Abstract
Background
Despite the high prevalence of HIV in correctional settings, the duration of therapy and response to various highly active antiretroviral therapy (HAART) regimens in this setting is unknown.
Method
Using a retrospective cohort study (1997−2002) of HIV-infected prisoners in Connecticut that linked demographic, pharmacy, and laboratory data, we compared HIV-1 RNA (VL) and CD4 lymphocyte responses to four treatment strategies at baseline and at the end of incarceration.
Results
Using an analysis of 1,044 incarceration periods or 1,099 subjects for whom ≥6 months of continuous data were available, HAART regimens that included a triple NRTI, two NRTIs + either a PI or NNRTI, or a three-class (NRTI+NNRTI+PI) strategy demonstrated no difference in virological and immunological outcomes. The proportion of subjects who were initiated with NRTI, NNRTI, PI, or three-class regimens were 14%, 32%, 46%, and 8%, respectively. For all study groups, the mean change from baseline in CD4 and VL was +74 cells/μL and −0.93 log10 copies/mL (p < .0001), respectively. Overall, 59% of subjects had an HIV-1 RNA level below the level of detection (<400 copies/mL) by the end of their incarceration. Using Kaplan-Meier curves to examine the time to change in the initial HAART strategy over the incarceration period, the three-class strategy was significantly more likely to be changed earlier than all others (p < .05).
Conclusion
Although the three-class strategy was less durable, initiating HAART with any strategy resulted in similar and impressive virological and immunological outcomes by the end of incarceration, further supporting prison as an important site for the initiation and provision of effective antiretroviral therapy.
Keywords: antiretroviral therapy, CD4 lymphocyte count, HAART, HIV/AIDS, HIV-1 RNA, incarceration, non-nucleoside reverse transcriptase inhibitors, nucleoside reverse transcriptase inhibitors, prisoners, protease inhibitors
In the United States, HIV infection is five times1,2 and AIDS incidence is four times3 greater among incarcerated persons compared to the general population. Similar to findings throughout the United States,4 AIDS-related mortality among prisoners in New York State reduced markedly from 1995 to 1998.5 Reductions in AIDS-related mortality in both settings have been attributed to the use of highly active antiretroviral therapy (HAART). Even though provision of health care in correctional settings has been reported to lag behind the community standard of care,6,7 98% of HIV-infected inmates in the state of Connecticut received recommended antiretroviral therapy regimens from 1997 to 2002 and 59% left the correctional setting with an HIV-1 RNA level below the level of detection.8 Systematic examination of the types of antiretroviral treatment strategies prescribed and their outcomes in correctional settings during the HAART era, however, have not been described.
We therefore conducted a retrospective cohort analysis that examined clinical outcomes of HIV-infected prisoners in Connecticut who were prescribed HAART that included either a triple nucleoside reverse transcriptase inhibitor (NRTI), non-nucleoside reverse transcriptase inhibitor (NNRTI)-based, protease inhibitor (PI)-based, or three-class regimen. The primary purpose of this study was to determine if significant differences exist among virological or immunological responses in prisoners prescribed various HAART combinations. Based upon 48-week data from prospective, randomized controlled trials, we hypothesized a priori that individuals receiving a triple-NRTI regimen would be less likely to achieve a virological response compared to NNRTI-9 or PI-based regimens and that those receiving a PI-based regimen would have improved immunological responses overall.10
METHOD
Study Site
The Connecticut Department of Correction (CT DOC) is an integrated correctional system with 18 (male = 17, female = 1) facilities that include both pretrial detainees and sentenced prisoners. The average daily census of the CT DOC in July 2003 was 19,171 (17,710 men and 1,411 women); of these, 15,243 are sentenced inmates. Blacks (44%) and Hispanics (27%) account for the majority of all inmates.11 Previous anonymous surveillance data suggest the HIV prevalence to be 6.1% among men12 and 9.2% among women.13 The University of Connecticut Correctional Managed Care provides all medical care, and HIV care is provided by the Yale University AIDS Program and other subcontracted HIV specialists. An HIV nurse specialist is available at each of the facilities to coordinate the HIV care. All antiretroviral medications approved by the US Food and Drug Administration are available on formulary for the treatment of HIV disease. HIV care is recommended in accordance with guidelines issued by the US Department of Health and Human Services (DHHS).14 HIV-1 RNA and CD4 lymphocyte counts are routinely obtained at least quarterly. The majority of correctional inmates with HIV in Connecticut self-administer their medications; however, in select cases the HIV specialist may prescribe antiretroviral therapy as directly observed therapy (DOT). The route of medication administration was not available for this analysis.
