Abstract
In this study, poplar (Populus alba) cellulase (PaPopCel1) was overexpressed in a tropical Leguminosae tree, sengon (Paraserianthes falcataria), by the Agrobacterium tumefaciens method. PaPopCel1 overexpression increased the length and width of stems with larger leaves, which showed a moderately higher density of green color than leaves of the wild type. The pairs of leaves on the transgenic plants closed more slowly during sunset than those on the wild-type plants. When main veins from each genotype were excised and placed on a paper towel, however, the leaves of the transgenic plants closed more rapidly than those of the wild-type plant. Based on carbohydrate analyses of cell walls, the leaves of the transgenic plants contained less wall-bound xyloglucan than those of the wild-type plants. In situ xyloglucan endotransglucosylase activity showed that the incorporation of whole xyloglucan, potentially for wall tightening, occurred in the parenchyma cells (motor cells) of the petiolule pulvinus attached to the main vein, although the transgenic plant incorporated less whole xyloglucan than the wild-type plant. These observations support the hypothesis that the paracrystalline sites of cellulose microfibrils are attacked by poplar cellulase, which loosens xyloglucan intercalation, resulting in an irreversible wall modification. This process could be the reason why the overexpression of poplar cellulase both promotes plant growth and disturbs the biological clock of the plant by altering the closing movements of the leaves of the plant.
Overexpression of plant cellulase in plants does not lead to a lack of cellulose; rather, it modifies the cell walls by trimming off disordered Glc chains from the microfibrils. This action has been demonstrated in Arabidopsis (Arabidopsis thaliana) overexpressing poplar (Populus alba) cellulase (Park et al., 2003). Transgenic poplar overexpressing Arabidopsis cellulase (cel1) had longer internodes and longer fiber cells (Shani et al., 2004). Overexpression does not increase xyloglucan depolymerization (Harpster et al., 2002), because the reaction efficiency of plant cellulase for xyloglucan is very low compared with that for amorphous 1,4-β-glucans (the amorphous regions of cellulose microfibrils). This is confirmed by the fact that the enzyme has high reactive efficiency for artificial substrates such as carboxymethylcellulose, phospho-swollen cellulose, and (1→3),(1→4)-β-glucan (Nakamura and Hayashi, 1993). Nevertheless, the trimming of microfibrils by cellulase might solubilize some xyloglucan that would otherwise have been intercalated within the disordered paracrystalline domains of the microfibrils (Hayashi, 1989). This kind of cell wall modification in Arabidopsis causes an increase in the size of cells in the petioles and blades of rosette leaves and stems (Park et al., 2003). Based on relative load extension curves, a cross-linking component appeared to be reduced in the walls of the transgenic plants compared with those of the wild-type plants. The decreased cross-linking component is attributable to a decreased amount of tethering xyloglucan and could in turn accelerate growth by increasing plastic extensibility under turgor pressure. Thus, the overexpression of cellulase could affect wall dynamics, particularly the turgor-related movements of plant organs such as the opening and closing movements of leaves and stomata, et cetera.
Poplar cellulase cDNA with 35S promoter has been used in sengon (Paraserianthes falcataria) because the overexpression of poplar cellulase in Arabidopsis and poplar produced more visible effects in their leaves (Ohmiya et al., 2003; Park et al., 2003). In these species, leaf length and width were increased to the same extent as the length of the blade and petiole. The question, therefore, is whether Leguminosae plants that overexpress cellulase (in this case, sengon) show any phenotype for leaf movements related to the walls of motor cells.
Sengon belongs to the subfamily Mimosoideae of Leguminosae and is native to Haiti, Indonesia, and Papua New Guinea. The sengon variety used for reforestation is the fastest growing tree in industrial forests. It even thrives in marginal land, where it grows symbiotically with nitrogen-fixing Rhizobium and phosphorus-promoting mycorrhizal fungi. Therefore, it is a suitable species for industrial timber estates in southeast Asian countries (Binkley et al., 2003; Shively et al., 2004; Kurinobu et al., 2007; Siregar et al., 2007). The sengon tree typically gains 7 m in height per year and reaches a mean height of 25.5 m and a bole diameter of 17 cm after 6 years. After 15 years, it reaches 39 m in height and 63.5 cm in diameter. The tree is useful not only for timber material but also for pulp and paper. Due to its soft timber and leaves that can be used as animal feed (Merkel et al., 2000), the tree has a wider range of end uses than Acacia species (Otsamo, 1998). Therefore, it is expected to be one of the most useful tree species for industrial forests. However, despite attempts with tree cuttings, tissue culture techniques with multiple propagations, and stable gene transfer using Agrobacterium tumefaciens, attempts to propagate the tree clonally have failed. In this article, we demonstrate, to our knowledge for the first time, the production of transgenic sengon.
Cellulose is an important component of plants and serves as the most abundant biopolymer on earth, with about 100 billion tons produced annually. In addition, it is a significant biological sink for CO2. It has been suggested that cellulases may have originally been involved in either the repair or arrangement of cellulose microfibrils during their biosynthesis, rather than in cellulose degradation (Hayashi et al., 2005). It has also been reported that membrane-bound cellulase (Korrigan) is required for cellulose biosynthesis (Nicol et al., 1998), but nothing is known about its role in cellulose biosynthesis.
