Abstract
Responses to environmental stresses in higher plants are controlled by a complex web of abscisic acid (ABA)-dependent and independent signaling pathways. To perform genetic screens for identification of novel Arabidopsis (Arabidopsis thaliana) loci involved in the control of abiotic stress responses, a complementary DNA (cDNA) expression library was created in a Gateway version of estradiol-inducible XVE binary vector (controlled cDNA overexpression system [COS]). The COS system was tested in three genetic screens by selecting for ABA insensitivity, salt tolerance, and activation of a stress-responsive ADH1-LUC (alcohol dehydrogenase-luciferase) reporter gene. Twenty-seven cDNAs conferring dominant, estradiol-dependent stress tolerance phenotype, were identified by polymerase chain reaction amplification and sequence analysis. Several cDNAs were recloned into the XVE vector and transformed recurrently into Arabidopsis, to confirm that the observed conditional phenotypes were due to their estradiol-dependent expression. Characterization of a cDNA conferring insensitivity to ABA in germination assays has identified the coding region of heat shock protein HSP17.6A suggesting its implication in ABA signal transduction. Screening for enhanced salt tolerance in germination and seedling growth assays revealed that estradiol-controlled overexpression of a 2-alkenal reductase cDNA confers considerable level of salt insensitivity. Screening for transcriptional activation of stress- and ABA-inducible ADH1-LUC reporter gene has identified the ERF/AP2-type transcription factor RAP2.12, which sustained high-level ADH1-LUC bioluminescence, enhanced ADH1 transcription rate, and increased ADH enzyme activity in the presence of estradiol. These data illustrate that application of the COS cDNA expression library provides an efficient strategy for genetic identification and characterization of novel regulators of abiotic stress responses.
Adverse environmental conditions, such as drought, low temperature, and high soil salinity are among the most challenging factors for plant growth and survival. Adaptation to abiotic stress requires coordinate changes in metabolism, cell growth, division, and differentiation, which depend on a large set of genes controlling complex regulatory mechanisms. Cloning of genes whose expression is up-regulated by salt, cold, or drought stress identified several targets and regulators of stress signaling (Serrano and Gaxiola, 1994; Ingram and Bartels, 1996; Hasegawa et al., 2000). However, genetic approaches are better suited for the identification of regulatory genes than differential display techniques. In fact, the majority of gene functions controlling responses to high salinity, drought, and cold were discovered using forward genetic screens of mutagenized Arabidopsis (Arabidopsis thaliana) populations. For example, uncovering the SOS (salt overly sensitive) signaling pathway, which controls NaCl homeostasis and salt tolerance (Zhu, 2002), was aided by the isolation of salt hypersensitive mutants assisting the characterization of key determinants of salt tolerance, including the SOS1 Na+/H+ antiporter (Shi et al., 2000), the SnRK3 kinase SOS2 (Guo et al., 2001), and the SOS2-interacting EF-hand Ca2+ binding protein SOS3 (Halfter et al., 2000). Similarly, forward genetic approaches unraveled important components of drought, cold, and abscisic acid (ABA) signaling pathways (for review, see Thomashow, 1999; Finkelstein et al., 2002; Yamaguchi-Shinozaki and Shinozaki, 2006).
Genetic screens for deregulated expression of stress-responsive reporter gene constructs, such as RD29A-LUC, provide an alternative to isolate mutations in regulatory genes that either activate or repress the activity of the reporter (Xiong et al., 1999). Combined with nondestructive bioluminescence imaging, the RD29A-LUC reporter gene system was instrumental in identifying regulatory components of salt, cold, and ABA signaling pathways (Ishitani et al., 1997; Xiong et al., 2001, 2002; Lee et al., 2002; Zhu, 2002). As multiple regulatory networks control the expression of stress-induced genes (Yamaguchi-Shinozaki and Shinozaki, 2006), diverse reporter gene constructs are designed to dissect each of these regulatory cascades.
Whereas chemical mutagenesis followed by map-based cloning provides a strategy for identification of weak mutant alleles of essential regulatory genes, wide-scale exploitation of T-DNA and transposon insertion mutagenesis facilitates the isolation of loss-of-function mutations affecting various stress responses (Koiwa et al., 2006). The utility of locus-specific mutations is however limited when a phenotype is controlled by a multigene family. Activation tagging, employing strong enhancers or promoters to induce ectopic expression of genes adjacent to the insertion sites, is used for generation of dominant mutations that may reveal the functions of gene family members (Weigel et al., 2000; Nakazawa et al., 2003). Functional identification of the cytokinin receptor kinase (Kakimoto, 1996), various microRNA genes (Palatnik et al., 2003), and isolation of dominant mutations conferring enhanced salt tolerance (Koiwa et al., 2006) illustrate the potential of this experimental system. Nonetheless, a disadvantage of activation tagging is that gene activation might not be restricted to a single gene located in the vicinity of the T-DNA or transposon insertion site, and therefore multiple gene activation events may lead to complex and confusing phenotypes (Ichikawa et al., 2003). Combination of activation tagging with screening for induction (or repression) of promoter-driven luciferase reporters provides a more specific technique for activation tagging of regulatory genes. Screening for enhanced luminescence of a pathogen responsive PR1-luciferase reporter gene was thus used for tagging of the Arabidopsis ADR1 pathogen resistance gene (Grant et al., 2003). Analogous genetic screens are facilitated by the gene trapping systems that offer a collection of promoter trap insertions in stress-responsive genes that control the expression of GFP, GUS, or luciferase reporters (Alvarado et al., 2004).
Expression of complementary DNA (cDNA) libraries in plants provides an additional strategy to screen for gain of function phenotypes. This strategy is comparable to the so-called multicopy suppressor screen, which was first invented in yeast to identify functions suppressing salt sensitivity (Bender and Pringle, 1991; Masson and Ramotar, 1998). cDNA libraries driven by constitutive promoters have also been used to generate transgenic Arabidopsis and rice (Oryza sativa) lines showing altered developmental traits (LeClere and Bartel, 2001; Ichikawa et al., 2006; Nakamura et al., 2007). Large-scale transformation of Arabidopsis roots with a cDNA library led to the identification of the ESR1 gene, overexpression of which stimulates cytokinin-independent plant regeneration (Banno et al., 2001). By screening a collection of cDNA overexpressing plants, Kuhn et al. (2006) found that protein phosphatase AtPP2AC plays an important regulatory role in ABA-controlled closure of gas exchange cells and thereby controls drought sensitivity.
The cDNA library transformation approach may also produce dominant loss of function phenotypes, which result from cosuppression of endogenous genes by overexpression of truncated or antisense cDNAs (LeClere and Bartel, 2001). Recently, this possible disadvantage has been overcome by the design of the FOX (full-length cDNA overexpresser) gene hunting system (Ichikawa et al., 2006; Nakamura et al., 2007). Nevertheless, constitutive activation of stress regulatory genes can disturb cell proliferation and development, resulting e.g. in dwarf and sterile plants (Kasuga et al., 1999; Gilmour et al., 2000). To resolve this problem, we created a controlled cDNA overexpression system (COS) by Gateway cloning of an Arabidopsis cDNA library into the chemically inducible XVE expression cassette of pER8 plant transformation vector (Zuo et al., 2000). The cDNA library was introduced into wild-type Arabidopsis, as well as into an ADH1-LUC reporter line, to screen for salt tolerance, ABA insensitivity, and activation of the ADH1 (stress-responsive alcohol dehydrogenase) promoter. Here we describe the identification of a set of cDNAs conferring dominant stress-tolerance phenotypes and initial characterization of three regulatory functions identified in the different genetic screens. Our data indicate that overexpression of HSP17.6 cDNA confers ABA insensitivity, whereas activation of 2AER (for 2-alkenal reductase) results in improved salt tolerance, and induction of the RAP2.12 transcription factor stimulates the expression of the ADH1-LUC reporter gene. These examples illustrate that the COS technology can be exploited as a simple and versatile genetic tool to screen for regulators of stress responses.
RESULTS
Construction and Testing of the COS
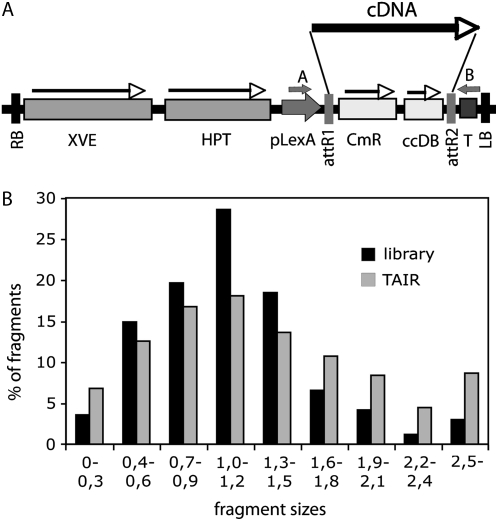
An Arabidopsis cDNA library was constructed in the pDONR201 vector using a SuperSMART cDNA synthesis system (Clontech; Supplemental Fig. S1) in combination with the Gateway cloning technology and RNA templates from different Arabidopsis organs, dark-grown, green, and salt-stressed seedlings, and cultured cells (Supplemental Table S1). The cDNA library was subsequently transferred into pER8GW, a Gateway version of estradiol-inducible expression vector pER8 (Zuo et al., 2000), which carries an attR1 and attR2 recombination cassette between XhoI and SpeI cloning sites of pER8 (I. Sommsich and B. Ülker, unpublished data; Fig. 1A). In the resulting COS cDNA library each clone carried a cDNA and the average insert size was 1.1 kb, similarly to the cDNA expression library of LeClere and Bartel (2001). The size distribution of cDNA inserts in the COS library and full-length cDNA sequences deposited in The Arabidopsis Information Resource (TAIR) database were similar to that described for the full-length FOX cDNA library (Fig. 1B; Ichikawa et al., 2006). Random sequencing of 50 cDNA clones indicated that about 60% of clones carried full-length cDNAs, whereas in the library of LeClere and Bartel (2001) this ratio was 43%. The COS library was introduced into Agrobacterium GV3101 (pMP90; Koncz and Schell, 1986) by electroporation and used subsequently for transformation of wild-type ecotype Columbia of Arabidopsis (Col-0) plants, as well as a transgenic line carrying the ADH1-LUC reporter gene construct. T1 progeny of infiltrated plant populations was used for the subsequent screening procedures.