Data Sources
Data were retrospectively obtained on all HIV-infected inmates who had received antiretroviral therapy within the CT DOC during the time period of January 1, 1997 through December 31, 2002. Three primary sources of data were linked to establish the longitudinal cohort. First, pharmacy data that included all antiretroviral therapy were linked to all laboratory data that included HIV-1 RNA levels and CD4 lymphocyte counts. Once merged, these data were linked to the statewide correctional database system that included demographic information and dates of incarceration and release. When all three sources of data were merged into one complete dataset, all unique identifiers were removed and provided on a password-protected disc for analysis. The following were not available to incorporate into the analysis: genotypic resistance testing, adverse events due to antiretroviral therapy or drug interactions, laboratory tests other than HIV-1 RNA levels and CD4 lymphocyte counts, past medical history, co-infection with other viral diseases such as hepatitis C and B, history of treatment experience, or reasons for switching antiretroviral therapy. The institutional review boards at the Yale University School of Medicine, the University of Connecticut Health Sciences Center, and the CT DOC independently approved the research.
Study Population
Only prisoners who met the following criteria were analyzed: (a) HIV seropositive, (b) inmate within the CT DOC, (c) incarcerated for 6 or more consecutive months and having initiated antiretroviral therapy (ART) during the prison sentence, (d) available baseline and follow-up viral load (VL) and CD4 counts, and (e) available pharmacy prescriptions for HAART. The denominator of interest included incarcerations that exceeded 6 or more months of consecutive HAART and included some individuals with more than one incarceration indicated as “incarceration period.”
Study Definitions
A HAART regimen strategy was defined by the type of regimen that was first initiated during the eligible incarceration period. The rationale for this definition is that it delineated a clinical strategy by the HIV specialist that was based on the subject's clinical status and previous antiretroviral history. These strategies included: (a) PI-based regimens with two NRTIs and at least one PI (no NNRTI included); (b) NNRTI-based regimens with two NRTIs and at least one NNRTI (no PI included); (c) NRTI-only regimens with three or more NRTIs (no PI or NNRTI included); and (d) three-class regimens (multiple) with at least one NRTI and one NNRTI and one PI. Baseline VL and CD4 was defined as the laboratory value just prior to the first prescription date of a HAART regimen for each incarceration period. Data regarding treatment experience or genotypic resistance or tolerance to medications were, however, unavailable.
Data Scheme
There were 1,866 HIV-infected inmates within the CT DOC who received ≥6 consecutive months of HAART during the study period. Basic demographic characteristics are provided for this group in the results section of this article. Of these 1,866 inmates, 1,099 had complete pharmacy, demographic, laboratory, and admission and discharge dates. Of these 1,099 subjects, data were censored to include only the 1,044 incarceration periods for these inmates that comprised at least a 6-month continuous incarceration period; 88% (n = 919) of the subjects had one and 11% (n = 125) had two incarceration periods ≥6 months. The characteristics of the individuals who were excluded for missing information did not differ from the final cohort for whom only incarceration periods are analyzed (data not shown).
Analyses
HIV-1 RNA levels were measured using the Roche Amplicor 1.0 assay (Roche Diagnostic Systems, Inc., Branchburg, New Jersey, USA), and CD4 counts were measured with flow cytometry using a facscaliber by Becton Dickensen at the University of Connecticut's Health Science Center Laboratory. All data analyses were conducted at the Yale School of Medicine using SAS version 8.2 (SAS Institute, Inc., Cary, North Carolina, USA). Mean values for HIV-1 RNA levels were calculated by (a) mean value of the absolute HIV-1 RNA level for absolute mean, and (b) transformation to log10 before imputing the mean log value. Initial univariate analyses were conducted on all demographic indicators and outcome variables using Student t test and chi-square testing. Data were analyzed based on first prescribed HAART regimen regardless of HAART regimen changes for incarceration periods of at least 6 continual months. Analyses of variance (ANOVAs) were conducted to determine the overall effect of HAART regimen. Analyses were conducted to compare the effects of four prescribed HAART regimen strategies on CD4 and VL outcome measures. Baseline CD4 and VL was defined as result prior to or at the same date as prescribed HAART regimen. F tests were used when controlling for baseline CD4 count and baseline VL within analyses. Kaplan-Meier test was utilized to determine time to switch strategy curves. Log-rank test, Wilcoxon test, and log likelihood tests were also used to determine comparisons between the curves.
