlings expressing CspA and CspB were tested for improved growth under low temperature conditions, using nontransgenic seedlings as controls. Following a 6-week treatment of 8°C under constant light, seedlings were visually scored for relative growth. At the end of the treatment period, a visual improvement in growth was noted for both transgenes, relative to their negative controls. This proof-of-concept for CSPs, demonstrating improved cold tolerance in a dicot plant species, was followed by similar abiotic stress experiments in transgenic rice (Oryza sativa).
Figure 1.
Transgenic Arabidopsis seedlings demonstrate improved growth under constant light conditions at 8°C for 6 weeks. Transgenic Arabidopsis seedlings expressing CspA or CspB are displayed on the right and exhibit more growth under these conditions relative to nontransgenic controls. A, CspA-negative control. B, CspA-positive transgenic. C, CspB-negative control. D, CspB-positive transgenic. The positive growth effect was only observed under the chilling stress conditions and not at 25°C (data not shown). Experimental details are described in Supplemental Materials and Methods S1.
RICE COLD, HEAT, AND WATER-DEFICIT TOLERANCE
Transgenic rice plants expressing CspA and CspB manifest improved stress tolerance for a number of abiotic stresses, including cold, heat, and water deficits. Improved tolerance was documented by demonstrating improved plant growth rates of transgenic plants relative to their nontransgenic controls, as measured by plant height (Table I).
Table I.
Transgenic rice plants expressing CspA or CspB demonstrated improved growth characteristics under cold, heat, and water-deficit treatments
CspA transgenics were subjected to a cold stress of 3 d at 10°C and a heat stress of 50°C for 3 h, followed by a 14-d recovery period. CspB transgenics were subjected to a cold stress of 1 h at 8°C and a heat stress of 53°C for 1 h, followed by a 14-d recovery period. CspB transgenics were also subjected to water-deficit treatments by growth under 25% soil saturation for 15 d. Final plant height (defined as the distance between the soil and the upper-most point of the leaf blade) for individual seedlings was determined at the end of the recovery period. Results shown for each event are the mean values for 10 transgene-positive or control plants per treatment. Events have been designated as Os1, Os2, etc., to indicate the crop species O. sativa (Os). Experimental details are described in Supplemental Materials and Methods S1. Nt, Not tested.
| Gene-Event | Plant Height
|
||
|---|---|---|---|
| Cold Treatment | Heat Treatment | Drought Treatment | |
| cm | |||
| CspA-Os1 | 28.8 ± 3.1a | 26.7 ± 5.0 | Nt |
| CspA-Os2 | 29.5 ± 2.9a | 26.2 ± 3.5a | Nt |
| CspA-Os3 | 15.8 ± 2.9 | 25.2 ± 1.9a | Nt |
| CspA-Os4 | 26.1 ± 3.8 | 20.8 ± 1.2 | Nt |
| CspA-Os5 | 27.2 ± 2.3a | 23.2 ± 1.8 | Nt |
| CspA-Os6 | 29.6 ± 3.5a | 29.3 ± 5.0a | Nt |
| CspA-Os7 | 24.6 ± 3.4 | 24.7 ± 2.8 | Nt |
| Nontransgenic | 20.6 ± 1.7 | 18.5 ± 3.5 | Nt |
| No treatment | 37.9 ± 8.6 | 37.9 ± 8.6 | Nt |
| CspB-Os1 | 28.8 ± 2.9 | 34.5 ± 2.1a | 17.4 ± 2.2 |
| CspB-Os2 | 30.2 ± 3.2 | 32.4 ± 1.5a | Nt |
| CspB-Os3 | 30.4 ± 2.2a | 28.8 ± 4.2 | Nt |
| CspB-Os4 | 32.1 ± 3.4a | 33.3 ± 3.9a | Nt |
| CspB-Os5 | 29.5 ± 3.6 | 34.0 ± 2.1a | 18.1 ± 1.6a |
| CspB-Os6 | 27.1 ± 3.4 | 33.8 ± 3.7a | Nt |
| CspB-Os7 | 23.8 ± 2.9 | 25.7 ± 4.3 | 18.5 ± 2.2a |
| CspB-Os8 | 33.8 ± 3.5a | 34.8 ± 1.7a | 19.5 ± 2.0a |
| Nontransgenic | 23.9 ± 3.7 | 25.5 ± 3.0 | 12.8 ± 3.2 |
| No treatment | 36.7 ± 4.0 | 36.7 ± 4.8 | 24.6 ± 1.6 |
Indicates significant (P < 0.05) improvement relative to nontransgenic control.
On average, the cold and heat treatments led to 35% and 31% reductions, respectively, in the final plant height of nontransgenic control plants. In the cold treatment, three of eight CspB-positive events demonstrated significantly greater plant heights (P < 0.05) at the end of the recovery period when compared to nontransgenic control plants. In the heat treatment, six of eight CspB-positive events demonstrated significantly greater plant heights (P < 0.05) at the end of the recovery period when compared to nontransgenic control plants.
