Abstract
The stomata of the fern Adiantum capillus-veneris lack a blue light-specific opening response but open in response to red light. We investigated this light response of Adiantum stomata and found that the light wavelength dependence of stomatal opening matched that of photosynthesis. The simultaneous application of red (2 μmol m−2 s−1) and far-red (50 μmol m−2 s−1) light synergistically induced stomatal opening, but application of only one of these wavelengths was ineffective. Adiantum stomata did not respond to CO2 in the dark; the stomata neither opened under a low intercellular CO2 concentration nor closed under high intercellular CO2 concentration. Stomata in Arabidopsis (Arabidopsis thaliana), which were used as a control, showed clear sensitivity to CO2. In Adiantum, stomatal conductance showed much higher light sensitivity when the light was applied to the lower leaf surface, where stomata exist, than when it was applied to the upper surface. This suggests that guard cells likely sensed the light required for stomatal opening. In the epidermal fragments, red light induced both stomatal opening and K+ accumulation in guard cells, and both of these responses were inhibited by a photosynthetic inhibitor, 3-(3,4-dichlorophenyl)-1,1-dimethylurea. The stomatal opening was completely inhibited by CsCl, a K+ channel blocker. In intact fern leaves, red light-induced stomatal opening was also suppressed by 3-(3,4-dichlorophenyl)-1,1-dimethylurea. These results indicate that Adiantum stomata lack sensitivity to CO2 in the dark and that stomatal opening is driven by photosynthetic electron transport in guard cell chloroplasts, probably via K+ uptake.
Stomata are small adjustable pores located on the surface of leaves. They allow the exchange of CO2 and water between the leaf interior and the atmosphere. Through the regulation of stomatal apertures, plants obtain the CO2 required for photosynthesis and prevent excessive water loss through transpiration. Stomata are found in mosses, ferns, and higher plants, and several works have shown their morphological and/or functional diversity (Willmer and Fricker, 1996; Franks and Farquhar, 2007). Such differences may have arisen as the plants evolved to adapt to different environmental factors. While several environmental stimuli, such as light, CO2, humidity, and temperature, have been shown to regulate stomatal movements in higher plants (Willmer and Fricker, 1996), little is known about how these stimuli affect the stomata of ferns.
In higher plants, stomata open in response to light through at least two light signaling cascades, so-called blue light-specific and red light-induced responses (Shimazaki et al., 2007). Because the blue light-specific response is mediated by guard cells, this response is observed in both the detached epidermis and guard cell protoplasts (Zeiger and Hepler, 1977; Shimazaki et al., 1986). The response is initiated by blue light absorption by blue light receptors, phototropins, followed by the activation of the plasma membrane H+-ATPase via the signal transduction cascade, which is not yet fully understood (Kinoshita and Shimazaki, 1999; Kinoshita et al., 2001; Shimazaki et al., 2007). The blue light-specific response is largely enhanced by background red light irradiation (Ogawa et al., 1978), and blue light at low intensity is effective in the presence of red light.
In contrast, a high intensity of red light is required for inducing stomatal opening, and the light wavelength dependence matches photosynthetically active radiation. The action spectrum for stomatal opening resembles that for leaf photosynthesis (Sharkey and Raschke, 1981). In accord with these results, some studies have indicated that a photosynthetic electron transport inhibitor, 3-(3′,4′-dichlorophenyl)-1,1-dimethylurea (DCMU), inhibits red light-induced stomatal opening (Sharkey and Raschke, 1981; Schwartz and Zeiger, 1984; Tominaga et al., 2001; Olsen et al., 2002). Mesophyll and guard cells have photosynthetically active chloroplasts (Zeiger et al., 1981; Shimazaki et al., 1982; Shimazaki and Zeiger, 1985; Cardon and Berry, 1992; Wu and Assmann, 1993; Lawson et al., 2002), and their photosynthesis has been suggested to induce stomatal opening in intact plants.
Because stomata open in response to a low concentration of ambient CO2 (Ca) and close in response to elevated levels of CO2 (Morison, 1987; Assmann, 1999; Roelfsema and Hedrich, 2005; Vavasseur and Raghavendra, 2005), it is generally accepted that guard cells sense intercellular CO2 concentration (Ci; Mott, 1988). This suggests that the decrease in Ci brought about by mesophyll photosynthesis induces the stomatal opening. Furthermore, it has been suggested that the ratio of Ci to Ca is kept constant under various conditions of the leaves (Wong et al., 1979), although the ratio is not always conserved in the plants (von Caemmerer et al., 2004; Baroli et al., 2008). In accord with these, red light applied to a large area of the leaf, including mesophyll cells, triggered stomatal opening, probably due to mesophyll photosynthesis. However, the responses were not found when a localized beam of red light was applied only to individual guard cells, and thus Ci was proposed to have a role as an intermediate in the stomatal response (Roelfsema et al., 2002). Moreover, red light did not induce stomatal opening in albino leaf patches (Roelfsema et al., 2006).
By contrast, several lines of evidence have suggested that guard cell chloroplasts have a direct role in stomatal opening. Red light induces stomatal opening in isolated epidermis (Sharkey and Raschke, 1981; Schwartz and Zeiger, 1984; Olsen et al., 2002). Red light is suggested to activate the H+ pump in the plasma membrane of guard cells (Serrano et al., 1988), although the pump activation has not been reproduced (Roelfsema et al., 2001; Taylor and Assmann, 2001). A recent report indicates that cocklebur stomata open in response to red light independent of reduced Ci (Messinger et al., 2006). However, it is unclear whether or not guard cell chloroplasts are responsible for stomatal opening in intact plant leaves. Furthermore, phytochrome has been suggested to be involved in stomatal response to red light (Talbott et al., 2003).