RESULTS
The demographic characteristics for the 1,099 cohort subjects who were included in the final analysis are the following. The mean age for men was greater than for women (men = 39 years; women = 36 years; p < .001). The majority of subjects were men (81%, n = 890; 19% women, n = 280), were persons of color (83%, n = 912), and had used opiates, cocaine, and/or alcohol (82%, n = 901) prior to incarceration. The mean time in prison for the 1,099 inmates during this study period was 478 days (interquartile range [IQR] 286−917 days). The majority of inmates were prescribed PI-based HAART (46%) as their initial regimen, followed by NNRTI (32%), NRTI only (14%), and three-class (8%) HAART regimens. Of note, all NRTI regimens consisted of abacavir/lamivudine/retrovir (Trizivir®). These four regimen strategies accounted for 100% of all prescribed antiretroviral regimens during the study period, and 98% of these were either preferred or alternative regimens in the contemporaneous DHHS guidelines.
Overall, 59% of subjects had an HIV-1 RNA level below the level of detection (BLD) by the end of their incarceration.8 The median time to the first VL <400 copies was 93 days and was similar for the PI, NNRTI, and NRTI-only regimens. There were no baseline or follow-up differences among the four treatment strategies with respect to VL and CD4. These results are depicted in Tables 1 and 2.
Table 1.
Overall analyses of baseline and change in outcomes from first incarceration to end of sentence (at least 6 month continual sentence)
| Baseline values | Last value | Change in value from baseline | p | |
|---|---|---|---|---|
| Mean CD4 count (cells/mL) | 330 | 404 | +74 | <.0001 |
| Absolute mean HIV-1 RNA (copies/mL) | 59,820 | 14,200 | −45,620 | <.0001 |
| Log transformed mean HIV-1 RNAa | 3.76 log10 | 2.83 log10 | −0.93 log10 | <.0001 |
log10 mean refers to the log transformed mean and not the log10 of the absolute mean in the previous row.
Table 2.
Analysis of baseline and mean change in HIV-1 RNA and CD4 lymphocyte count to end of sentence (at least 6 month continual sentence)a
| Initial prescribed HAART regimen | Baseline mean HIV-1 RNA (log10) | Mean change in HIV-1 RNA (log10) | Baseline mean CD4 (cells/mL) | Change in mean CD4 (cells/mL) |
|---|---|---|---|---|
| PI | 3.66 | −0.89 | 336 | 82 |
| NNRTI | 3.72 | −0.86 | 358 | 68 |
| NRTI | 4.11 | −1.26 | 285 | 70 |
| Multiple | 3.90 | −0.88 | 259 | 54 |
No statistical difference between prescribed HAART regimens.
Only 16% of the subjects remained on their originally prescribed HAART regimen over the length of their incarceration study period (1997−2002). Among the 506 initially prescribed a PI-based regimen, 21% (n = 106) remained on their original treatment regimen at the end of the study period; the remainder changed to an alternative regimen. Of the 154 who initiated a triple-NRTI regimen, 17% (n = 26) remained on their original regimen at the end of the study period. Of the 353 who had NNRTI-based regimens prescribed initially, 42% (n = 148) switched to an alternative HAART regimen, leaving only 12% (n = 42) on the original regimen at the end of the study period. Of the 87 who were originally prescribed a three-class HAART regimen, 61% (n = 53) had changed to an alternative HAART regimen prior to the end of the study period and only 5% (n = 4) remained on their original treatment regimen.
