Abstract
Background Recent studies of the Hiroshima and Nagasaki A-bomb survivors, together with some (but not all) cohorts exposed occupationally or medically to ionizing radiation, have found an increasing trend in mortality from non-malignant disease with increasing radiation dose. The aim of this study was to establish whether such a trend could be found in a large cohort of employees in the UK nuclear industry.
Methods The cohort comprised 64 937 individuals ever employed at the study sites between 1946 and 2002, followed up to 2005; radiation exposures as measured by personal dosimeters (‘film badges’) were available for 42 426 individuals classified as ‘radiation workers’. Poisson regression models were used to investigate the relationship between excess mortality rates and cumulative radiation exposure, using both relative and additive risk models.
Results The cohort shows a pronounced ‘healthy worker’ effect. Overall, socio-economic status as indicated by employment status has a greater influence on mortality than does radiation exposure status. For male radiation workers, there is an apparent dose response for mortality from circulatory system disease [P < 0.001, ERR = 0.65 (90% CI 0.36–0.98) Sv−1]. However there is evidence for inhomogeneity in the apparent dose response (P = 0.016), arising principally at cumulative doses in excess of 300 mSv, when the four categories of employment and radiation exposure status are examined separately.
Conclusions We have found evidence for an association between mortality from non-cancer causes of death, particularly circulatory system disease, and external exposure to ionizing radiation in this cohort. However, the tentative nature of biological mechanisms that might explain such an effect at low chronic doses and the above inhomogeneities in apparent dose–response, mean that the results of our analysis are not consistent with any simple causal interpretation. Further work is required to explain these inhomogeneities, and on the possible role of factors associated with socio-economic status and shift working, before any further conclusions can be drawn.
Keywords: Ionizing radiation, non-cancer mortality, circulatory disease, cohort study, occupational exposure
Introduction
A causative link has long been established between exposure to ionizing radiation and the risk of mortality from many forms of cancer. More recently, an association between radiation exposure and non-cancer mortality has been shown for the survivors of the A-bomb attacks on Hiroshima and Nagasaki1–4 and also in some, but not all, occupationally and medically exposed cohorts.5,6 Evidence of association is strongest for circulatory system disease, although associations for other non-cancer diseases are observed in the A-bomb survivors. Taking account of the plausibility of possible biological mechanisms, evidence for a causative link between acute high-dose exposures (greater than about 5 Gy) and mortality from circulatory system disease is convincing, whereas that for lower acute doses, or chronic exposure generally, is equivocal.5,7–9
The cohort of employees at sites formerly operated by British Nuclear Fuels plc (BNFL) in the UK comprises a large and important study group. Ascertainment of vital status is high (99.3%); individual external radiation exposures are well characterized and include, particularly in the earlier years, exposures at a substantial fraction of the regulatory limits. The cancer mortality and morbidity experience of this cohort have been studied extensively.10–16
In the present study, we examine the non-cancer mortality experience of male radiation workers in this cohort in relation to individual recorded exposures to external sources of ionizing radiation.
This study was originally commissioned by BNFL, with the approval of employees and their trade union representatives. Since April 1, 2006 the study has been overseen by a Governance Group with workforce, employer, expert and funding body representatives. The study has been given exemption from the requirement for individual informed consent in respect of all workers entering the cohort prior to 2003 by the Patient Information Advisory Group of the UK Department of Health.
Methods
The cohort comprises 64 937 employees of BNFL, the United Kingdom Atomic Energy Authority and the former Ministry of Supply ever employed at the Springfields, Sellafield, Capenhurst or Chapelcross sites between the beginning of 1946 and the end of 2002. Of these, 119 were excluded from the analysis because of incompleteness or inconsistency in their personal data. Vital status was followed to December 31, 2005 through the Office for National Statistics (ONS), Southport and the General Register Office (GRO), Edinburgh. Deaths from 1991 onwards were identified by electronic searches, and earlier deaths by manual searches, accessing both the National Health Services Central Register and (for deaths prior to 1952) the National Register. A further 434 individuals were either unable to be flagged or lost to follow-up during this process; those lost to follow-up are included in the analysis up to their termination of employment at the study sites. Underlying and contributory causes of death were coded by ONS according to the International Classification of Disease (ICD) codes current at the time of death; codes for the disease categories discussed in this paper are given in Supplementary Data A.
Vital status was traced for 99.3% of the cohort and 1 894 225 person-years of experience were accumulated (Table 1); the largest proportion of experience by radiation workers is accumulated at Sellafield (50.5%), followed by Springfields (37%), Capenhurst (6.8%) and Chapelcross (5.6%).
Table 1.
Vital status of the cohort as of 31 December 2005
| Male | Female | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NRW | RW | NRW | RW | ||||||
| Ind | Nonind | Ind | Nonind | Ind | Nonind | Ind | Nonind | all | |
| Alive | 4073 | 3558 | 14 709 | 11 963 | 2006 | 3430 | 973 | 2348 | 43 060 |
| Dead | 5420 | 1221 | 8560 | 2460 | 1292 | 370 | 121 | 138 | 19 582 |
| Embarked | 357 | 204 | 605 | 334 | 79 | 105 | 13 | 45 | 1742 |
| LTFU | 126 | 81 | 102 | 46 | 35 | 35 | 3 | 6 | 434 |
| Total | 9976 | 5064 | 23 976 | 14 803 | 3412 | 3940 | 1110 | 2537 | 64 818 |
| Person-years | 331 596 | 160 428 | 667 316 | 414 254 | 116 140 | 121 027 | 24 762 | 58 703 | 1 894 225 |
LTFU: lost to follow-up; NRW: non-radiation workers; RW: radiation workers; Ind: industrial employees; Nonind: nonindustrial employees.
Operations at the sites studied include uranium processing and nuclear fuel manufacture (Springfields); uranium enrichment (Capenhurst); nuclear fuel reprocessing, radioactive waste management and plutonium processing (Sellafield); nuclear power generation (Sellafield and Chapelcross); and tritium production and processing (Sellafield, Chapelcross, and Capenhurst). The employment status of each individual worker was classified as ‘industrial’ or ‘non-industrial’ according to whether they were weekly paid or salaried, and the radiation exposure status as ‘radiation worker’ or ‘non-radiation worker’ according to whether monitoring and recording of their radiation exposure for regulatory purposes had been deemed necessary. All radiation workers were issued with personal dosimeters, usually ‘film badges’, to measure external radiation dose; detailed records are available for each individual worker.
Radiation workers are further sub-categorized into ‘external radiation workers’ and ‘internal radiation workers’. The latter category is defined as those external radiation workers who had ever provided a biological sample for the purpose of assessing internal doses. Only the external doses are considered in this analysis. The radiation doses accumulated by the cohort are summarized in Table 2.
Table 2.
Distribution of cumulative external radiation dose (radiation workers only)
| Male | Female | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ext RW | Int RW | Ext RW | Int RW | ||||||
| Ind | Nonind | Ind | Nonind | Ind | Nonind | Ind | Nonind | all | |
| Number | 10 717 | 8170 | 13 259 | 6633 | 622 | 1935 | 488 | 602 | 42 426 |
| Mean (mS v) | 32.8 | 18.4 | 85.0 | 87.1 | 9.0 | 6.2 | 25.8 | 18.0 | 53.0 |
| Median (mS v) | 5.0 | 3.9 | 32.6 | 33.2 | 3.7 | 2.3 | 13.3 | 10.7 | 12.1 |
| 99th% (mS v) | 439.4 | 276.9 | 728.6 | 704.4 | 103.5 | 54.2 | 161.9 | 98.8 | 589.5 |
| Person S v | 351.4 | 150.7 | 1127.4 | 577.5 | 5.6 | 12.1 | 12.6 | 10.8 | 2248 |
Ext RW: external radiation worker; Int RW: internal radiation worker.