Adiantum capillus-veneris belongs to the Leptosporangiopsida, which are newly diversified fern species that grow in shadow beneath angiosperms (Schneider et al., 2004), and usually makes its habitat beneath the canopy, where the ambient light is weak and rich in far-red. The stomata are found only on the lower surface of leaves and lack subsidiary cells, and their guard cells contain densely arranged chloroplasts. Recently, we reported that the stomata of the fern A. capillus-veneris lacked a blue light-specific opening response but did open in response to red light (Doi et al., 2006). A lack of blue light-specific response has commonly been seen in other species of Leptosporangiopsida. We also showed that phy3 (neochrome 1; Suetsugu et al., 2005), a chimeric protein of phototropin and phytochrome, did not work as a photoreceptor for red light-induced stomatal opening in Adiantum. Therefore, the red light-induced stomatal opening in Adiantum could be explained by the photosynthetic activity and/or by the response via phytochrome. Here, we investigated the stomatal responses of Adiantum to light and CO2 in the dark. We demonstrated that the guard cells are insensitive to CO2 and that the guard cell chloroplasts are responsible for stomatal opening in response to light in this plant species.
RESULTS
Stomatal Opening in Response to Photosynthetically Active Radiation
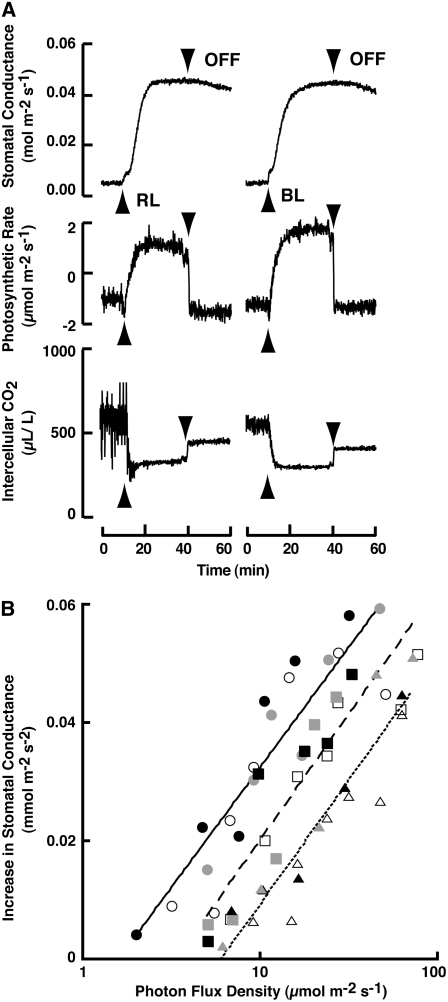
Stomatal responses to light were investigated in intact Adiantum leaves using gas exchange techniques (Fig. 1). As previously reported, the application of photosynthetically saturating red light at 600 μmol m−2 s−1 induced a fast increase in stomatal conductance (Fig. 1A; Doi et al., 2006). The same intensity of blue light (600 μmol m−2 s−1) brought about a similar increase in conductance. The initial rates of conductance increase by red and blue light were 0.061 ± 0.011 and 0.063 ± 0.009 (means of five measurements ± se) mmol m−2 s−2, respectively. When the light was turned off, the stomatal conductance decreased very slowly, but the photosynthetic CO2 fixation ceased instantly. The Ci decreased immediately upon light irradiation but did not return to the original levels after the light was turned off, probably due to the inability of the stomata to close rapidly. Neither a brief pulse of red light nor a brief pulse of blue light induced stomatal opening; rather, continuous irradiation was required for the opening (data not shown).
Figure 1.
Stomatal responses to light in intact leaves of A. capillus-veneris. A, Stomatal conductance, photosynthetic rate, and Ci in response to red (left) and blue (right) light. Each light at an intensity of 600 μmol m−2 s−1 was applied to the upper surface of a leaf as indicated by the upward-pointing arrowhead, and turned off as indicated by the downward-pointing arrowhead. B, PFD response curves for stomatal opening. For each type of light, opening rates were measured at five to seven PFDs with separate leaves of the same plant. Circles, Blue light; squares, red light; triangles, green light. Black, gray, and white symbols represent the separate experiments that were conducted on the different days, respectively. Solid, broken, and dotted lines represent the regression curves for blue, red, and green lights, respectively.
To characterize the mechanisms underlying the stomatal responses to light in Adiantum, we determined the light dependence of the conductance increase using red, green, and blue light (Fig. 1B). The rates of stomatal conductance increase occurred at around 5 μmol m−2 s−1. The response was saturated at 80 μmol m−2 s−1, at which wavelength mesophyll photosynthesis was not saturated (see Fig. 4). Similarly to red light, both blue and green light increased stomatal conductance at low intensity. In contrast, far-red light did not induce any increase in the conductance up to 300 μmol m−2 s−1. A linear regression was obtained for each light wavelength that fell in the linear response range (Fig. 1B). The slopes of the linear regression lines in blue, green, and red light were 0.043 ± 0.003, 0.042 ± 0.003, and 0.048 ± 0.006 (average of three measurements ± se), respectively. The lines were parallel to each other. Using these data, we determined the effectiveness of each light wavelength (Table I). Blue, red, and green light were effective at inducing an increase in conductance, with blue light being the most effective and green light the least, and this pattern of efficacy was in accord with the hypothesis that the photosynthetic pigments acted as photoreceptors for the responses (Taiz and Zeiger, 2006).
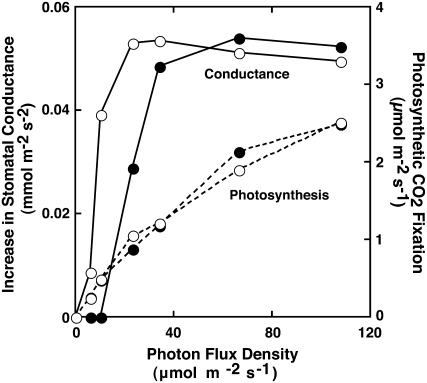
Figure 4.
Dependencies of PFD for stomatal conductance increase and photosynthetic CO2 fixation. Solid and dotted lines represent the increase in stomatal conductance and photosynthetic CO2 fixation, respectively. Red light was irradiated onto the upper (black circles) or lower side (white circles) of the leaf.
Table I.