A water-limited treatment led to a 50% reduction in the final plant height of nontransgenic control plants relative to well-watered controls. CspB conferred improved tolerance to water deficits as demonstrated by greater final plant heights in transgene-positive plants. At the end of the water-limited treatment, three of four CspB events tested were significantly taller (P < 0.05) than their nontransgenic controls. One event demonstrated significant improvements under all three stress treatments, and several other events showed positive trends across multiple treatments. Overall, this demonstrated that expression of CspB in rice results in tolerance to multiple abiotic stresses, and this tolerance occurs with fairly high frequency in the individual stress treatments.
For CspA-positive rice plants, cold and heat treatments also resulted in improved growth, as measured by greater final plant height. The treatment impact on plant height at the end of the recovery period resulted in a 46% and 51% reduction in plant height of nontransgenic control plants for the cold and heat treatments, respectively. Four of seven CspA events demonstrated significantly greater final plant height than their nontransgenic controls in the cold treatment, while three of seven CspA events demonstrated significantly greater final plant height compared to their nontransgenic control plants under the heat stress treatment. CspA rice plants were not evaluated in a water-deficit treatment.
Thus, the cold tolerance we demonstrated in transgenic Arabidopsis, a dicot, was also observed in rice, a monocot. Furthermore, the improved stress tolerance was extended beyond the cold tolerance that we observed in Arabidopsis and shown to also include heat and water-deficit tolerance. The rice water-deficit results, in particular, present a compelling outcome that drove further abiotic stress testing of transgenic CspA and CspB in maize.
MAIZE VEGETATIVE WATER-DEFICIT TOLERANCE
An important factor in the success of a field testing program is the ability to accurately collect phenotypic data under predictable and consistent water deficit conditions in a managed stress environment. This has been achieved through a field testing network located in rain-free environments, allowing precise control over irrigation levels. Herein, we have reported improved vegetative and reproductive performance in field-grown maize exposed to water deficits that were introduced during periods of vegetative growth and ovule and kernel development.
We have demonstrated that transgenic expression of CspB in maize plants contributes to improved vegetative performance. Twenty-two CspB events were evaluated in water-limited field trials using commercial grade hybrid corn in environments that received no rainfall during the target period for the water-deficit treatment, a span of 10 to 14 d immediately prior to flowering. The water-deficit treatment resulted in an average reduction in growth rates to 50% of the well-watered rate. Using an across-event analysis, the CspB transgenics demonstrated a 3.6% increase in leaf extension rates relative to nontransgenic controls (Table II). The best performing events demonstrated growth rate increases of 12% and 24%. This growth rate improvement under water-limited conditions indicated that the CspB transgene was having a substantial positive impact on plant productivity during the vegetative phase of plant growth and development. The CspB-expressing plants also demonstrated significant improvements in chlorophyll content and photosynthetic rates (Table II). Across all events, chlorophyll content was increased by 2.5%, with the top two events exhibiting increases of 4.4% and 3.3%. The improvements to the photosynthetic rates were 3.6% across all events, with increases of 8.5% and 7.7% for the top two performing events. Similar observations have been made with transgenic maize plants expressing CspA under greenhouse conditions (data not shown). These measures of vegetative performance are key indicators of plant productivity and would be expected to enhance the overall yield potential of the crop. When plants were grown under nonstressed, fully irrigated, or rain-fed conditions in both the greenhouse and field, we did not detect any appreciable difference between CspA- or CspB-expressing lines and the isogenic control for plant growth rate or plant height measured at different stages of development (data not shown).
Table II.
Stable transgenic maize seedlings demonstrate improved growth, chlorophyll content, and photosynthetic rates under water-deficit stress test
Water-deficit conditions were created in the field by reducing irrigation for a 14-d period during the late vegetative stage of development, immediately prior to flowering. The treatment reduced the relative growth rates during the treatment and the average end of season yields by approximately 50% of well-watered levels. Relative differences in measures were determined by comparison with appropriate nontransgenic controls. Experimental details are described in Supplemental Materials and Methods S1.
| Gene Event | LER | Chlorophyll | Photosynthesis |
|---|---|---|---|
| % increase | |||
| CspB-Event | 3.6%a | 2.5%b | 3.6%c |
| Grouping | (n = 756) | (n = 432) | (n = 432) |
| CspB-Zm | 12%a | 4.4%a | 8.5%b |
| Event 1 | (n = 36) | (n = 72) | (n = 72) |
| CspB-Zm | 24%a | 3.3%b | 7.7%c |
| Event 2 | (n = 36) | (n = 72) | (n = 72) |
Indicates significant (P < 0.05) improvement relative to nontransgenic control.
Indicates significant (P < 0.10) improvement relative to nontransgenic control.
Indicates significant (P < 0.20) improvement relative to nontransgenic control.