Only 8.6% of the radiation workers are female, and they have accumulated only 7.1% of person-years follow-up for radiation workers and 1.8% of the collective dose. Accordingly, this study has concentrated on the analysis of the mortality experience of male workers. The mortality experience of female workers has been reported in a separate study.16
Standardized Mortality Ratios (SMRs) were calculated in order to compare mortality rates for male workers with those expected on the basis of mortality statistics for the northwest region of England,17 after stratification for age, gender and calendar year. Mortality experience in bands of cumulative radiation dose was also compared with the background rate estimated by stratification on gender, site of employment, employment status, radiation exposure status, birth year and attained age. Details of the stratification are provided in Supplementary Data B.
Poisson regression models were used to investigate the relationship between cumulative radiation dose and cause specific mortality for male radiation workers, using a relatively simple model for excess relative risk of the form:
Here, R is the cause specific mortality rate and λ is the background mortality rate in the absence of any effects from radiation exposure. The subscripts b, a, r, i and s refer respectively to birth cohort, attained age, radiation exposure status, employment status and site of employment. ERR(d) is a function of lagged cumulative external dose, d, describing the excess relative risk.
The excess relative risks were investigated by use of the AMFIT routine within the Epicure program,18 using stratification as the means of estimating background mortality rates in each cell of the person-year matrix. This method was used to analyse mortality in terms of both diseases as underlying cause and, for certain conditions, diseases as either underlying or contributory cause.
The results discussed below are for the simplest model, in which excess relative risk is assumed to be a linear time invariant function of lagged cumulative radiation dose only. The results of some more complex analyses, in which excess relative risk is allowed to vary with attained age, or in which excess additive risk models are assumed, are presented as Supplementary Data D.
Results
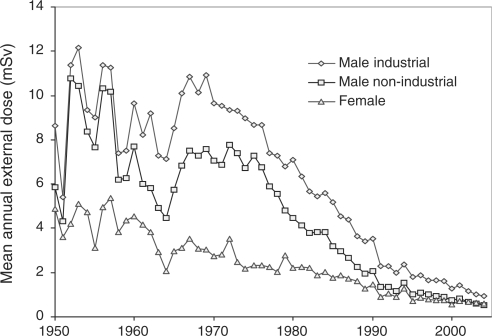
Internal radiation workers have accumulated higher external doses, both collectively and individually, than external radiation workers (Table 2). This reflects the fact that a high proportion of workers at the Sellafield site, where both external radiation exposure levels and the number of individuals exposed are high relative to the other sites, are monitored for internal exposure. Mean annual external radiation doses have declined substantially since 1970 (Figure 1).
Figure 1.
Mean annual external radiation dose by year
The performance of the radiation dosimeters used has been reviewed as part of a large international study.19,20 Prior to the early 1960s, the dosimeter type in use was known to be inaccurate for low-energy gamma radiation (<200 keV), although at that time radiation of this energy only accounted for a small proportion of the doses received by workers. The newer dosimeters performed better at low energies, but at the same time exposures to 17 and 60 keV X-rays in plutonium processing facilities increased, and in these areas the doses at depths >10 mm in the body are likely to have been overestimated.
Mortality rates for major causes of death (Table 3) clearly show the expected ‘healthy worker’ effect,21 with SMRs for major causes of death being markedly lower than standardized regional rates; the reduction is most marked for respiratory disease. Within the cohort the major discrimination in mortality rates is seen for employment status, with industrial employees having significantly higher mortality, by a factor of 1.3 (circulatory diseases) to 1.9 (respiratory diseases), than non-industrial employees.
Table 3.
Standardized mortality ratios for major causes of death in male workers
| Standardized mortality ratio (compared with rates for NW England) | ||||||
|---|---|---|---|---|---|---|
| All non-cancer | Circulatory diseases | Respiratory diseases | ||||
| SMR | 95% CI | SMR | 95% CI | SMR | 95% CI | |
| Male industrial employees | 85 | 83.1–86.5 | 89 | 86.7–91.0 | 72 | 68.9–76.2 |
| Non-radiation workers | 85 | 82.7–88.2 | 87 | 83.8–90.8 | 78 | 72.0–84.0 |
| External radiation workers | 85 | 82.1–88.6 | 91 | 86.8–95.2 | 69 | 62.2–75.9 |
| Internal radiation workers | 84 | 80.8–86.5 | 89 | 85.4–92.8 | 69 | 63.2–75.5 |
| Male non-industrial employees | 62 | 59.5–64.4 | 70 | 66.5–73.0 | 38 | 33.4–42.8 |
| Non-radiation workers | 66 | 61.5–70.5 | 75 | 69.4–81.6 | 41 | 33.3–50.4 |
| External radiation workers | 62 | 57.9–65.9 | 69 | 63.7–74.6 | 34 | 26.7–41.7 |
| Internal radiation workers | 58 | 54.3–62.5 | 65 | 59.8–70.8 | 39 | 31.3–48.4 |
| All male employees | 79 | 77.3–80.1 | 84 | 82.1–85.7 | 64 | 61.1–67.0 |
Much less variation is seen with radiation exposure status, although there is some indication that the mortality for non-radiation workers is higher than that for external or internal radiation workers, particularly for respiratory disease.
Exploratory analyses22 indicated that higher levels of significance were attached to trends when calculated with cumulative doses lagged between 10 and 20 years, and that there was significant inhomogeneity between the excess relative risks for doses accumulated over the preceding 10–15 years when compared with that for doses accumulated over the preceding 15–20 or 20+ years. A 15-year lag in cumulative dose has therefore been used in all the main analyses. For underlying causes of death in male radiation workers, strong evidence for trends with cumulative dose were seen for diseases of the circulatory system [5319 deaths, P (one-sided) < 0.001], driven largely by ischaemic heart disease (3567 deaths, P < 0.001) and particularly acute myocardial infarction (2051 deaths, P < 0.001). When deaths were analysed by both underlying and contributory cause, evidence for trends was also observed for cerebrovascular disease (1365 deaths, P = 0.0085), chronic ischaemic heart disease (2752 deaths, P = 0.0023) and diabetes (359 deaths, P = 0.0029). The trend for cerebrovascular disease was driven by ‘ill-defined cerebrovascular disease’, probably reflecting the lack of precision in death certificates of distinguishing between ischaemic and haemorrhagic strokes. Based on these observations, we consider that most weight should be placed on the results for excess relative risk of mortality from circulatory system disease in this cohort as a function of cumulative radiation dose, although we also present results for other disease groupings to aid comparison with other studies.
For all male radiation workers, the excess relative risk for mortality from circulatory system disease is 0.65 (90% CI 0.36–0.98) Sv−1 on 5319 deaths, and that for ischaemic heart disease is 0.70 (0.33–1.11) Sv−1 on 3567 deaths (Table 4). The excess relative risk of mortality from all non-cancer causes, at 0.52 (0.29–0.77) Sv−1 on 7345 deaths, is driven largely by that for circulatory system disease.
Table 4.