In this study, we used the overexpression of poplar cellulase in the sengon tree in order to increase its growth rate. We hope, thereby, to increase the plant's production of raw material, not only for timber, pulp, and paper, but also for use as a biofuel (Ragaukas et al., 2006). Ultimately, experiments like this that result in an increased deposition of cellulose in the stem could produce the fastest growing tree in the world.
RESULTS
Transformation
Two-week-old hypocotyls germinated from seeds were cut into 2- to 4-mm-long stems, the explants of which were used for transformation in the same manner as leaf discs for the Agrobacterium-mediated transformation. The explants from the cut hypocotyls formed a callus-like tissue, which was followed by green color and shoot formation on Murashige and Skoog (MS) medium containing 4 μm benzylaminopurine. The transformants were selected in MS medium containing kanamycin.
During several transfers of explants to fresh medium each month, shoots were induced by the addition of benzylaminopurine (Fig. 1A) under light (4,000 lux). Benzylaminopurine alone was used as a plant hormone to induce shoots because auxin did not affect the formation of callus tissue or shoots in the presence of benzyladenine (Bon et al., 1998).
Figure 1.
Regenerating shoots and roots in transgenic and wild-type plants. A, Regenerating shoots of the transgenic plants. Arrows indicate adventitious buds. Bar = 3 mm. B, Growth of regenerated transgenic shoots. The shoots had pinnate leaflets during elongation. Bar = 2.0 cm. C, Regenerating transgenic roots. The plantlets producing roots had pinnate leaves. Bar = 1 cm. D, Regenerated plantlets of wild-type plants. Bar = 1 cm.
During direct adventitious shoot formation, parts of the explants turned green and produced green nodular structures to form adventitious buds at the apical ends. The adventitious shoots (5 mm long) were transplanted into the medium in the absence of benzylaminopurine. After the shoots elongated to 3 cm (Fig. 1B), they were again transplanted into fresh MS medium in the absence of any plant hormone for the induction of roots (Fig. 1C). No plant hormone was used for rooting because auxin and cytokinin prevented the induction of roots (Bon et al., 1998).
Pinnate leaflets were formed during shoot elongation and root formation, although young trees and shoot apical meristems in adult trees form pinnate leaves. About 30 shoots were regenerated from 400 cocultivated explants; 10 of these shoots produced roots. Ultimately, seven independent seedlings were obtained. Shoots and roots were also induced from the young shoots of wild-type plants in the presence and absence of benzylaminopurine, respectively (Fig. 1D).
It should be noted that it took about 5 to 6 months to produce transgenic seedlings and about 2 to 3 months to produce wild-type seedlings.
Cellulase Expression
To study the effects of cellulase on cell wall structure and growth, we generated transgenic sengon plants that expressed poplar cellulase (PaPopCel1) under the control of a constitutive promoter. To assay the expression of the transgene, we performed reverse transcription-PCR Southern-blot analysis of mRNAs derived from small sections excised from the petiolule pulvinus (Fig. 2A). PopCel1 mRNA was accumulated in transgenic lines 1 to 7 (trg1–trg7), and weak signals were detected in trg4 to trg7. Also, we used an antibody against a 15-amino acid sequence (163-CWERPEDMDTPRNVY-167) for the PaPopCel1 gene product (Ohmiya et al., 2003).
Figure 2.
Analysis of PaPopCel1 expression in the main vein with petiolule pulvinus. A, Reverse transcription-PCR Southern-blot analysis of PaPopCel1 mRNA. The relative amounts of mRNAs by reverse transcription-PCR analysis at 15 cycles are shown. B, Western-blot analysis of cell wall proteins. Five micrograms of protein was used for each. C, Level of cellulase activity. D, Level of cello-oligosaccharides. Three separate main veins for each plant were used for the determination.
In each transgenic line (trg1–trg7), the antibody recognized a single, 50-kD band on a western blot, present in the petiolule pulvinus, running at a position corresponding to the expected and actual size of the mature cellulase (Fig. 2B). No signal was detected in the wild-type plants.
In the pulvinus attached to the veins of the transgenic plants, cell wall fractions showed cellulase activity that was approximately 1.05- to 5.25-fold higher than that of the wild type (Fig. 2C). The activity of cellulase was also assessed by measuring soluble cello-oligosaccharides, which are presumably released by the enzyme (Fig. 2D). These oligosaccharides accumulated in the transgenic plants, as all of the transgenic plants were found to contain far more oligosaccharides than the wild-type plants contained. The amount of oligosaccharides was closely related to cellulase activity levels in each of the seven nic lines. Thus, the levels of expression and activity varied among the transgenic plants: they were relatively high in trg1 to trg3 and relatively low in trg7, although a trace of cello-oligosaccharides were detected even in trg7. This was probably because the poplar cellulase expressed was discretely localized in the cellulose microfibrils of the apoplastic spaces.
Based on the carbohydrate analyses of cell walls, it appears that the petiolule pulvinus and the main vein in the transgenic plants contained less wall-bound xyloglucan than those in the wild-type plants (Table I). Increased cellulase activity in the wall did not decrease the levels of cellulose; rather, cellulose content per plant increased with plant growth (i.e. cellulose per milligram dry weight was not changed). The methylated sugars due to the minor components consisted of 4-linked Xyl, 4-linked Gal, and 4-linked Man at a constant proportion in both the transgenic and the wild-type plants (data not shown). These methylated sugars are probably derived from xylan, galactan, and mannan at a ratio of 4.7:3.2:1. Therefore, the transgenic plants differ from the wild-type plants only in the amount of xyloglucan present in the cell walls.