PFD required for achieving a 50% increase in the rate of stomatal conductance increase
Values are averages of three measurements ± se.
| Blue Light | Green Light | Red Light | |
|---|---|---|---|
| μmol m−2 s−1 | |||
| PFD | 10.4 ± 1.2 | 37.5 ± 5.7 | 19.2 ± 2.3 |
Synergistic Effect of Red and Far-Red Light on Stomatal Opening
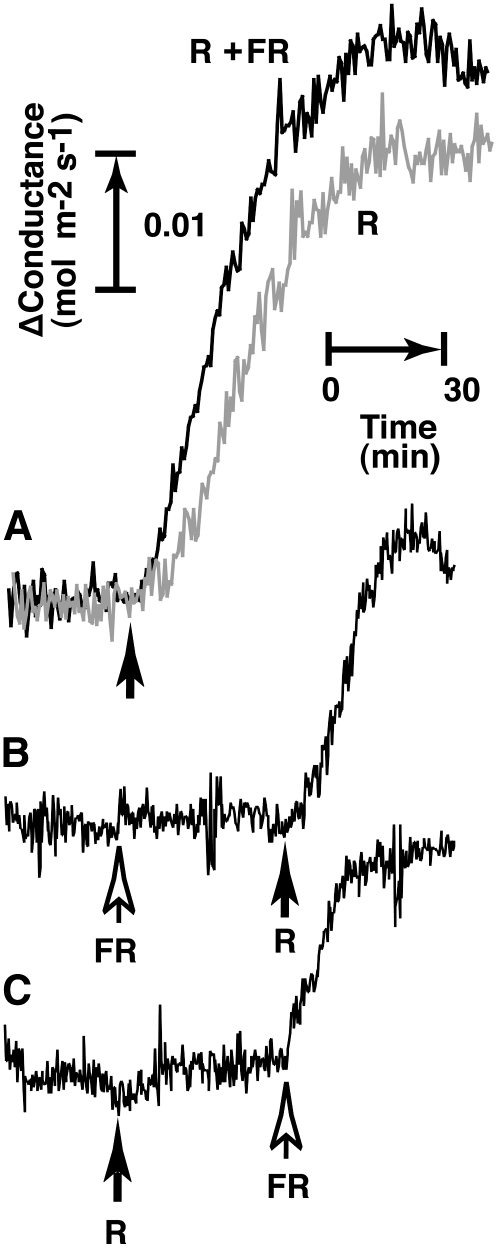
We showed that phy3, a chimeric protein of phototropin and phytochrome, did not function as a photoreceptor for red light-induced stomatal opening in Adiantum (Doi et al., 2006). However, Adiantum has at least three phytochromes besides phy3 (Wada et al., 1997). To determine whether or not these phytochromes are responsible for red light-induced stomatal opening in Adiantum, we investigated the effect of far-red light on stomatal opening. Red light at 50 μmol m−2 s−1 applied to the upper surface of leaves induced stomatal opening (Fig. 2A, gray line). When this red light was applied together with far-red light at 300 μmol m−2 s−1, the added far-red light increased stomatal conductance by 15% (Fig. 2A, black line).
Figure 2.
Stomatal responses to red and far-red light in Adiantum. A, Increase in stomatal conductance by red (R; 50 μmol m−2 s−1) plus far-red (FR; 300 μmol m−2 s−1) light (black line) or by red light alone (R; gray line). Lights were applied to the upper surface of the leaf as indicated by the upward-pointing arrow. B and C, Increase in stomatal conductance by a sequential application of red (R; 2 μmol m−2 s−1) and far-red (FR; 50 μmol m−2 s−1) light. Red and far-red lights were applied as indicated by the black and white arrows, respectively.
The remarkable synergistic effect between red and far-red light on stomatal opening was found in Adiantum when the light intensities were decreased. When far-red light at 50 μmol m−2 s−1 was applied to the leaf, no increase in stomatal conductance was found (Fig. 2B). However, when red light at 2 μmol m−2 s−1, which alone could not induce any detectable stomatal opening (Fig. 2C), was superimposed on the far-red light, the conductance increased markedly (Fig. 2B). Furthermore, when far-red light at 50 μmol m−2 s−1 was applied to leaves superimposed on the preceding red light at 2 μmol m−2 s−1, a similarly large increase in conductance was observed (Fig. 2C). Because neither red light nor far-red light alone increased stomatal conductance, the combined effects of red and far-red light on stomatal conductance were synergistic. In a trial in which the leaves were preirradiated with red or far-red light, this pretreatment had no effects on the subsequent stomatal response elicited by far-red or red light, respectively (data not shown). As shown in Figure 2A, far-red light did not suppress red light-induced stomatal opening but rather enhanced it. These results suggest that light induces stomatal opening through photosynthesis and that phytochromes are not involved in the response of Adiantum. The synergistic effect between red and far-red light on stomatal opening may be attributable to the so-called enhancement effect (the Emerson effect) in photosynthesis, where PSI and PSII operate in series (Taiz and Zeiger, 2006).