Poisson regression analysis for all male radiation workers, with background stratified on birth cohort, attained age, employment status, site of employment and radiation exposure status
| Observed deaths [expected deaths] by cumulative external dose (mSv), 15-year lag | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <10 | 10–20 | 20–50 | 50–100 | 100–200 | 200–400 | >400 | Total | P(H0: ERR = 0) | ERR Sv−1 (90% CI) | |
| Underlying causes | ||||||||||
| All non-cancersa | 3054 [3122.14] | 825 [795.10] | 1276 [1254.68] | 878 [813.52] | 596 [551.48] | 429 [359.01] | 287 [224.80] | 7345 [7120.73] | <0.001 | 0.52 (0.29–0.77) |
| All non-cancersa ex circulatory disease | 847 [840.94] | 225 [225.11] | 367 [364.52] | 236 [237.48] | 168 [155.56] | 99 [103.00] | 84 [69.03] | 2026 [1995.95] | 0.28 | 0.24 (−0.11–0.66) |
| Respiratory diseases | 431 [429.21] | 119 [121.85] | 200 [199.62] | 137 [130.79] | 86 [82.10] | 55 [51.21] | 44 [35.04] | 1072 [1049.81] | 0.30 | 0.33 (−0.17–0.99) |
| Lung Cancer | 442 [439.94] | 108 [104.56] | 154 [161.04] | 96 [106.47] | 86 [76.45] | 56 [53.81] | 28 [32.99] | 970 [975.26] | >0.5 | −0.09 (−0.53–0.49) |
| Digestive diseases | 160 [155.89] | 30 [34.43] | 56 [50.50] | 34 [29.86] | 15 [19.84] | 12 [12.85] | 11 [6.91] | 318 [310.30] | 0.42 | 0.50 (−0.40–1.95) |
| Circulatory disease | 2207 [2279.60] | 600 [569.50] | 909 [889.23] | 642 [575.17] | 428 [394.90] | 330 [255.29] | 203 [155.64] | 5319 [5119.33] | <0.001 | 0.65 (0.36–0.98) |
| IHD | 1532 [1574.35] | 394 [375.71] | 585 [577.44] | 420 [374.77] | 287 [258.55] | 221 [167.62] | 128 [100.43] | 3567 [3428.86] | <0.001 | 0.70 (0.33–1.11) |
| CeVD | 387 [401.62] | 119 [112.51] | 200 [186.28] | 120 [121.97] | 91 [82.14] | 59 [53.25] | 42 [32.80] | 1018 [990.57] | 0.196 | 0.43 (−0.10–1.12) |
| Other circulatory diseases | 288 [303.18] | 87 [81.16] | 124 [125.33] | 102 [78.29] | 50 [54.04] | 50 [34.34] | 33 [22.32] | 734 [698.66] | 0.07 | 0.83 (−0.10–1.12) |
| Circulatory ex CeVD | 1820 [1877.49] | 481 [456.88] | 709 [702.81] | 522 [453.09] | 337 [312.61] | 271 [201.97] | 161 [122.78] | 4301 [4127.62] | <0.001 | 0.72 (0.39–1.10) |
| Underlying and contributory causes | ||||||||||
| Diabetes | 125 [133.13] | 48 [35.68] | 54 [57.60] | 42 [39.86] | 40 [28.99] | 26 [20.85] | 24 [14.54] | 359 [330.64] | 0.038 | 1.15 (0.20–2.56) |
| Circulatory diseases | 2663 [2731.73] | 732 [692.83] | 1106 [1095.65] | 769 [717.81] | 549 [496.16] | 400 [327.50] | 257 [203.17] | 6476 [6264.83] | <0.001 | 0.54 (0.30–0.82) |
| IHD | 1745 [1786.72] | 473 [439.96] | 678 [682.23] | 492 [448.09] | 354 [312.77] | 259 [204.48] | 164 [123.20] | 4165 [3997.44] | <0.001 | 0.70 (0.37–1.07) |
| CeVD | 506 [521.21] | 152 [146.90] | 261 [244.80] | 169 [164.34] | 130 [110.50] | 79 [72.25] | 68 [46.97] | 1365 [1306.96] | 0.02 | 0.66 (0.17–1.27) |
| Respiratory diseases | 1060 [1086.44] | 327 [314.05] | 522 [513.13] | 356 [344.74] | 259 [228.95] | 164 [152.59] | 125 [99.04] | 2813 [2738.93] | 0.04 | 0.40 (0.07–0.79) |
IHD: ischaemic heart disease; CeVD: cerebro vascular disease.
aExcluding accidents and violence.
As internal radiation workers will have also received doses from internally incorporated radionuclides, and as our stratification according to industrial or non-industrial employment status may only crudely reflect differences in background mortality due to socio-economic factors, we have also examined the mortality experience of the four subgroups of radiation exposure and employment status separately (Table 5, Supplementary Data C).
Table 5.
Linear excess relative risk coefficients for the four categories of employment and radiation exposure status
| ERR Sv−1 (number of deaths, 90% CI) | |||||
|---|---|---|---|---|---|
| External | Internal | ||||
| Cause of death | Male Ind | Male non-Ind | Male Ind | Male non-Ind | Test for homogeneity χ2 (3 df) [P] |
| Underlying causes | |||||
| All non-cancersa | 0.63 (2600, 0.12–1.22) | 0.51 (848, −0.48–1.79) | 0.70 (3155, 0.38–1.08) | −0.03 (742, −0.39–0.44) | 4.49 [0.21] |
| All non-cancersa ex circulatory disease | −0.60 (745, <−1.63–0.18) | −0.68 (214, <−1.72–0.96) | 0.55 (880, 0.006–1.27) | 0.61 (187, −0.19–1.92) | 5.35 [0.15] |
| Respiratory disease | −1.00 (398, <−1.00–−0.11) | 1.41 (83, −1.02–6.71) | 0.94 (505, 0.10–2.14) | 0.28 (86, <−0.66–2.02) | 6.50 [0.090] |
| Lung cancer | 0.06 (348, <−1.2–1.98) | 3.78 (72, −0.31–12.29) | −0.22 (468, −0.69–0.45) | −0.11 (82, <−1.10–1.65) | 2.45 [0.49] |
| Digestive diseases | 1.48 (117, <−0.86–5.72) | −1.54 (45, <−1.54–0.35) | 0.30 (124, −0.77–2.49) | 2.20 (32, −0.43–18.01) | 3.91 [0.27] |
| Circulatory disease | 1.25 (1855, 0.56–2.08) | 1.38 (634, −0.05–3.27) | 0.76 (2275, 0.37–1.23) | −0.29 (555, <−0.66–0.21) | 10.3 [0.016] |
| IHD | 1.69 (1261, 0.78–2.82) | 1.45 (434, −0.28–3.91) | 0.52 (1494, 0.09–1.06) | 0.05 (378, −0.48–0.84) | 5.94 [0.11] |
| CeVD | −0.20 (362, −0.94–1.04) | 0.31 (105, <−1.99–4.49) | 1.47 (456, 0.49–3.00) | −0.61 (95, <−0.61–0.11) | 8.07 [0.045] |
| Other circulatory disease | 2.23 (232, 0.25–6.44) | 2.53 (95, <−1.62–10.33) | 0.83 (325, 0.02–2.40) | −0.61 (82, <−0.61–0.45) | 5.63 [0.13] |
| Circulatory disease ex CeVD | 1.81 (1493, 0.94–2.87) | 1.68 (529, −0.02–3.94) | 0.60 (1819, 0.20–1.10) | −0.12 (460, −0.56–0.52) | 9.62 [0.022] |
| Underlying and contributory causes | |||||
| Diabetes | 4.88 (111, 1.26–11.50) | 0.58 (37, <−2.71–7.87) | 0.73 (169, −0.21–2.27) | −0.15 (42, <−0.15–3.66) | 3.94 [0.27] |
| Circulatory disease | 0.92 (2256, 0.34–1.59) | 1.67 (750, 0.38–3.33) | 0.55 (2801, 0.22–0.92) | −0.007 (669, −0.36–0.86) | 6.37 [0.095] |
| IHD | 1.69 (1462, 0.89–2.69) | 1.22 (495, −0.24–3.22) | 0.50 (1763, 0.11–0.98) | 0.14 (445, −0.44–1.03) | 6.65 [0.084] |
| CeVD | −0.35 (464, <−1.00–0.68) | 0.20 (145, <−1.67–3.41) | 1.52 (625, 0.63–2.73) | 0.16 (131, −0.51–1.39) | 5.86 [0.12] |
| Respiratory disease | 0.24 (1010, −0.43–1.11) | 1.55 (266, −0.23–4.24) | 0.38 (1301, −0.03–0.88) | 0.42 (236, −0.28–1.54) | 1.09 [>0.5] |
IHD: ischaemic heart disease; CeVD: cerebro vascular disease.
aExcluding accidents and violence.