Reproductive performance was evaluated for CspB plants by harvesting all kernel-bearing ears from six replicates (34 plants per replicate) for each of six events selected for harvest based on the magnitude of their improved vegetative performance. CspB-positive plants were compared to nontransgenic control plants grown in an adjacent row. An across-event analysis demonstrated significant improvements (P < 0.05) in the number of plants with kernel-bearing ears (+4.0%) and the number of kernels per plant (+11.7%; data not shown). There were no significant differences observed for individual kernel weight. The nature of the improvements, primarily more kernels on ears and more plants with kernel-bearing ears, was consistent with expectations based on the timing of the limited-water treatment, which occurred during late vegetative stages and early immature ear development and was relieved with sufficient water available to the plants during pollination and grain fill periods.
MAIZE REPRODUCTIVE TOLERANCE AND YIELD UNDER WATER-DEFICIT CONDITIONS
Grain yield trials were performed under water-deficit stress and nonstress conditions on 10 CspA and 10 CspB-positive events, most of which had previously demonstrated improved vegetative performance in either greenhouse screens or field trials. Grain yield data was collected from four field sites where water was limited during the late vegetative phase of development, a treatment similar to the initial water-deficit field trial. Mean yield at the water-limited sites was 6.8 tons (t)/ha, representing an approximately 50% reduction in yield relative to the average mean yield of crops in the Midwest. An across-event analysis demonstrates that the CspA transgenic entries provide a yield increase of 4.6% (P < 0.2) under water stress, with the two best performing events demonstrating advantages of 30.8% and 18.3% (Table III). Yield averages of CspB-positive plants as a group were significantly greater than controls, by 7.5% (P < 0.01). A number of individual events exhibited significant yield advantages as well; the best two performing events, CspB-Zm event 1 and event 2, demonstrated yield improvements of 20.4% and 10.9%, respectively. These are the same two events that demonstrated significant improvements in leaf growth, chlorophyll content, and photosynthetic rates, providing evidence that these improvements in vegetative productivity will translate into improvements in reproductive performance and grain yield.
Table III.
CspA and CspB transgenic maize plants demonstrate improved end-of-season grain yield under water-limiting conditions
Mean yield values (tons/hectare) of nontransgenic and transgene-positive plots are shown for groupings of CspA and CspB events and for two individual transgenic events from each construct. Experimental details are described in Supplemental Materials and Methods S1.
| Event-Event Pool | Yield | Yield Improvement |
|---|---|---|
| t/ha | ||
| CspA nontransgenic mean | 6.38 | |
| CspA-event group mean | 6.68 | 4.6% (P < 0.2) |
| CspA-Zm event 1 | 8.35 | 30.8% (P < 0.1) |
| CspA-Zm event 2 | 7.52 | 18.3% (P < 0.1) |
| CspB nontransgenic mean | 6.86 | |
| CspB-event group mean | 7.38 | 7.5% (P < 0.1) |
| CspB-Zm event 1 | 8.26 | 20.4% (P < 0.1) |
| CspB-Zm event 2 | 7.61 | 10.9% (P < 0.1) |
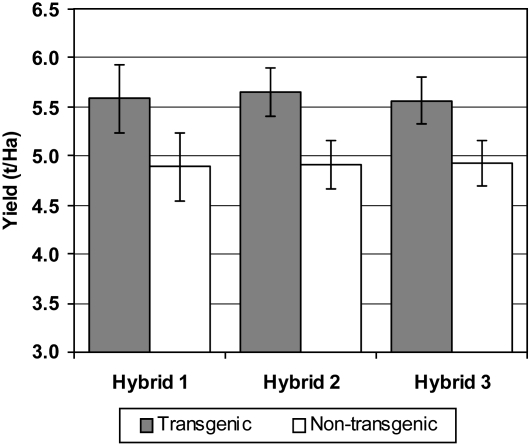
Several years of field trials have been conducted with a single CspB-expressing event, CspB-Zm event 1, to further investigate the ability of this gene to provide tolerance to water deficits during the late vegetative and reproductive developmental stages. Field trials were conducted under controlled water-deficit conditions where two distinct stress treatments were conducted by limiting water during these two stages of development. This single CspB event was deployed in three different hybrid backgrounds and evaluated under these stress regimes at five replicated locations. The two treatments resulted in decreases in the overall yield of the experiment by approximately 50% relative to well-watered treatments planted at the same locations. The CspB-positive entries exhibited improvements in end-of-season grain yield across the different hybrid entries and under both water stress regimes when compared to a conventional wild-type control of the same genetic background (Table IV). Yield benefits in these experiments ranged from 11% to as much at 21% across yield values that averaged 6.4 to 8.5 t/ha. The transgenic CspB event consistently out-yielded the nontransgenic controls by at least 0.5 t/ha across 12 out of 15 reproductive stress treatments and 13 out of 15 vegetative stress treatments, highlighting the potential agricultural benefits that this technology can deliver.
Table IV.
