Abstract
Feline immunodeficiency virus (FIV) is an important viral pathogen worldwide in the domestic cat, which is the smallest animal model for the study of natural lentivirus infection. Thus, understanding the molecular mechanisms by which FIV carries out its life cycle and causes an acquired immune deficiency syndrome (AIDS) in the cat is of high priority. FIV has an overall genome size similar to HIV, the causative agent of AIDS in man, and shares with the human virus genomic features that may serve as common targets for development of broad-based intervention strategies. Specific targets include enzymes encoded by the two lentiviruses, such as protease (PR), reverse transcriptase (RT), RNAse H, and integrase (IN). In addition, both FIV and HIV encode Vif and Rev elements essential for virus replication and also share the use of the chemokine receptor CXCR4 for entry into the host cell. The following review is a brief overview of the current state of characterization of the feline/FIV model and development of its use for generation and testing of anti-viral agents.
Keywords: feline immunodefiency virus, receptor, CD134, CXCR4
Introduction
FIV Pathology
FIV is a lentivirus associated with an AIDS-like syndrome in the domestic cat (Pedersen, 1993). Like HIV, FIV can be transmitted via mucosal exposure, blood transfer, and vertically via prenatal and postnatal routes (Pedersen, 1987; Rideout, 1992; O’Neil, 1995; O’Neil, 1996; Obert, 2000;). Progression of the disease follows a pattern typical of that observed with primate lentiviruses, starting with a relatively short (weeks) acute phase denoted by increasing viral loads, febrile episodes, weight loss, lymphadenopathy, and neutropenia. The acute phase is followed by an of t-protracted (years) asymptomatic phase denoted by relatively strong antiviral immune responses, lower viral titers, a gradual decline in CD4+ cells, and minimal clinical symptoms. The terminal phase is marked by immunologic decompensation, exacerbation of plasma viral load, and clinical symptoms of immunodeficiency with opportunistic infections (Pedersen, 1993; English, 1994). Lymphoid tissue alterations are consistent with those in HIV and SIV infections and include thymic depletion, lymphoid hyperplasia, plasmacytosis, and terminal lymphoid depletion (Callanan, 1993; Bach, 1994; Beebe, 1994; English, 1994; Parodi, 1994; Woo, 1997; Rogers, 1998). Neurological manifestations are also evident (Lafrado, 1993; Phillips, 1994; Prospero-Garcia, 1994; Phillips, 1996) including delayed auditory evoked and visual evoked potential changes (Lafrado, 1993; Phillips, 1994) and marked alterations in sleep patterns (Prospero-Garcia, 1994a). Many of these early symptoms resolve as the animals proceed into the latent phase of the disease, although the neurological abnormalities persist. As the disease progresses, decline in the number of CD4+ T cells continues, with ultimate increase in viral load in the later stages of the disease (O’Neil, 1996; Obert, 2000). Animals, if not euthanized, generally die of opportunistic infections.
Considerable advances have been made in understanding the molecular workings of FIV since its discovery 20 years ago, both from the standpoint of understanding the rudiments of the virus life cycle as well as in establishing the molecular basis for the observed pathogenic phenotypes outlined above. Although FIV is of direct concern to veterinary medicine, the majority of the work has involved comparison to HIV, with the hope that the cat model can contribute to the development of intervention strategies effective against both lentivirus infections. As will be shown below, many parallels exist in both the pathology and molecular structure of FIV and HIV and substantial progress has been made in use of the cat model for development of both drug and vaccine treatments.
FIV Genomic Structure
The overall genomic structure of FIV is markedly similar to HIV, although there are important distinctions (Olmsted, 1989; Talbott, 1989; Phillips, 1990) (Figure 1). The length of the FIV genome is around 9400 nucleotides, approximating that of HIV and other lentiviruses. The integrated provirus is bordered by long terminal repeats (LTRs) and possesses gag, pol, and env genes as all other retroviruses. As with other lentiviruses, FIV uses a tRNALys primer binding site to prime first strand synthesis by reverse transcriptase (RT). Transport of unspliced and singly spliced mRNAs is regulated by Rev interaction with the distinction that Rev Response Element (RRE) and second coding exon of Rev are located 3′ of env instead of overlapping it as in primate lentiviruses (Phillips, 1992). FIV lacks Vpr, Vpu, and Nef “accessory” genes that are present in HIV. FIV has an apparent transactivator, termed OrfA (or Orf2), which promotes a net increase in translation of gene products whose transcription is driven by the FIV LTR (Sparger, 1992; Waters, 1996; de Parseval, 1999). However, OrfA does not act via a TAR element, as is the case with HIV-1 Tat and promotes transcription/translation via mechanisms distinct from that of other lentiviruses (Chatterji, 2002). Sparger’s laboratory has presented evidence that there is no wholesale increase in transcription in the presence of Orf A and thus, the increase in net translation may be the consequence of downstream action (Gemeniano, 2003, 2004). They also showed that OrfA may have relatedness to Vpr and present indications of involvement in virus release from the cell and influence on cell cycle, similar to HIV Vpr (Gemeniano, 2003). Overall, the findings suggest that OrfA may be a multi-functional protein, which would certainly be in keeping with the need for versatility of gene products encoded by a relatively small viral genome.