Figure 1.
Characterization of the COS cDNA library. A, Schematic map of the T-DNA region of pER8GW vector. XVE, Chimeric XVE fusion gene, encoding the chimeric transcription activator for the pLexA promoter (Zuo et al., 2000); HPT, hygromycin phosphotransferase gene; pLexA, LexA operator fused to a minimal promoter of the cauliflower mosaic virus 35S gene; attR1 and attR2, Gateway recombination sites; CmR, chloramphenicol resistance gene; ccdB, suicide marker for bacterial contraselection; T, Rubisco rbcsS3A polyA sequence; RB and LB, T-DNA left and right border sequences, respectively; cDNA, randomly inserted cDNA clone; A and B, positions of T-DNA specific PCR primers used for amplification of inserted cDNAs. B, Comparison of size distribution of DNA fragments in the cDNA library and in the database of predicted full-length transcripts at TAIR (http://www.Arabidopsis.org). Fragment sizes are indicated in kilobase pair.
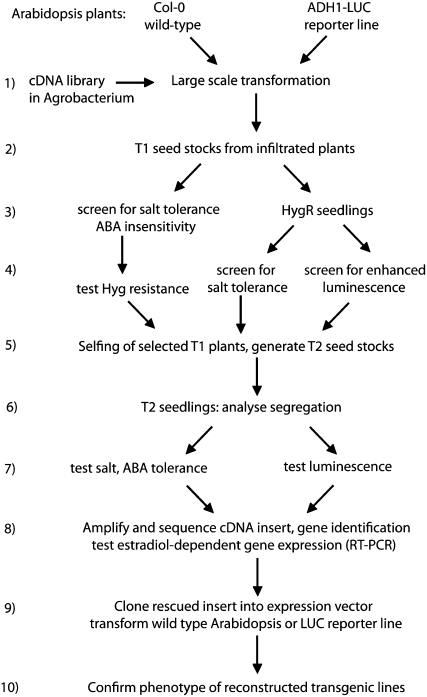
To test the utility of the COS system, three screening strategies were employed by selecting for transformants showing ABA insensitivity in germination screens, salt tolerance in seedling growth assays, and activation of a stress-inducible ADH1-LUC reporter gene in seedlings (Fig. 2). Twenty-thousand to 40,000 transgenic seeds and seedlings were screened in each of these assays using estradiol in the growth medium for transient induction of cDNA expression. Upon selection, the transgenic plants were transferred into estradiol-free medium and then into soil to set seed. The segregation of selected phenotype in the T2 offspring was recurrently assayed by germinating and growing seedlings both in the presence and absence of estradiol, and testing for cosegregation of estradiol-induced conditional phenotype with the hygromycin resistance marker of the pER8GW T-DNA insert. Subsequently, cDNA inserts present in the selected Arabidopsis lines were isolated by PCR amplification from genomic DNA templates using the ER8A and ER8B primers that anneal to the pER8 vector T-DNA sequences flanking the attB recombination sites (Supplemental Fig. S2; Supplemental Table S2). Subsequently, the isolated cDNAs were sequenced and characterized by performing BLAST homology searches with the Arabidopsis sequence database (www.Arabidopsis.org). As each cDNA was flanked by attB1 and attB2 recombination sites, their PCR fragments could easily be recloned in the Gateway entry vector pDONR201 and subsequently into pER8GW for recurrent transformation of Arabidopsis to confirm the phenotype conferred by their estradiol-inducible expression (Supplemental Fig. S2). Using the Gateway technology, the rescue, identification, and subsequent confirmatory recloning of cDNAs required less than 2 weeks providing a high-throughput technology for functional analysis.
Figure 2.
Flow chart of the screening strategies employed for testing the COS technology and to identify stress regulatory functions in Arabidopsis. Consecutive steps of cDNA library transformation, different screening procedures, gene identification, and characterization of the selected transgenic lines are illustrated schematically.
Identification of Factors Affecting ABA Sensitivity of Seed Germination
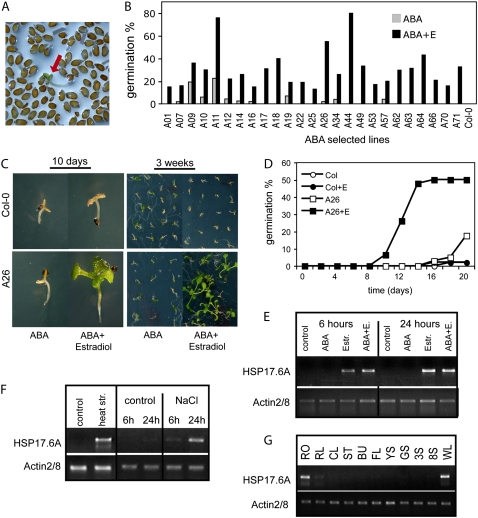
To identify cDNAs that confer ABA-insensitive seed germination when expressed conditionally during estradiol induction, one million of the T1 seeds containing about 20,000 transformants were plated on half-strength Murashige and Skoog (MS) agar medium containing 2.5 μm ABA and 4 μm estradiol. Under this selective condition, the germination of wild-type seeds was completely blocked. ABA-insensitive seeds, which germinated within 7 to 10 d producing seedlings with open green cotyledons and emerged radicles, were transferred onto ABA and estradiol free, hygromycin containing medium (Fig. 3A). Seventy-four ABA-insensitive, hygromycin-resistant plants were identified. T2 progeny of these plants was retested for the ABA insensitive seed germination phenotype in the presence and absence of estradiol along with parallel scoring for single locus segregation of the T-DNA-encoded hygromycin resistance marker. For further analysis, we chose 25 lines, which showed different degrees of estradiol-dependent ABA insensitivity in the germination assay (Fig. 3B). From these, ABA insensitive germination of 19 lines was completely estradiol dependent, whereas six lines displayed some degree of ABA-insensitive germination also in the absence of estradiol. From a subset of selected lines, we have rescued and sequenced 11 cDNAs, and found that they code for proteins that were previously reported to play various roles in different abiotic stress responses, including a glutathione-S-transferase, a SNF1-related kinase regulatory subunit, a lipid transfer protein, a subtilase, and a dehydrin type protein (Table I; A lines). Although most ABA-insensitive lines carried full-length cDNA inserts, several of them were truncated at the 5′ end (Supplemental Fig. S6).
Figure 3.
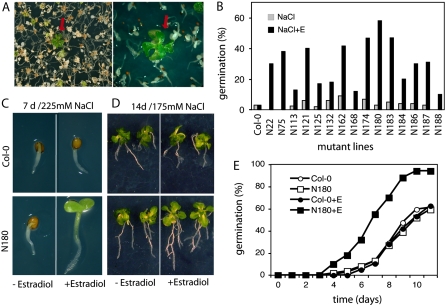
Identification of Arabidopsis cDNAs conferring estradiol-inducible conditional ABA insensitivity. A, Screening for ABA insensitive seed germination in the presence of 2.5 μm ABA and 4 μm estradiol. Red arrow shows a germinated seedling within the nongerminating seed population. B, Testing ABA insensitivity of 25 T2 lines. Seeds were germinated on half-strength MS medium containing 2.5 μm ABA in the presence or absence of 4 μm estradiol. Germination efficiencies were determined at day 14. C, ABA insensitivity phenotype of line A26. Seeds were germinated in the presence of 3 μm ABA in the presence or absence of 4 μm estradiol. Seedlings are shown 10 d (left) and 3 weeks (right) after germination. D, Comparison of germination efficiencies of wild-type (Col-0) and A26 seeds in the presence and absence of estradiol on ABA-containing plates. The graph shows typical germination data derived from three independent experiments. E to G, Semiquantitative RT-PCR analysis of HSP17.6A transcript levels in 2-week-old A26 seedlings using actin2/8 as standard internal reference. E, Activation of HSP17.6A transcription by 4 μm estradiol treatment with or without 50 μm ABA. F, Induction of HSP17.6A transcription with heat shock and salt stress in leaves of wild-type plants. G, Expression of HSP17.6 in different organs of wild-type Arabidopsis plants. RT-PCR analysis was performed with RNA templates prepared from roots (RO), rosette leaf (RL), cauline leaf (CL), stem (ST), unopened buds (BU), flowers (FL), young siliques (4 d after pollination; YS), developed, green siliques (10 d after pollination; GS), 3- and 8-d-old seedlings growing in vitro on solid half-strength MS medium under short day photoperiod (3S; 8S), and wilted rosette leaves (WL).
Table I.