There is evidence for inhomogeneity in apparent dose–response for circulatory disease (P = 0.016) and cerebrovascular disease (P = 0.045) although the directions of the differences within the four employment and radiation exposure status categories vary; there is no evidence of inhomogeneity in the apparent dose–response for circulatory system disease amongst industrial external, industrial internal and non-industrial external workers [χ2(2 df) = 1.13, P > 0.5] and for these three groups the common ERR is 0.93 (0.52–1.40) Sv−1.
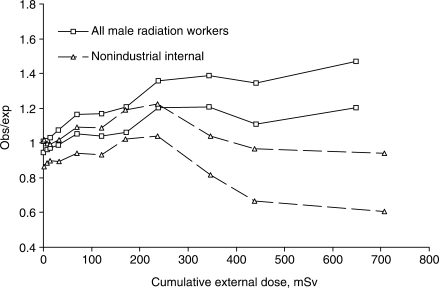
It can be seen that much of the inhomogeneity discussed above is driven by the mortality experience of non-industrial employees, particularly in the case of non-industrial internal radiation workers at high cumulative dose (Figure 2). It should be noted that, because of the dose distributions at the different sites, person-years of follow-up for non-industrial employees at cumulative doses above 300 mSv are accumulated almost entirely at Sellafield, particularly for internal workers, and account for only 0.36% and 2.8% of the follow-up for external and internal workers, respectively.
Figure 2.
Loess smoothers (±1 standard error) on point estimates of the ratio of observed to expected mortality from circulatory system disease, for non-industrial internal radiation workers compared to all radiation workers
For circulatory system disease, we have examined the sensitivity of the result to use of differing lag periods and the alternatives of stratifying for length of service, or not stratifying for employment status (Table 6). The results are relatively insensitive to these changes, with the exception of the results for the four cohort subcategories when analysed without stratification for employment status; the differences here simply reflect the different background mortality rates for industrial and non-industrial employees, and the proportionally greater contribution of industrial employees to person-years of follow-up at high cumulative doses.
Table 6.
Sensitivity analyses for lag period and stratification
| Circulatory disease ERR Sv−1 (90% CI) | ||||
|---|---|---|---|---|
| Cohort subdivision | Main analysis | 10-year lag | Stratification on length of servicea | No stratification on employment statusb |
| Industrial external | 1.25 (0.44–2.25) | 0.89 (0.32–1.57) | 1.25 (0.44–2.25) | 1.85 (1.10–2.72) |
| Non-industrial external | 1.38 (−0.28–3.70) | 1.42 (0.12–3.13) | 0.54 (−0.72–2.40) | 0.01 (−0.86–1.11) |
| Industrial internal | 0.76 (0.30–1.32) | 0.58 (0.25–0.97) | 0.76 (0.28–1.34) | 1.02 (0.62–1.46) |
| Non-industrial internal | −0.29 (<−0.73–0.33) | −0.25 (<−0.76–0.22) | −0.39 (<−0.86–0.11) | −0.51 (<−0.77–−0.19) |
| All radiation workers | 0.65 (0.31–1.05) | 0.50 (0.26–0.79) | 0.54 (0.21–0.92) | 0.65 (0.36–0.97) |
aTwo strata used <15 years or >= 15years service.
bBackground rates determined from all radiation workers, with no stratification for employment status.
We have undertaken numerous additional analyses to explore alternative empirical descriptions of the data. These include consideration of attained age as an effect modifier for excess relative risk, and the use of excess additive risk models. Details are provided in Supplementary Data D. However, none of these satisfactorily resolve the observed inhomogeneity in apparent dose–response according to employment and radiation exposure status.
Discussion
SMRs
As already noted, the overall SMRs for the cohort show clear evidence of a ‘healthy worker’ effect, and there is a clear difference in the mortality experience of the industrial and non-industrial employees. In terms of the definitions from the UK census office in the 1990s, industrial and non-industrial grades would correspond broadly to social classes II and IIIM;23 the observed factor of 1.3–1.9 difference in mortality between employment grades is consistent with that reported for these social classes in NW England.24 The somewhat more marked ‘healthy worker’ effect for respiratory disease, together with the reduced mortality from respiratory disease in radiation workers compared with non-radiation workers, suggests that the restriction placed on smoking in the workplace for radiation workers has been a significant factor in reducing the mortality of the cohort. Overall, it is clear that socio-economic status has a more significant influence on mortality in the cohort than does radiation exposure status.
Analysis for trend, linear excess relative risk co-efficients and linear excess additive risk co-efficients
The results of the analysis for male radiation workers show evidence for an association between cumulative external radiation dose and mortality from circulatory system disease.
If this association were to reflect a causal effect of ionizing radiation, it would be a significant factor to be taken into account in radiation protection as the implied co-efficient of excess relative risk for mortality from circulatory system disease amongst all male radiation workers (0.65 Sv−1, 90% CI 0.36–0.98) is several times higher than that seen in the A-bomb survivors, and comparable with the co-efficient of excess relative risk for mortality from all solid cancers in the survivors,1 on which current radiological protection standards are largely based.
However, the inhomogeneity we have observed in the apparent dose–response amongst the subgroups divided according to employment and radiation exposure status requires explanation if a causal interpretation is to be made. Additional analyses, including attained age as an effect modifier, and using an excess additive risk model, rather than an excess relative risk model, do not satisfactorily resolve the inhomogeneity (Supplementary Data D). It therefore seems unlikely that the inhomogeneity is an artefact of our approach to the analysis.
We have considered a number of other possible explanations for these differences in apparent dose–response. First, it is important to recognize that the differences in dose–response for non-industrial employees arise primarily during the person-years of follow-up at cumulative external doses in excess of 300 mSv. As already noted, this is only a small proportion of the total follow-up of the cohort and is, moreover, accumulated almost entirely by workers at Sellafield. As a result, factors uncontrolled for in the present analysis need only act on a specific and relatively small proportion of the cohort to produce the inhomogeneity observed.
The cohort shows evidence of a pronounced healthy worker effect. Whilst the initial effects of selection into employment may diminish with time,21 there may also be a continuing selection process such that those who remain employed tend to be healthier than those who leave employment (the ‘healthy worker survivor effect’), leading to attenuation of the exposure–response relationship.25 Internal radiation workers tend to have greater length of service than external workers and could be more affected by this differential selection, but stratification on length of service has no material effect either on the overall dose–response for circulatory system disease, or the apparent differences in dose response between groups (Table 6).