Yield results from managed irrigation water-deficit conditions
Yield results from replicated, multi-location evaluation of a single CspB transgenic event (CspB-Zm event 1) under vegetative or reproductive water-deficit stress. Three hybrids expressing the CspB event were evaluated using 20 replications of data across five locations for each stress treatment window. Experimental details are described in Supplemental Materials and Methods S1.
| Stress Class | Entries | Mean Yield Positive | Mean Yield Check | Difference | Difference |
|---|---|---|---|---|---|
| t/ha | % | ||||
| Vegetative | Hybrid 1 (positive) | 10.1 | 8.5 | 1.6 | 19 |
| Reproductive | Hybrid 1 (positive) | 9.0 | 7.7 | 1.3 | 16 |
| All stress | Hybrid 1 (positive) | 9.1 | 7.9 | 1.1 | 14 |
| Vegetative | Hybrid 2 (positive) | 7.7 | 6.5 | 1.2 | 18 |
| Reproductive | Hybrid 2 (positive) | 8.1 | 6.8 | 1.3 | 19 |
| All stress | Hybrid 2 (positive) | 7.7 | 6.4 | 1.3 | 21 |
| Vegetative | Hybrid 3 (positive) | 8.3 | 7.2 | 1.1 | 16 |
| Reproductive | Hybrid 3 (positive) | 8.9 | 8.0 | 0.9 | 11 |
| All stress | Hybrid 3 (positive) | 8.8 | 7.9 | 0.9 | 12 |
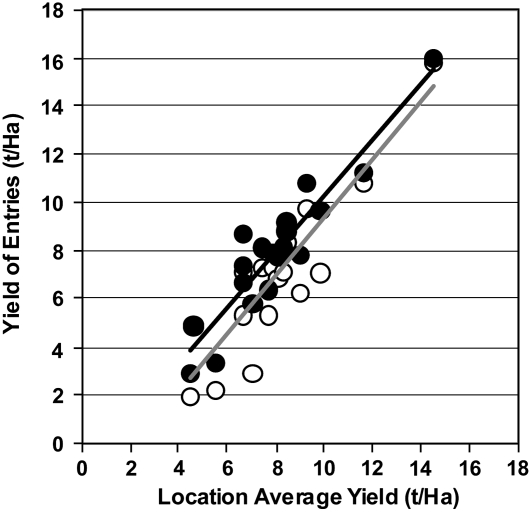
A multi-year analysis was also conducted with CspB-Zm event 1 to assess the stability of the yield advantages across locations under water-limiting conditions. Locations that had experienced some level of water stress, where yield reductions ranged from 20% to 80%, were compiled and analyzed across years. Figure 2 indicates the yield stability observed for the transgenic entry and its control across 3 years of water stress testing in a single hybrid background. Yield advantages are evident across a wide range of environments with varying degrees of water-deficit stress, with yield levels ranging from as low as 2 t/ha to as high as 16 t/ha, indicating that this technology could have broad utility across the U.S. growing regions.
Figure 2.
A single transgenic CspB event demonstrates yield improvements across multiple years of testing under water-stressed environments. Performance of CspB-Zm event 1 (black diamonds) and the appropriate control (white circles) were plotted against the location average yields across the past three seasons of testing under managed stress environments. x axis, Average yield (tons/hectare) at each location; y axis, mean yield (tons/hectare) of the entries, by location. Experimental details are described in Supplemental Materials and Methods S1.
We have assessed the average performance of this material over the past several years, combining yield performance across three hybrid test-crosses that have been under evaluation in each of the prior 4 years. Across 4 years of testing, this CspB event provides an average yield benefit of 10.5% across three hybrid test-crosses under managed stress environmental testing. The average yield advantage each year was 0.89, 0.48, 0.49, and 0.79 t/Ha, representing percentage increases of 13.4%, 6.7%, 10.5%, and 11.3%, respectively.
This same transgenic CspB event was tested under western dryland maize conditions without supplemental water. Hybrid entries were planted as 100-foot-long 4-row plots to better simulate normal agronomic conditions for this region, planting at appropriate population densities for those regions, and were paired with appropriate nontransgenic controls. Environmental data was collected and seasonal weather patterns, including rainfall accumulation, were utilized to classify the dryland locations as to whether each of the locations experienced water-deficit stress during the season. In the final analysis, 12 of the locations planted across the western dryland were categorized as having experienced water stress during the late vegetative through reproductive developmental stages and were utilized for analysis. As depicted in Figure 3, yield benefits were observed in the same three hybrid backgrounds that were evaluated under controlled water-deficit conditions described in Table IV. When compared to the nontransgenic control, the CspB transgenic event provides yield benefits of up to 0.75 t/ha, or 15%. These dryland growing conditions created a lower yielding environment (average yield of the controls were 4.9 t/ha) than the controlled water-deficit locations where the overall yields of the controls ranged from 6.4 to 8.5 t/ha. The results from the managed stress environment treatments were very similar to the observations made under dryland growing conditions, highlighting the utility of the managed stress environmental testing platform.
Figure 3.
Yield results from Midwest evaluations under water-deficit conditions. Three different hybrids carrying a single transgenic event expressing CspB were evaluated in yield trials across the western dryland market. Yield results were averaged across locations that experienced water-deficit stress during the late vegetative or grain fill periods of the season. Experimental details are described in Supplemental Materials and Methods S1.