Figure 1.
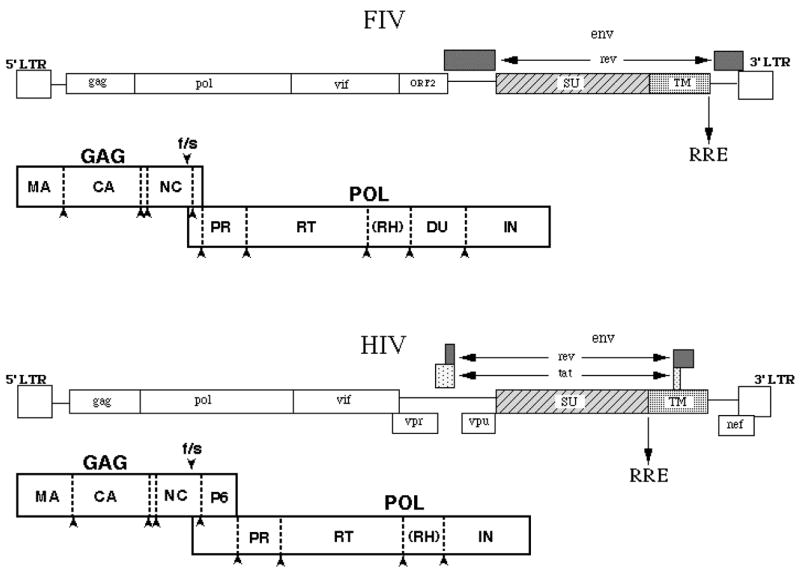
Comparison of the genomic structures of Feline Immunodeficiency Virus (FIV) and Human Immunodeficiency Virus (HIV). Diagrams are shown for each virus genome as present in the integrated provirus, with organization of each respective Gag-Pol polyprotein shown below. LTR, long terminal repeat; f/s, frameshift; RRE, rev response element; Orf2, open reading frame 2; Vif, viral infectivity factor.
Typical of most other lentiviruses, the Gag polyprotein is expressed via ribosomal frame-shifting (Morikawa, 1992) and is comprised of a myristoylated matrix (MA) protein, a capsid (CA) protein, and a nucleocapsid (NC) protein that has two copies of the zinc finger motif (Elder, 1992) (Figure 1). FIV lacks a P6 protein between Gag and Pol, but contains instead a P2 protein (Elder, 1993) that has a P(S/T)AP domain necessary for virus budding (Manrique, 2004). Thus, it is likely that P2 serves the same purpose as HIV-1 P6. It is now recognized that the PTAP domain in HIV P6 recruits TSG 101, a cellular protein involved in the virus budding process (Garrus, 2001; Martin-Serrano, 2001; VerPlank, 2001; Demirov, 2002; Freed, 2002). As to whether similar interactions are critical for FIV budding remains to be determined, but this appears likely. The FIV Pol polyprotein is comprised of protease (PR), reverse transcriptase (RT), and integrase (IN) genes, but in addition contains a gene encoding deoxyuridine pyrophosphatase (DU) between RT and IN (Elder, 1993; Lerner, 1995) (Figure 1). FIVs lacking DU are incapable of successful propagation in cells that are not undergoing division, such as primary macrophages, whereas wild type FIV will productively infect such cells (Lerner, 1995). These findings are also true for equine infectious anemia virus (EIAV), which is also a DU+ lentivirus (Threadgill, 1993; Steagall, 1995). DU is not necessary for replication in rapidly dividing cells, due to high endogenous levels of DU in the replicating cell (Lerner, 1995). The primary role of DU is to prevent mis-incorporation of uracil into DNA by limiting the concentration of dUTP through conversion to dUMP, a precursor for dTTP synthesis. DU− FIV accumulates five- to eight-fold more base changes, primarily G->A transitions, than wild type FIV during replication in macrophages in vivo, consistent with the mis-incorporation of uracil into viral DNA through utilization by RT of undegraded dUTP during viral DNA synthesis. Importantly, when HIV-1 infects non-dividing cells, it must also deal with the problem of uracil misincorporation, although it does not encode DU. Findings of others suggest that Vpr may compensate for the lack of DU in HIV-1, both by its necessity for growth of macrophage-tropic isolates in primary macrophages and by an apparent association with uracil N-glycosylase (Ung), the enzyme responsible for excision of uracil mis-incorporated into DNA (Bouhamdan, 1996). Mutations of Vpr that knock out Ung association causes a phenotype remarkably similar to the DU−phenotype noted in FIV and EIAV (Mansky, 2000).