Table I.
Xyloglucan and cellulose content in cell walls
Xyloglucan was determined from 24% KOH-soluble fractions. Three separate main veins for each plant were used for the determination. se values were calculated from three samples per line.
| Plant | Petiolule Pulvinus
|
Main Vein
|
||
|---|---|---|---|---|
| Xyloglucan | Cellulose | Xyloglucan | Cellulose | |
| μg mg−1dry weight | ||||
| Wild type | 41.1 ± 2.2 | 370 ± 32 | 16.0 ± 2.2 | 454 ± 23 |
| trg1 | 9.1 ± 1.4 | 371 ± 18 | 8.1 ± 0.9 | 460 ± 55 |
| trg2 | 8.3 ± 1.3 | 372 ± 20 | 9.1 ± 1.2 | 455 ± 32 |
| trg3 | 8.8 ± 1.8 | 378 ± 21 | 8.2 ± 0.8 | 461 ± 31 |
| trg4 | 10.4 ± 1.5 | 377 ± 28 | 8.7 ± 1.6 | 451 ± 43 |
| trg5 | 11.6 ± 1.8 | 374 ± 32 | 8.9 ± 0.9 | 452 ± 53 |
| trg6 | 10.1 ± 2.1 | 385 ± 38 | 7.9 ± 1.3 | 460 ± 47 |
| trg7 | 12.0 ± 2.8 | 366 ± 24 | 8.2 ± 1.4 | 458 ± 44 |
Admittedly, there is no correlation between cellulase activity (Fig. 2B) and the extent of xyloglucan solubilization (Table I). Nevertheless, the levels of soluble cello-oligosaccharides, which could be related to in vivo cellulase activity, were closely related to cellulase activity levels across the seven transgenic lines and were consistently higher in the transgenic plants than in the wild-type plants, which again corresponds to cellulase activity.
Growth Response of Transgenic Plants
We generated seven independent transgenic sengon lines, four of which (trg1–trg4) grew significantly better than the wild type, although the overall morphology of these transgenic plants was similar to that of the wild type (Fig. 3). Two other transgenic lines (trg5 and trg6) grew slightly better than the wild type, and one (trg7) grew about as well as the wild type. Based on the expression of the transgene, the four lines that showed a high growth rate (approximately 20-cm stem length) were selected for further analysis.
Figure 3.
Effects of PaPopCel1 transgenes on stem growth. A, Increase in stem length. B, Increase in stem diameter. Black circles, trg1; white circles, trg2; black squares, trg3; white squares, trg4; white triangles, wild type.
The transgenic sengon plants (trg1–trg4) grew faster than the wild-type plants, although the young seedlings (less than 30 cm in height) of both types sometimes grew at the same rate. The stems of the transgenic plants elongated faster than those of the wild-type plants and had larger diameters (Fig. 3). The overall morphology of the transgenic plants was similar to that of the wild type (Fig. 4A). As with stem growth, the leaves of the transgenic plants were greener and larger than those of the wild type (Fig. 4B). In both types, the length and width of the leaves increased to the same extent as the length of the main and minor veins, and this increase in size was even distributed among all leaves of the plant. Parenchyma cells in leaves of both types were identified in the central part of the petioles. Finally, both palisade and epidermal cells were a little larger in the leaves of the transgenic plants than in the leaves of the wild-type plants (data not shown).
Figure 4.
Wild-type and transgenic (trg1) plants. The wild-type and transgenic plants are shown on the left and right, respectively, at 390 d after adventitious shoot formation. A, Whole plants. Bar = 10 cm. B, Leaves. Bar = 1.5 cm.
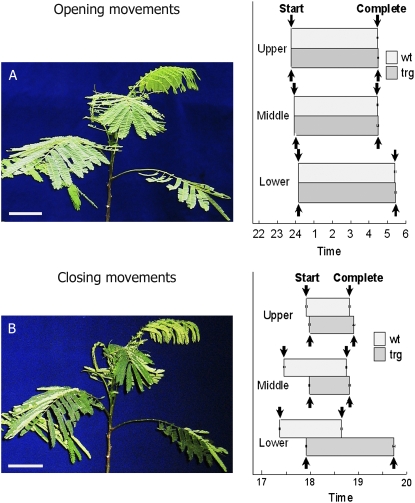
Figure 5 shows the times at which the transgenic and wild-type plants started leaf opening before sunrise and completed leaf closure during sunset. There was no difference between transgenic and wild-type plants in the starting time of leaf opening (Fig. 5A); the leaves in both types of plants started opening around midnight and completed opening by 5:25 am. In contrast, the leaf pairs closed more slowly in the transgenic plants than in the wild-type plants. This difference was visible in the upper, middle, and lower parts of the petioles (Fig. 5B). In the transgenic plants, older leaves located at the middle and bottom part of the stems started closing 30 min later than the corresponding leaves in the wild-type plants. Likewise, in the transgenic plants, leaves at the bottom part of the stem completed closing more than 1 h later than the corresponding wild-type leaves. However, both the transgenic and wild-type plants closed their leaves within a few minutes when they were placed in darkness at noon (data not shown). In spite of this change to their normal conditions, they also started opening their leaves at almost the same time (midnight) and finished opening completely by 5:25 am (Fig. 5A).