Lack of Stomatal Response to Ci in Adiantum under Darkness
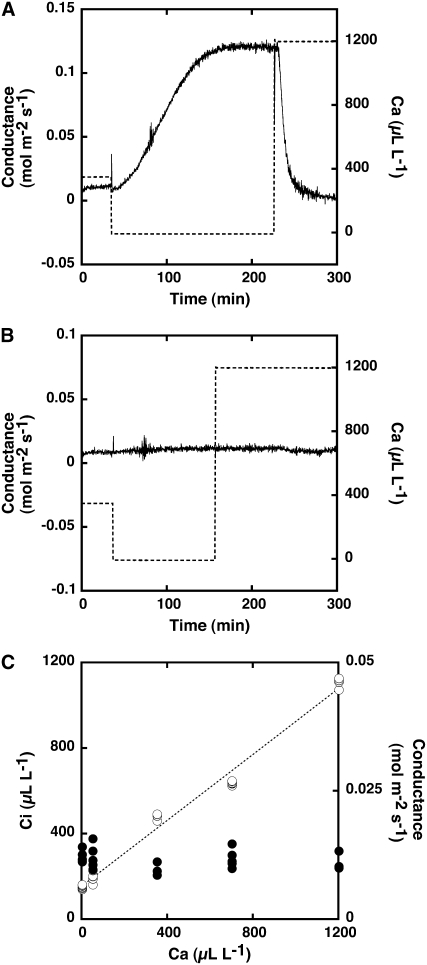
It is likely that red light induces stomatal opening through the reduction of Ci in Adiantum leaves, because a high Ca causes stomatal closure via the activation of anion channels in guard cells of higher plants (Hanstein and Felle, 2002; Roelfsema et al., 2002; Roelfsema and Hedrich, 2005). We previously reported that the Ci was 250 to 275 μL L−1 under saturated red light in Adiantum leaves in which photosynthetic CO2 fixation proceeded (Fig. 1A; Doi et al., 2006) when Ca was 350 μL L−1. If light-induced stomatal opening occurred via the reduction of Ci brought about by mesophyll photosynthesis, the stomata in Adiantum should open at a Ci below 250 μL L−1. To test this, we investigated the stomatal responses under different Ci values, which we manipulated by changing Ca in the dark. First, we verified the stomatal response to Ca using Arabidopsis leaves as a control (Fig. 3A). In Arabidopsis, stomatal conductance was 0.0114 ± 0.0005 mol m−2 s−1 (average of three measurements ± se) when a leaf was exposed to Ca at 350 μL L−1 in the dark, which was close to the atmospheric concentration of CO2. When Ca was decreased to 0 μL L−1, stomatal conductance increased substantially, peaking at 0.1213 ± 0.007 mol m−2 s−1 (average of three measurements ± se), and it decreased to a new steady state when Ca was increased from 0 to 1,200 μL L−1. The Ci values were determined to be 85.4 ± 0.7, 455.6 ± 3.4, and 1,200.0 ± 6.3 μL L−1 (average of three measurements ± se) at Ca of 0, 350, and 1,200 μL L−1, respectively. Thus, Arabidopsis stomata had sensitivity to CO2; they opened when Ca was reduced below atmospheric concentration in the dark and closed under high Ca. Surprisingly, stomata in the Adiantum leaves did not open when Ca was decreased from 350 μL L−1 to 0 μL L−1 (Fig. 3B) and did not close when Ca was increased from 0 μL L−1 to 1,200 μL L−1. These results indicate that stomata in Adiantum leaves are not sensitive to CO2 in the dark. However, it was suspected that the tight closing of the stomata prevented any changes in Ci despite the drastic changes in Ca. To test this possibility, we changed the Ca over a wide range and determined both Ci and stomatal conductance in Adiantum leaves (Fig. 3C). The Ci changed with the changes of Ca and showed a linear correlation with Ca, whereas stomatal conductance did not change in the Ca range of 0 to 1,200 μL L−1. Ci was <200 μL L−1 and >1,000 μL L−1 under darkness when leaves were exposed to CO2-free air and air having Ca of 1,200 μL L−1, respectively. The Ci under the CO2-free air was lower than that of the Adiantum leaves under light, in which stomata opened (Fig. 1A).
Figure 3.
Stomatal responses to Ca in the dark. A, Stomatal conductance in intact leaves of Arabidopsis. B, Stomatal conductance in intact leaves of A. capillus-veneris. Leaves were held in a leaf chamber for 1 h under darkness at a CO2 concentration of 350 μL L−1 and then exposed to low (0) and high (1,200 μL L−1) concentrations of CO2 by changing the concentration in the chamber. Dotted lines show the CO2 concentration in the chamber. C, Relationship between Ca and Ci (white circles) and stomatal conductance (black circles) in the range of 0 to 1,200 μL L−1 Ca. The dotted line represents the linear relationship between Ca and Ci.
Stomatal Opening Is Dependent on Guard Cell Photosynthesis
As described above, photosynthetic active radiation increased stomatal conductance in the fern Adiantum. Because a decrease in Ci did not induce stomatal opening (Fig. 3), photosynthesis in guard cells seems to drive stomatal opening in Adiantum. To examine the role of chloroplasts in guard cells, we utilized the fact that guard cells are localized exclusively to the lower surface of Adiantum leaves. If guard cell chloroplasts are responsible for stomatal opening, it would be expected that light applied to the lower leaf surface would be much more effective at increasing stomatal conductance than light applied to the upper surface. And indeed, when red light was applied to the lower surface, stomatal conductance increased at a light intensity of <5 μmol m−2 s−1 and continued to increase with increasing light intensity, reaching a maximum at about 20 μmol m−2 s−1 (Fig. 4). By contrast, no conductance increase was found at 5 μmol m−2 s−1 when red light was applied to the upper surface. The half-saturation for the conductance increase was 20 μmol m−2 s−1 under this condition. Despite the difference in the requirement of light intensity for stomatal opening, the maximum rates of conductance increase were the same irrespective of the difference in the irradiated leaf surfaces (Fig. 4). We also measured the photosynthetic CO2 fixation of Adiantum leaves. The CO2 fixation rate increased with photon flux density (PFD) and became saturated above 100 μmol m−2 s−1. The light dependence of the CO2 fixation did not differ irrespective of the irradiation direction. Interestingly, there was a clear difference in the PFD dependence between the conductance increase and the photosynthetic CO2 fixation (Fig. 4). These results indicate that stomatal opening in Adiantum is most likely mediated by guard cell chloroplasts and is not dependent on the decreased Ci via mesophyll CO2 fixation.
Inhibition of Stomatal Opening by a Photosynthetic Electron Transport Inhibitor
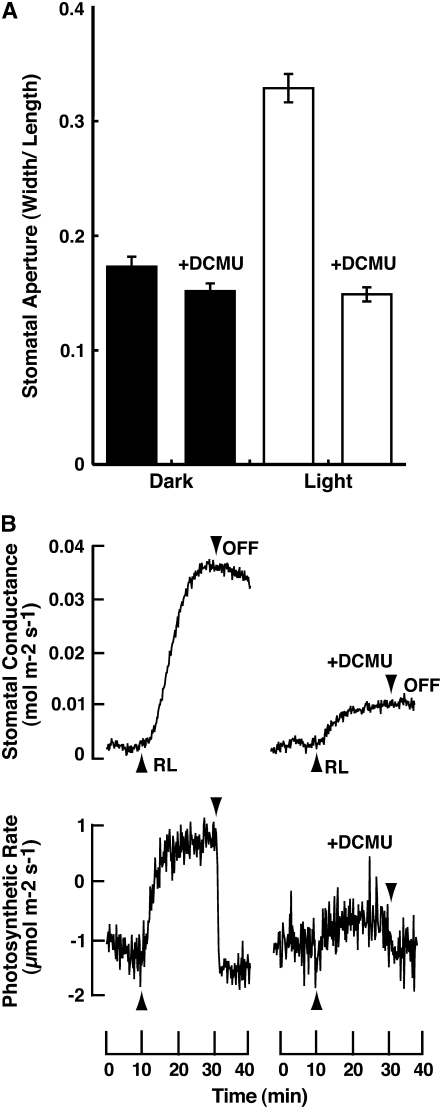
To test whether or not light-dependent stomatal opening is driven by photosynthesis of guard cell chloroplasts, we determined stomatal responses using well-sonicated epidermal fragments devoid of mesophyll and epidermal cells (Fig. 5A). Stomata opened when the epidermis was irradiated by red light at 30 μmol m−2 s−1 for 3 h. The red light-induced stomatal opening was completely inhibited by a photosynthetic electron transport inhibitor, DCMU, at 20 μm. We further tested the effect of DCMU on the increase in stomatal conductance by red light in intact Adiantum leaves (Fig. 5B). Stomatal opening and photosynthetic CO2 fixation were similarly inhibited by DCMU.