A gene encoding viral infectivity factor (Vif) is present 3′ of pol, as in other lentiviruses except EIAV. Interestingly, findings indicate that HIV Vif also acts to reduce G->A transition mutations, but by preventing cytidine deamination by the cellular deaminase, APOBEC-3G (Mangeat, 2003; Zhang, 2003). The role of Vif in the FIV life cycle is currently being investigated to determine if parallels exist between Vif function in FIV and HIV.
The env gene encodes heavily glycosylated SU and TM proteins and exhibits considerable amino acid sequence variation, with 5 consensus major variable regions (V1–V5) in SU and 3 (V6–V8) in TM (Phillips, 1990; Pancino, 1993). The mechanism of virus entry dictated by interactions of the FIV envelope with binding and entry receptors closely parallels SU/receptor interactions noted with HIV. Characterization of receptor binding and entry of FIV has progressed markedly in the last few years and may offer an additionally target for development antiviral therapies in both cats and man (see below and also Willett, this issue). Four env subtypes (clades) plus numerous outliers have been defined via Env sequences (Bachmann, 1997). The genetic-clade variation of FIV is remarkably similar to that found in HIV (Delwart, 1997), although the bias towards non-synonymous site changes is not as great, especially for the B clade (Bachmann, 1997; Delwart, 1997). As with HIV, heteroduplex mobility assay has been used to define the diversity of FIV envelope sequence subtypes, as well as assist in the identification of numerous inter-subtype recombinants. Evolutionary trends, as assessed by rates of synonymous and non-synonymous base changes and level of mutational saturation, differ between the most commonly found FIV env clades, A and B (Sodora, 1994, 1995). FIV is tropic for T cells (Pedersen, 1987; Yamamoto, 1988; Sparger, 1989; Novotney, 1990; Brown, 1991; Beebe, 1994) macrophages (Brunner, 1989; Rideout, 1992; Callanan, 1993; Parodi, 1994), and central nervous system (CNS) cells. In vivo tissue tropism studies have demonstrated viral RNA in T cells, macrophages, and CNS cells. FIV RNA has also been demonstrated in association with follicular dendritic cells (FDC) (Toyosaki, 1993; Bach, 1994; Hurtrel, 1994). Although CD4+ cell decline is a hallmark of FIV infection, FIV has a broader lymphocyte tropism than CD4+ T cells, with infection also evident in at least a subset of CD8+ T cells and B cells in vitro and in vivo (Brown, 1991; Willett, 1993; de Parseval, 2000).