Subjects who were initially started on a three-class HAART regimen were the least likely to remain on their regimen at the end of their incarceration period, compared to any of the other treatment strategies (p < .05). Figure 1 depicts the Kaplan-Meier curve for time to switch to an alternative HAART strategy. There were no statistical differences in time to switch of the initial HAART regimen among the PI-, NNRTI-, and triple-NRTI–based regimen strategies.
Figure 1.
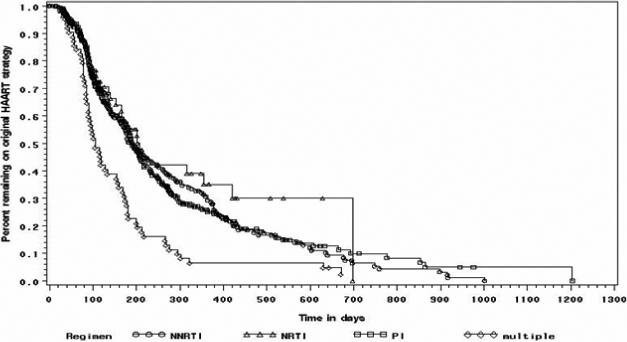
Kaplan-Meier curve demonstrating time until switch to alternative HAART regimen. Multiple (three-class) HAART regimen strategy was statistically significantly more likely to discontinue initial strategy compared to other regimens (p < .05).
Virological outcomes and time to failure analyses were similar between the triple NRTI, NNRTI-based, PI-based, and three-class regimens (see Table 2). The percentage of persons with an undetectable VL (HIV-1 RNA <400 copies/mL) and percent with >100 CD4 cell increase over their ≥6 month incarceration period were also not statistically different among treatment strategies (see Figure 2).
Figure 2.
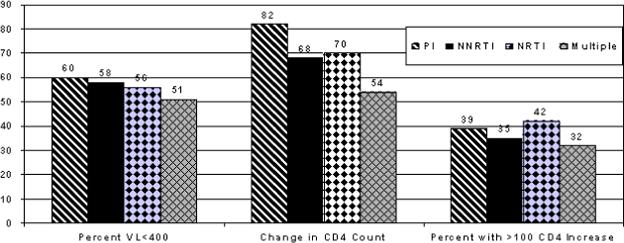
HIV-1 RNA and CD4 count outcomes among four different prescribed antiretroviral regimens. There were no statistical difference between strategies. VL = viral load.
DISCUSSION
This study represents the first systematic analysis of responses to various antiretroviral regimens among an incarcerated population. This study found that although all regimen strategies were effective, the three-class HAART strategy was not as durable as the other treatment options and was changed earlier than any of the three other antiretroviral regimen strategies. These data, however, are unable to determine causality for the inferior durability of the three-class strategy. Possible explanations for inferior durability of this strategy may be selection bias by the clinician who had prior knowledge of baseline resistance, intolerability to one or more antiretroviral medications, or anticipation that a more potent regimen would be needed to achieve virological suppression. Additionally, the reasons for early change in therapy may be similar to those described among nonincarcerated populations, such as increased toxicity15,16 or increased rates of virological failure.17,18 Furthermore, the three-class regimen group likely represented a more heavily pretreated group as compared to the other regimen groups and likely had more antiretroviral resistance.
Only 16% of this sample remained on their originally prescribed HAART regimen. Some studies suggest that viral suppression is likely to persist for long periods of time if the individual has achieved a nondetectable viral load after 1 year of treatment.19–21 Other studies of antiretroviral therapy switch strategies, however, suggest results similar to those found in this study of prisoners.22–24 In one cohort of 1,056 HIV-infected women with substance use disorder, almost 46% had switched their HAART regimen and 18% reported discontinuing their HAART altogether within a 1-year period.25 Furthermore, in another evaluation of the impact of antiretroviral regimen switches on adherence in a community-based sample, approximately 66% of HIV-infected patients had reported changing their regimens with the most common reasons for switch including changing capsule to liquid ritonavir, gastrointestinal intolerance, and virologic failure, followed by peripheral neuropathy.15 Our study, with a longer duration of follow-up, demonstrated higher rates of medication changes.