As the radiation dose from internally incorporated radionuclides has not been included in the present analysis, there may well be a greater degree of exposure misclassification for the internal workers than is the case for the external workers. Substantial non-differential misclassification of exposure often (but not always) attenuates the association between exposure and mortality,26,27 so this effect may at least contribute to the apparent discrepancy. However, as the cumulative radiation doses to internal workers must be negatively biased due to the exclusion of their internal dose, and the currently estimated excess relative risk coefficients therefore positively biased by this effect, it seems unlikely that exposure misclassification alone could explain the inhomogeneity.
As already noted, it is likely that the external doses recorded for workers in plutonium plants at Sellafield from the 1960s onwards are likely to be overestimates of the true dose at depth in the body; levels of individual dose were reduced substantially in the 1980s as newer process plant with better shielding against low-energy X-rays was introduced. This would certainly be an important effect for some of the internal workers with high cumulative doses, as the recorded dose rates in the older plutonium process plants were close to the statutory limit of 50 mSv a−1. If non-industrial employees from these areas made a disproportionate contribution to follow-up at high cumulative doses, this may provide a partial explanation for the observed inhomogeneity.
Selection effects may provide an alternative explanation for the observed inhomogeneity. During the early 1980s employees who had been working in a number of areas at the Sellafield site, and previously classified as external radiation workers, were designated as requiring monitoring for internal exposure to plutonium. As a consequence, in terms of our analysis, they changed from ‘external’ to ‘internal’ workers. Although the number of individuals involved is small relative to the cohort as a whole, it is just possible that selection effects from this change may have influenced the mortality statistics for internal radiation workers with high cumulative doses.
These latter two possible explanations cannot be investigated rigorously with the current structure of our database, as details of the work location of individuals are not captured; further work is required to investigate these issues.
Possible confounding factors not controlled for in the present analysis
Confounding may account for some or all of the apparent dose–response for ionizing radiation, and if that were to be the case it would not be surprising to see inhomogeneities in the apparent response to radiation for the four subgroups.
The absence of a trend in lung cancer mortality with external radiation dose makes it unlikely that smoking is a significant confounder in the trend for circulatory system disease, particularly as smoking increases the risk of lung cancer by a much greater factor than it increases the risk of circulatory system disease.28 As diabetes and ischaemic heart disease have many lifestyle risk factors in common, the significant trend for diabetes as an underlying and contributory cause of death for all radiation workers does raise the possibility of confounding by lifestyle factors other than smoking.
As already noted, socio-economic status as indicated by the employment status of ‘industrial’ or ‘non-industrial’ is an important discriminating factor for mortality experience in this cohort. Socio-economic status is an important indicator for many of the known risk factors for circulatory system disease, including smoking, diabetes, obesity, elevated blood pressure and high levels of blood low-density lipoprotein.7 In the absence of detailed information on these specific risk factors, the present study has controlled for socio-economic status to some degree by using the two categories of employment status, but there will inevitably be gradation within them that may lead to residual confounding.
Many studies have associated shift work with an increased risk of mortality from circulatory disease.29–32 Although socio-economic status may account in whole or in part for this association33,34 there is evidence that shift work affects metabolism, leading to low levels of HDL cholesterol and elevated levels of triglycerides in blood, which are important markers of risk for circulatory system disease.35 Shift work is also commonly associated with elevated levels of stress,36 which is an additional risk factor for circulatory system disease. Although a case–control study of employees at Sellafield failed to find evidence of enhanced circulatory system mortality in shift workers,37 the number of cases studied was only a small fraction of the total number of deaths from circulatory system disease in the present cohort. Shift work is likely to be correlated with radiation dose, and is therefore a potential confounder in this study; information on shift work status is not held in our present database.
In addition to shift work and workplace stress, linkages between occupational exposure and circulatory system disease have been identified, or suggested, for low-frequency electromagnetic fields,38,39 carbon disulphide,40–43 carbon monoxide, polycyclic aromatic hydrocarbons and airborne particulates,44–49 solvents,50–55 noise,56,57 lead58,59 and (for environmental exposure) arsenic.60,61 However, there is no reason to suppose that exposures of the cohort to these substances are both substantial and correlated with external radiation dose. For the earliest joiners, the linkage between exposure to nitroglycerine and nitrotoluenes in munitions manufacture and heart disease31,62 may be of some relevance as the sites were all either used for, or located close to, major World War II munitions facilities. However, it appears that effects may not persist for long after exposure ceases63–65 so that a lasting effect on the mortality experience of the cohort is unlikely.
In summary, it is possible that a combination of gradation in the adverse lifestyle factors associated with socio-economic status, together with stress and other factors possibly associated with shift work, may at least contribute to the apparent dose–response for cumulative external radiation dose. Significant confounding by other occupational factors is unlikely.
Relationship to other studies of non-cancer mortality and ionizing radiation
The principal evidence for an association between radiation dose and non-cancer mortality comes from the Life Span Study of the survivors of the A-bomb attacks at Hiroshima and Nagasaki.1–4 The A-bomb survivors were acutely exposed to gamma and neutron radiation, with doses in the study group ranging up to 4 Sv. For those exposed closest to the hypocentres of the explosions, a pronounced ‘healthy survivor’ effect, in which baseline mortality rates were markedly reduced in the first two decades following the attacks, was seen. Having adjusted for this effect, the dose–response was adequately described by a linear excess relative risk model, and risk coefficients were comparable with the major non-cancer causes of death, with coefficients (Sv−1) and 90% confidence intervals as follows: heart disease, 0.17 (0.08–0.26); cerebrovascular disease 0.12 (0.02–0.22); respiratory disease 0.18 (0.06–0.32); digestive disease 0.15 (0.00–0.32).1 This broad pattern of response is similar to that seen for all male radiation workers, albeit with higher-risk co-efficients, in the present study (Table 4).
The radiation doses received by the A-bomb survivors were acute and ranged up to an order of magnitude higher than the chronic cumulative external exposures received in the present study. Three recent reviews have examined the epidemiological evidence on circulatory system disease at low and moderate doses of radiation, including chronic exposures.5,7,8 In essence, the epidemiological evidence is not consistent. Thus, McGale and Darby5 conclude that out of the six studies identified with appreciable power to detect effects comparable with those seen in the atomic bomb survivors, and apparently free of substantial bias or confounding, only one66 shows clear evidence of a trend in circulatory system disease with increasing radiation dose. Whilst some limited support is provided by other studies with lower power and/or the possible presence of confounding factors,67–69 McGale and Darby were unable to reach any firm conclusion on the evidence then available. Likewise, Little et al.7 find the epidemiological evidence inconsistent. Considering many of the studies reviewed by McGale and Darby, they note that the risk co-efficients derived, or implied, by the studies vary over two orders of magnitude. Little et al. draw particular attention to the lack of adjustment for socio-economic status in the majority of the studies reviewed, which leaves them vulnerable to confounding. UNSCEAR8 conclude that the scientific data are not at present sufficient to conclude that there is a causative relationship between circulatory system disease and exposure to ionizing radiation at doses less than about 1–2 Gy; in its most recent review the US Committee on the Biological Effects of Ionizing Radiation concluded that non-cancer risks at low and moderate doses were especially uncertain, and developed no risk estimates for them.9
Most recently, Vrijheid et al.6 have reported on the non-cancer mortality experience of a combined cohort of occupationally exposed subjects from 15 countries; the mortality experience of external radiation workers within the BNFL cohort over the period 1955–1992 is included within the UK subcohort in their study. Vrijheid et al. report apparent excess relative risks per Sv of cumulative exposure that are positive, but with confidence intervals that include zero [at 15 years lag all non-cancer causes 0.53 (95% CI –0.09–1.25) Sv−1; circulatory diseases 0.48 (95% CI –0.23–1.31) Sv−1]. They conclude that their study gives little evidence for a relation between mortality from non-malignant diseases and external radiation dose in these occupationally exposed cohorts, but that risks of the same order of magnitude as those found in the A-bomb survivors cannot be excluded. Our results for the apparent excess relative risk per Sv, whilst higher than those of Vrijheid et al., appear to be statistically compatible with them. Vrijheid et al. exclude workers monitored for internal radiation from their analysis; these workers contribute a high proportion of the experience at high cumulative dose in our study. Moreover, our follow-up period is more extended than that of Vrijheid et al.; a per caput average of 27.5 compared with 14.8 years, and a mean age at end of follow up of 57.6 years, compared with 46 years.
