Abstract
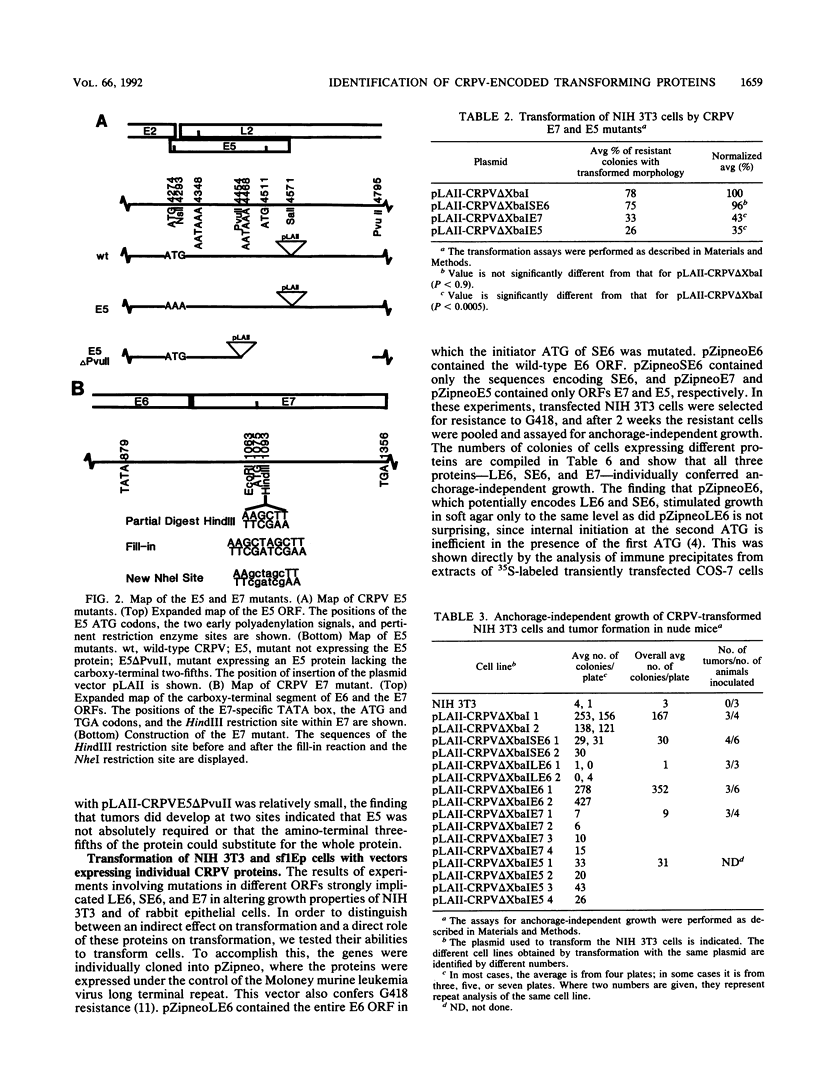
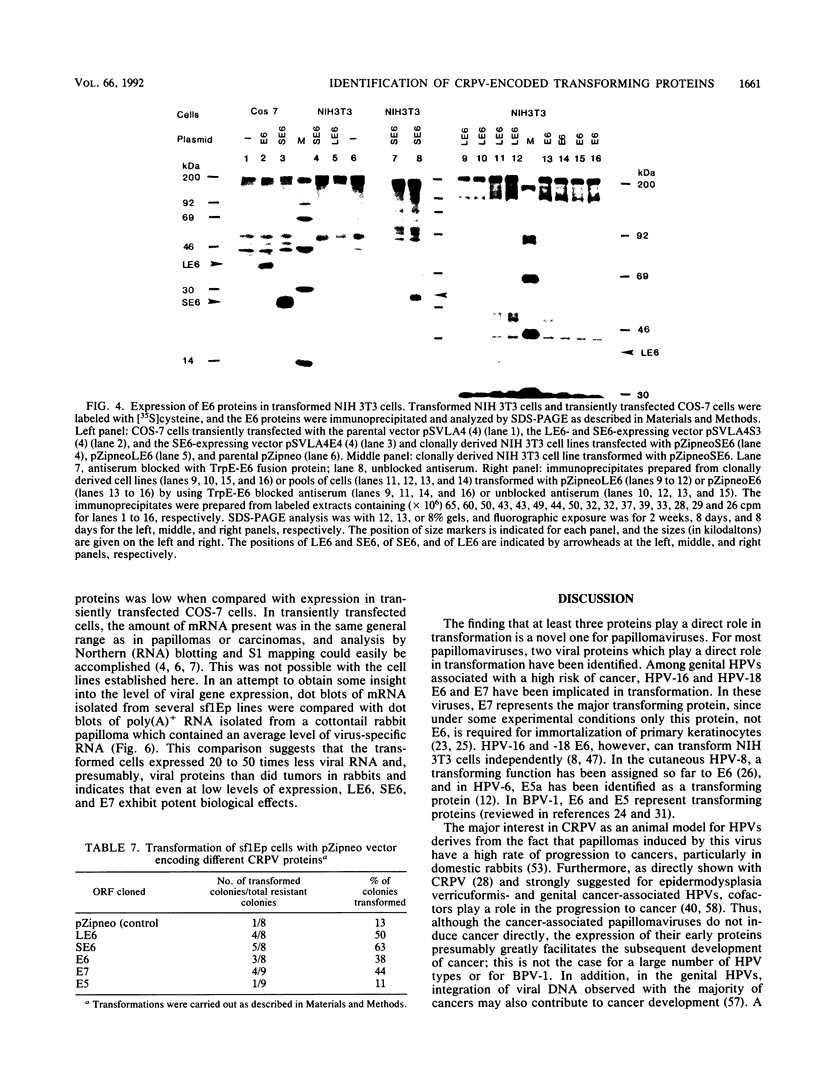
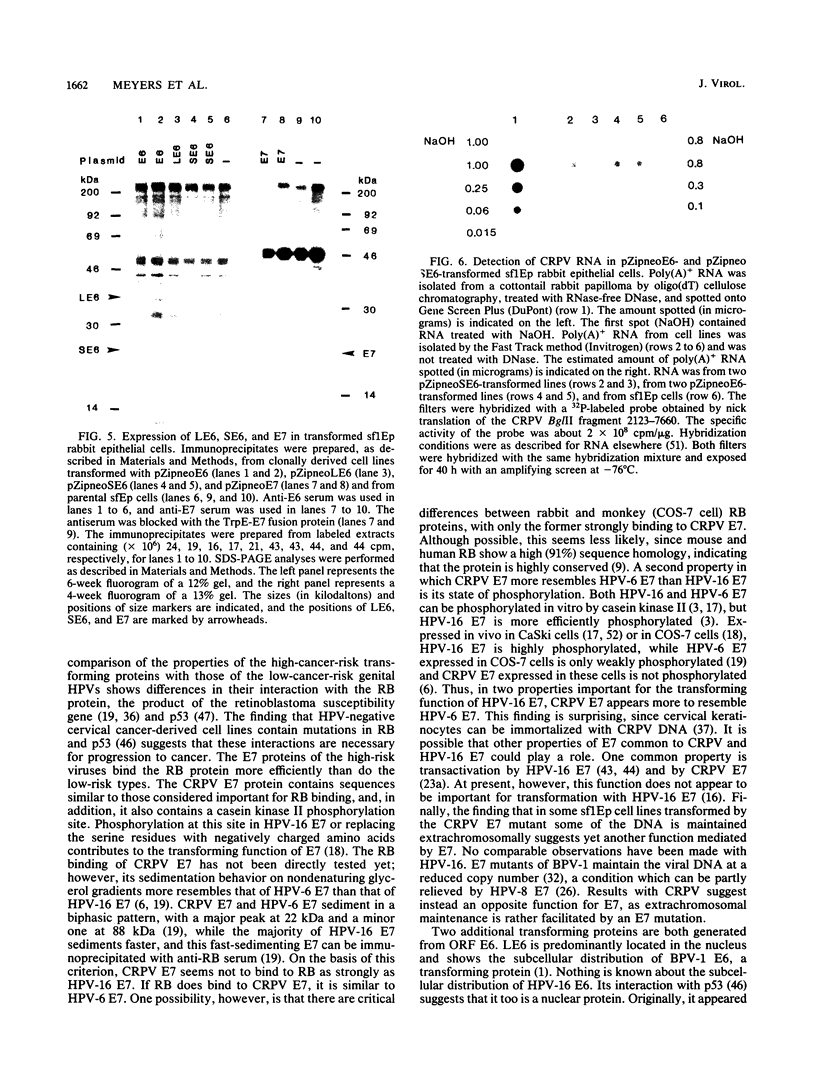
Cottontail rabbit papillomavirus (CRPV) provides an animal model for human papillomaviruses associated with a high risk of cancer development. So far, nothing is known about the transforming functions of CRPV genes because of the lack of an assay system. We have recently developed two systems to assay for CRPV transforming functions. One is based on the finding that transformation of NIH 3T3 cells by CRPV is considerably increased by deleting sequences in open reading frame L2. The second one is based on the use of a cottontail rabbit skin epithelial cell line, sf1Ep (C. Meyers and F. O. Wettstein, Virology 181:637-646, 1991). Mutations were introduced which abolished expression of the full-length E6 protein (LE6), the short E6 protein (SE6) initiated at the second ATG of E6, the E7 protein, or the E5 protein. Mutations affecting LE6 or E7, but not SE6, reduced transformation of NIH 3T3 and sf1Ep cells. Transformed NIH 3T3 cell lines with mutations in LE6 and E7 did not grow in soft agar, while those with mutations in SE6 and E5 grew with a reduced efficiency. The cell lines with mutations in LE6, SE6, or E7 still did induce tumors in nude mice. These mutations, however, abolished the ability to induce papillomas in rabbits. When expressed individually with a retroviral vector, LE6, SE6, or E7, but not E5, conferred anchorage-independent growth. The level of viral protein expression in these cell lines was generally low, and a comparison of the abundance of virus-specific mRNA showed that cell lines contained 20 to 50 times less mRNA than a cottontail rabbit papilloma. These data demonstrate that CRPV encodes at least three transforming proteins.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Androphy E. J., Schiller J. T., Lowy D. R. Identification of the protein encoded by the E6 transforming gene of bovine papillomavirus. Science. 1985 Oct 25;230(4724):442–445. doi: 10.1126/science.2996134. [DOI] [PubMed] [Google Scholar]
- Barbosa M. S., Edmonds C., Fisher C., Schiller J. T., Lowy D. R., Vousden K. H. The region of the HPV E7 oncoprotein homologous to adenovirus E1a and Sv40 large T antigen contains separate domains for Rb binding and casein kinase II phosphorylation. EMBO J. 1990 Jan;9(1):153–160. doi: 10.1002/j.1460-2075.1990.tb08091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa M. S., Wettstein F. O. E2 of cottontail rabbit papillomavirus is a nuclear phosphoprotein translated from an mRNA encoding multiple open reading frames. J Virol. 1988 Sep;62(9):3242–3249. doi: 10.1128/jvi.62.9.3242-3249.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa M. S., Wettstein F. O. Identification and characterization of the CRPV E7 protein expressed in COS-7 cells. Virology. 1988 Jul;165(1):134–140. doi: 10.1016/0042-6822(88)90666-6. [DOI] [PubMed] [Google Scholar]