Figure 5.
Leaf movements of upper, middle, and lower parts of petioles in the wild-type and transgenic plants. A, Opening. All of the leaves are open (left). Bar = 4 cm. B, Closing. Closing leaves are distinguishable as yellow and white leaves (left). Bar = 4 cm. The lower part of the petiole was defined as the second petiole from the bottom, the middle part as the fifth or sixth petiole from the bottom, and the upper part as the ninth or tenth (or newest) petiole. All of the leaves in the petiole were observed to determine the opening and closing times of leaf pairs from start to finish. se values were calculated from four lines of trg1, trg2, trg3, and trg4.
Interestingly, the transgene had the opposite effect on excised main veins. When the main vein with leaves was excised and placed on a paper towel, the pairs of leaves from the transgenic plants closed faster than those of the wild-type plants (Fig. 6). The younger leaves in the upper part of each plant started closing immediately; leaves in the middle part completed closing more than 1 h later; and leaves in the lower part completed closing 2 h later. The older leaves in the middle and lower parts of the transgenic plants completed closing more than 30 min earlier than those of the wild-type plants. Thus, when the main vein was excised, closing was faster in the leaves of the transgenic plants than in those of the wild-type plants, whereas in vivo, closing was slower in the transgenic leaves.
Figure 6.
Closing movements of leaves whose main vein was excised. Leaves in the vein at the upper, middle, and lower parts of petioles are shown from left to right. se values were calculated from four lines of trg1, trg2, trg3, and trg4. Bar = 2 cm.
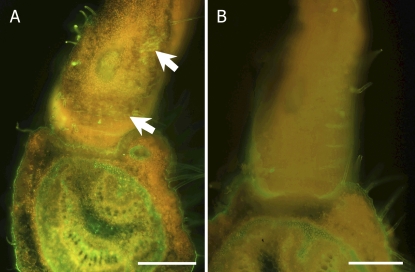
Xyloglucan endotransglucosylase activity was detected in situ on the transverse sections of the petiolule pulvinus attached to the main vein using either fluorescent whole xyloglucan (50 kD) or fluorescent xyloglucan heptasaccharide (XXXG; Takeda et al., 2002). Whole xyloglucan was incorporated into the parenchyma cells (motor cells) of the pulvinus in both genotypes, although the transgenic plants incorporated less xyloglucan than the wild-type plants (Fig. 7). The incorporation of whole xyloglucan into the vascular bundle of the main vein was also greater in the wild type than in the transgenic plants, although it was incorporated into the connection between the petiolule pulvinus and the main vein at the same rate in both genotypes.
Figure 7.
In situ xyloglucan endotransglucosylase activity incorporating green fluorescent whole xyloglucan for 15 min on the transverse section (200 μm) of the petiolule pulvinus attached to the main vein of trg1. The tissues of the pulvinus and vein are shown in the upper and lower areas, respectively, in the images of the wild-type (A) and transgenic (B) plants. The arrows indicate the incorporated whole xyloglucan in the parenchyma motor cells. The red color is due to the autofluorescence of chloroplasts. Bars = 0.5 mm.
In contrast, the incorporation of XXXG, either into the parenchyma cells of the pulvinus or into the vascular bundle of the main vein, was not observed in either genotype. These results show that the walls of the parenchyma cells (motor cells) can incorporate whole xyloglucan but not XXXG. The level of incorporation was higher in the wild-type plants than in the transgenic plants, probably because the walls of the transgenic plants contain less endogenous xyloglucan molecules to act as donors. Another possible explanation is that the increased cellulase in the transgenic plants cleaves the glucan chains to which xyloglucan binds, so that the glucan chains are washed out, making it more difficult for xyloglucan molecules to attach firmly to the wall.
DISCUSSION
We have succeeded in producing transgenic sengon plants for the first time. Seven independent shoots regenerated from 400 cocultivated explants were demonstrated to be transgenic; this represents a transformation frequency of 1.75%. So far, we have not succeeded in either multiple propagation of the transgenic shoots or clonal propagation by cutting their stems. Nevertheless, shoots and roots were induced in MS medium in both the presence and absence of 4 μm benzylaminopurine. The shoots always formed from the callus-like tissues of explants from the cut hypocotyls, although the shoots and roots formed directly from the explants of hypocotyls in the case of Acacia sinuate (Vengadesan et al., 2006). Now sengon can be genetically modified to exhibit desirable traits, such as easy degradation of cellulose microfibrils as well as pathogen and insect resistance, qualities that are difficult to achieve by traditional breeding. It would also be expected to increase the transformation frequency, particularly the induction of roots from shoots. The increased frequency of root induction should accelerate the biotechnological application of sengon as a biomass resource. It should be noted that wild-type sengon is already one of the fastest growing tree species in the world; the transgenic sengon developed in this study grows even faster and may ultimately be the fastest growing tree in the world.