It is surprising and inconsistent with prospective randomized controlled trials9 that the prisoners in this cohort initially prescribed a triple-NRTI regimen resulted in similar levels of virological suppression compared to the other treatment approaches. One explanation for this finding is that this cohort study did not have a fixed duration of study (e.g., 48 weeks) and individuals who might not have achieved a nondetectable HIV-1 RNA level in this cohort may have been changed to a more suppressive regimen later in the course of their treatment. This notion is partially supported by the high rates of change to an alternative regimen during the course of the study. Additionally, we anticipated that subjects on a PI-based regimen might have a more robust immunological response10; however, in our study, it was similar for all groups. One potential explanation is that we were unable to distinguish between boosted and nonboosted PIs in our cohort due to the study period examined. Boosted-PI regimens with the co-administration of low-dose ritonavir have been shown to be more effective than unboosted PI regimens.26
Limitations of this study include its retrospective, cohort design, possible selection bias of HAART strategy by clinicians, inability to determine causality for outcomes, and inability to explain the reasons for prisoners changing their original regimen. Issues of previous antiretroviral treatment history, toxicity, adherence, and drug interactions were not available in this study. Notwithstanding these limitations, the majority of these inmates achieved impressive virological and immunological outcomes both early during their incarceration and certainly by the end of their incarceration and did so irrespective of the initial antiretroviral regimen prescribed.
As previously reported, 98% of this cohort of prisoners were prescribed DHHS-approved HAART regimens and 59% of subjects maintained an HIV-1 RNA level <400 copies/mL.8,27 This reduction is significantly greater than that found in a reported cohort of prisoners in Texas28 and is comparable to, if not greater than, that described in a subsequent small cohort of incarcerated persons in North Carolina.29 More important, these benefits are derived by a population of individuals (primarily injection drug users, people of color, and the mentally ill) who in community settings would have decreased access to antiretroviral therapy30,31 and would be less likely to adhere to treatment.25,32 Notwithstanding the questionable benefit from existing drug policy and mandatory sentencing legislation, these data, at a minimum, support the notion that while individuals are remanded to the correctional system, impressive medical outcomes for initiating and maintaining HIV therapy can be achieved if the infrastructure is sufficient to address the complex HIV and other medical needs of these patients.2,33,34 We believe that this study supports the idea that all HIV-infected prisoners should be evaluated for antiretroviral treatment initiation from the beginning of their incarceration period. Effective antiretroviral therapy that is initiated early will reduce morbidity and mortality and, it is hoped, decrease transmission of HIV infection if and when this population engages in high-risk behavior within or after release from prison.
CONCLUSION
The adherence to DHHS guidelines in the prescription of HAART in this correctional system is better than reported in correctional systems elsewhere.28,29 Impressive early and at end-of-incarceration virological and immunological outcomes were similar, irrespective of initial HAART regimen prescribed. These data suggest that to maintain a sup- pressed viral load over a longer time period than observed in most clinical trials, regimens can be salvaged by alternative treatment approaches and more potent combinations over a period of years rather than months. The data do not, however, support a change in the current treatment recommendations for people living with HIV/AIDS or a different standard of care for HIV-infected prisoners. To delineate further the durability and reasons for switching their initial regimen in this setting, prospective studies are necessary. As newer, more simplified, and more potent regimens become available, it may be possible to further improve virological, immunological, and clinical outcomes in HIV-infected prisoners. In highly structured settings like prisons, the appropriate use of HAART may result in health benefits for a population of individuals who have otherwise not shared equally in the benefit of HIV treatment.
ACKNOWLEDGMENTS
Funding for this research was provided through the Bristol-Myers Squibb HIV Virology Fellowship Award and from the National Institute on Drug Abuse (K24-DA017072, Altice) and (K23-DA019381, Springer).
REFERENCES
- 1.National Commission on Correctional Health Care [March 27, 2006];The Health Status of Soon-to-be-Released Inmates: A Report to Congress March. 2002 Available at: http://www.ncchc.org/pubs/pubs_stbr.html.