Identification of COS cDNAs conferring estradiol-dependent ABA insensitivity, NaCl tolerance, and activation of the ADH1-LUC reporter gene
| Line | Gene | Encoded Protein | Screen | 5′ End from ATG | Insert |
|---|---|---|---|---|---|
| bp | kb | ||||
| A11 | At1g78380 | ATGSTU19 (glutathione-S-transferase 8) | ABA | −69 | 0.97 |
| A14 | At1g67360 | REF (rubber elongation factor) family protein | ABA | −178 | 1.19 |
| A17 | At2g47900 | ATTLP3 (tubby-like protein 3) | ABA | +768 | 0.4 |
| A18 | At5g59960 | Unknown expressed protein | ABA | −49 | 1.42 |
| A26 | At5g12030 | ATHSP17.6A (cytosolic small heat shock protein) | ABA | −54 | 0.63 |
| A49 | At1g71950 | Subtilase family protein | ABA | +67 | 0.61 |
| A53 | At3g48530 | AKING1 (SNF1-related protein kinase γ regulatory subunit) | ABA | −118 | 1.56 |
| A57 | At2g17690 | F-box family protein | ABA | +860 | 0.4 |
| A62 | At2g45180 | LTP (seed storage/lipid transfer protein) | ABA | −26 | 0.65 |
| A64 | At1g71950 | Subtilase family protein | ABA | +67 | 0.61 |
| A66 | At1g54410 | Dehydrin family protein, similar to aldose 1-epimerase | ABA | −62 | 0.62 |
| ADH121 | At1g53910 | RAP2.12 (ERF/AP2 transcription factor family) | LUC | −98 | 1.42 |
| ADH242 | At3g46080 | Zinc finger (C2H2 type) protein, similar to ZAT7 | LUC | +1 | 0.49 |
| N022 | At3g05050 | Ser/Thr kinase, similar to CRK1 protein | NaCl | −274 | 2.08 |
| N075 | At2g45180 | LTP | NaCl | −30 | 0.65 |
| N121a | At4g01320 | ATSTE24 (CAAX protease) | NaCl | −300 | 1.56 |
| N121b | At2g30860 | ATGSTF9 (glutathione-S-transferase 9) | NaCl | +1 | 0.83 |
| N125 | At1g13520 | Unknown protein | NaCl | −24 | 1.26 |
| N162 | At1g27020 | Unknown protein | NaCl | +527 | 0.67 |
| N168 | At4g22220 | ATISU1 (iron-sulfur cluster assembly protein) | NaCl | −50 | 0.84 |
| N174 | At3g22230 | RPL27B (60S ribosomal protein L27) | NaCl | +114 | 0.46 |
| N180 | At5g16970 | AT-2AER | NaCl | −45 | 1.2 |
| N183 | At4g15550 | IAGLU (indole-3-acetate β-d-glucosyltransferase) | NaCl | +659 | 1.05 |
| N184 | At3g17420 | GPK1 (glyoxysomal protein kinase 1) | NaCl | +528 | 0.52 |
| N186 | At2g19310 | Heat-shock protein, similar to HSP18.2 | NaCl | −13 | 0.6 |
| N187 | At3g53740 | RPL36B (60S ribosomal protein L36) | NaCl | −179 | 0.6 |
| N188 | At2g25450 | Unknown protein, similar to ACC oxidase | NaCl | +1150 | 0.3 |
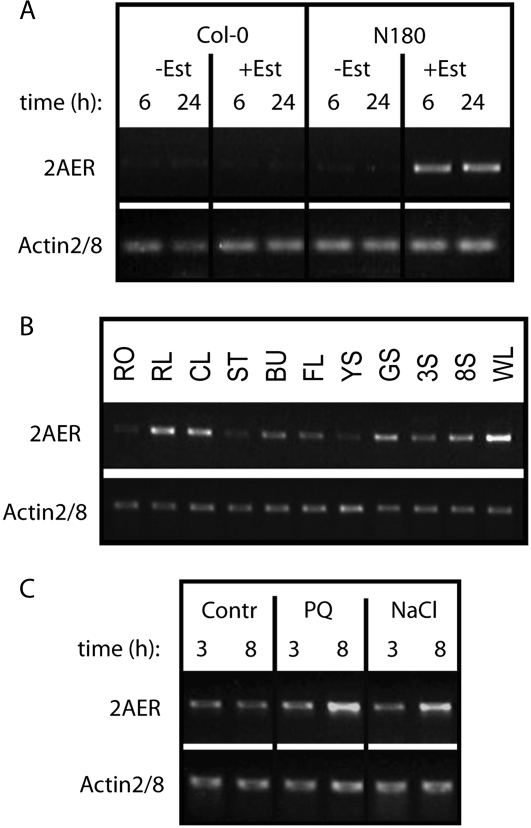
Line A26 carried a full-length cDNA of class II small heat shock protein 17.6A (HSP17.6A, At5g12030) gene, including a 5′-untranslated leader of 53 bp and 3′-UTR sequences of 127 bp (Supplemental Fig. S3). Because HSP17.6A has not been implicated so far in the control of ABA response of seed germination, we have performed further characterization of line A26. In the T2 generation, the conditional ABA insensitivity of line A26 was dominant, and 3:1 segregation of hygromycin-resistant and sensitive offspring indicated that this trait is linked to a single T-DNA insertion. A26 seeds germinated in the presence of 3 μm ABA and 4 μm estradiol, while their ABA sensitivity was similar to wild-type seeds in the absence of estradiol (Fig. 3, C and D). Semiquantitative reverse transcription (RT)-PCR analysis confirmed that HSP17.6A expression in line A26 was indeed induced only by estradiol, but not by ABA (Fig. 3E). As expected, HSP17.6A transcription was activated by heat shock and salt stress in wild-type plants (Fig. 3F). HSP17.6A showed very low expression in most organs except for roots and wilted leaves (Fig. 3G).
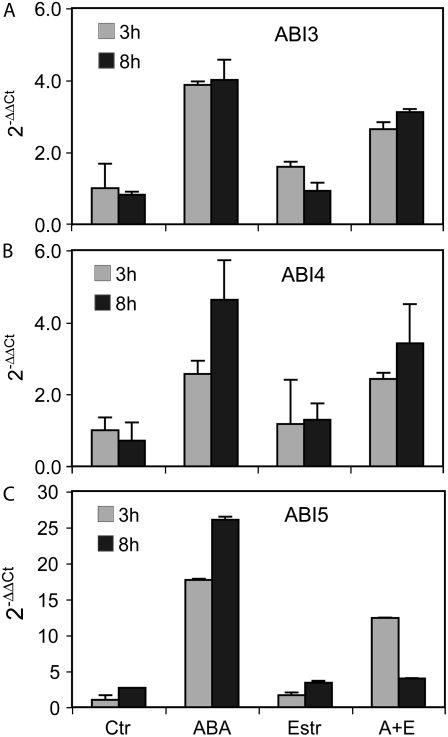
ABA sensitivity of seed germination is controlled by the transcription factors ABI3, ABI4, and AB5 (Finkelstein et al., 2002). Therefore, we tested the transcript levels of these key transcription factors in A26 plants treated with or without ABA and estradiol. As in wild type, ABA treatment of A26 seeds lead to the induction of expression of ABI3, ABI4, and ABI5 genes, whereas estradiol treatment alone had no effect on their transcription. However, combined ABA and estradiol treatment, triggering coexpression of HSP17.6A with these transcription factors, resulted in 85% reduction of ABI5 transcription compared to ABA treatment alone, and resulted in 15% to 25% lower ABI3 and ABI4 transcript levels (Fig. 4). Nevertheless, heat stress and salt treatment (150 mm NaCl) did not suppress or enhance the capability of line A26 to germinate in the presence of ABA and estradiol, whereas 3 μm ABA in combination with heat or salt stress inhibited similarly the germination of wild-type and A26 seeds in the absence of estradiol (data not shown; see “Materials and Methods”).
Figure 4.
A to C, Comparison of regulation of ABI3, ABI4, and ABI5 transcript levels in A26 seedlings (A, ABI3; B, ABI4; C, ABI5). Quantitative RT-PCR analysis was performed with RNA templates isolated from 3-d-old A26 seedlings treated with either 20 μm ABA, or 4 μm estradiol, or their combination (A+E) for 3 and 8 h. Ctr, Untreated control. Relative values are shown using GAPDH2 as internal reference.
Identification of a cDNA Conferring Increased Salt Tolerance
To screen for enhanced salt tolerance of seed germination and seedling growth, a population of 40,000 transgenic T1 seed was germinated on plates containing hygromycin, and resistant plantlets were transferred to selective half-strength MS agar plates supplemented with 225 mm NaCl and 4 μm estradiol. Alternatively, 20,000 seeds were germinated on half-strength MS agar plates containing 225 mm NaCl and 4 μm estradiol. Under this condition, wild-type seeds either did not germinate or the seedlings died after germination. Lines displaying salt-tolerant germination and subsequent development of green seedlings within 15 to 20 d on the selective medium (Fig. 5A) were transferred to soil to set seed. Salt tolerance of T2 offspring of selected lines was recurrently tested in germination and growth assays using salt selection in the presence or absence of 4 μm estradiol. Estradiol-dependent conditional salt tolerance characterized by at least 2-fold higher germination rate compared to wild-type seeds was confirmed for 14 lines (Fig. 5B), from which the cDNA inserts were subsequently PCR amplified. Nine from the sequenced cDNAs carried full-length coding regions of a CDK2-related Ser/Thr kinase, a seed storage/lipid transfer protein (LTP), AtSTE24 CAAX protease, AtISU1 (iron-sulfur cluster assembly complex protein), 60S ribosomal protein L27 (RPL27B), and several unknown proteins (Table I; N lines). Five lines carried inserts with 5′ truncated cDNAs (Supplemental Fig. S6). From this population, line N180 showing high salt tolerance and 3:1 segregation of hygromycin resistance marker of a single copy pER8GW T-DNA insert was characterized. In the presence of estradiol and 225 mm NaCl the T2 offspring of line N180 germinated at least 2 d earlier than wild-type and estradiol-untreated N180 seeds, and the germination efficiency reached nearly 100% already 10 d after sawing. By contrast, line N180 displayed only the emergence of radicles and similar germination efficiency as control wild-type seed on estradiol-free selective medium containing 225 mm NaCl (Fig. 5, C and E). When 5-d-old seedlings were germinated on half-strength MS medium and were transferred to medium containing 175 mm NaCl, N180 plantlets continued to grow only in the presence of estradiol (Fig. 5D). From line N180 a single cDNA insert was PCR amplified and proved to carry the full-length coding region of the At5g16970 2-alkenal reductase enzyme (2AER, EC 1.3.1.74; Supplemental Fig. S4). Compared to wild-type and estradiol-untreated N180 seedlings, semiquantitative RT-PCR analysis indicated that the transcription of 2AER is estradiol induced and correlates with conditional salt tolerance of line N180 (Fig. 6A). However, upon increasing the sensitivity of RT-PCR detection (i.e. higher cycle number) transcription of the 2AER gene could also be detected in all tested organs with highest abundance in cauline and rosette leaves, wilted leaves, and developed siliques (Fig. 6B).