Consideration of possible biological mechanisms for non-cancer mortality arising from radiation exposure, as addressed in two recent reviews,7,70 has been limited to cardiovascular disease. For acute radiation doses above 1–2 Gy a number of mechanisms, including damage to the endothelial cells in capillaries or larger blood vessels and the up-regulation of inflammatory processes that initiate or promote the process of atherosclerosis, can account for the enhanced mortality seen in both the A-bomb survivors with higher doses and patients receiving high doses to the heart in radiotherapy procedures. Mechanisms that could account for increases in cardiovascular mortality arising from protracted exposure at occupational levels are at present tentative. Those that have been suggested include the observation that the proliferation of smooth muscle cells in atherosclerotic plaques is monoclonal in nature,71,72 raising the possibility that radiation induced mutagenesis may be a causative pathway;7 or the finding that atherosclerotic plaques unrelated to radiation show evidence of genomic instability,73–75 raising the possibility that radiation induced genomic instability may be a causative pathway.70 Against these possible mechanisms, it may also be significant that, whilst acute doses above 1 Gy are pro-inflammatory, lower doses have long been recognized as having a beneficial anti-inflammatory action and could conceivably slow the progression of circulatory system disease.7,76
In summary, epidemiological evidence for enhanced non-cancer mortality in other studies of chronic exposure to radiation is inconsistent, and biological mechanisms that could account for such enhancement, whilst conceivable, are at present tentative.
Conclusions
We have found evidence for an association between mortality from non-cancer causes of death, particularly circulatory system disease, and external exposure to ionizing radiation in this cohort. As such, this adds to the evidence of similar associations from other studies. However, the tentative nature of biological mechanisms that might explain such an effect at low chronic doses, and the inhomogeneity in apparent dose–response according to employment and radiation exposure status, mean that the results of our analysis are not consistent with any simple causal interpretation. Before any firm conclusions can be drawn, further work is required; this is likely to involve a detailed investigation of the possible role of factors associated with socio-economic status and shift working, more detailed examination of work histories and the potential for measurement biases in dosimetry, and consideration of the dose from internally incorporated radionuclides.
Supplementary data
Supplementary data are available at IJE online.
Supplementary Material
Acknowledgements
Funding for this study up until April 1, 2006 has been provided by British Nuclear Fuels plc; subsequently funding has been provided by the Nuclear Decommissioning Authority. We are grateful to the employees of British Nuclear Group and BNFL, particularly Dr David Macgregor, the Data Custodian, for their support and co-operation in this study; and to the Governance Group for guidance during the course of this work. We also thank the many staff at NHSCR, Smedley Hydro, Southport, and the General Register Office, Scotland, who have assisted in establishing the tracing status of the employees in this study cohort and provided us with mortality rates. We gratefully acknowledge the help of Pat Walker, Sharon Maxwell and our Data Administrators, Marge Williams, Deborah Ritson and Judith Brereton; as well as Les Scott and Andrew Wilson for data management and IT support. We also thank Mark Little and Richard Wakeford for their advice and comments during the course of this work; and Tony Swerdlow, Sarah Darby and Colin Muirhead for their comments on an earlier version of this manuscript.
Conflict of interest: None declared.
References
- 1.Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950-1997. Radiat Res. 2003;160:381–407. doi: 10.1667/rr3049. [DOI] [PubMed] [Google Scholar]
- 2.Shimizu Y, Kato H, Schull WJ, Hoel DG. Studies of the mortality of A-bomb survivors. 9. Mortality, 1950-1985: Part 3. Noncancer mortality based on the revised doses (DS86) Radiat Res. 1992;130:249–66. [PubMed] [Google Scholar]
- 3.Shimizu Y, Pierce DA, Preston DL, Mabuchi K. Studies of the mortality of atomic bomb survivors. Report 12, part II. Noncancer mortality: 1950-1990. Radiat Res. 1999;152:374–89. [PubMed] [Google Scholar]
- 4.Shimizu Y. Life Span Study Report 11, part 3. Noncancer mortality, 1950-85, based on the revised dosimetry (DS86). Hiroshima: Radiation Effects Research Foundation; 1993. Studies of the mortality of the atomic bomb survivors. [Google Scholar]
- 5.McGale P, Darby SC. Low doses of ionizing radiation and circulatory diseases: a systematic review of the published epidemiological evidence. Radiat Res. 2005;163:247–57. doi: 10.1667/rr3314. [DOI] [PubMed] [Google Scholar]
- 6.Vrijheid M, Cardis E, Ashmore P, et al. Mortality from diseases other than cancer following low doses of ionizing radiation: results from the 15-Country Study of nuclear industry workers. Int J Epidemiol. 2007;36:1126–35. doi: 10.1093/ije/dym138. [DOI] [PubMed] [Google Scholar]
- 7.Little MP, Tawn EJ, Tzoulaki I, et al. A systematic review of epidemiological associations between low and moderate doses of ionizing radiation and late cardiovascular effects, and their possible mechanisms. Radiat Res. 2008;169:99–109. doi: 10.1667/RR1070.1. [DOI] [PubMed] [Google Scholar]
- 8.UNSCEAR. New York: United Nations; 2006. Report of the fifty-fourth session of the United Nations Scientific Committee on the Effects of Atomic Radiation. [Google Scholar]
- 9.BEIR. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington DC: National Acadamies Press; 2006. 0-309-09156-X. [PubMed] [Google Scholar]
- 10.Douglas AJ, Omar RZ, Smith PG. Cancer mortality and morbidity among workers at the Sellafield plant of British Nuclear Fuels. Br J Cancer. 1994;70:1232–43. doi: 10.1038/bjc.1994.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGeoghegan D, Binks K. The mortality and cancer morbidity experience of workers at the Capenhurst uranium enrichment facility 1946-95. J Radiol Prot. 2000;20:381–401. doi: 10.1088/0952-4746/20/4/303. [DOI] [PubMed] [Google Scholar]
- 12.McGeoghegan D, Binks K. The mortality and cancer morbidity experience of workers at the Springfields uranium production facility, 1946-95. J Radiol Prot. 2000;20:111–37. doi: 10.1088/0952-4746/20/2/301. [DOI] [PubMed] [Google Scholar]
- 13.McGeoghegan D, Binks K. The mortality and cancer morbidity experience of employees at the Chapelcross plant of British Nuclear Fuels plc, 1955-95. J Radiol Prot. 2001;21:221–50. doi: 10.1088/0952-4746/21/3/302. [DOI] [PubMed] [Google Scholar]