- Barbosa M. S., Wettstein F. O. The two proteins encoded by the cottontail rabbit papillomavirus E6 open reading frame differ with respect to localization and phosphorylation. J Virol. 1988 Mar;62(3):1088–1092. doi: 10.1128/jvi.62.3.1088-1092.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa M. S., Wettstein F. O. Transcription of the cottontail rabbit papillomavirus early region and identification of two E6 polypeptides in COS-7 cells. J Virol. 1987 Sep;61(9):2938–2942. doi: 10.1128/jvi.61.9.2938-2942.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell M. A., Jones K. H., Grossman S. R., Laimins L. A. Identification of human papillomavirus type 18 transforming genes in immortalized and primary cells. J Virol. 1989 Mar;63(3):1247–1255. doi: 10.1128/jvi.63.3.1247-1255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards R., Shackleford G. M., Schackleford G. M., Gerber M. R., Horowitz J. M., Friend S. H., Schartl M., Bogenmann E., Rapaport J. M., McGee T. Structure and expression of the murine retinoblastoma gene and characterization of its encoded protein. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6474–6478. doi: 10.1073/pnas.86.17.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandsma J. L., Yang Z. H., Barthold S. W., Johnson E. A. Use of a rapid, efficient inoculation method to induce papillomas by cottontail rabbit papillomavirus DNA shows that the E7 gene is required. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4816–4820. doi: 10.1073/pnas.88.11.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Chen S. L., Mounts P. Transforming activity of E5a protein of human papillomavirus type 6 in NIH 3T3 and C127 cells. J Virol. 1990 Jul;64(7):3226–3233. doi: 10.1128/jvi.64.7.3226-3233.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danos O., Georges E., Orth G., Yaniv M. Fine structure of the cottontail rabbit papillomavirus mRNAs expressed in the transplantable VX2 carcinoma. J Virol. 1985 Mar;53(3):735–741. doi: 10.1128/jvi.53.3.735-741.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorbar J., Parton A., Hartley K., Banks L., Crook T., Stanley M., Crawford L. Detection of novel splicing patterns in a HPV16-containing keratinocyte cell line. Virology. 1990 Sep;178(1):254–262. doi: 10.1016/0042-6822(90)90401-c. [DOI] [PubMed] [Google Scholar]
- Edmonds C., Vousden K. H. A point mutational analysis of human papillomavirus type 16 E7 protein. J Virol. 1989 Jun;63(6):2650–2656. doi: 10.1128/jvi.63.6.2650-2656.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firzlaff J. M., Galloway D. A., Eisenman R. N., Lüscher B. The E7 protein of human papillomavirus type 16 is phosphorylated by casein kinase II. New Biol. 1989 Oct;1(1):44–53. [PubMed] [Google Scholar]