Figure 5.
Identification of cDNAs conferring enhanced salt tolerance. A, The screening for enhanced salt tolerance in plant growth assays was performed in the presence of 225 mm NaCl and 4 μm estradiol. Red arrows indicate salt-tolerant plants within the population of salt-sensitive seedlings. B, Conditional salt tolerance of seed germination of 15 selected COS lines. Germination efficiencies are shown 7 d after sowing the seeds on half-strength MS medium containing 225 mm NaCl in the presence (+E) or absence of 4 μm estradiol. C, Comparison of salt tolerance of N180 and wild-type (Col-0) seedlings germinated in the presence of 225 mm NaCl with or without estradiol. D, Salt tolerance test in plant growth assay. Seven-day-old wild-type (Col-0) and N180 seedlings were transferred from half-strength MS medium to selective medium containing 175 mm NaCl with or without estradiol and grown for an additional 7 d. E, Quantitative analysis of germination efficiencies of N180 and wild-type (Col-0) seeds on half-strength MS plates containing 225 mm NaCl with (+E, 4 μm) or without estradiol. The data represent values of a typical germination assay of three independent experiments.
Figure 6.
Analysis of AER (2-alkenal reductase, At5g16970) transcription by semiquantitative RT-PCR. A, Transcription of AER cDNA is induced by estradiol only in line N180 but not in the control wild-type (Col-0) seedlings. RT-PCR (30 cycles) was performed with RNA templates purified from 3-week-old seedlings treated with 4 μm estradiol for 6 or 24 h. B, RT-PCR (35 cycles) comparison of AER transcript levels in different organs of wild-type (Col-0) plants (for abbreviations, see Fig. 3G legend). C, Effects of paraquat (4 μm) and NaCl (200 mm) treatments on the transcription of AER gene. The RNA templates were standardized for equal level of actin2/8 mRNA as internal control.
Oxidative stress imposed by 4 μm paraquat treatment for 3 and 8 h on 14-d-old, in vitro germinated wild-type seedlings (plantlets with four leaves), as well as treatment of similar seedlings with 200 mm NaCl for 3 and 8 h, resulted in moderate increase of 2AER transcript levels (Fig. 6C). Similarly to our results, inspection of public transcript profiling data (http://www.genevestigator.ethz.ch) indicated that transcription of the 2AER At5g16970 gene is up-regulated by hydrogen peroxide, senescence, and wounding.
Screening for ABA insensitivity and salt tolerance has also resulted in the identification of several lines carrying truncated cDNA inserts (Table I). As in all other cases examined, the estradiol-dependent ABA insensitivity and salt tolerance phenotypes of four of these lines were repeatedly tested in three independent experiments. Sequence analysis showed that the identified cDNA inserts contained in frame ATG codons for potential translation of N-terminally truncated proteins carrying some functionally important regulatory domains (Supplemental Fig. S6). However, in the absence of suitable antibodies against these proteins, it remained an open question whether the observed estradiol-dependent dominant stress tolerance phenotypes resulted from overproduction of truncated proteins, or from dominant cosuppression mediated by the corresponding truncated transcripts as suggested by LeClere and Bartel (2001), or both.
Identification of Factors Conferring Trans-Activation of the ADH1-LUC Reporter Gene
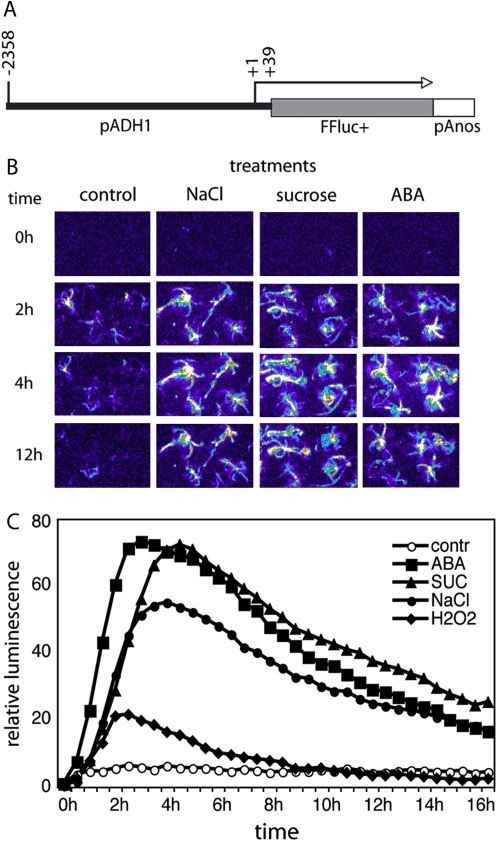
To test the applicability of COS technology in screening for factors that confer trans-activation of a stress-regulated promoter, we have constructed an ADH1-LUC luciferase reporter gene, the activation of which can be monitored using nondestructive low-light imaging (Alvarado et al., 2004). Transcription of the Arabidopsis ADH1 gene (At1g77120) is induced by both ABA-dependent (dehydration, high salinity) and independent (cold and anoxia) regulatory pathways (de Bruxelles et al., 1996). The 2,358-bp-long ADH1 promoter, which has previously been characterized by site-specific mutagenesis and in vivo footprinting studies (Dolferus et al., 1994), was fused to a promoterless firefly luciferase Ffluc+ reporter gene in pBINluc+ (Fig. 7A; Mullineaux et al., 1990) to generate an ADH1-LUC reporter construct, which was introduced into Arabidopsis. Lines carrying single locus insertions of ADH1-LUC reporter in homozygous form were obtained and tested for basal level of ADH1-LUC conferred light emission in seedlings and various organs of developing plants (data not shown). In the parental line chosen for COS library transformation, low light imaging showed that the ADH1-LUC activity was low, but detectable, under standard culture conditions (e.g. germination and growth on half-strength MS medium), whereas treatment of seedlings with either 50 μm ABA, or 200 mm NaCl or 400 mm Suc resulted in reproducible, high level induction of light emission conferred by the activation of ADH1-LUC reporter (Fig. 7B). Due to nondestructive nature of bioluminescence detection, temporal changes in luciferase activity could be measured using a series of automatic exposures. Standard monitoring the induction kinetics indicated that activation of ADH1-LUC by either 200 mm NaCl or 400 mm Suc has reached its maximum 3 to 5 h after initiation of the treatment leading to nearly 100-fold increase of measured luminescence levels (Fig. 7C). Activation of ADH1-LUC was considerably faster by ABA than by salt or sugar, supporting the view that ABA is a mediator of sugar and salt stress responses (Finkelstein et al., 2002). Analogously, hydrogen peroxide treatment resulted in fast (about 2 h) but rather moderate (i.e. 10- to 20-fold) activation of ADH1-LUC reporter.
Figure 7.
Characterization of the ADH1-LUC reporter construct. A, Schematic map of ADH1 promoter and firefly luciferase reporter gene fusion. Position of the transcription start is +1, whereas the position of promoter junction is +39. B, Temporal changes of bioluminescence in the parental ADH1-LUC line during treatments with 200 mm NaCl, 400 mm Suc, and 20 μm ABA for 2, 4, and 12 h. C, Time kinetics of luciferase-mediated light emission in the ADH1-LUC line during treatments with 20 μm ABA, 400 mm Suc, 200 mm NaCl, and 10 mm H2O2. Values of total light emission were recorded at every 30 min throughout a period of 18 h. Relative luminescence values are shown, where 1 is the luminescence of untreated control at time 0.
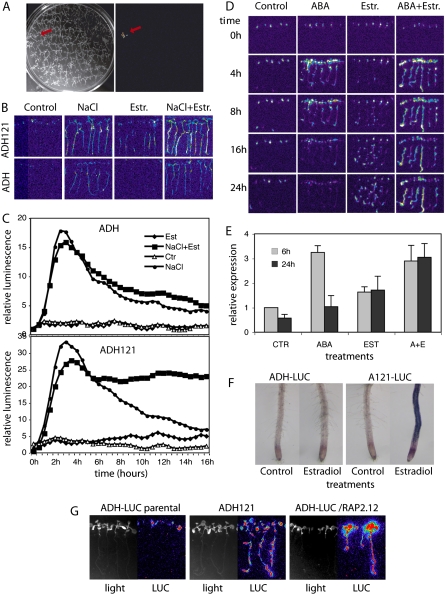
Upon transformation of ADH1-LUC reporter line with the COS cDNA library, we screened 20,000 hygromycin-resistant T1 seedlings and identified 11 plants displaying enhanced LUC activity in the presence of 4 μm estradiol (Fig. 8A). Recurrent assays confirmed estradiol-dependent LUC activation in the T2 progeny of two lines (Table I; ADH lines), whereas all other candidates found in the primary screen showed constitutive estradiol-independent LUC activity. One of the two estradiol-inducible lines, ADH121, showed 3:1 segregation of hygromycin-resistant versus sensitive progeny, and inducible expression of the ADH1-LUC reporter in all hygromycin-resistant T2 seedlings.
Figure 8.