Transgenic sengon overexpressing poplar cellulase (PaPopCel1) increased the size of leaves by increasing cell volume, as other authors have demonstrated in Arabidopsis leaves (Park et al., 2003). This phenomenon has been attributed to an increase in the specific activity of cellulase, similar to the increase observed in transgenic Arabidopsis and poplar (Park et al., 2003; Shani et al., 2004). Nevertheless, no bulk degradation of cellulose was confirmed, because the amount of cellulose per dry weight was nearly the same in the transgenic and the wild-type plants. Therefore, we believe that cellulase promotes increased growth in transgenic sengon by trimming off disordered Glc chains from cellulose microfibrils, where some xyloglucan would otherwise be solubilized. Residual xyloglucan can adhere tightly to cellulose microfibrils, perhaps as a monolayer coating the surface, but the transgenic plants contained less xyloglucan bound to cellulose microfibrils. This agrees with a previous finding that a decrease in xyloglucan tethers accelerates cell elongation (Takeda et al., 2002).
We have found that leaf movements are somehow disturbed by the transgene expression. The transgenic plants opened their leaf pairs at the same time as the wild-type plants (midnight) but started closing their leaves 30 min later and completed closing them more than 1 h later (Fig. 5). Nevertheless, transgenic sengon still retains a type of circadian rhythm in the opening and closing movements of leaves, although sterilized sengon (in vitro culture) did not show closing movements at night, even if the plant was placed in darkness. The opening and closing movements of leaves occur in the leaf bases (petiolule pulvinus) and are caused by the expansion and contraction of the motor cells. Motor cells occupy most of the space in the pulvinus, surrounding the central ring of the vascular bundle. The expansion and contraction of these cells is believed to result from changes in their turgor, which is regulated in turn by the flow of K+ ions across the cells' thin walls.
In the case of transgenic sengon that overexpresses poplar cellulase, an increase in wall plasticity may cause changes in normal leaf movements, because the movements correspond to the balance between turgor pressure and wall pressure. Therefore, the turgor pressure in transgenic sengon motor cells might increase during the day and decrease at night, while the wall pressure remains constant. In the case of pea (Pisum sativum) hypocotyls, the wall pressure in growing cells is decreased during elongation, while the turgor pressure remains constant. Since the walls of the motor cells in the pulvinus incorporated whole xyloglucan but not XXXG, wall tightening rather than loosening could be required to prevent the expansion of the cells at a cut surface (Takeda et al., 2002).
In this case, the xyloglucan endotransglucosylase activity could occur between high-molecular-size xyloglucans in the cell walls, where the enzyme has both enzyme-donor and enzyme-acceptor complexes. Ueda and Nakamura (2007) suggested that leaf movements are controlled by the balance of the concentrations of chemical substances involving an aglycon or a glucoside, which induce opening and closing, respectively. Nakanishi et al. (2005) showed that in excised leaves of Oxalis corymbosa, the opening movement was sensitive to blue light but not to red light. It should be noted that cellulase tends to affect the down-regulation of leaf movements, whereas chemical substances and light tend to affect the up-regulation of leaf movements.
Darwin (1880) postulated that the nyctinastic movements of plants occurred in order to hide the upper surface of the leaves at night, because the upper leaf epidermis was believed to be a sense organ for adaptive responses. Bunning and Moser (1969), on the contrary, suggested that adaptive leaf movements occurred to protect the plant's photoperiodic rhythm against radiation from moonlight rather than against radiation from the leaf surfaces into the sky. This cannot be considered a satisfactory explanation in the case of sengon, however, because pairs of sengon leaves start to open at night. It should be noted that light does not induce the opening movements during normal growth.
It is possible that the transgenic sengon plants have slightly higher photosynthetic capability than the wild-type plants, since the leaf pairs of the transgenic plants close more slowly than those of the wild-type plants. The sun always sets in Indonesia by 6:40 pm, while the transgenic plants keep their leaf pairs open for about 1 h after the normal sunset time. We are performing detailed analyses of the plant's leaf movements to determine their photosynthetic efficiency.
MATERIALS AND METHODS
Transgenic Constructs
The PaPopCel1 cDNA fragment was excised from pBluescript SK+ by digestion with BamHI and KpnI (Nakamura et al., 1995). The GUS-coding sequence of pBE2113 was removed from the fragment by digestion with BamHI and SacI, and the cDNA fragment was inserted into the pBE vector between the cauliflower mosaic virus 35S promoter and the Agrobacterium tumefaciens NOS transcription terminator. The chimeric construct was introduced into the disarmed Agrobacterium strain LBA4404.
Plant Transformation
Agrobacterium carrying plasmid-harboring poplar (Populus alba) cellulase cDNA (PaPopCel1) and selectable marker NPTII genes were cultured in YES medium (0.1% yeast extract, 0.5% polypeptone, 0.5% Suc, and 0.0246% MgSO4) containing 50 μg mL−1 kanamycin. The bacterial suspension was pelleted and resuspended with sterilized water. Seeds were germinated for 2 weeks to produce hypocotyls elongating 1 to 2 cm in length.
Pieces of sengon (Paraserianthes falcataria) stems (2–4 mm in length) excised from their hypocotyls were dipped in diluted Agrobacterium solution (optical density at 600 nm = 0.1) for 5 to 10 min and put on sterile filter paper, then cocultivated for 1 d on half-strength hormone-free MS medium. Next, the pieces of stems were placed on MS agar medium containing 600 μg mL−1 kanamycin for 2 weeks, after which they were washed with a water solution containing 400 μg mL−1 Claforan. The stems were cultured several times by transplantation on MS agar medium containing 600 μg mL−1 kanamycin and 4 μm benzylaminopurine for 2 to 4 months, under 14-h-day (4,000 lux)/10-h-night cycles, after which they were placed on a medium containing 300 μg mL−1 kanamycin for 2 weeks. Shoots 5 mm in length were excised from the medium and cultured again on a medium containing 300 μg mL−1 kanamycin in the absence of plant hormone. Roots were then formed in the medium for 2 to 4 weeks, and the plantlets were further cultured for growth in the medium for 2 months. Plantlets approximately 10 to 15 cm long were planted in soil.