- 2.Spaulding A, Stephenson B, Macalino G, Ruby W, Clarke JG, Flanigan TP. Human immunodeficiency virus in correctional facilities: a review. Clin Infect Dis. 2002;35(3):305–312. doi: 10.1086/341418. [DOI] [PubMed] [Google Scholar]
- 3.Maruschak L. HIV in Prisons. Department of Justice; Washington, DC: 2000. [March 27, 2006]. [Bureau of Justice Statistics bulletin]. October 2002. Available at: http://www.ojp.gov/bjs/pub/pdf/hivp00.pdf. [Google Scholar]
- 4.Palella FJ, Delaney K, Moorman A, HIV Outpatient Study Investigators Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control (CDC) Decrease in AIDS-related mortality in a state correctional system–New York, 1995−1998. MMWR Morb Mortal Wkly Rep. 1999;47(51−52):1115–1117. [PubMed] [Google Scholar]
- 6.Pollack H, Khoshnood K, Altice FL. Health care delivery strategies for criminal offenders. J Health Care Finance. 1999;26(1):63–77. [PubMed] [Google Scholar]
- 7.Restum ZG. Public health implications of substandard correctional health care. Am J Public Health. 2005;95(10):1689–1691. doi: 10.2105/AJPH.2004.055053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Springer SA, Pesanti E, Hodges J, Macura T, Doros G, Altice FL. Effectiveness of antiretroviral therapy among HIV-infected prisoners: reincarceration and the lack of sustained benefit after release to the community. Clin Infect Dis. 2004;38(12):1754–1760. doi: 10.1086/421392. [DOI] [PubMed] [Google Scholar]
- 9.Gulick RM, Ribaudo HJ, Shikuma CM, et al. Three- vs four-drug antiretroviral regimens for the initial treatment of HIV-1 infection: a randomized controlled trial. JAMA. 2006;296(7):769–781. doi: 10.1001/jama.296.7.769. [DOI] [PubMed] [Google Scholar]
- 10.Riddler S, Haubrich R, DiRienzo G, et al. AIDS Clinical Trials Group 5142 Study Team. A prospective, randomized, phase III trial of NRTI-, PI- and NNRTI-sparing regimens for initial treatment of HIV-1 infection-ACTG 5142.. Paper presented at: XVI International AIDS Conference; Toronto, Ontario, Canada. August 13−18, 2006. [Google Scholar]
- 11.Connecticut Department of Correction Statistics. 2003 July 1; Available at: http://www.doc.state.ct.us/report/stat20030701.htm.
- 12.Altice FL, Mostashari F, Selwyn PA, et al. Predictors of HIV infection among newly sentenced male prisoners. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(5):444–453. doi: 10.1097/00042560-199808150-00005. [DOI] [PubMed] [Google Scholar]
- 13.Altice FL, Khoshnood K, Blankenship KM, et al. Determinants of HIV seroprevalence and seroincidence of new entrants to a women's prison.. Paper presented at: XI International Conference on AIDS; Vancouver, Canada. 1996. [Google Scholar]
- 14.US Department of Health and Human Services (DHHS) [November 10, 2006];Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Panel on Clinical Practices for Treatment of HIV infection convened by the Department of Health and Human Services. 2006 October; Available at: http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
- 15.Smeaton L, DeGruttola V, Robbins G, Shafer R, ACTG 384 (AIDS Clinical Trials Group) A strategy trial comparing consecutive treatments for HIV-1. Control Clin Trials. 2001;22(2):142–159. doi: 10.1016/s0197-2456(00)00126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas D, Zala C, Schrader S, et al. Therapy with atazanavir plus saquinavir in patients failing highly active antiretroviral therapy: a randomized comparative pilot trial. AIDS. 2003;17(9):1339–1349. doi: 10.1097/00002030-200306130-00008. [DOI] [PubMed] [Google Scholar]
- 17.Hammer S, Vaida F, Bennett K, et al. Dual vs. single protease inhibitor therapy following antiretroviral treatment failure: a randomized trial. JAMA. 2002;288(2):169–180. doi: 10.1001/jama.288.2.169. [DOI] [PubMed] [Google Scholar]