Identification of cDNAs conferring estradiol-inducible activation of ADH1-LUC reporter. A, Image of a COS cDNA transformed ADH1-LUC seedling showing enhanced bioluminescence in the presence of 4 μm estradiol. B, Salt and estradiol activation of luminescence in the parental ADH1-LUC (ADH) and pER8GW-cDNA transformed ADH121 lines after exposing the seedlings to 200 mm NaCl, 4 μm estradiol, and combined salt and estradiol treatment for 10 h. C, Time kinetics of luciferase activities in the parental ADH1-LUC (ADH) and ADH121 lines during 200 mm NaCl, 4 μm estradiol, and combined salt and estradiol treatments. Light emission was recorded at every 30 min for 16 h and the values were normalized to luminescence detected in untreated plants at time 0. D, Temporal changes of bioluminescence in line ADH121 following exposure to 20 μm ABA, 4 μm estradiol (Estr.), and combined ABA+estradiol spraying. Images were recorded 4, 8, 16, and 24 h after the treatments. E, Quantitative RT-PCR analysis of endogenous ADH1 transcript levels in line ADH121. Two-week-old seedlings were treated with 20 μm ABA, 4 μm estradiol, and 20 μm ABA + 4 μm estradiol. GAPDH2 served as internal quantitative RT-PCR reference. F, Histochemical detection of ADH enzyme activity in roots of parental ADH1-LUC and ADH121 lines shows that estradiol (Est) induces ADH1 expression only in the roots of ADH121 seedlings. G, Confirmatory experiment showing that estradiol induction of RAP2.12 confers activation of the ADH1-LUC reporter. The rescued cDNA insertion from line ADH121 was recloned into ER8GW and retransformed into the parental ADH1-LUC line. Bioluminescence (LUC) of 10-d-old seedlings is shown after spraying with 4 μm estradiol.
Whereas estradiol did not induce the ADH1-LUC reporter in the parental control plants, the ADH121 line showed gradually increasing bioluminescence after estradiol treatment (Fig. 8, B and C). In the absence of estradiol, transfer of the parental ADH1-LUC and ADH121 seedlings to medium containing 200 mm NaCl led to transient increase of bioluminescence within 3 to 4 h followed by gradual decrease of ADH1-LUC expression (Fig. 8, B and C). Whereas the parental ADH1-LUC seedlings showed a similar pattern of luciferase expression also in response to combined treatment with both estradiol and 200 mm NaCl, the COS cDNA transformed ADH121 line displayed persistent maintenance of high level luciferase expression for at least 16 h (Fig. 8, B and C).
Similarly to salt treatment, spraying of ADH121 plantlets with 50 μm ABA in the absence of estradiol yielded a transient luminescence peak within 4 h in leaves and roots followed by a gradual decline of LUC activity. This reflected normal ABA-mediated activation of the ADH1-LUC reporter. By contrast, the parental ADH-LUC line showed no response to estradiol induction, whereas treatment of ADH121 seedlings with 4 μM estradiol triggered gradual increase and long-term maintenance of LUC activity in roots but not in leaves. Combined ABA and estradiol treatment of line ADH121 resulted in sustained LUC activation without apparent decline of light emission for at least 24 h (Fig. 8D). These results indicated that activation of a cDNA encoded function conferred root-specific activation of the ADH1-LUC reporter in the ADH121 line, which was superimposed onto the ABA- and salt-induced activation pattern of ADH-LUC (i.e. leaves and roots) in plants subjected to combined treatment with estradiol and ABA or salt.
To compare the induction of the endogenous ADH1 gene with activation of the ADH1-LUC reporter, we have monitored the ABA- and estradiol-induced changes in ADH1 mRNA levels by quantitative RT-PCR in the ADH121 line. In seedlings harvested 6 h after treatment with 50 μm ABA a 3-fold increase of ADH1 transcript levels was observed, but following 24 h the ADH1 mRNA levels declined and were comparable to those of untreated control plants. Upon estradiol treatment, the endogenous ADH1 transcript level was increased 1.8- to 2-fold and also remained at the same level 24 h following the treatment. This indicated that estradiol-induced expression of a cDNA in line ADH121 conferred limited activation of the endogenous ADH1 gene. Combination of estradiol with ABA treatment resulted in a 3-fold increase of ADH1 mRNA levels as seen upon ABA induction, but the transcript levels failed to decline even 24 h after the induction (i.e. due to synergistic effect of estradiol-induced cDNA overexpression; Fig. 8E).
To confirm that estradiol-mediated induction of a cDNA construct in ADH121 also leads to root-specific activation of endogenous ADH1 gene (i.e. as seen for estradiol-induced activation of the ADH-LUC reported in the ADH121 line in Fig. 8D), we have compared the ADH enzyme activities in roots of parental ADH1-LUC and cDNA transformed ADH121 lines using a histochemical assay (Baud and Graham, 2006). Whereas estradiol treatment failed to stimulate endogenous ADH activity in roots of parental ADH1-LUC plants, strong histochemical staining revealed estradiol-induced activation of the ADH enzyme in roots of cDNA transformed ADH121 seedlings (Fig. 8F).
PCR amplification and sequence analysis revealed that a single pER8GW T-DNA insert in the ADH121 line carried a full-length cDNA of the At1g53910 gene encoding RAP2.12, a yet uncharacterized member of the AP2/ERF (ethylene responsive element binding factor) transcription factor family (Supplemental Fig. S5). AP2/ERF-like transcription factors carry one or two AP2-type DNA binding domains and are represented by 122 genes in Arabidopsis (Nakano et al., 2006). RAP2.12 has a single AP2 domain and belongs to the B-2 subfamily, which includes five members sharing high sequence identity, some of them known to control stress responses (Fig. 9A; Sakuma et al., 2002). AtEBP/RAP2.3, which is closely related to RAP2.12, is thus known to confer resistance to H2O2 and heat stress, and to activate a number of defense genes (Ogawa et al., 2005). Other AP2/ERF transcription factors recognize so-called GCC-box sequences and control ethylene responsiveness of several PR gene promoters (Gu et al., 2000; Ogawa et al., 2005). To test ethylene-dependent activation of the ADH1-LUC reporter in the parental and ADH121 lines, 4-d-old seedlings and 2-week-old plantlets were treated with 100 μm ethephon in the presence or absence of 4 μm estradiol. Ethephon treatment had no effect on luciferase activity in either line. In dark germination assays, ethephon treatment triggered similar triple ethylene response of etiolated seedlings of both parental and ADH121 lines and estradiol-dependent activation of RAP2.12 in ADH121 had no effect on the ethylene-induced triple response (data not shown).
Figure 9.
RAP2.12 is a member of the B-2 subfamily of AP2/ERF transcription factors and shows ABA-independent transcriptional regulation. A, Multiple sequence alignment of the B-2 subfamily of ERF family transcription factors. Region of the conserved AP domain is framed. B, RT-PCR (30 cycles) analysis of estradiol-induced activation of RAP2.12 transcription in line ADH121. Seedlings of the parental ADH1-LUC and the ADH121 line were treated by 20 μM ABA, 4 μm estradiol, and both for 6 and 24 h. Note that RAP2.12 transcription is induced by estradiol but not by ABA. C, Comparative RT-PCR (35 cycles) analysis of RAP2.12 transcript levels in various organs of wild-type (Col-0) plants. Actin2/8 was used as internal control (for abbreviations, see Fig. 3G legend).
To determine whether RAP2.12 expression correlates with the induction of the ADH1-LUC reporter, we monitored the RAP2.12 mRNA levels in the parental ADH1-LUC and pER8-cDNA transformed ADH121 lines by RT-PCR. High levels of RAP2.12 transcript was exclusively detected in estradiol-treated ADH121 seedlings, whereas in the absence of estradiol or in the presence of ABA only very low levels of RAP2.12 RNA could be detected in ADH121 seedlings and the parental ADH1-LUC line (Fig. 9B). This data thus showed that RAP2.12 transcription was not induced by ABA and could only be activated by estradiol treatment in ADH121. At higher sensitivity, the RAP2.12 transcript was detected in all organs of wild-type plants, showing the highest levels in roots, flower buds, and stems. The fact that the RAP2.12 mRNA level was somewhat higher in leaves of wilted than well-watered plants suggested possible drought regulation of RAP2.12 (Fig. 9C). These results well agreed with the available transcript profiling data (http://www.genevestigator.ethz.ch) indicating that transcription of the RAP2.12 (At1g53910) gene is not affected by ABA, ethylene, and other plant hormones, and is only slightly up-regulated by senescence and osmotic stress.
To confirm that estradiol-inducible activation of RAP2.12 transcription was indeed responsible for activation of the ADH1-LUC reporter in the ADH121 line, we have recloned the isolated cDNA into the pDONR201 plasmid, and upon moving it into the pER8GW vector (Supplemental Fig. S2) we introduced it by transformation into the parental ADH1-LUC line again. Most of the ADH1-LUC transformants (10 out of 13) carrying the pER8GW-RAP2.12 construct showed estradiol-inducible luciferase activity, which was similar to the activity of the ADH121 line described above (Fig. 8G). In conclusion, this reconstruction experiment confirmed that RAP2.12 is a positive regulator of the ADH1 gene, which is known to be induced in Arabidopsis by anoxia, high salinity, cold, and drought stress.
DISCUSSION
The COS offers a simple and powerful technology to screen for gene functions implicated in the regulation of specific stress responses. The COS cDNA library was prepared in a chemically inducible expression vector using the Gateway technology, which offers precise transcriptional control and easy recloning of the cDNA inserts. The rationale of opting for an inducible system was that constitutive overexpression of cDNAs encoding regulatory factors in stress signaling was observed to result frequently in severe developmental deficiencies. For example, constitutive overexpression of DREB1-type transcription factors resulted in growth retardation, abnormal development, late flowering, and reduced fertility (Liu et al., 1998; Kasuga et al., 1999; Gilmour et al., 2000). The estradiol-inducible XVE/pER8 expression vector (Zuo et al., 2000) was therefore chosen for construction of the COS library to secure conditional and controlled expression of cDNAs to avoid potentially deleterious effects of their overexpression.