- Gage J. R., Meyers C., Wettstein F. O. The E7 proteins of the nononcogenic human papillomavirus type 6b (HPV-6b) and of the oncogenic HPV-16 differ in retinoblastoma protein binding and other properties. J Virol. 1990 Feb;64(2):723–730. doi: 10.1128/jvi.64.2.723-730.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri I., Danos O., Yaniv M. Genomic structure of the cottontail rabbit (Shope) papillomavirus. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1580–1584. doi: 10.1073/pnas.82.6.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri I., Yaniv M. Study of the E2 gene product of the cottontail rabbit papillomavirus reveals a common mechanism of transactivation among papillomaviruses. J Virol. 1988 May;62(5):1573–1581. doi: 10.1128/jvi.62.5.1573-1581.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Halbert C. L., Demers G. W., Galloway D. A. The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J Virol. 1991 Jan;65(1):473–478. doi: 10.1128/jvi.65.1.473-478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J. B., Bedell M. A., McCance D. J., Laiminis L. A. Immortalization and altered differentiation of human keratinocytes in vitro by the E6 and E7 open reading frames of human papillomavirus type 18. J Virol. 1990 Feb;64(2):519–526. doi: 10.1128/jvi.64.2.519-526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iftner T., Bierfelder S., Csapo Z., Pfister H. Involvement of human papillomavirus type 8 genes E6 and E7 in transformation and replication. J Virol. 1988 Oct;62(10):3655–3661. doi: 10.1128/jvi.62.10.3655-3661.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Evans C. A. INDUCTION OF TUMORS IN DOMESTIC RABBITS WITH NUCLEIC ACID PREPARATIONS FROM PARTIALLY PURIFIED SHOPE PAPILLOMA VIRUS AND FROM EXTRACTS OF THE PAPILLOMAS OF DOMESTIC AND COTTONTAIL RABBITS. J Exp Med. 1961 Sep 30;114(4):485–500. doi: 10.1084/jem.114.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Effects of intercistronic length on the efficiency of reinitiation by eucaryotic ribosomes. Mol Cell Biol. 1987 Oct;7(10):3438–3445. doi: 10.1128/mcb.7.10.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. F., Byrne J. C., Howley P. M. A stable bovine papillomavirus hybrid plasmid that expresses a dominant selective trait. Mol Cell Biol. 1983 Nov;3(11):2110–2115. doi: 10.1128/mcb.3.11.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Genetic analysis of bovine papillomavirus type 1 trans-acting replication factors. J Virol. 1985 Mar;53(3):955–965. doi: 10.1128/jvi.53.3.955-965.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers C., Wettstein F. O. The late region differentially regulates the in vitro transformation by cottontail rabbit papillomavirus DNA in different cell types. Virology. 1991 Apr;181(2):637–646. doi: 10.1016/0042-6822(91)90897-k. [DOI] [PubMed] [Google Scholar]