In spite of the targeting of CD4+ cells, CD4 is not used as a binding receptor for FIV. However, all domestic cat FIVs examined to date bind and utilize the chemokine receptor, CXCR4 as an entry receptor (Poeschla, 1998; Willett, 1998) similar to T celltropic HIVs. Recent studies (de Parseval, 2001; Joshi, 2005) have indicated that FIV infection of certain cells may occur solely mediated via CXCR4 if expression of the chemokine receptor is sufficiently high. However, feline CD4+ T cells express a 43 kDa glycoprotein that past studies had demonstrated is a binding receptor for FIV SU (de Parseval, 1999). Studies of Shimojima et al (Shimojima, 2004) have now shown that this molecule is the activation marker, CD134, and we have now confirmed that the SU binding molecule previously reported was, indeed, CD134 (de Parseval, 1999; also see below). The demonstration that CD134 is up-regulated on activated CD4+ T cells (de Parseval, 2004) explains how FIV targets this cell population in vivo in spite of failure to bind CD4. Furthermore, soluble CD134 can interact with the virus to facilitate productive infection of CD134− CXCR4+ target cells (de Parseval, 2005), indicating that the binding receptor alters the conformation of SU to promote high affinity binding to CXCR4 (Figure 2). This parallels findings with CD4 binding to HIV SU and indicates that although different binding receptors are involved, both viruses use very similar mechanisms to infect target cells. Furthermore, neutralizing monoclonal antibodies have been identified that only neutralize the virus when soluble CD134 is present (de Parseval, 2006). Again, these findings parallel observations of such masked neutralizing epitopes on HIV that become available upon interaction of SU with the CD4 binding receptor (Kwong, 2002; Moulard, 2002; Labrijn, 2003; Lusso, 2005; Xiang, 2005). The striking similarity and conservation of entry mechanisms between the two divergent lentiviruses likely is the result of common immunological pressures in the two hosts. Thus, the feline lentivirus offers a valuable venue to study the mechanisms of lentivirus infection of T cells and for development of strategies to compromise the virus’ ability to escape immune surveillance.
Figure 2.
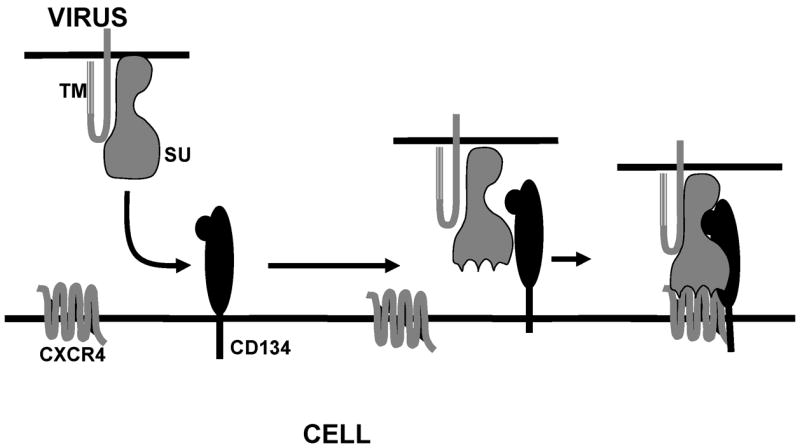
Illustration depicting receptor binding events during FIV infection. Primary binding to CD134 causes a conformational change in the FIV surface glycoprotein (SU), which facilitates higher affinity binding to the chemokine receptor, CXCR4. Subsequent fusion events involving the host cell membrane and the viral transmembrane protein, TM, occur to facilitate virus entry (not shown).
FIV as a system for development of intervention strategies
As detailed above, there are many parallels between the outcomes of infection with FIV in the cat and infection of humans by HIV. Additionally, the target cell populations are similar and thus both viruses are confronted with similar obstacles for replication and perpetuation of species. These two lentiviruses have evolved along unique pathways that have led to development of alternative mechanisms to deal with certain aspects of replication, including transcriptional transactivation and uracil misincorporation. However, there are sufficient similarities to make the cat/FIV model a valuable tool for several lines of direct experimentation. The utilization of CXCR4 by FIV as one of the receptors used to enter target cells is an important similarity to HIV that can be explored in development of intervention strategies. The parallels in the role of the binding receptor in facilitating interaction with CXCR4 in the two systems are striking and imply a strong selection for similar mechanisms of entry and evasion of immune surveillance. The Gag and Pol gene products have common functions in the two viruses and in many cases, respond to the same inhibitors. Use of the cat for development of broad-based protease inhibitors has been successful (Lee, 1998, 1999; also see below). Nucleoside analogs that interact with the active site of reverse transcriptase have been found efficacious against both FIV and HIV (North, 1989). Given the high relatedness of integrase proteins in the two lentiviruses, it is likely that FIV will serve as a valuable system for development of anti-integrase drugs. The structural proteins of Gag may also provide broad-based targets, since all lentiviruses share common morphological features. Elements of the virus core are likely to maintain commonalities in their mechanisms of action and orientations in the particles. The matrix, capsid, and nucleocapsid proteins may thus present effective targets for broad-based intervention strategies. As pointed out above, the P2 protein of FIV (Elder, 1993) is an apparent functional homologue to the P6 protein of HIV and shares late domain homologies (Demirov, 2002; Freed, 2002). Both HIV and FIV encode Vif proteins, which may provide an additional target for intervention strategies to be used in both lentivirus systems. Importantly, the cat offers an economical, low biohazard, and readily manipulated venue for in vivo testing of potential therapeutic modalities that cannot be economically tested in primate models. Thus, anti-viral approaches, both drug and vaccine treatments (see Yamamoto, this issue), can be carried forward directly into the natural host species.