The COS system was tested in several screening strategies, each of them aiming at a particular aspect of a stress response. Screening for salt tolerance at seedling level permitted the identification of genes which, upon overexpression, could enhance the germination rate or increase the survival of seedlings in saline environment. As an example, line N180 was characterized to show that overexpression of 2AER cDNA confers salt tolerance to transgenic plants. Previous studies document that the 2AER enzyme has a NADPH-dependent oxidoreductase activity, which probably plays a role in the detoxification of reactive carbonyls, and hence in the protection of cells against oxidative stress (Mano et al., 2005). As high salinity and drought is accompanied by enhanced production of reactive oxygen species (ROS; Price et al., 1989; Mittler, 2002), the functions of antioxidant enzymes, such as 2AER, are important in mounting salt tolerance by reducing the amount of reactive radicals (Jithesh et al., 2006). In fact, expression of Arabidopsis 2AER cDNA in yeast was found to confer tolerance to diamide, a drug that generates oxidative damage (Babiychuk et al., 1995; Kushnir et al., 1995), whereas 2AER overexpression in transgenic tobacco (Nicotiana tabacum) was reported to confer tolerance to photooxidative injury (Mano et al., 2005). Therefore, enhanced salt tolerance correlating with estadiol-induced expression of 2AER cDNA in the N180 line indicates that low level transcription of 2AER in young wild-type seedlings is a limiting factor of salt tolerance, which is presumably related to the detoxification function of 2-alkenal reductase.
Screening for ABA insensitive germination aimed at the identification of novel negative regulators of ABA signaling. Isolation of numerous lines displaying estradiol-dependent ABA insensitivity indicates that the COS technology could also effectively support this screening strategy. As an example, we showed that regulated overexpression of small heat shock protein gene AtHSP17.6A in line A26 conferred conditional ABA insensitivity, pointing to a novel function of this gene. It is well documented that expression of small heat shock proteins is induced by high temperature but some of them, including AtHSP17.6A, are also produced in developing seeds and in response to water stress (Vierling, 1991; Wehmeyer et al., 1996). In particular, AtHSP17.6A was reported to be highly expressed during seed maturation and its seed specific expression was shown to be dependent on the ABI3 transcription factor (Wehmeyer et al., 1996; Sun et al., 2001). Public transcript profiling data (http://www.genevestigator.ethz.ch/) suggest that AtHSP17.6A expression is strongly induced by heat shock and several other environmental stresses, but only slightly influenced by ABA. Sun et al. (2001) reported that overexpression of AtHSP17.6A confers salt and drought tolerance in transgenic plants. In our assays, estradiol-dependent overexpression of HSP17.6A correlated with conditional ABA insensitivity of A26 seeds suggesting that this heat shock protein is also implicated in the control of ABA sensitivity of seed germination, which is controlled by the transcription factors ABI3, ABI4, and ABI5 (Finkelstein et al., 2002). ABI5 is a basic leucin zipper (bZIP) transcription factor that regulates ABA signaling during seed development and germination by modulating the expression of a subset of ABA-induced genes. Transcription of ABI5 is autoregulated and controlled by both ABI3 and ABI4, and ABI5 also shows molecular interaction with ABI3 (Brocard et al., 2002; Finkelstein et al., 2002). Reduction of ABA-mediated induction of ABI5 by estradiol-dependent coactivation of HSP17.6A suggests that this small heat shock protein interferes with transcriptional activation of ABI5, leading to a partial loss of function abi5 mutant phenotype and ABA insensitivity of A26 seeds in the germination assay (Finkelstein et al., 2002).
The application of luciferase reporter gene constructs driven by different stress-induced promoters facilitates nondestructive detection of gene activation in mutant screens, as well as the identification of transcription factors controlling the expression of a particular target gene. Expression of the alcohol dehydrogenase gene ADH1 is controlled by multiple regulatory pathways, including ABA and ethylene signaling (Jarillo et al., 1993; de Bruxelles et al., 1996; Peng et al., 2001). Whereas AtMYB2 is known to be a regulator of ADH1 in response to hypoxia (Hoeren et al., 1998), the activation of ADH1 by dehydration through ABA signaling is mediated by the G-box1 promoter element, which is independent of low-oxygen response (Dolferus et al., 1994; de Bruxelles et al., 1996). Our data show that the estradiol-dependent overproduction of the AP2/ERF transcription factor RAP2.12 can also activate ADH1 expression. The AP2/ERF transcription factor family includes key regulators of abiotic and biotic stress responses. AtEBP/RAP2.3 controls responses to heat and oxidative stress, and activates defense genes (Ogawa et al., 2005), while other AP2/ERF transcription factors control ethylene responses and activate PR gene promoters (Gu et al., 2000; Ogawa et al., 2005). The CBF/DREB subfamily of AP2/ERF factors is demonstrated to regulate transcription of cold and dehydration responsive genes through binding to conserved DRE promoter motives (Stockinger et al., 1997; Liu et al., 1998; Thomashow, 1999; Sakuma et al., 2002; Yamaguchi-Shinozaki and Shinozaki, 2006), and overexpression of several CBF/DREB factors is shown to confer enhanced tolerance to drought, salt stress, and freezing (Liu et al., 1998; Kasuga et al., 1999; Gilmour et al., 2000). Whereas overexpression of RAP2.12 can activate the transcription of the ADH1 gene, according to our data this transcription factor probably acts independently of ABA and ethylene regulation. Thus, RAP2.12 appears to perform a positive signaling function, which has not been linked so far to known regulators of ADH1 transcription. Identification of RAP2.12 as novel regulator of ADH1 promoter illustrates that the COS technology is also applicable to screen for promoter activation and identify response specific transcription factors.
The above-described examples illustrate the COS library transformation method facilitates high-throughput screening for phenotypes conferred by inducible overexpression of Arabidopsis transcripts in an Arabidopsis genetic background. Although the COS approach utilizes artificial overexpression of mRNAs in analogy to multicopy suppressor screens, in the example of the ADH1-luc reporter activation experiment we illustrated that RAP2.12 can also control the expression of the native endogeneous ADH1 gene. In any case, further analysis of functions identified by the COS approach should be supported logically by a series of confirmatory studies using e.g. insertion mutations, inducible artificial RNAi, microarray transcript profiling, and chromatin immunoprecipitation to thoroughly characterize and validate the newly identified regulatory functions. It is also evident that application of the COS technology is not restricted to intraspecies studies using Arabidopsis as the model but can also be extended to interspecies library screens, in which cDNAs from natural variants of drought-, salt-, or cold-tolerant plant species are tested in Arabidopsis or other model species. This extended COS approach provides the possibility for identification of natural sequence variations in known regulatory genes (i.e. based on cross-species sequence comparisons) that confer either increase or decrease in stress tolerance, or are associated with characteristically altered regulatory functions of signaling factors (i.e. transcription factors, protein kinases, protein phosphatases, etc.) controlling a set of target genes in response to well-defined stress or hormonal stimuli.
MATERIALS AND METHODS
Construction of the COS Library
Flow chart of the cDNA library construction is shown in Supplemental Figure S1. The library was constructed from Arabidopsis (Arabidsopsis thaliana; Col-0) RNA samples, which were collected from 10 different tissue sources (Supplemental Table S1). RNA was isolated according to Chomczynski and Sacchi (1987). Equal amounts of RNA samples were pooled and used for cDNA synthesis following the protocol of Super SMART PCR cDNA synthesis kit (Clontech). PCR amplification of the primary cDNA was performed with the Advantage-2 polymerase mix and PCR PrimerIIA as recommended by the manufacturer (1× 95°C/90 s, 65°C/30 s, 68°C/6 min, 18× 95°C/10 s, 65°C/10 s, 68°C/6 min, 1× 68°C/10 min). Using 10 μL aliquot as the template, five more cycles of amplification were performed with a combination of ATTSM1 and ATTSM2 primers (Supplemental Table S2). The PCR product was purified with QIAquick PCR purification kit (Qiagen) and then cloned into pDONR201 using an overnight BP clonase reaction (Invitrogen) as recommended by the manufacturer. Aliquots of the reaction mix were transformed into electrocompetent Escherichia coli DH10 cells. Plasmid DNA was isolated from one million colonies and aliquots were used for transferring cDNA inserts into the pER8GW vector using the LR clonase reaction (Invitrogen). Upon transformation of the pER8GW cDNA library into E. coli DH10, plasmid DNA was isolated from half-million colonies and introduced in aliquots into electrocompetent Agrobacterium GV3101 (pMP90) cells (Koncz et al., 1994).
Generation and Testing of ADH1-LUC Reporter Gene Construct
Promoter region of the Arabidopsis ADH1 gene (At1g77120) was amplified by PCR using the gene-specific primers ADH-1 and ADH-2 (Supplemental Table S2). The amplified fragment contains the 5′ region of the ADH1 gene extending from position −2,385 to −20 upstream of the ATG codon (position +39 downstream of the transcription start; Fig. 7A). Following sequence verification, the amplified promoter fragment was inserted into the HindIII site of the promoter test vector pBinLuc+ (Mullineaux et al., 1990; a kind gift of F. Nagy, BRC, Szeged, Hungary) generating a transcriptional fusion with the firefly luciferase (LUC) reporter gene. The resulting ADH1-LUC reporter construct was introduced into Arabidopsis (Col-0 ecotype) by Agrobacterium-mediated gene transfer. Twenty independent transformants were selected and tested for segregation of the kanamycin resistance marker in the T2 generation, as well as for the activity of the ADH1-LUC reporter using bioluminescence imaging (Alvarado et al., 2004). Induction of ADH1-LUC by ABA was performed by spraying seedlings with 50 μm ABA solution or transferring seedlings on culture medium supplemented by 200 mm NaCl, 400 mm Suc, or 10 mm H2O2 and measuring bioluminescence in 30-min intervals for 18 h. Luminescence values were analyzed with the Metaview software (Universal Imaging). For graphical presentation, luminescence values were normalized to background.