- Mincheva A., Gissmann L., zur Hausen H. Chromosomal integration sites of human papillomavirus DNA in three cervical cancer cell lines mapped by in situ hybridization. Med Microbiol Immunol. 1987;176(5):245–256. doi: 10.1007/BF00190531. [DOI] [PubMed] [Google Scholar]
- Münger K., Phelps W. C., Bubb V., Howley P. M., Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989 Oct;63(10):4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münger K., Werness B. A., Dyson N., Phelps W. C., Harlow E., Howley P. M. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989 Dec 20;8(13):4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasseri M., Gage J. R., Lorincz A., Wettstein F. O. Human papillomavirus type 16 immortalized cervical keratinocytes contain transcripts encoding E6, E7, and E2 initiated at the P97 promoter and express high levels of E7. Virology. 1991 Sep;184(1):131–140. doi: 10.1016/0042-6822(91)90829-z. [DOI] [PubMed] [Google Scholar]
- Nasseri M., Meyers C., Wettstein F. O. Genetic analysis of CRPV pathogenesis: the L1 open reading frame is dispensable for cellular transformation but is required for papilloma formation. Virology. 1989 May;170(1):321–325. doi: 10.1016/0042-6822(89)90388-7. [DOI] [PubMed] [Google Scholar]
- Nasseri M., Wettstein F. O. Differences exist between viral transcripts in cottontail rabbit papillomavirus-induced benign and malignant tumors as well as non-virus-producing and virus-producing tumors. J Virol. 1984 Sep;51(3):706–712. doi: 10.1128/jvi.51.3.706-712.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps W. C., Leary S. L., Faras A. J. Shope papillomavirus transcription in benign and malignant rabbit tumors. Virology. 1985 Oct 15;146(1):120–129. doi: 10.1016/0042-6822(85)90058-3. [DOI] [PubMed] [Google Scholar]
- Phelps W. C., Yee C. L., Münger K., Howley P. M. The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to those of adenovirus E1A. Cell. 1988 May 20;53(4):539–547. doi: 10.1016/0092-8674(88)90570-3. [DOI] [PubMed] [Google Scholar]
- Rawls J. A., Pusztai R., Green M. Chemical synthesis of human papillomavirus type 16 E7 oncoprotein: autonomous protein domains for induction of cellular DNA synthesis and for trans activation. J Virol. 1990 Dec;64(12):6121–6129. doi: 10.1128/jvi.64.12.6121-6129.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggenbuck B., Larsen P. M., Fey S. J., Bartsch D., Gissmann L., Schwarz E. Human papillomavirus type 18 E6*, E6, and E7 protein synthesis in cell-free translation systems and comparison of E6 and E7 in vitro translation products to proteins immunoprecipitated from human epithelial cells. J Virol. 1991 Sep;65(9):5068–5072. doi: 10.1128/jvi.65.9.5068-5072.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SYVERTON J. T. The pathogenesis of the rabbit papilloma-to-carcinoma sequence. Ann N Y Acad Sci. 1952 Jul 10;54(6):1126–1140. doi: 10.1111/j.1749-6632.1952.tb39983.x. [DOI] [PubMed] [Google Scholar]