RNA Isolation and Reverse Transcription-PCR Analysis
Total RNA was isolated from the main vein with petiolule pulvinus (Ohmiya et al., 2000). The first-strand cDNA was synthesized using 5 μg of total RNA at 42°C for 1 h using oligo(dT) (n = 20) and SuperScript II reverse transcriptase (Gibco BRL). PCR was performed with final volumes of 20 μL containing 0.5 unit of cDNA polymerase mix (Clontech), 0.2 mm dNTPs, 3.5 mm Mg(OAc)2, and 0.4 μm gene-specific primers. The forward primer was 5′-CACCACGCAATGTGTACAAAGTAACCATC-3′ (nucleotide positions 512–540), and the reverse primer was 5′-GGGTGTATATTGGGCCTGGAAACTAGAAGTT-3′ (nucleotide positions 993–1,023) with the first-strand cDNA. The PCR was initially denatured at 94°C for 5 min and in the subsequent cycles at 94°C for 30 s. Annealing and elongation cycles were both performed for 3 min at 68°C. PCR products were size separated by electrophoresis on a 0.9% agarose gel and blotted onto nylon membranes (Hybond-N+; Amersham-Pharmacia Biotech).
Membranes were hybridized in 5× SSC, with 1.0% blocking reagent, 0.1% lauroylsarcosine, and 0.02% SDS at 42°C, to digoxigenin-dUTP-labeled probes. Probes were labeled using the DIG-DNA labeling kit (Roche Diagnostics) and were synthesized from PopCel1 cDNA by gene-specific primers.
Following hybridization, the membranes were washed in 2× SSC for 5 min at room temperature and then two times in 0.1× SSC with 0.1% SDS at 68°C for 15 min each time. The washed membranes were developed using the DIG-DNA detection kit (Roche Diagnostics) for chemiluminescent detection.
Western-Blot Analysis
After the leaves were removed, the main vein with petiolule pulvinus in the middle part of the petiole was homogenized in 20 mm sodium phosphate buffer (pH 6.2) in a mortar, and the wall residue was washed three times. The wall-bound proteins were extracted from the wall residue with a buffer containing 1 m NaCl. The proteins were then subjected to electrophoresis with 10% SDS-PAGE, electrotransferred to Hybond-C Extra (Amersham), and probed with an antibody against the PopCel sequence, followed by a second antibody using the Toyobo ABC High-HRP immunostaining kit. Seven lines of transgenic plants were assayed.
Cellulase Activity Assay
Each enzyme preparation was obtained from the wall residue of the main vein with petiolule pulvinus in the middle part of the petiole with a buffer containing 1 m NaCl, and its activity was assayed viscometrically at 35°C for 2 h, using 0.1 mL of the enzyme preparation plus 0.9 mL of 10 mm sodium phosphate buffer (pH 6.2) containing 0.65% (w/v) carboxymethylcellulose in semimicroviscometers from Cannon Instruments. One unit of activity is defined as the amount of enzyme required to cause 0.1% loss in viscosity in 2 h under such conditions (Ohmiya et al., 1995). Protein was determined using the Coomassie Plus protein assay reagent (Pierce), according to the method described by Bradford (1976).
Determination of Cello-Oligosaccharides
After the leaves were removed, the main vein with petiolule pulvinus in the middle part of the petiole was homogenized in 20 mm sodium phosphate buffer (pH 6.2) in a mortar. The soluble extract was boiled for 5 min and left at room temperature for 24 h to equilibrate the anomer configuration between α- and β-types. After centrifugation, the amount of cello-oligosaccharides was determined by cellobiose dehydrogenase purified from conidia spores of Phanerochaete chrysosporium (Tominaga et al., 1999). The reaction mixture contained 90 mU (10 μL) of cellobiose dehydrogenase, 50 μm cytochrome c (10 μL), and sample solution (70 μL) in 100 mm sodium acetate buffer, with a pH of 4.2. After incubation for 5 min at room temperature, the A550 was determined. A linear standard curve was obtained with a standard cellobiose solution, and an absorbance of 0.5% corresponded to approximately 270 ng per 100 μL of reaction mixture for cellobiose.
Fractionation and Measurement of Wall Components
The main vein and petiolule pulvinus in the middle part of the petiole were separated after the leaves had been removed. Each sample was ground in liquid nitrogen and freeze-dried before its dry weight was determined. The sample was successively extracted six times with 10 mm sodium phosphate buffer (pH 7.0) and three times with 24% KOH containing 0.1% NaBH4 at less than 45°C for 3 h in an ultrasonic bath. The insoluble wall residue (the cellulose fraction) was washed with water and solubilized with ice-cold 72% sulfuric acid. Total sugar in each fraction was determined by the phenol/sulfuric acid method (Dubois et al., 1956). The xyloglucan level was determined by the iodine/sodium sulfate method (Kooiman, 1961).