Figure 5.
Inhibition of stomatal opening by DCMU in epidermal fragments and intact leaves of Adiantum. Adiantum plants were kept in the dark overnight. A, The epidermal fragments were obtained from the dark-adapted plants, and were kept in the dark for 1 h and irradiated by red light at 30 μmol m−2 s−1 for 2 h. The sizes of stomatal apertures of 60 to 70 stomata were determined microscopically. Values are the means ± se. DCMU was administered to the epidermal fragments at 20 μm in 0.02% ethanol. B, An Adiantum plant was irradiated by red light at 50 μmol m−2 s−1 from the upper side of the leaf, and stomatal conductance and photosynthetic CO2 fixation were determined. DCMU at 50 μm in 0.05% ethanol was administered to Adiantum leaves via the petiole under a fluorescent light at 30 μmol m−2 s−1 for 1 h, and then the leaves were left in the dark for 3 h before the measurement.
Accumulation of K+ in Guard Cells
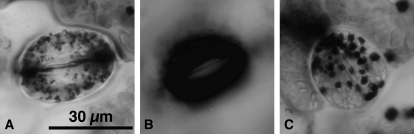
We determined the amount of K+ in guard cells of isolated epidermis to elucidate the underlying mechanisms of the light response (Fig. 6). Small amounts of K+ were detected in guard cells of the epidermal fragments prepared from leaves that had been kept in the dark for 12 h. When the fragments were irradiated by red light for 2 h at 30 μmol m−2 s−1, substantial amounts of K+ were found in the guard cells (Fig. 6B). The light-induced K+ accumulation was abolished by 20 μm DCMU, suggesting that K+ uptake was driven by photosynthetic electron transport in guard cells of Adiantum.
Figure 6.
K+ accumulation in guard cells of Adiantum epidermis. Epidermal fragments prepared from the dark-adapted leaves were kept in the dark for 1 h in 5 mm MES, 50 mm KCl, and 0.1 mm CaCl2, pH 6.5. The fragments were then irradiated by red light at 30 μmol m−2 s−1 for 2 h in the same solution. K+ was stained with sodium hexanitrocobaltate (III). A, Kept in darkness; B, irradiated by red light; C, irradiated by red light in the presence of 20 μm DCMU. Scale bar in A = 30 μm.
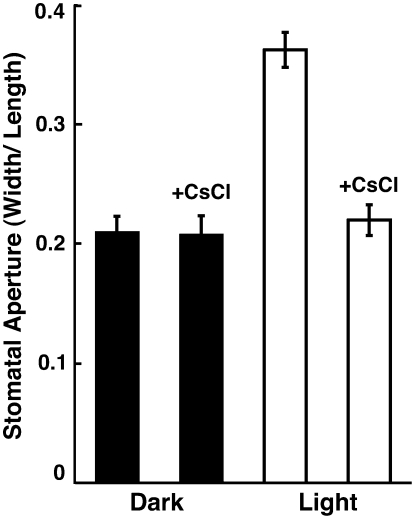
To test whether or not K+ is the major osmotica in Adiantum guard cells, we investigated the effect of Cs+, a known K+ channel blocker in plant cells (Ichida et al., 1997; Bei and Luan, 1998), on light-induced stomatal opening. Red light-induced stomatal opening was completely inhibited by Cs+ at 10 mm (Fig. 7). These results indicate that K+ uptake is essential for the stomatal opening in epidermal fragments.
Figure 7.
Inhibition of stomatal opening by CsCl in epidermal fragments of Adiantum. Epidermal fragments were prepared and irradiated by red light as in Figure 5. CsCl was added at 10 mm to the epidermal fragments, which were then kept in the dark for 1 h. Red light was irradiated onto the epidermal fragments for 2 h. The sizes of stomatal apertures of 50 to 60 stomata were determined by microscopic examination. Values are the means ± se.
DISCUSSION
Lack of CO2 Sensitivity in Adiantum
Although there are differences in the CO2 sensitivity of stomatal movement among plant species and/or their growth conditions, stomatal responses to Ca have been found in more than 50 higher plant species (Morison, 1985, 1987). High concentrations of CO2 stimulate stomatal closure, probably due to activation of anion channels in the plasma membrane, and low concentrations of CO2 induce stomatal opening by inactivating the channels (Brearley et al., 1997; Hanstein and Felle, 2002; Roelfsema et al., 2002; Roelfsema and Hedrich, 2005). The guard cells in higher plants are equipped with a mechanism to sense CO2 and may directly respond to CO2, and we confirmed that Arabidopsis stomata responded to Ca in the dark (Fig. 3). In this study, we found that Adiantum stomata lacked the sensitivity to CO2 in the dark. The Adiantum stomata neither opened at low concentrations nor closed at high concentrations of CO2 (Fig. 3). The fern Adiantum likely impairs the CO2 sensor and/or the components in the CO2 signaling cascade, such as anion channels, found in higher plant guard cells (Hashimoto et al., 2006; Marten et al., 2007). The lack of CO2 sensitivity in Adiantum stomata would result in the extremely slow stomatal closure upon darkness, as indicated in this study (Fig. 1A). It is interesting to note the very recent findings that SLOW ANION CHANNEL-ASSOCIATED1 is essential for stomatal closure in response to CO2, and the impairment of this gene greatly decreased stomatal closure upon darkness (Negi et al., 2008; Vahisalu et al., 2008). Adiantum is a plant whose stomata lack CO2 sensitivity, and conditional CO2-insensitive plant has been reported (Raschke et al., 1976; Hashimoto et al., 2006).