PR as a target for antiviral therapies
Much of our laboratory’s efforts to develop broad-based antivirals using the cat model have centered around use of PR as a target for drug development and testing. The aspartic protease has the critical responsibility for the processing of viral Gag and Gag-Pol polyproteins into individual structural and enzymatic proteins during assembly and maturation (Pettit, 1991; Elder, 1993; Dunn, 1994; Katz, 1994; Tozser, 1997; Palella, 1998). This proteolytic step is highly specific, ordered, and essential for producing mature and infectious retrovirus particles (Pettit, 1994; Tomasselli, 1994; Swanstrom, 1997; Pettit, 1998). Therefore, PR has been a very important target for antiviral therapies (Vacca, 1997; Swanstrom, 2000). There are several approved protease inhibitors available that are effective for treating HIV-1 infection (Vacca, 1997; Swanstrom, 2000; Richman, 2004; Johnson, 2005). Combination drug therapies have been used successfully in the treatment of AIDS brought on by infection of individuals with HIV-1. In particular, the use of highly specific inhibitors of PR in combination with RT inhibitors, referred to as highly active anti-retroviral therapy (HAART), has proven to suppress HIV-1 replication to undetectable levels in patients (Collier, 1996; Gulick, 1997; Kirk, 1999; Richman, 2004; Johnson, 2005). However, HIV-1 variants frequently evolve that are resistant to these inhibitors (Condra, 1995; Jacobsen, 1996; Molla, 1996; Zhang, 1997; Palella, 1998; Young, 1998; Lawrence, 1999; Kutilek, 2003; Kozal, 2004). As many as 40% of the patients receiving HAART have a viral rebound within the first 3 years and this number is likely to be higher outside of controlled studies (Cohen, 1998). In addition, transmission of resistant HIV has been observed and is likely to increase with the increase of patients on combination therapy (Cohen, 1998). Also, poor tolerance to current protease inhibitors by a significant number of patients may lead to increased non-compliance, which may be the leading reason for cases of failure of HAART therapy. Side effects resulting from long-term drug treatment have also been observed. Both of the latter problems might be allayed by development of drugs with better bioavailability and length of efficacy per dosage, which would reduce the drug regimen. Thus, there is a need to develop novel inhibitors with activities against drug-resistant isolates that exhibit delayed resistance development and show a high degree of specificity (Kutilek, 2003). Defining the determinants of substrate specificity of the lentiviral PRs is a logical first step in the development of such broad-based inhibitors.
FIV protease, like HIV-1 protease, is a homodimeric aspartic proteinase and the two enzymes are strikingly similar at the crystallographic level, particularly within the substrate binding region (Wlodawer, 1995; Laco, 1997) (Figure 3). However, FIV is distinct in that each monomer is comprised of 116 amino acids, as opposed to 99 amino acids for HIV-1 protease, with only 27 conserved amino acids between FIV and HIV-1 PRs. Like HIV protease, FIV PR is responsible for processing Gag and Gag-Pol polyproteins (Elder, 1993). Similar to SIV and HIV-1 PRs, autoproteolysis of FIV protease is observed in vitro (Laco, 1997). Despite these similarities, FIV PR is specific to its respective substrates and inhibitors of HIV-1 protease currently employed in clinic do not inhibit FIV protease (Slee, 1995; Schnolzer, 1996; Dunn, 1999). FIV protease cleaves the FIV MA/CA cleavage junction efficiently. However, it does not appreciably cut the HIV-1 MA/CA cleavage junction, despite the presence of four identical residues in the P3-P3′ position. HIV-1 protease prefers its own substrates as well, but can cleave FIV MA/CA cleavage junction to some degree. Important to the present discussion, there are at least 6 mutations found in HIV-1 proteases associated with drug resistances that are identical to structurally equivalent residues of wild type FIV protease (Slee, 1995). Two particularly interesting resistance mutations of HIV-1 protease, Val32→Ile (FIV Ile37) and Ile50→Val (FIV Val59), are located in the substrate binding pockets of the protease (Figure 3), which suggests they may play an important role in the inhibitor and substrate selectivity of retroviral protease. Recent studies (Lee, 1998; Lee, 1999) have shown that a major structural distinction between FIV and HIV-1 PRs is that the combined S1/S3 substrate binding pocket is restricted in size relative to the same site in HIV-1 PR. This finding offers a structural explanation for the failure of the current HIV-1 PR inhibitors, which possess bulky P3 groups, to inhibit FIV PR (Lee, 1998, 939–44). Importantly, many drug-resistant HIV-1 PRs appear to have more restricted S1/S3 subsites as well (Lee, 1999, 1145–1155), reducing inhibitor binding affinities in a manner similar to the feline enzyme. In addition, the nature of S2/S2′ amino acids is particularly critical in directing PR substrate specificity as well as certain inhibitor efficacies. Thus, studies directed at understanding the structural basis for inhibitor and substrate specificity in the feline and human systems may lead to development of broad-based inhibitors with efficacy for a range of HIV variants.