Growth Measurements
The growth response of the transgenic plants was monitored after they were transplanted in soil and habituated for 3 weeks under nonsterile conditions. Each stem (around 15 cm) was marked at a height of 5 cm, which was used as a reference point for measuring the height, diameter, and number of internodes every third day. The length of the stem was determined from the top to the reference point. Dry weight was determined after freeze drying the samples.
The timing of the leaf movement was determined by observing the movements every day for 20 d during both the rainy (January) and dry (May) seasons. The closing movements of the leaves with the base of their main vein excised occurred between 10 and 11 am during both the rainy and dry seasons. The veins with leaves attached were placed on paper towels immediately after excision.
Four transgenic lines, trg1, trg2, trg3, and trg4, were used to observe the opening and closing movements of leaves. These plants had nine or 10 petioles each, all of which were more than 10 cm long. The lower part of the petiole was defined as the second petiole from the bottom, the middle part as the fifth or sixth petiole from the bottom, and the upper part as the ninth or tenth (or newest) petiole. All of the pairs of leaves attached to the main vein in the petiole were observed to determine the opening and closing movements.
Fluorescent Xyloglucans
Fluorescent xyloglucan (Takeda et al., 2002) was prepared by dissolving 10 mg of cyanogen bromide and 20 mg of pea (Pisum sativum) xyloglucan (50 kD) in 1 mL of water and adjusting the pH to 11.0 by adding NaOH. The activated polysaccharide was incubated with 4 mg of fluoresceinamine overnight at room temperature. The fluorescein-labeled xyloglucan was purified by gel filtration on a Sephadex G-50 column (Amersham-Pharmacia Biotech). Calculations showed that 1 μmol of fluorescein incorporated into 110 μmol of sugar residues, corresponding to a substitution rate of 3.7 mol of fluorescein per mole of xyloglucan. To prepare fluorescent XXXG (Fry, 1997), 40 mg of XXXG was aminated with 2 mL of 0.4 m sodium cyanotrihydroborate in 2.0 m ammonium chloride at 100°C for 120 min. The aminated oligosaccharide was purified by gel filtration on Bio-Gel P-2 and incubated with 50 mg of fluorescein isothiocyanate in sodium carbonate bicarbonate buffer (pH 9.0) for 2 h at room temperature. The oligosaccharide-fluorescein isothiocyanate conjugate was purified by gel filtration on Bio-Gel P-2.
In Situ Xyloglucan Endotransglucosylase Activity
Transverse sections (200 μm) of the main vein including petiolule pulvinus with fluorescent derivatives were incubated for 15 min in 300 μL of 2 mm MES/KOH buffer (pH 6.2) containing 0.2 mm fluorescent whole xyloglucan or 9 mm fluorescent XXXG while being shaken in darkness at 23°C. The sections that were incubated with whole xyloglucan were washed three times in 0.01 m NaOH for 30 min. Those incubated with XXXG were washed with 5% formic acid in 90% ethanol for 5 min followed by 5% formic acid for 5 min (Takeda et al., 2002). The sections were washed twice with water and examined using a Zeiss Axioscope microscope equipped with epifluorescence illumination.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number D32166 (PopCel1 cDNA).
Acknowledgments
We thank Takahide Tsuchiya and Nobuyuki Kanzawa (Department of Chemistry, Sophia University) for valuable discussions during the final preparation of this article.
This work was supported by the Program for the Promotion of Basic Research Activities for Innovative Biosciences and by JSPS KAKENHI (grants nos. 19208016 and 19405030). This work is also part of the outcome of the JSPS Global COE Program (E–04): In Search of Sustainable Humanosphere in Asia and Africa.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Takahisa Hayashi (taka@rish.kyoto-u.ac.jp).
Open Access articles can be viewed online without a subscription.