Plant Transformation and Screening Procedures
The pER8GW COS cDNA library was introduced into Arabidopsis (Col-0) by large-scale in planta transformation (Clough and Bent, 1998). T1 seed of 1,000 infiltrated plants were collected in bulk. To select for estradiol-inducible dominant gain-of-function phenotype, three selection schemes were employed. To select for salt tolerance in growth assays, T1 seeds were first germinated on agar-solidified half-strength MS medium (0.5 MS) containing 0.5% Suc, 20 mg/L of hygromycin, and 100 mg/L of claforan. Subsequently, 40,000 hygromycin-resistant seedlings were transferred onto selective 0.5 MS medium supplemented with 225 mm NaCl and 4 μm estradiol. Plantlets that survived salt stress and remained green for at least 2 weeks under these conditions were rescued, transferred to 0.5 MS medium for 2 weeks, and subsequently into soil to produce seed. Alternatively, T1 seeds were germinated on selective (i.e. salt and estradiol containing) medium in the presence of claforan and salt-tolerant seedlings were subsequently tested for hygromycin resistance. Screening for ABA insensitivity in germination assay was performed by germinating T1 seed on solid 0.5 MS medium containing 0.5% Suc, 100 mg/L of claforan, 4 μm estradiol, and 2.5 μm ABA. Five days after sawing, germinated seedlings with emerged radicles and open green cotyledons were transferred to hygromycin-containing plates, and then 2 weeks later into soil. Based on the previously determined transformation frequencies, approximately 20,000 transformed seeds were screened in each of the salt and ABA germination screens. To screen for activation of the ADH1-LUC reporter, the characterized parental line (see “Results”) was transformed with the cDNA expression library. Subsequently, T1 seeds were germinated on hygromycin plates and 20,000 Hyg resistant seedlings were assayed on 4 μm estradiol-containing medium for luciferase activity. Seedlings showing enhanced luminescence were transferred onto 0.5 MS medium for 2 weeks and then into soil to obtain T2 progeny. Flow chart of the screening and testing procedure is depicted in Figure 2.
Rescue of cDNA Inserts from Transgenic Plants
cDNAs carried by the pER8GW T-DNA inserts were rescued by PCR amplification using genomic DNA templates prepared from transgenic plants according to Dellaporta et al. (1983), and the ER8A and ER8B primers that are complementary to vector sequences flanking the attR1 and attR2 sites (Supplemental Table S2). The cDNAs were sequenced with the same primer pair and the sequences were analyzed by BlastN homology searches. To verify the phenotype conferred by estradiol-inducible cDNA expression, the PCR-amplified cDNA was cloned into pDONR201 vector using the Gateway BP clonase reaction (Invitrogen) and then moved into the binary vector pER8GW by Gateway LR clonase reaction. A flow chart of the cloning procedure is depicted in Supplemental Figure S2.
RT-PCR Analysis of Gene Expression
To monitor estradiol-induced production of cDNA encoded transcripts, either real-time or semiquantitative RT-PCR was performed. Hormone and stress treatments were carried out with 3-week-old plants grown in sterile culture in vitro under short day photoperiod (8-h light/16-h dark) by transferring them into liquid culture medium supplemented by different additives. If not stated otherwise, the following treatments were employed: 20 μm ABA, 200 mm NaCl, 400 mm Suc, 10 mm H2O2, 4 μm paraquat in liquid half-strength MS medium for 3 to 24 h. Control plants were incubated for the same time period in half-strength MS medium. Heat shock was performed at 37°C for 3 h in a humid chamber, while control plants were kept under similar conditions at 22°C for the same time. To induce transcription of the inserted cDNAs, plants were sprayed with 4 μm 17-β-estradiol (prepared in dimethyl sulfoxide [DMSO] as 4 mm stock and then diluted in water; Sigma) and harvested at a defined time point following the treatment. Control plants were sprayed with 0.1% DMSO in water. For comparative analysis of transcript levels in plant tissues and organs, the samples were harvested either at the same time or within the same light period of the day. Leaves were collected from 4-week-old greenhouse-grown plants. Siliques were removed from flowering plants 4 and 10 d after pollination. Wilted leaves were collected from 4-week-old greenhouse-grown plants, which were kept without watering for 5 d. Roots samples were collected from 4-week-old plants grown in the greenhouse.
Total RNA was isolated from plant tissues using the Tri-reagent method (Chomczynski and Sacchi, 1987). DNase-treated RNA (1 μg) was used for RT (high-capacity cDNA RT kit; Applied Biosystems). Semiquantitative PCR reactions were performed in 50 μL volume using 1 μL cDNA (1/20 volume of reverse transcriptase reaction) as template and Dupla-Taq polymerase (Zenon Bio) employing the following protocol: one cycle of 94°C/2 min, 25 to 35 cycles of 94°C/30 s, 60°C/30 s, and 72°C/30 s. Real-time quantitative RT-PCR reactions were prepared with SYBR Green JumpStart Taq ReadyMix (Sigma) employing the following protocol: denaturation 95oC/10 min, 40 to 45 cycles of 95°C/10 s, and 60°C/1 min, with ABI PRISM 7700 sequence detection system (Applied Biosystems). Gene-specific primers, used for RT-PCR analysis, are described in Supplemental Table S2. Actin2/8 (At3g18780) and GAPDH2 (At1g13440) used as internal reference (An et al., 1996). Experiments were repeated at least twice.
ADH Enzyme Assay
Detection of ADH1 enzyme by histochemical staining was performed as described (Baud and Graham, 2006). Three-week-old seedlings from the parental ADH1-LUC and pER8GW-cDNA transformed ADH121 lines were treated with liquid 0.5 MS medium containing either 4 μm estradiol and 0.1% DMSO, or 0.1% DMSO as control, for 24 h. Subsequently, the seedlings were transferred into the ADH1 reaction buffer containing 100 mm sodium phosphate (pH 7.5), 400 μm NAD+, 100 μm Nitro Blue Tetrazolium (Sigma-Aldrich), and 3% ethanol as substrate, and incubated at 30°C for 10 min. The enzyme reaction was subsequently stopped by removing the reaction mixture and rinsing the plants with distilled water.
Sequence Analyses
Sequence homology searches were performed using the TAIR BLAST service (http://www.Arabidopsis.org/Blast/index.jsp). PCR primers were designed with the Primer3 software (http://biotools.umassmed.edu/bioapps/primer3_www.cgi). Multiple sequence alignments were generated using the ClustalW program (http://www.ebi.ac.uk/clustalw/index.html). Protein domain analyses were performed using the SMART service (http://smart.embl-heidelberg.de/). Analysis of publicly available transcript profiling data was performed using the Genevestigator service and database (http://www.genevestigator.ethz.ch/).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Strategy for generating the COS library.
Supplemental Figure S2. Gene identification and cloning.
Supplemental Figure S3. Sequence of the A026 cDNA insert.
Supplemental Figure S4. Sequence of the N180 cDNA insert.
Supplemental Figure S5. Sequence of the ADH121 cDNA insert.
Supplemental Figure S6. Analysis of truncated cDNA inserts.
Supplemental Table S1. Arabidopsis organs used as RNA source.
Supplemental Table S2. Oligonucleotides used in this study.
Supplementary Material
Acknowledgments
The authors thank Annamária Király for her technical assistance, Mihály Dobó for growing the plants, and Imre Sommsich and Bekir Ülker for providing the pER8GW vector.