- 18.Gulick R, Hu X, Fiscus S, et al. Durability of response to treatment among antiretroviral-experienced subjects: 48-week results from AIDS Clinical Trials Group Protocol 359. J Infect Dis. 2002;186(5):626–633. doi: 10.1086/342681. [DOI] [PubMed] [Google Scholar]
- 19.Palella FJ, Chmiel J, Moorman A, et al. Durability and predictors of success of highly active antiretroviral therapy for ambulatory HIV-infected patients. AIDS. 2002;16(12):1617–1626. doi: 10.1097/00002030-200208160-00007. [DOI] [PubMed] [Google Scholar]
- 20.Phillips A, Miller V, Sabin C, et al. Durability of HIV-1 viral suppression over 3.3 years with multi-drug antiretroviral therapy in previously drug-naive individuals. AIDS. 2001;15(18):2379–2384. doi: 10.1097/00002030-200112070-00005. [DOI] [PubMed] [Google Scholar]
- 21.Paredes R, Mocroft A, Kirk O, et al. Predictors of virological success and ensuing failure in HIV-positive patients starting highly active antiretroviral therapy in Europe: results from the EuroSIDA study. Ann Intern Med. 2000;160(8):1123–1132. doi: 10.1001/archinte.160.8.1123. [DOI] [PubMed] [Google Scholar]
- 22.Celantano D, Vlahov D, Cohn S, et al. Self-reported antiretroviral therapy in injection drug users. JAMA. 1998;280(6):544–546. doi: 10.1001/jama.280.6.544. [DOI] [PubMed] [Google Scholar]
- 23.Ostrop N, Hallett K, Gill M. Long-term patient adherence to antiretroviral therapy. Ann Pharmacother. 2000;34(6):703–709. doi: 10.1345/aph.19201. [DOI] [PubMed] [Google Scholar]
- 24.Miller LG, Golin CE, Hays R, et al. Impact of antiretroviral regimen switches on adherence. HIV Clin Trials. 2002;3(5):355–360. doi: 10.1310/NNK4-GAGD-5C1Y-K9QT. [DOI] [PubMed] [Google Scholar]
- 25.Wood E, Montaner JS, Yip B, et al. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. CMAJ. 2003;169(7):656–661. [PMC free article] [PubMed] [Google Scholar]
- 26.Bartlett J, Fath M, DeMasi R, et al. An updated meta-analysis of triple combination therapy in antiretroviral–naïve HIV-infected adults.. Paper presented at: 12th Conference on Retroviruses and Opportunistic Infections (CROI); Boston. February 22−25, 2005. [Google Scholar]
- 27.Altice FL, Springer S, et al. Reply to Babudieri. Clin Infect Dis. 2005;40:322–323. [Google Scholar]
- 28.Baillargeon J, Borucki MJ, Zepeda S, Jenson HB, Leach CT. Antiretroviral prescribing patterns in the Texas prison system. Clin Infect Dis. 2000;31(6):1476–1481. doi: 10.1086/317478. [DOI] [PubMed] [Google Scholar]
- 29.Stephenson BL, Wohl DA, Golin CE, Tien HC, Stewart P, Kaplan AH. Effect of release from prison and re-incarceration on the viral loads of HIV-infected individuals. Public Health Rep. 2005;120(1):84–88. doi: 10.1177/003335490512000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Celentano D, Vlahov D, Cohn S, et al. Self-reported antiretroviral therapy in injection drug users. JAMA. 1998;280(6):544–546. doi: 10.1001/jama.280.6.544. [DOI] [PubMed] [Google Scholar]
- 31.Strathdee S, Palepu A, Cornelisse P, et al. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280(6):547–549. doi: 10.1001/jama.280.6.547. [DOI] [PubMed] [Google Scholar]
- 32.Lucas GM, Gebo KA, Chaisson RE, Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16(5):767–774. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- 33.Altice FL, Mostashari F, Friedland G. Trust and acceptance of and adherence to antiretroviral therapy. J Acquir Immune Defic Syndr Hum Retrovirol. 2001;28(1):47–58. doi: 10.1097/00042560-200109010-00008. [DOI] [PubMed] [Google Scholar]
- 34.Flanigan T, Rich J, Spaulding A. HIV care among incarcerated persons: a missed opportunity. AIDS. 1999;13:2475–2476. doi: 10.1097/00002030-199912030-00020. [DOI] [PubMed] [Google Scholar]