Demonstration of significant yield improvements with transgenic CspB events under controlled drought environments as well as under water-stressed western dryland conditions represents a major advancement in the field of abiotic stress research. Continued evaluation of these materials across additional genetic backgrounds will be important ongoing work in the development of products for the U.S. corn belt.
A Functional RNA Binding Site Is Required to Confer Yield Benefits to Field-Grown Maize under Water Stress
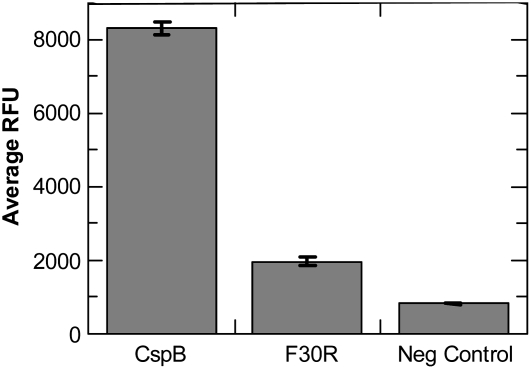
A well-documented functional aspect of CSPs, including CspB, is their ability to bind single-stranded DNA or single-stranded RNA that can be measured by the protein's ability to open a stem-loop or double-stranded dual-labeled fluorescence probe in vitro. By mutating a key residue in the RNA binding domain of B. subtilis CspB (CspB_F30R), we have reduced the ability of this protein to open a double-stranded stem loop structure engineered into the probe (Fig. 4). There was an approximately 10-fold reduction in fluorescence observed with the CspB_F30R mutant, suggesting that this residue is important for maintaining RNA chaperone activity. A similar result has been obtained through mutation of the identical residue in the E. coli CspE protein (Phadtare et al., 2002), and its activity in antitermination was similarly lost. The comparable mutation in the F31 residue of the E. coli CspA protein (Schröder et al., 1995) resulted in a similar loss of function. Moreover, the protein containing the F30R mutation no longer complements an E. coli mutant (BX04; Xia et al., 2001) that lacks four native CSPs and is highly sensitive to cold stress (data not shown). These results suggest that this protein has lost its ability to function as an RNA chaperone in E. coli.
Figure 4.
Single-stranded DNA probe opening ability for B. subtilis CspB and the CspB_F30R mutant. Probe opening assay for BsCspB, BsCspBF30R, and controls. Negative control contained all elements that Csp samples contained except protein. The average of three independent replications is represented (±sd). The y axis is labeled as the average relative fluorescence units (RFU). Experimental details are described in Supplemental Materials and Methods S1.
By expressing the CspB_F30R mutant protein constitutively in maize, we were able to assess the requirement of effective RNA chaperone activity to confer yield benefits under water stress. Of 11 CspB_F30R events tested, none was able to provide an increase in yield under water-deficit conditions when compared to nontransgenic controls, and results were effectively neutral when an across-event analysis was performed (data not shown). Field trial conditions were similar to those described in previous studies. Transgenic maize plants expressing CspA and CspB in the same field trials continued to confer yield benefits (data not shown). These data suggest that effective RNA chaperone activity of CspB is critical for providing tolerance to water-deficit stress and that the mode of action of CspB in maize occurs through its predicted function as an RNA chaperone.
DATA SUMMARY AND CONCLUSIONS
Future progress toward more sustainable agricultural practices will be accelerated through the systematic analysis of gene function important for yield under water-deficit conditions. Model systems research has provided a greater understanding of pathways of plant stress acclimation that is further providing valuable insights into crop responses under environmental stress conditions. Rapidly moving to large-scale analyses of candidate gene function in crops, under standard agronomic practices and where the influences of environmental factors and the ultimate impact on yield can be assessed, is critical for developing improved crops through biotechnology.
We have demonstrated that constitutive expression of two members of a family of bacterial RNA chaperones, E. coli CspA and B. subtilus CspB, was shown to confer abiotic stress tolerance in transgenic Arabidopsis, rice, and maize. Importantly, the improvements in water-limited field trials were not associated with a yield penalty in high-yielding environments. Consistent with the timing of the water deficit, the positive yield impact of the transgenes was predominately on kernel numbers, not on kernel weight (data not shown). This technology has been observed under different stress regimes and across environments as clearly providing performance benefits under late vegetative/flowering water deficit as well as during the grain fill period. During these two periods, water-deficit stress leading to three consecutive days of wilting can lead to 30% to 50% reductions in grain yields (Claassen and Shaw, 1970). The sensitivity of maize yield at these developmental stages and the frequency with which these conditions occur across the corn belt are consistent with the observed higher correlation of grain yield to kernel number and not kernel weight (Duvick, 2005). A multi-year analysis across the full range of stress conditions suggests that this technology provides broad yield stability under water-limiting conditions. In addition to the managed stress environment testing, a CspB transgenic event was tested under dryland conditions as well. Demonstration of consistent yield benefits under managed water environments and dryland conditions is an important advancement in the development of this technology. These yield improvements further outline the potential of this technology to provide a significant agricultural benefit. As these hybrids were configured with an elite transformation germplasm, a component of future evaluation of this technology will be testing of these transgenic events in germplasm that is adapted for the marketplace.