This work was supported by the EU FP5 (no. QLRT–2001–00841), Marie-Curie Action (no. 020232), OTKA grants (nos. K–68226 and F–68598), and joint research project DFG–436UNG–13/172/01 between the Deutsche Forschungsgemeinschaft and the Hungarian Academy of Sciences.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: László Szabados (szabados@brc.hu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Alvarado MC, Zsigmond LM, Kovács I, Cséplö Á, Koncz C, Szabados LM (2004) Gene trapping with firefly luciferase in Arabidopsis. Tagging of stress-responsive genes. Plant Physiol 134 18–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An YQ, McDowell JM, Huang S, McKinney EC, Chambliss S, Meagher RB (1996) Strong, constitutive expression of the Arabidopsis ACT2/ACT8 actin subclass in vegetative tissues. Plant J 10 107–121 [DOI] [PubMed] [Google Scholar]
- Babiychuk E, Kushnir S, Belles-Boix E, Van Montagu M, Inzé D (1995) Arabidopsis NADPH oxidoreductase homologs confer tolerance of yeasts toward the thiol-oxidizing drug diamide. J Biol Chem 270 26224–26231 [DOI] [PubMed] [Google Scholar]
- Banno H, Ikeda Y, Niu QW, Chua NH (2001) Overexpression of Arabidopsis ESR1 induces initiation of shoot regeneration. Plant Cell 13 2609–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud S, Graham IA (2006) A spatiotemporal analysis of enzymatic activities associated with carbon metabolism in wild-type and mutant embryos of Arabidopsis using in situ histochemistry. Plant J 46 155–169 [DOI] [PubMed] [Google Scholar]
- Bender A, Pringle JR (1991) Use of a screen for synthetic lethal and multicopy suppressee mutants to identify two new genes involved in morphogenesis in Saccharomyces cerevisiae. Mol Cell Biol 11 1295–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocard IM, Lynch TJ, Finkelstein RR (2002) Regulation and role of the Arabidopsis abscisic acid-insensitive 5 gene in abscisic acid, sugar and stress response. Plant Physiol 129 1533–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruxelles GL, Peacock WJ, Dennis ES, Dolferus R (1996) Abscisic acid induces the alcohol dehydrogenase gene in Arabidopsis. Plant Physiol 111 381–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162 156–159 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 1 19–21 [Google Scholar]
- Dolferus R, Jacobs M, Peacock WJ, Dennis ES (1994) Differential interactions of promoter elements in stress responses of the Arabidopsis Adh gene. Plant Physiol 105 1075–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SSL, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell (Suppl) 14 S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour SJ, Sebolt AM, Salazar MP, Everard JD, Thomashow MF (2000) Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol 124 1854–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JJ, Chini A, Basu D, Loake GJ (2003) Targeted activation tagging of the Arabidopsis NBS-LRR gene, ADR1, conveys resistance to virulent pathogens. Mol Plant Microbe Interact 16 669–680 [DOI] [PubMed] [Google Scholar]
- Gu YQ, Yang C, Thara VK, Zhou J, Martin GB (2000) Pti4 is induced by ethylene and salicylic acid, and its product is phosphorylated by Pto kinase. Plant Cell 12 771–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Haftner U, Ishitani M, Zhu JK (2001) Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell 13 1383–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter U, Ishitani M, Zhu JK (2000) The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc Natl Acad Sci USA 97 3735–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51 463–499 [DOI] [PubMed] [Google Scholar]
- Hoeren FU, Dolferus R, Wu Y, Peacock WJ, Dennis ES (1998) Evidence for a role for AtMYB2 in the induction of the Arabidopsis alcohol dehydrogenase gene (ADH1) by low oxygen. Genetics 149 479–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa T, Nakazawa M, Kawashima M, Iizumi H, Kuroda H, Kondou Y, Tsuhara Y, Suzuki K, Ishikawa A, Seki M, et al (2006) The FOX hunting system: an alternative gain-of-function gene hunting technique. Plant J 48 974–985 [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Nakazawa M, Kawashima M, Muto S, Gohda K, Suzuki K, Ishikawa A, Kobayashi H, Yoshizumi T, Tsumoto Y, Tsuhara Y, Iizumi H, et al. (2003) Sequence database of 1172 T-DNA insertion sites in Arabidopsis activation-tagging lines that showed phenotypes in T1 generation. Plant J 36 421–429 [DOI] [PubMed] [Google Scholar]
- Ingram J, Bartels D (1996) The molecular basis of dehydration tolerance in plants. Annu Rev Plant Physiol Plant Mol Biol 47 377–403 [DOI] [PubMed] [Google Scholar]
- Ishitani M, Xiong L, Stevenson B, Zhu JK (1997) Genetic analysis of osmotic and cold stress signal transduction in Arabidopsis: interactions and convergence of abscisic acid-dependent and abscisic acid-independent pathways. Plant Cell 9 1935–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarillo JA, Leyva A, Salinas J, Martinez-Zapater JM (1993) Low temperature induces the accumulation of alcohol dehydrogenase mRNA in Arabidopsis, a chilling-tolerant plant. Plant Physiol 101 833–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jithesh MN, Prashanth SR, Sivaprakash KR, Parida AK (2006) Antioxidative response mechanisms in halophytes: their role in stress defence. J Genet 85 237–254 [DOI] [PubMed] [Google Scholar]
- Kakimoto T (1996) CKI1, a histidine kinase homolog implicated in cytokinin signal transduction. Science 74 982–985 [DOI] [PubMed] [Google Scholar]
- Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1999) Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotechnol 17 287–291 [DOI] [PubMed] [Google Scholar]
- Koiwa H, Bressan RA, Hasegawa PM (2006) Identification of plant stress-responsive determinants in Arabidopsis by large-scale forward genetic screens. J Exp Bot 57 1119–1128 [DOI] [PubMed] [Google Scholar]
- Koncz C, Schell J (1986) The promoter of TL-DNA gene 5 controls the tissue specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet 204 383–396 [Google Scholar]
- Koncz C, Martini N, Szabados L, Hrouda M, Bachmair A, Schell J (1994) Specialized vectors for gene tagging and expression studies. In SB Gelvin, RA Schilperoort, DPS Verma, eds, Plant Molecular Biology Manual, Ed 2, Vol B. Kluwer Academic Publisher, Dordrecht, The Netherlands, pp 1–22
- Kuhn JM, Boisson-Dernier A, Dizon MB, Maktabi MH, Schroeder JI (2006) The protein phosphatase AtPP2CA negatively regulates abscisic acid signal transduction in Arabidopsis, and effects of abh1 on AtPP2CA mRNA. Plant Physiol 140 127–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnir S, Babiychuk E, Kampfenkel K, Belles-Boix E, Van Montagu M, Inzé D (1995) Characterization of Arabidopsis cDNAs that render yeasts tolerant toward the thiol-oxidizing drug diamide. Proc Natl Acad Sci USA 92 10580–10584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClere S, Bartel B (2001) A library of Arabidopsis 35S-cDNA lines for identifying novel mutants. Plant Mol Biol 46 695–703 [DOI] [PubMed] [Google Scholar]
- Lee H, Guo Y, Ohta M, Xiong L, Stevenson B, Zhu JK (2002) LOS2, a genetic locus required for cold-responsive gene transcription encodes a bi-functional enolase. EMBO J 21 2692–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10 1391–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano J, Belles-Boix E, Babiychuk E, Inzè D, Torii Y, Hiraoka E, Takimoto K, Slooten L, Asada K, Kushnir S (2005) Protection against photooxidative injury of tobacco leaves by 2-alkenal reductase. Detoxication of lipid peroxide-derived reactive carbonyls. Plant Physiol 139 1773–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson JY, Ramotar D (1998) The transcriptional activator Imp2p maintains ion homeostasis in Saccharomyces cerevisiae. Genetics 149 893–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7 405–410 [DOI] [PubMed] [Google Scholar]
- Mullineaux PM, Guerineau F, Accotto GP (1990) Processing of complementary sense RNAs of Digitaria streak virus in its host and in transgenic tobacco. Nucleic Acids Res 18 7259–7265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Hakata M, Amano K, Miyao A, Toki N, Kajikawa M, Pang J, Higashi N, Ando S, Toki S, et al. (2007) A genome-wide gain-of-function analysis of rice genes using the FOX-hunting system. Plant Mol Biol 65 357–371 [DOI] [PubMed] [Google Scholar]
- Nakano T, Suzuki K, Fujimura T, Shinshi H (2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140 411–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa M, Ichikawa T, Ishikawa A, Kobayashi H, Tsuhara Y, Kawashima M, Suzuki K, Muto S, Matsui M (2003) Activation tagging, a novel tool to dissect the functions of a gene family. Plant J 34 741–750 [DOI] [PubMed] [Google Scholar]
- Ogawa T, Pan L, Kawai-Yamada M, Yu LH, Yamamura S, Koyama T, Kitajima S, Ohme-Takagi M, Sato F, Uchimiya H (2005) Functional analysis of Arabidopsis ethylene-responsive element binding protein conferring resistance to Bax and abiotic stress-induced plant cell death. Plant Physiol 138 1436–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatnik JF, Allen E, Wu X, Schommer C, Schwab R, Carrington JC, Weigel D (2003) Control of leaf morphogenesis by microRNAs. Nature 425 257–263 [DOI] [PubMed] [Google Scholar]
- Peng HP, Chan CS, Shih MC, Yang SF (2001) Signaling events in the hypoxic induction of alcohol dehydrogenase gene in Arabidopsis. Plant Physiol 126 742–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AH, Atherton NM, Hendry GAF (1989) Plants under drought-stress generate activated oxygen. Free Radic Res Commun 8 61–66 [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaki K (2002) DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem Biophys Res Commun 290 998–1009 [DOI] [PubMed] [Google Scholar]
- Serrano R, Gaxiola R (1994) Microbial models and salt stress tolerance in plants. Crit Rev Plant Sci 13 121–138 [Google Scholar]
- Shi H, Ishitani M, Kim C, Zhu JK (2000) The Arabidopsis salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci USA 97 6896–6901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger EJ, Gilmour SJ, Thomashow MF (1997) Arabidopsis CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA 94 1035–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Bernard C, van de Cotte B, Van Montagu M, Verbruggen N (2001) At-HSP17.6A, encoding a small heat-shock protein in Arabidopsis, can enhance osmotolerance upon overexpression. Plant J 27 407–415 [DOI] [PubMed] [Google Scholar]
- Thomashow MF (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol 50 571–99 [DOI] [PubMed] [Google Scholar]
- Vierling E (1991) The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 42 579–620 [Google Scholar]
- Wehmeyer N, Hernandez LD, Finkelstein RR, Vierling E (1996) Synthesis of small heat-shock proteins is part of the developmental program of late seed maturation. Plant Physiol 112 747–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D, Ahn JH, Blázquez MA, Borevitz JO, Christensen SK, Fankhauser C, Ferrándiz C, Kardailsky I, Malancharuvil EJ, Neff MM, et al. (2000) Activation tagging in Arabidopsis. Plant Physiol 122 1003–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, David L, Stevenson B, Zhu JK (1999) High throughput screening of signal transduction mutants with luciferase imaging. Plant Mol Biol Rep 17 159–170 [Google Scholar]
- Xiong L, Lee BH, Ishitani M, Lee H, Zhang C, Zhu JK (2001) FIERY1 encoding an inositol polyphosphate 1-phosphatase is a negative regulator of abscisic acid and stress signalling in Arabidopsis. Genes Dev 15 1971–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Lee H, Ishitani M, Zhu JK (2002) Regulation of osmotic stress-responsive gene expression by the LOS6/ABA1 locus in Arabidopsis. J Biol Chem 277 8588–8596 [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57 781–803 [DOI] [PubMed] [Google Scholar]
- Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53 247–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, Niu QW, Chua NH (2000) An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J 24 265–273 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.