Figure 3.
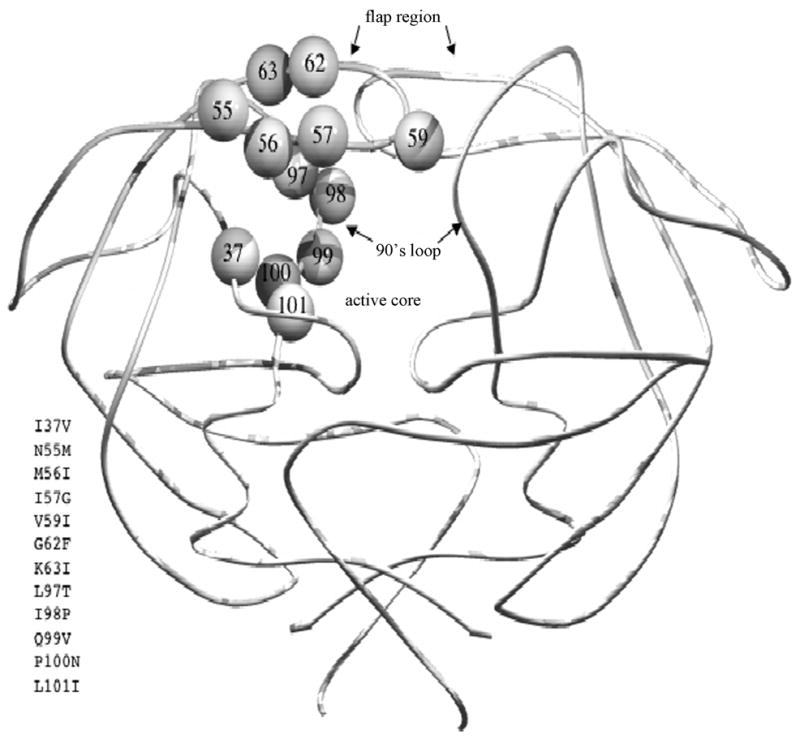
Structural locations of substituted residues in FIV PR for defining both substrate and inhibitor specificities. Residues substituted in FIV/HIV chimeric PRs are shown on only one chain of the homodimeric FIV PR. Substitutions for equivalent residues of HIV-1 PR (FIV numbering with HIV-1 numbering in superscript) include I3732V in the active core, N5546M, M5647I, I5748G, V5950I, G6253F, and K6354I in the flap, L9780T, I9881P, Q9982V, P10083N, and L10184I in 90’s loop.