- 14.Omar RZ, Barber JA, Smith PG. Cancer mortality and morbidity among plutonium workers at the Sellafield plant of British Nuclear Fuels. Br J Cancer. 1999;79:1288–301. doi: 10.1038/sj.bjc.6690207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith PG, Douglas AJ. Mortality of workers at the Sellafield plant of British Nuclear Fuels. Br Med J (Clin Res Ed) 1986;293:845–54. doi: 10.1136/bmj.293.6551.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGeoghegan D, Gillies M, Riddell AE, Binks K. Mortality and cancer morbidity experience of female workers at the British Nuclear Fuels Sellafield plant, 1946-1998. Am J Ind Med. 2003;44:653–63. doi: 10.1002/ajim.10316. [DOI] [PubMed] [Google Scholar]
- 17.ONS. CD-ROM (with updates). London: Office for National Statistics; 2004. 20th Century mortality - 100 years of mortality data in England and Wales by age, sex, year and underlying cause. [Google Scholar]
- 18.Hirosoft. Seattle, WA: Hirosoft International Corporation; 1998. Epicure: Fast, interactive software for the analysis of medical, public health, epidemiologic, econometric, and reliability data v. 2.10. [Google Scholar]
- 19.Thierry-Chef I, Marshall M, Fix JJ, et al. The 15-Country Collaborative Study of Cancer Risk among radiation workers in the nuclear industry: study of errors in dosimetry. Radiat Res. 2007;167:380–95. doi: 10.1667/RR0552.1. [DOI] [PubMed] [Google Scholar]
- 20.Thierry-Chef I, Pernicka F, Marshall M, Cardis E, Andreo P. Study of a selection of 10 historical types of dosemeter: variation of the response to Hp(10) with photon energy and geometry of exposure. Radiat Prot Dosimetry. 2002;102:101–13. doi: 10.1093/oxfordjournals.rpd.a006078. [DOI] [PubMed] [Google Scholar]
- 21.Wen CP, Tsai SP, Gibson RL. Anatomy of the healthy worker effect: a critical review. J Occup Med. 1983;25:283–89. [PubMed] [Google Scholar]
- 22.Hakulinen T. A Mantel-Haenszel statistic for testing the association between a polychotomous exposure and a rare outcome. Am J Epidemiol. 1981;113:192–97. doi: 10.1093/oxfordjournals.aje.a113083. [DOI] [PubMed] [Google Scholar]
- 23.Bunting J. Sources and methods. In: Drever F, Whitehead M, editors. Health Inequalities. London: The Stationery Office; 1997. [Google Scholar]
- 24.Griffiths C, Fitzpatrick J. London: Office for National Statistics; 2001. Geographic variations in health. Report No. DS16. [Google Scholar]
- 25.Arrighi HM, Hertz-Picciotto I. The evolving concept of the healthy worker survivor effect. Epidemiology. 1994;5:189–96. doi: 10.1097/00001648-199403000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong BG. Effect of measurement error on epidemiological studies of environmental and occupational exposures. Occup Environ Med. 1998;55:651–56. doi: 10.1136/oem.55.10.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jurek AM, Greenland S, Maldonado G, Church TR. Proper interpretation of non-differential misclassification effects: expectations vs observations. Int J Epidemiol. 2005;34:680–87. doi: 10.1093/ije/dyi060. [DOI] [PubMed] [Google Scholar]
- 28.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boggild H, Knutsson A. Shift work, risk factors and cardiovascular disease. Scand J Work Environ Health. 1999;25:85–99. doi: 10.5271/sjweh.410. [DOI] [PubMed] [Google Scholar]
- 30.Knutsson A. Health disorders of shift workers. Occup Med (Lond) 2003;53:103–8. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- 31.Steenland K. Epidemiology of occupation and coronary heart disease: research agenda. Am J Ind Med. 1996;30:495–99. doi: 10.1002/(SICI)1097-0274(199610)30:4<495::AID-AJIM16>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 32.Steenland K, Fine L, Belkic K, et al. Research findings linking workplace factors to CVD outcomes. Occup Med. 2000;15:7–68. [PubMed] [Google Scholar]
- 33.Boggild H, Suadicani P, Hein HO, Gyntelberg F. Shift work, social class, and ischaemic heart disease in middle aged and elderly men; a 22 year follow up in the Copenhagen Male Study. Occup Environ Med. 1999;56:640–45. doi: 10.1136/oem.56.9.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yadegarfar G, McNamee R. Shift work, confounding and death from ischaemic heart disease. Occup Environ Med. 2007 doi: 10.1136/oem.2006.030627. doi: 10.1136/oem2006.030267. [DOI] [PubMed] [Google Scholar]
- 35.Karlsson BH, Knutsson AK, Lindahl BO, Alfredsson LS. Metabolic disturbances in male workers with rotating three-shift work. Results of the WOLF study. Int Arch Occup Environ Health. 2003;76:424–30. doi: 10.1007/s00420-003-0440-y. [DOI] [PubMed] [Google Scholar]
- 36.Virkkunen H, Harma M, Kauppinen T, Tenkanen L. The triad of shift work, occupational noise, and physical workload and risk of coronary heart disease. Occup Environ Med. 2006;63:378–86. doi: 10.1136/oem.2005.022558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNamee R, Binks K, Jones S, Faulkner D, Slovak A, Cherry NM. Shiftwork and mortality from ischaemic heart disease. Occup Environ Med. 1996;53:367–73. doi: 10.1136/oem.53.6.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savitz DA, Liao D, Sastre A, Kleckner RC, Kavet R. Magnetic field exposure and cardiovascular disease mortality among electric utility workers. Am J Epidemiol. 1999;149:135–42. doi: 10.1093/oxfordjournals.aje.a009779. [DOI] [PubMed] [Google Scholar]
- 39.Hakansson N, Gustavsson P, Sastre A, Floderus B. Occupational exposure to extremely low frequency magnetic fields and mortality from cardiovascular disease. Am J Epidemiol. 2003;158:534–42. doi: 10.1093/aje/kwg197. [DOI] [PubMed] [Google Scholar]
- 40.Kotseva KP, De Bacquer D. Cardiovascular effects of occupational exposure to carbon disulphide. Occup Med (Lond) 2000;50:43–47. doi: 10.1093/occmed/50.1.43. [DOI] [PubMed] [Google Scholar]
- 41.Nurminen M. Survival experience of a cohort of carbon disulphide exposed workers from an eight-year prospective follow-up period. Int J Epidemiol. 1976;5:179–85. doi: 10.1093/ije/5.2.179. [DOI] [PubMed] [Google Scholar]
- 42.Partanen T, Hernberg S, Nordman CH, Sumari P. Coronary heart disease among workers exposed to carbon disulphide. Br J Ind Med. 1970;27:313–25. doi: 10.1136/oem.27.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sweetnam PM, Taylor SW, Elwood PC. Exposure to carbon disulphide and ischaemic heart disease in a viscose rayon factory. Br J Ind Med. 1987;44:220–27. doi: 10.1136/oem.44.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burstyn I, Kromhout H, Partanen T, et al. Polycyclic aromatic hydrocarbons and fatal ischemic heart disease. Epidemiology. 2005;16:744–50. doi: 10.1097/01.ede.0000181310.65043.2f. [DOI] [PubMed] [Google Scholar]
- 45.Herbert R, Schechter C, Smith DA, et al. Occupational coronary heart disease among bridge and tunnel officers. Arch Environ Health. 2000;55:152–63. doi: 10.1080/00039890009603401. [DOI] [PubMed] [Google Scholar]