Guard Cell Chloroplasts Mediate Stomatal Opening in Adiantum Leaves
Stomatal response to light has been much less examined in ferns than in other plants despite the importance and prevalence of ferns in the terrestrial ecosystem, which includes over 10,000 living fern species. Recently, we demonstrated that the stomata of Leptosporangiopsida ferns lack a blue light-specific opening response (Doi et al., 2006). Phototropins do not function for stomatal opening as blue light receptors in the ferns, whereas the stomata open by red light. Thus, photosynthesis and/or phytochromes seem to be responsible for the light-induced stomatal opening in the ferns. The efficiency of light from the blue-to-red region at inducing stomatal opening indicates that photosynthesis is most likely responsible for the response. The remarkable synergistic effect between red and far-red light on stomatal opening also suggests the essential role of linear photosynthetic electron transport.
In higher plants, mesophyll photosynthesis decreases Ci, and the decreased Ci most likely induces stomatal opening (Roelfsema and Hedrich, 2005; Vavasseur and Raghavendra, 2005). As a typical case, stomata in Arabidopsis open under low Ci in the dark (Fig. 3A). We therefore suspected that the decrease in Ci induced by mesophyll photosynthesis brought about stomatal opening in the fern. However, this was not the case, because the stomata in the fern did not open in response to decreased Ci (Fig. 3, B and C). This result suggests that guard cell chloroplasts may have an important role in stomatal opening. In accord with this idea, the light was much more effective when it was applied to the lower epidermis than to the upper one and the guard cells existed exclusively in the lower epidermis in Adiantum. We next utilized epidermal fragments devoid of mesophyll and epidermal cells, in which guard cell chloroplasts are the sole photosynthetic machinery, and investigated the stomatal response. We found that the stomata opened in response to red light, and this opening was completely inhibited by DCMU (Fig. 5A). Furthermore, we applied DCMU to intact Adiantum leaves via the petiole and inspected the stomatal opening in response to red light (Fig. 5B). The red light-induced stomatal opening was inhibited by DCMU, supporting the functional importance of guard cell chloroplasts in the stomatal opening of intact leaves.
Adiantum guard cells have large and dense chloroplasts. This enables the chloroplasts to capture the light efficiently for guard cell photosynthesis in their habitat, where the ambient light is weak and enriched in far-red light. To meet the demand of CO2 for photosynthesis in mesophyll cells, Adiantum stomata are required to open in response to low-intensity light. In accord with this, we found that the stomata in Adiantum opened at the maximum rate in response to a weak red light of 20 μmol m−2 s−1 (Fig. 4), which is almost the same value as the compensation point of Arabidopsis photosynthesis (Takemiya et al., 2005).
K+ Is the Osmotica in Stomatal Response in Adiantum
We found that the stomatal opening was driven by guard cell chloroplasts in Adiantum epidermis (Fig. 5A) and was completely inhibited by DCMU. K+ accumulated in guard cells concomitantly with stomatal opening (Fig. 6), and such K+ accumulation was suppressed by DCMU and CsCl. These results suggested that K+ worked as the main osmotica for stomatal opening and that K+ was taken up via the K+ channels in the fern guard cells. Furthermore, we found that the stomatal opening in intact Adiantum leaves was inhibited by DCMU (Fig. 5B), implying that photosynthetic electron transport by guard cell chloroplasts provided energy for K+ uptake.
However, whether or not K+ is the major osmotica in stomatal opening of intact Adiantum has yet to be determined. The mechanism by which guard cells accumulate K+ remains unknown in Adiantum. According to the established mechanisms operating in higher plants, K+ accumulation is driven by the increase in membrane hyperpolarization that is brought about by activation of the H+-ATPase and deactivation of the anion channels in the plasma membrane (Schroeder et al., 2001; Marten et al., 2007; Merlot et al., 2007; Shimazaki et al., 2007). H+-ATPase activation in the fern may be the principal mechanism generating the driving force for the uptake of K+. In accord with this, we showed the presence of fusicoccin-activated H+-ATPase in the fern guard cells (Doi et al., 2006). However, the activation mechanism of the H+-ATPase by photosynthetic electron transport in the fern remains unclear. The photosynthesis-induced membrane hyperpolarization by H+-ATPase has been reported in green algae Chara (Takeshige et al., 1992) and in aquatic angiosperm Vallisneria leaves (Harada et al., 2002). In Vallisneria, the H+-ATPase is activated by a decrease in the Km for ATP through photosynthetic active radiation and is suppressed by DCMU. In Vicia guard cells, provision of ATP from guard cell chloroplasts to the cytoplasm stimulates the H+-ATPase in the plasma membrane (Tominaga et al., 2001). Further investigation is needed to address the mechanism of K+ uptake by guard cells in Adiantum.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
The wild type of Adiantum capillus-veneris was kindly provided by Professor Masamitsu Wada (National Institute for Basic Biology, Okazaki, Japan). Adiantum was grown in a growth room under a white fluorescent lamp (30 μmol m−2 s−1) on a 12-h-light/-dark cycle at 24°C.
Gas Exchange Measurements
Photosynthetic CO2 fixation and stomatal conductance were measured using a gas exchange system (LI-6400; LI-COR) equipped with an Arabidopsis (Arabidopsis thaliana) chamber (LI-COR) as previously described (Doi et al., 2004). Red light was provided from a tungsten lamp (MHF-G150LR; Moritex) by passing the light through a red cutoff filter (2-61; Corning) or from an LED Lamp Drive unit (SS-001; Shimatec) equipped with a red LED (WBS-Φ-30-16R, λ = 656 ± 18 nm). Blue light was provided by a metal halide lamp (LS-M250; Moritex) by passing the light through a blue filter (5-60; Corning) or an LED Lamp Drive unit equipped with a blue LED (WBS-Φ-30-16B, λ = 467 ± 19 nm). Green light was provided from a tungsten lamp by passing the light through a green interference filter (λ = 500 ± 26 nm; Optical Coatings Japan). Far-red light was provided from an LED Lamp Drive unit equipped with a far-red LED (WBS 720; λ = 723 ± 12 nm). PFD was measured using an LI-2500 light meter with an LI190SA quantum sensor (LI-COR) and an SKR 110 sensor (Skye Instruments). To administer DCMU to Adiantum leaves, we cut off the leaf with its petiole and placed the petiole in water under darkness. The next morning, the leaf was transferred to 0.05% ethanol solution that contained 50 μm DCMU and was kept under fluorescent light at 30 μmol m−2 s−1 for 1 h. Then, the Adiantum leaf was returned to the darkness for 3 h and used for the measurement.