- Scheffner M., Münger K., Byrne J. C., Howley P. M. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5523–5527. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M., Werness B. A., Huibregtse J. M., Levine A. J., Howley P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990 Dec 21;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Schneider-Gädicke A., Schwarz E. Different human cervical carcinoma cell lines show similar transcription patterns of human papillomavirus type 18 early genes. EMBO J. 1986 Sep;5(9):2285–2292. doi: 10.1002/j.1460-2075.1986.tb04496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedman S. A., Barbosa M. S., Vass W. C., Hubbert N. L., Haas J. A., Lowy D. R., Schiller J. T. The full-length E6 protein of human papillomavirus type 16 has transforming and trans-activating activities and cooperates with E7 to immortalize keratinocytes in culture. J Virol. 1991 Sep;65(9):4860–4866. doi: 10.1128/jvi.65.9.4860-4866.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shope R. E., Hurst E. W. INFECTIOUS PAPILLOMATOSIS OF RABBITS : WITH A NOTE ON THE HISTOPATHOLOGY. J Exp Med. 1933 Oct 31;58(5):607–624. doi: 10.1084/jem.58.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotkin D., Prokoph H., Wettstein F. O. Oncogenic and nononcogenic human genital papillomaviruses generate the E7 mRNA by different mechanisms. J Virol. 1989 Mar;63(3):1441–1447. doi: 10.1128/jvi.63.3.1441-1447.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotkin D., Wettstein F. O. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4680–4684. doi: 10.1073/pnas.83.13.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettstein F. O., Barbosa M. S., Nasseri M. Identification of the major cottontail rabbit papillomavirus late RNA cap site and mapping and quantitation of an E2 and minor E6 coding mRNA in papillomas and carcinomas. Virology. 1987 Aug;159(2):321–328. doi: 10.1016/0042-6822(87)90470-3. [DOI] [PubMed] [Google Scholar]
- Wettstein F. O., Stevens J. G. Variable-sized free episomes of Shope papilloma virus DNA are present in all non-virus-producing neoplasms and integrated episomes are detected in some. Proc Natl Acad Sci U S A. 1982 Feb;79(3):790–794. doi: 10.1073/pnas.79.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. C., Okayama H., Howley P. M. Bovine papillomavirus contains multiple transforming genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1030–1034. doi: 10.1073/pnas.82.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers E. M. Heterogeneity of the human papillomavirus group. J Virol. 1989 Nov;63(11):4898–4903. doi: 10.1128/jvi.63.11.4898-4903.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology. 1991 Sep;184(1):9–13. doi: 10.1016/0042-6822(91)90816-t. [DOI] [PubMed] [Google Scholar]