The potential utility of CSPs as transgenes is strongly supported by the demonstration that the two CSPs tested, which share only 61% overall identity, were both capable of improving stress tolerance in plants. As tested, we were not able to distinguish the performance of CspA from CspB, and we speculate that they are likely acting by similar mechanisms. The myriad of CSPs known in nature, and available for testing, and the possibility of improving on the current expression pattern and coding regions of these transgenes present intriguing possibilities for even further improvements.
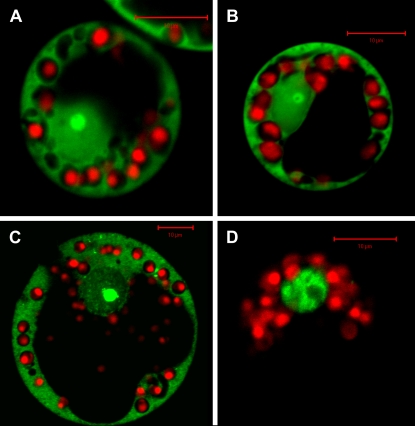
Previous reports demonstrated that CSD protein functionality in vivo is significantly impaired by mutations that affect RNA binding. Nakaminami et al. (2006), for example, reported that site-directed mutagenesis of WCSP1 ribonucleoprotein (RNP) domains abolishes the ability of the protein to complement the E. coli cold-sensitive mutant BX04. Our observation that maize plants expressing the CspB_F30R single amino acid mutation do not exhibit improved yield under water-deficit conditions further supports the prediction that the biochemical function of these proteins as defined in bacterial systems is also important in plants. Testing the ability of other known CSP mutants for their ability to confer tolerance to water-deficit in plants will further define the structure-function relationships and improve our understanding of the mode of action of these proteins. Consistent with these hypotheses, we have evaluated the subcellular location of GFP protein fusions with CspA, CspB, and a cotton (Gossypium hirsutum) CSP transiently expressed in maize protoplasts. As depicted in Figure 5, we observed GFP fluorescence in the cytosol, nucleus, and nucleolar region, consistent with observations of subcellular localization of plant and animal CSD proteins (Nakaminami et al., 2006; Sasaki et al., 2007). By comparison, expression of an Arabidopsis AP2-type transcription factor is restricted to the nucleus and nucleolar region, consistent with its function. Nucleoli are largely comprised of proteins and the ribosomal DNA sequences of chromosomes, surrounded by a layer of condensed chromatin. Because nucleoli are involved in the production and maturation of ribosomes, these suborganellar structures are extremely important during phases of rapid growth and development. Concentrations of CspA and CspB proteins in these regions as well as in the cytosolic regions of the cell are highly consistent with their proposed role in RNA binding and participation in RNPs.
Figure 5.
CspA (A), CspB (B), and cotton CSP-like 1 (C) GFP fusion proteins accumulate in the cytoplasm, nucleus, and nucleolus when expressed in maize protoplasts. D, Results obtained with an AtCBF3 transcription factor:GFP fusion protein that localizes exclusively to the nucleus. Experimental details are described in Supplemental Materials and Methods S1.
New insights into the functions of plant CSPs suggest that we are tapping into a conserved mechanism of cellular function. Plant CSPs and other plant RNA binding proteins have been shown to function as RNA chaperones in Arabidopsis and wheat (Nakaminami et al., 2006; Kim et al., 2007; Sasaki et al., 2007). CSD-containing proteins from other eukaryotic organisms, Chlamydomonas, Xenopus, and mammalian systems, have also been demonstrated to play a role in RNA metabolism, protein translation, and regulation of gene expression, coupling the transcription of mRNA to its translational fate (Matsumoto et al., 1996; Matsumoto and Wolffe, 1998; Manival et al., 2001; Mussgnug et al., 2005). Eukaryotic mRNPs likely play a central role in integrating various cellular signals by providing a physical context for the control of mRNA translation, stabilization, and sequestration. CSD-containing proteins are likely components of mRNPs and important for this level of regulation. Further work is required to establish the cause and effect relationship linking RNA structure stabilization and posttranscriptional control with stress acclimation mechanisms at the cellular level. However, these observations as well as several other reports in the literature, suggest that posttranscriptional control is an important level of regulation, allowing plants to adjust protein synthesis and fine-tune expression under periods of long-term stress.
Based on the yield and stress benefits we have observed from expression of CSD-containing proteins in plants and our current understanding of the common RNA chaperone function of CSD proteins in prokaryotes and eukaryotes, expression of CSD proteins from a variety of plant and bacterial sources represents a promising approach for stimulating growth and productivity in plants under conditions of abiotic stress. The fact that expression of CSP proteins did not result in detrimental effects on plant size, development, or productivity as previously observed for other transgenic approaches further supports the utility of these proteins. It is interesting to speculate why CSP functionality was not selected for over the years of conventional breeding. However, the low heritability of yield, the challenges associated with controlled environment testing, and the quantitative nature of the trait are likely contributing factors.