Preparation of Epidermal Fragments and Determination of Stomatal Aperture
Adiantum plants were kept in the dark for 16 h before the preparation of epidermal fragments to induce stomatal closing. Ten to 15 leaves were harvested and blended three times in cold water using a Waring blender for 30 s at full speed. The blender contents were filtered through a 200-μm nylon mesh, and the retained samples were washed with 5 mm MES, 50 mm KCl, and 0.1 mm CaCl2, pH 6.5. The resultant epidermal fragments were floated in the same solution in petri dishes and kept in the dark for 1 h. The epidermal fragments were irradiated for 2 h, then 1% (w/v) fluorescein diacetate in acetone was added at 0.1 μg mL−1 to determine the viability of the guard cells. Micrographs of over 50 viable stomata were obtained using a Nikon fluorescence microscope equipped with a CCD camera. The stomatal aperture surrounded by viable guard cells was defined as the width to length ratio of the stomatal pores.
Cytochemical Detection of K+ in Guard Cells
Accumulation of K+ by guard cells was determined with sodium hexanitrocobaltate (III) staining as described previously (Green et al., 1990; Willmer and Fricker, 1996).
This work was supported in part by the Ministry of Education, Science, Sports, and Culture of Japan (grant no. 17084005 to K.S.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Ken-ichiro Shimazaki (kenrcb@mbox.nc.kyushu-u.ac.jp).
Open Access articles can be viewed online without a subscription.
References
- Assmann SM (1999) The cellular basis of sensing of rising CO2. Plant Cell Environ 22 629–637 [Google Scholar]
- Baroli I, Price GD, Badger MR, von Caemmerer S (2008) The contribution of photosynthesis to the red light response of stomatal conductance. Plant Physiol 146 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei Q, Luan S (1998) Functional expression and characterization of a plant K+ channel gene in a plant cell model. Plant J 13 857–865 [DOI] [PubMed] [Google Scholar]
- Brearley J, Venis MA, Blatt MR (1997) The effect of elevated CO2 concentrations on K+ and anion channels of Vicia faba L. guard cells. Planta 203 145–154 [Google Scholar]
- Cardon ZG, Berry J (1992) Effect of O2 and CO2 concentration on the steady-state fluorescence yield of single guard cell pairs in intact leaf discs of Tradescantia albiflora. Plant Physiol 99 1238–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M, Shigenaga A, Emi T, Kinoshita T, Shimazaki K (2004) A transgene encoding a blue-light receptor, phot1, restores blue-light responses in the Arabidopsis phot1 phot2 double mutant. J Exp Bot 55 517–523 [DOI] [PubMed] [Google Scholar]
- Doi M, Wada M, Shimazaki K (2006) The fern Adiantum capillus-veneris lacks stomatal responses to blue light. Plant Cell Physiol 47 748–755 [DOI] [PubMed] [Google Scholar]
- Franks PJ, Farquhar GD (2007) The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol 143 78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DB, Dodge SM, Lee JR, Tallman G (1990) Effect of sodium hexanitrocobaltate (III) decomposition on its staining of intracellular potassium ions. Stain Technol 65 15–24 [DOI] [PubMed] [Google Scholar]
- Hanstein SM, Felle HH (2002) CO2-triggered chloride release from guard cells in intact fava bean leaves. Kinetics of the onset of stomatal closure. Plant Physiol 130 940–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A, Okazaki Y, Takagi S (2002) Photosynthetic control of the plasma membrane H+-ATPase in Vallisneria leaves. I. Regulation of activity during light-induced membrane hyperpolarization. Planta 214 863–869 [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Negi J, Young J, Israelsson M, Schroeder JI, Iba K (2006) Arabidopsis HT1 kinase controls stomatal movement in response to CO2. Nat Cell Biol 8 391–397 [DOI] [PubMed] [Google Scholar]
- Ichida AM, Pei Z, Baizabal-Aguirre VM, Turner KJ, Schroeder JI (1997) Expression of a Cs+-resistant, light-induced stomatal opening in transgenic Arabidopsis. Plant Cell 9 1843–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K (2001) phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414 656–660 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki K (1999) Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J 18 5548–5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T, Oxborough K, Morison IL, Baker NR (2002) Responses of photosynthetic electron transport in stomatal guard cells and mesophyll cells in intact leaves to light, CO2, and humidity. Plant Physiol 128 52–62 [PMC free article] [PubMed] [Google Scholar]
- Marten H, Hedrich R, Roelfsema MRG (2007) Blue light inhibits guard cell plasma membrane anion channels in a phototropin-dependent manner. Plant J 50 29–39 [DOI] [PubMed] [Google Scholar]
- Merlot S, Leonhardt N, Fenzi F, Valon C, Costa M, Vavasseur A, Genty B, Boivin K, Mueller A, Giraudat J, et al (2007) Constitutive activation of a plasma membrane H+-ATPase prevents abscisic acid-mediated stomatal closure. EMBO J 26 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger SM, Buckley TN, Mott KA (2006) Evidence for involvement of photosynthetic processes in the stomatal response to CO2. Plant Physiol 140 771–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morison JIL (1985) Sensitivity of stomata and water use efficiency to high CO2. Plant Cell Environ 8 467–474 [Google Scholar]
- Morison JIL (1987) Intercellular concentration and stomatal response to CO2. In E Zeiger, GD Farquhar, I Cowan, eds, Stomatal Function. Stanford University Press, Palo Alto, CA, pp 229–251
- Mott KA (1988) Do stomata respond to CO2 concentration other than intercellular? Plant Physiol 86 200–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai-Yamada M, Uchimiya H, Hashimoto M, Iba K (2008) CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452 483–486 [DOI] [PubMed] [Google Scholar]