References
- Binkley D, Senock R, Bird S, Cole TG (2003) Twenty years of stand development in pure and mixed stands of Eucalyptus saligna and nitrogen-fixing Facaltaria moluccana. For Ecol Manage 182 93–102 [Google Scholar]
- Bon MC, Bonal D, Goh DK, Monteuuis O (1998) Influence of different macronutrient solutions and growth regulators on micropropagation of juvenile Acacia mangium and Paraserianthes falcataria explants. Plant Cell Tissue Organ Cult 53 171–177 [Google Scholar]
- Bradford MN (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–254 [DOI] [PubMed] [Google Scholar]
- Bunning E, Moser I (1969) Interference of moonlight with the photoperiodic measurement of time by plants, and their adaptive reaction. Proc Natl Acad Sci USA 62 1018–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C (1880) The Power of Movement in Plants. John Murray, London
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28 350–356 [Google Scholar]
- Fry SC (1997) Novel ‘dot-blot’ assays for glycosyltransferases and glycosylhydrolases: optimization for xyloglucan endotransglycosylase (XET) activity. Plant J 11 1141–1150 [Google Scholar]
- Harpster MH, Dawson DM, Nevins DJ, Dunsmuir P, Brummell DA (2002) Constitutive overexpression of ripening-related pepper endo-1,4-beta glucanase in transgenic tomato fruit does not increase xyloglucan depolymerization of fruit softening. Plant Mol Biol 50 357–369 [DOI] [PubMed] [Google Scholar]
- Hayashi T (1989) Xyloglucans in the primary cell wall. Annu Rev Plant Physiol Plant Mol Biol 40 139–168 [Google Scholar]
- Hayashi T, Yoshida K, Park YW, Konishi T, Baba K (2005) Cellulose metabolism in plants. Int Rev Cytol 247 1–34 [DOI] [PubMed] [Google Scholar]
- Kooiman P (1961) The constitution of Tamarindus-amyloid in plant seeds. Recl Trav Chim Pays Bas 80 849–865 [Google Scholar]
- Kurinobu S, Prehatin D, Mohanmad N, Matsune K, Chigira O (2007) A provisional growth model with a size-density relationship for a plantation of Paraserianthes falcataria derived from measurements taken over 2 years in Pare, Indonesia. J For Res 12 230–236 [Google Scholar]
- Merkel RC, Pond KR, Burns JC, Fisher DS (2000) Rate and extent of dry matter digestibility in sacco of both oven- and freeze-dried Paraserianthes falcataria, Calliandra calothyrsus, and Gliricidia sepium. Trop Agric 77 1–5 [Google Scholar]
- Nakamura S, Hayashi T (1993) Purification and properties of extracellular endo-1,4-β-glucanase from suspension-cultured poplar cells. Plant Cell Physiol 34 1009–1013 [PubMed] [Google Scholar]
- Nakamura S, Mori H, Sakai F, Hayashi T (1995) Cloning and sequencing of cDNA for poplar endo-1,4-β-glucanase. Plant Cell Physiol 36 1229–1235 [PubMed] [Google Scholar]
- Nakanishi F, Nakazawa M, Katayama N (2005) Opening and closing of Oxalis leaves in response to light stimuli. J Biol Educ 39 87–91 [Google Scholar]
- Nicol F, His I, Jauneau A, Vernhettes S, Canut H, Hofte H (1998) A plasma membrane-bound putative endo-1,4-β-glucanase is required for normal wall assembly and cell elongation in Arabidopsis. EMBO J 17 5563–5576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmiya Y, Nakai T, Park YW, Aoyama T, Oka A, Sakai F, Hayashi T (2003) The role of PopCel1 and PopCel2 in poplar leaf growth and cellulose biosynthesis. Plant J 33 1087–1097 [DOI] [PubMed] [Google Scholar]
- Ohmiya Y, Nakamura S, Sakai F, Hayashi T (1995) Purification and properties of wall-bound endo-1,4-β-glucanase from suspension-cultured poplar cells. Plant Cell Physiol 36 607–614 [PubMed] [Google Scholar]
- Ohmiya Y, Samejima M, Amano Y, Kanda T, Sakai F, Hayashi T (2000) Evidence that endo-1,4-β-glucanase act on cellulose in suspension-cultured poplar cells. Plant J 24 147–158 [DOI] [PubMed] [Google Scholar]
- Otsamo R (1998) Effect of nurse tree species on early growth of Anisoptera marginata Korth. (Dipterocarpaceae) on an Imperata cylindrica (L.) Beauv. grassland site in south Kalimantan, Indonesia. For Ecol Manage 105 303–311 [Google Scholar]
- Park YW, Tominaga R, Sugiyama J, Furuta Y, Tanimoto E, Samejima M, Sakai F, Hayashi T (2003) Enhancement of growth by expression of poplar cellulase in Arabidopsis thaliana. Plant J 33 1099–1106 [DOI] [PubMed] [Google Scholar]
- Ragaukas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA, Frederick WJ Jr, Hallett JP, Leak DJ, Liotta CL, et al (2006) The path forward for biofuels and biomaterials. Science 311 484–489 [DOI] [PubMed] [Google Scholar]
- Shani Z, Dekel M, Tsabary G, Goren R, Shoseyov O (2004) Growth enhancement of transgenic poplar plants by overexpression of Arabidopsis thaliana endo-1,4-β-glucanase (cel1). Mol Breed 14 321–330 [Google Scholar]
- Shively GE, Zelek CA, Midmore DJ, Nissen TM (2004) Carbon sequestration in a tropical landscape: an economic model to measure its incremental cost. Agrofor Syst 60 189–197 [Google Scholar]
- Siregar UJ, Rachmi A, Massijaya MY, Ishibashi N, Ando K (2007) Economic analysis of sengon (Paraserianthes falcataria) community forest plantation, a fast growing species in East Java, Indonesia. For Policy Econ 9 822–829 [Google Scholar]
- Takeda T, Furuta Y, Awano T, Mizuno K, Mitsuishi Y, Hayashi T (2002) Suppression and acceleration of cell elongation by integration of xyloglucans in pea stem segments. Proc Natl Acad Sci USA 99 9055–9060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga R, Samejima M, Sakai F, Hayashi T (1999) Occurrence of cello-oligosaccharides in the apoplast of auxin-treated pea stems. Plant Physiol 199 249–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M, Nakamura Y (2007) Chemical basis of plant leaf movement. Plant Cell Physiol 48 900–907 [DOI] [PubMed] [Google Scholar]
- Vengadesan G, Amutha S, Muruganantham M, Anand RP, Ganapathi A (2006) Transgenic Acacia sinuata from Agrobacterium tumefaciens-mediated transformation of hypocotyls. Plant Cell Rep 25 1174–1180 [DOI] [PubMed] [Google Scholar]