- 46.Kalay N, Ozdogru I, Cetinkaya Y, et al. Cardiovascular effects of carbon monoxide poisoning. Am J Cardiol. 2007;99:322–24. doi: 10.1016/j.amjcard.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 47.Koskela RS. Cardiovascular diseases among foundry workers exposed to carbon monoxide. Scand J Work Environ Health. 1994;20:286–93. doi: 10.5271/sjweh.1396. [DOI] [PubMed] [Google Scholar]
- 48.Sjogren B. Occupational exposure to dust: inflammation and ischaemic heart disease. Occup Environ Med. 1997;54:466–69. doi: 10.1136/oem.54.7.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stern FB, Halperin WE, Hornung RW, Ringenburg VL, McCammon CS. Heart disease mortality among bridge and tunnel officers exposed to carbon monoxide. Am J Epidemiol. 1988;128:1276–88. doi: 10.1093/oxfordjournals.aje.a115081. [DOI] [PubMed] [Google Scholar]
- 50.Early Breast Cancer Trialists' Collaborative Group. Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Lancet. 2000;355:1757–70. [PubMed] [Google Scholar]
- 51.Blair A, Hartge P, Stewart PA, McAdams M, Lubin J. Mortality and cancer incidence of aircraft maintenance workers exposed to trichloroethylene and other organic solvents and chemicals: extended follow up. Occup Environ Med. 1998;55:161–71. doi: 10.1136/oem.55.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen R, Dick F, Seaton A. Health effects of solvent exposure among dockyard painters: mortality and neuropsychological symptoms. Occup Environ Med. 1999;56:383–87. doi: 10.1136/oem.56.6.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakamura K. Mortality patterns among cleaning workers. Sangyo Igaku. 1985;27:24–37. doi: 10.1539/joh1959.27.24. [DOI] [PubMed] [Google Scholar]
- 54.Wilcosky TC, Simonsen NR. Solvent exposure and cardiovascular disease. Am J Ind Med. 1991;19:569–86. doi: 10.1002/ajim.4700190503. [DOI] [PubMed] [Google Scholar]
- 55.Wilcosky TC, Tyroler HA. Mortality from heart disease among workers exposed to solvents. J Occup Med. 1983;25:879–85. doi: 10.1097/00043764-198312000-00010. [DOI] [PubMed] [Google Scholar]
- 56.Davies HW, Teschke K, Kennedy SM, Hodgson MR, Hertzman C, Demers PA. Occupational exposure to noise and mortality from acute myocardial infarction. Epidemiology. 2005;16:25–32. doi: 10.1097/01.ede.0000147121.13399.bf. [DOI] [PubMed] [Google Scholar]
- 57.Virkkunen H, Kauppinen T, Tenkanen L. Long-term effect of occupational noise on the risk of coronary heart disease. Scand J Work Environ Health. 2005;31:291–99. doi: 10.5271/sjweh.885. [DOI] [PubMed] [Google Scholar]
- 58.Moller L, Kristensen TS. Blood lead as a cardiovascular risk factor. Am J Epidemiol. 1992;136:1091–100. doi: 10.1093/oxfordjournals.aje.a116574. [DOI] [PubMed] [Google Scholar]
- 59.Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease–a systematic review. Environ Health Perspect. 2007;115:472–82. doi: 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen CJ, Chiou HY, Chiang MH, Lin LJ, Tai TY. Dose-response relationship between ischemic heart disease mortality and long-term arsenic exposure. Arterioscler Thromb Vasc Biol. 1996;16:504–10. doi: 10.1161/01.atv.16.4.504. [DOI] [PubMed] [Google Scholar]
- 61.Tseng CH, Chong CK, Tseng CP, et al. Long-term arsenic exposure and ischemic heart disease in arseniasis-hyperendemic villages in Taiwan. Toxicol Lett. 2003;137:15–21. doi: 10.1016/s0378-4274(02)00377-6. [DOI] [PubMed] [Google Scholar]
- 62.Levine RJ, Andjelkovich DA, Kersteter SL, et al. Heart disease in workers exposed to dinitrotoluene. J Occup Med. 1986;28:811–16. doi: 10.1097/00043764-198609000-00011. [DOI] [PubMed] [Google Scholar]
- 63.Ben-David A. Cardiac arrest in an explosives factory worker due to withdrawal from nitroglycerin exposure. Am J Ind Med. 1989;15:719–22. doi: 10.1002/ajim.4700150610. [DOI] [PubMed] [Google Scholar]
- 64.Klock JC. Nonocclusive coronary disease after chronic exposure to nitrates: evidence for physiologic nitrate dependence. Am Heart J. 1975;89:510–13. doi: 10.1016/0002-8703(75)90159-3. [DOI] [PubMed] [Google Scholar]
- 65.Lange RL, Reid MS, Tresch DD, Keelan MH, Bernhard VM, Coolidge G. Nonatheromatous ischemic heart disease following withdrawal from chronic industrial nitroglycerin exposure. Circulation. 1972;46:666–78. doi: 10.1161/01.cir.46.4.666. [DOI] [PubMed] [Google Scholar]
- 66.Hauptmann M, Mohan AK, Doody MM, Linet MS, Mabuchi K. Mortality from diseases of the circulatory system in radiologic technologists in the United States. Am J Epidemiol. 2003;157:239–48. doi: 10.1093/aje/kwf189. [DOI] [PubMed] [Google Scholar]
- 67.Cardis E, Gilbert ES, Carpenter L, et al. Effects of low doses and low dose rates of external ionizing radiation: cancer mortality among nuclear industry workers in three countries. IARC Technical Report 25 (Lyon) 1995 [PubMed] [Google Scholar]
- 68.Ivanov VK, Gorski AI, Maksioutov MA, Tsyb AF, Souchkevitch GN. Mortality among the Chernobyl emergency workers: estimation of radiation risks (preliminary analysis) Health Phys. 2001;81:514–21. doi: 10.1097/00004032-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 69.Ivanov VK, Maksioutov MA, Chekin SY, et al. The risk of radiation-induced cerebrovascular disease in Chernobyl emergency workers. Health Phys. 2006;90:199–207. doi: 10.1097/01.HP.0000175835.31663.ea. [DOI] [PubMed] [Google Scholar]
- 70.Schultz-Hector S, Trott KR. Radiation-induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys. 2007;67:10–18. doi: 10.1016/j.ijrobp.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 71.Benditt EP, Benditt JM. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci USA. 1973;70:1753–56. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murry CE, Gipaya CT, Bartosek T, Benditt EP, Schwartz SM. Monoclonality of smooth muscle cells in human atherosclerosis. Am J Pathol. 1997;151:697–705. [PMC free article] [PubMed] [Google Scholar]
- 73.Andreassi MG, Botto N. DNA damage as a new emerging risk factor in atherosclerosis. Trends Cardiovasc Med. 2003;13:270–75. doi: 10.1016/s1050-1738(03)00109-9. [DOI] [PubMed] [Google Scholar]
- 74.Hatzistamou J, Kiaris H, Ergazaki M, Spandidos DA. Loss of heterozygosity and microsatellite instability in human atherosclerotic plaques. Biochem Biophys Res Commun. 1996;225:186–90. doi: 10.1006/bbrc.1996.1151. [DOI] [PubMed] [Google Scholar]
- 75.Spandidos DA, Ergazaki M, Arvanitis D, Kiaris H. Microsatellite instability in human atherosclerotic plaques. Biochem Biophys Res Commun. 1996;220:137–40. doi: 10.1006/bbrc.1996.0370. [DOI] [PubMed] [Google Scholar]
- 76.Rodel F, Keilholz L, Herrmann M, Sauer R, Hildebrandt G. Radiobiological mechanisms in inflammatory diseases of low-dose radiation therapy. Int J Radiat Biol. 2007;83:357–66. doi: 10.1080/09553000701317358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.