The stress tolerance conferred by CSPs represents a novel, compelling approach toward engineering improved plant productivity in suboptimal growth conditions. These studies have confirmed that this family of proteins is capable of delivering broad stress tolerance, which also translates to improvements in grain yield under both managed stress studies and marketplace environments. As water resources become increasingly scarce and the global demands for grain continue to increase, the ability to bring yield stability across water-limiting environments presents an important advancement in the area of stress tolerance research. The opportunity exists for the drought-tolerant trait to be added to a growing set of germplasm and trait options that mitigate environmental stresses on the corn plant and provide the crop with a better opportunity to reach its yield potential in any environment.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers M30139 and U58859.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Materials and Methods S1.
Acknowledgments
This work could not have been accomplished without the assistance from Don Nelson, Tom R. Adams, Brendan Hinchey, and Adrian Lund. Valuable advice and consultation was received from Philip Miller, Tom H. Adams, Stan Dotson, Nordine Cheikh, Mark Lawson, Tom Peters, and Mike Stephens. We would also like to acknowledge the many people who supported this work, including the plant transformation, greenhouse staff, and farm network teams.
Mary Fernandes and Mark Abad contributed to the Arabidopsis work.
Ganesh Kumar, Jayaprakash Targolli, and Santanu Dasgupta contributed to the rice work.
Paolo Castiglioni, Don Anstrom, Robert Bensen, Dave Warner, Martin Stoecker, Jay Harrison, Christopher Bonin, Robert D'Ordine, Sara Salvador, Santiago Navarro, Stephanie Back, Michael Luethy, and Jacqueline Heard contributed to the maize work.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Jacqueline E. Heard (jacqueline.e.heard@monsanto.com).
The online version of this article contains Web-only data.
References
- Boyer JS (1982) Plant productivity and environment. Science 218 443–448 [DOI] [PubMed] [Google Scholar]
- Boyer JS, Westgate ME (2004) Grain yield with limited water. J Exp Bot 55 2385–2394 [DOI] [PubMed] [Google Scholar]
- Claassen MM, Shaw RH (1970) Water-deficit effects on corn. II. Grain composition. Agron J 62 652–655 [Google Scholar]
- Duvick DN (2005) The contribution of breeding to yield advances in maize. In DN Sparks, ed, Advances in Agronomy, Vol 86. Academic Press, San Diego, pp 83–145
- El-Sharoud WM, Graumann PL (2007) Cold shock proteins aid coupling of transcription and translation in bacteria. Sci Prog 90 15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchegaray JP, Jones PG, Inouye M (1996) Differential thermoregulation of two highly homologous cold-shock genes cspA and cspB of Escherichia coli. Genes Cells 1 171–178 [DOI] [PubMed] [Google Scholar]
- Etchegaray JP, Inouye M (1999) CspA, CspB and CspG, major cold shock proteins of Escherichia coli, are induced at low temperature and under conditions that completely block protein synthesis. J Bacteriol 181 1827–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusaro AF, Bocca SN, Ramos RL, Barrôco RM, Magioli C, Jorge VC, Coutinho TC, Rangel-Lima CM, De Rycke R, Inzé D, et al (2007) AtGRP2, a cold-induced nucleo-cytoplasmic RNA-binding protein, has a role in flower and seed development. Planta 225 1339–1351 [DOI] [PubMed] [Google Scholar]
- Goldstein J, Pollitt NS, Inouye M (1990) Major cold shock protein of Escherichia coli. Proc Natl Acad Sci USA 87 283–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualerzi CO, Giuliodori AM, Pon CL (2003) Transcriptional and post-transcriptional control of cold-shock genes. J Mol Biol 331 527–539 [DOI] [PubMed] [Google Scholar]
- Horn G, Hofweber R, Kremer W, Kalbitzer HR (2007) Structure and function of bacterial cold shock proteins. Cell Mol Life Sci 64 1457–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunger K, Beckering CL, Wiegeshoff F, Graumann PL, Marahiel MA (2006) Cold-induced putative DEAD box RNA helicases CshA and CshB are essential for cold adaptation and interact with Cold Shock Protein B in Bacillus subtilis. J Bacteriol 188 240–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Hou Y, Inouye M (1997) CspA, the major cold-shock protein of Escherichia coli, is an RNA chaperone. J Biol Chem 272 196–202 [DOI] [PubMed] [Google Scholar]
- Karlson D, Imai R (2003) Conservation of the cold shock domain protein family in plants. Plant Physiol 131 12–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlson D, Nakaminami K, Toyomasu T, Imai R (2002) A cold-regulated nucleic acid-binding protein of winter wheat shares a domain with bacterial cold shock proteins. J Biol Chem 277 35248–35256 [DOI] [PubMed] [Google Scholar]