- Ogawa T, Ishikawa H, Shimada K, Shibata K (1978) Synergistic action of red and blue light and action spectrum for malate formation in guard cells of Vicia faba. Planta 142 61–65 [DOI] [PubMed] [Google Scholar]
- Olsen RL, Pratt RB, Gump P, Kemper A, Tallman G (2002) Red light activates a chloroplast-dependent ion uptake mechanism for stomatal opening under CO2 concentrations in Vicia spp. New Phytol 153 497–508 [DOI] [PubMed] [Google Scholar]
- Raschke K, Pierce M, Popiela CC (1976) Abscisic acid content and stomatal sensitivity to CO2 in leaves of Xanthium strumariium L. after pretreatments in warm and cold growth chambers. Plant Physiol 57 115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema MRG, Hanstein S, Felle HH, Hedrich R (2002) CO2 provides an intermediate link in the red light response of guard cells. Plant J 32 65–75 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Hedrich R (2005) In the light of stomatal opening: new insights into ‘the watergate’. New Phytol 167 665–691 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Konrad KR, Marten H, Psaras GK, Hartung W, Hedrich R (2006) Guard cells in albino leaf patches do not respond to photosynthetically active radiation, but are sensitive to blue light, CO2, and abscisic acid. Plant Cell Environ 29 1595–1605 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Steinmeyer R, Staal M, Hedrich R (2001) Single guard cell recordings in intact plants: light-induced hyperpolarization of the plasma membrane. Plant J 26 1–13 [DOI] [PubMed] [Google Scholar]
- Schneider H, Schuettpels E, Pryer KM, Cranfill R, Magallón S, Lupia R (2004) Ferns diversified in the shadow of angiosperms. Nature 428 553–557 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D (2001) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52 627–658 [DOI] [PubMed] [Google Scholar]
- Schwartz A, Zeiger E (1984) Metabolic energy for stomatal opening: roles of photophosphorylation and oxidative phosphorylation. Planta 161 129–136 [DOI] [PubMed] [Google Scholar]
- Serrano EE, Zeiger E, Hagiwara S (1988) Red light stimulates an electrogenic proton pump in Vicia guard cell protoplasts. Proc Natl Acad Sci USA 85 436–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Raschke K (1981) Effect of light quality on stomatal opening in leaves of Xanthium strumarium L. Plant Physiol 68 1170–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki K, Doi M, Assmann SM, Kinoshita T (2007) Light regulation of stomatal movement. Annu Rev Plant Biol 54 219–248 [DOI] [PubMed] [Google Scholar]
- Shimazaki K, Gotow K, Kondo N (1982) Photosynthetic properties of guard cell protoplasts from Vicia faba. Plant Cell Physiol 23 871–880 [Google Scholar]
- Shimazaki K, Iino M, Zeiger E (1986) Blue light-dependent proton extrusion by guard-cell protoplasts of Vicia faba. Nature 319 324–326 [Google Scholar]
- Shimazaki K, Zeiger E (1985) Cyclic and non-cyclic photophosphorylation in isolated guard cell chloroplasts from Vicia faba L. Plant Physiol 78 211–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu N, Mittmann F, Wagner G, Hughes J, Wada M (2005) A chimeric photoreceptor gene, NEOCHROME, has arisen twice during plant evolution. Proc Natl Acad Sci USA 102 13705–13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiz L, Zeiger E (2006) Plant Physiology, Ed 4. Sinauer Associates, Sunderland, MA
- Takemiya A, Inoue S, Doi M, Kinoshita T, Shimazaki K (2005) Phototropins promote plant growth in response to blue light in low light environment. Plant Cell 17 1120–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshige K, Mitsumori F, Tazawa M, Mimura T (1992) Role of cytoplasmic inorganic phosphate in light-induced activation of H+-pumps in the plasma membrane and tonoplast of Chara corallina. Planta 186 466–472 [DOI] [PubMed] [Google Scholar]
- Talbott LD, Shmayevich IJ, Chung Y, Hammad JW, Zeiger E (2003) Blue light and phytochrome-mediated stomatal opening in the npq1 and phot1 phot2 mutants of Arabidopsis. Plant Physiol 133 1522–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AR, Assmann SM (2001) Apparent absence of a redox requirement for blue light activation of pump current in broad bean guard cells. Plant Physiol 125 329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Kinoshita T, Shimazaki K (2001) Guard-cell chloroplasts provide ATP required for H+ pumping in the plasma membrane and stomatal opening. Plant Cell Physiol 42 795–802 [DOI] [PubMed] [Google Scholar]
- Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan WY, Valerio G, Lamminmäki A, Broschë M, Moldau H, Desikan R, et al (2008) SLAC1 is required for plant guard cell S-type anion channels function in stomatal signaling. Nature 452 487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavasseur A, Raghavendra A (2005) Guard cell metabolism and CO2 sensing. New Phytol 165 665–682 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Lawson T, Oxborough K, Baker NR, Andrews TJ, Raines CA (2004) Stomatal conductance does not correlate with photosynthetic capacity in transgenic tobacco with reduced amounts of Rubisco. J Exp Bot 55 1157–1166 [DOI] [PubMed] [Google Scholar]
- Wada M, Kanegae T, Nozue K, Fukuda S (1997) Cryptogam phytochromes. Plant Cell Environ 20 685–690 [Google Scholar]
- Willmer CM, Fricker MD (1996) Stomata, Ed 2. Chapman & Hall, London
- Wong SC, Cowan IR, Farquhar GD (1979) Stomatal conductance correlates with photosynthetic capacity. Nature 282 424–426 [Google Scholar]
- Wu W, Assmann SM (1993) Photosynthesis by guard cell chloroplasts of Vicia faba L. Effect of factors associated with stomatal movement. Plant Cell Physiol 34 1015–1022 [Google Scholar]
- Zeiger E, Armond P, Melis A (1981) Fluorescence properties of guard cell chloroplasts. Plant Physiol 67 17–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger E, Hepler PK (1977) Light and stomatal function: Blue light stimulates swelling of guard cell protoplasts. Science 196 887–889 [DOI] [PubMed] [Google Scholar]