- Kim JS, Park SJ, Kwak KJ, Kim YO, Song J, Jang B, Jung CH, Kang H (2007) Cold shock domain proteins and glycine-rich RNA-binding proteins from Arabidopsis thaliana can promote cold adaptation process in Escherichia coli. Nucleic Acids Res 35 506–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishor P, Hong Z, Miao GH, Hu C, Verma D (1995) Overexpression of Δ-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol 108 1387–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manival X, Ghisolfi-Nieto L, Joseph G, Bouvet P, Erard M (2001) RNA-binding strategies common to cold-shock domain- and RNA recognition motif-containing proteins. Nucleic Acids Res 29 2223–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas J, Weber MH, Graumann PL (2001) Specific polar localization of ribosomes in Bacillus subtilis depends on active transcription. EMBO Rep 2 685–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Meric F, Wolffe AP (1996) Translational repression dependent on the interaction of the Xenopus Y-box protein FRGY2 with mRNA: role of the cold shock domain, tail domain, and selective RNA sequence recognition. J Biol Chem 271 22706–22712 [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Wolffe AP (1998) Gene regulation by Y-box proteins: coupling control of transcription and translation. Trends Cell Biol 8 318–323 [DOI] [PubMed] [Google Scholar]
- Morison JIL, Baker NR, Mullineaux PM, Davies WJ (2008) Improving water use in crop production. Philos Trans R Soc Lond B Biol Sci 363 639–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussgnug JH, Wobbe L, Elles I, Claus C, Hamilton M, Fink A, Kahmann U, Kapazoglou A, Mullineaux CW, Hippler M, et al (2005) NAB1 is an RNA binding protein involved in the light-regulated differential expression of the light-harvesting antenna of Chlamydomonas reinhardtii. Plant Cell 17 3409–3421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaminami K, Karlson DT, Imai R (2006) Functional conservation of cold shock domains in bacteria and higher plants. Proc Natl Acad Sci USA 103 10122–10127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DE, Repetti PP, Adams TR, Creelman RA, Wu J, Warner DC, Anstrom DC, Bensen RJ, Castiglioni PP, Donnarummo MG, et al (2007) Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proc Natl Acad Sci USA 104 16450–16455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadtare S, Tyagi S, Inouye M, Severinov K (2002) Three amino acids in Eschericia coli CspE surface-exposed aromatic patch are critical for nucleic acid melting activity leading to transcription antiterminations and cold acclimation of cells. J Biol Chem 277 46706–46711 [DOI] [PubMed] [Google Scholar]
- Rathinasabapathi B, McCue KF, Gage DA, Hanson AD (1994) Metabolic engineering of glycine betaine synthesis: plant betaine aldehyde dehydrogenases lacking typical transitpeptides are targeted to tobacco chloroplasts where they confer betaine aldehyde resistance. Planta 193 155–162 [DOI] [PubMed] [Google Scholar]
- Romero C, Belles JM, Vaya JL, Serrano R, Culianez-Maria FA (1997) Expression of the yeast trehalose-6-phosphate synthetase gene in transgenic tobacco plants: pleiotropic phenotypes include drought tolerance. Planta 201 293–297 [DOI] [PubMed] [Google Scholar]
- Sasaki K, Kim MH, Imai R (2007) Arabidopsis COLD SHOCK DOMAIN PROTEIN2 is a RNA chaperone that is regulated by cold and developmental signals. Biochem Biophys Res Commun 364 633–638 [DOI] [PubMed] [Google Scholar]
- Schröder K, Graumann P, Schnuchel A, Holak TA, Marahiel MA (1995) Mutational analysis of the putative nucleic acid-binding surface of the cold-shock domain, CspB, revealed an essential role of aromatic and basic residues in binding of single-stranded DNA containing the Y-box motif. Mol Microbiol 16 699–708 [DOI] [PubMed] [Google Scholar]
- Sheveleva E, Chmara W, Bohnert HJ, Jensen RG (1997) Increased salt and drought tolerance by D-ononitol production in transgenic Nicotiana tabacum L. Plant Physiol 115 1211–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taji T, Ohsumi C, Iuchi S, Seki M, Kasuga M, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K (2002) Important roles of drought- and cold-inducible genes for galactinal synthase in stress tolerance in Arabidopsis thaliana. Plant J 29 417–426 [DOI] [PubMed] [Google Scholar]
- Tarczynski MC, Jensen RG, Bohnert HJ (1993) Stress protection of transgenic tobacco by production of the osmolyte mannitol. Science 259 508–510 [DOI] [PubMed] [Google Scholar]
- Weber MH, Volkov AV, Fricke I, Marahiel MA, Graumann PL (2001) Localization of cold shock proteins to cytosolic spaces surrounding nucleoids in Bacillus subtilis depends on active transcription. J Bacteriol 183 6435–6443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MH, Marahiel MA (2003) Bacterial cold shock responses. Sci Prog 86 9–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia B, Ke H, Inouye M (2001) Acquirement of cold sensitivity by quadruple deletion of the cspA family and its suppression by PNPase S1 domain in Escherichia coli. Mol Microbiol 40 179–188 [DOI] [PubMed] [Google Scholar]
- Xu D, Duan X, Wang B, Hong B, Ho T, Wu R (1996) Expression or a late embryogenesis abundant protein gene, HVA1, from barley confers tolerance to water-deficit and salt stress in transgenic rice. Plant Physiol 110 249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]