Both FIV and HIV-1 PRs recognize, approximately, the P4-P4′ residues of peptide substrates via a long cavity in the middle of the protease, as analyzed by biochemical experiments (Moore, 1989; Weber, 1989; Tozser, 1991; Tozser, 1992; Silva, 1996; Tozser, 1997) and crystallographic analyses (Miller, 1989; Erickson, 1990; Swain, 1990). Both homodimeric PRs utilize an acid-base hydrolysis mechanism in which aspartic acids 25 and 25′ (of HIV-1 PR; 30 and 30′ for FIV PR) activate a water molecule to perform a nucleophilic attack on the amide carbonyl between the P1 and P1′ positions in various peptide substrates (Silva, 1996). Like most aspartic proteases, optimal substrate cleavage occurs at approximately pH 4–5 (Tozser, 1992; Polgar, 1994; Kutilek, 2003). There are three major structurally conserved regions that make up the substrate binding pockets of PR: 1) the active core region (residues 30–38 for FIV; 25–33 for HIV); 2) the flap (residues 54–60 for FIV; 45–51 for HIV); and 3) C-terminal “90’s loop” region (residues 98–101 for FIV; “80’s loop” for HIV, residues 80–84) (Figure 3). Within these regions, there are 11 amino acids that differ between FIV and HIV-1 proteases. These residues have proved to be good candidate targets for mutational studies of substrate selectivity. The 11 different amino acid residues in the S4 - S4′ subsites of FIV protease; Ile35, Ile37, Gln54, Asn55, Met56, Ile57, Val59, Ile98, Gln99, Pro100 and Leu101, most likely account for the specificity of the substrate/inhibitor binding. The corresponding residues in HIV-1 protease are Asp30, Val32, Lys45, Met46, Ile47, Gly48, Ile50, Pro81, Val82, Asn83 and Ile84, respectively. All the aforementioned residues have now been documented to mutate in response to protease inhibitor treatment (Schinazi, 1997, 129–142; Rhee, 2003, 298–303). We have prepared a series of mutant FIV PRs in which HIV-1 amino acid residues have been substituted into the FIV PR background at equivalent positions (highlighted in Figure 3). Confirmation of the involvement of several of these residues in both substrate and inhibitor specificities has been obtained (Lee, 1998; Lin, 2000, 2003, 2006). These wild type and mutant FIV PRs have, and will continue to serve, as a structural library for further defining substrate specificity and for inhibitor refinement in the proposed research.
Role of Gag-Pol polyprotein structure in processing
An increasing body of evidence points to a pivotal role of the polyprotein folding/conformation in the temporal cleavage of the Gag- and Gag-Pol proteins that is necessary for generation of infectious virus (Pettit, 1994; Vogt, 1996; Swanstrom, 1997; Wiegers, 1998; Gross, 2000). The studies of Petit et al. (Pettit, 1994; Swanstrom, 1997; Pettit, 1998, 2004, 2005, 2005) pointed out that polyprotein cleavage occurs in a specific order and that alteration of the order by site-directed mutagenesis of certain sites resulted in production of non-infectious HIV. In particular, cleavage at the N-terminus of NC appeared to be the earliest cleavage event, at least in vitro, and subsequent studies have shown early cleavage on either side of NC (Pettit, 2005; also see below). Differences in the rate of cleavage of synthetic substrates encompassing the cleavage sites suggested that the order of cleavage was in part, dictated by the relative cleavage efficacy of each junction. However, more recent studies have indicated that the availability of sites around NC, based on folding of the polyprotein relative to the “embedded” protease, is likely the critical trigger to the initiation of ordered processing. Interesting studies of Kaplan, Dunn, and colleagues have shown that subtle changes at the N-terminus of the embedded protease can markedly influence polyprotein processing in cis with no apparent influence on the ability of free protease to cleave the polyprotein in trans (Pettit, 2004, 2005). This finding underscores the role of polyprotein conformation in processing and the importance of the temporal cleavage of Gag-Pol in the generation of infectious virus. As recently reported (Lin, 2006), similar events occur during processing of FIV Gag-Pol polyprotein and the data again reinforce the requirements for early cleavage around NC to generate infectious virus. Additional studies are in progress to investigate factors that dictate ordered polyprotein cleavage. Given the critical nature of the proper temporal cleavage of Gag/Pol in generating infectious virus, that process offers yet another venue to disrupt the virus life cycle, independent of direct inhibition of PR.
Conclusion
In summary, great strides have been made in dissecting the inner workings of the FIV genome and in defining the parallels and distinctions between the feline lentivirus and HIV. FIV is sufficiently diverse from HIV so as to allow extensive ex vivo and in vivo work with low biohazard and the cost of maintaining this small animal model is a fraction of that required for non-human primate models. Of particular importance, FIV and HIV share many features in their life cycles, host cell targets, and protein functions so as to make the cat a particularly valuable system for developing antiviral therapies at several levels.
Acknowledgments
Work summarized in this review was supported from grants R01AI025825 and R01AI040882 of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health. We thank Ms. Karen Tam for technical assistance and Ms. Nancy Dorman for manuscript preparation.
Footnotes
Conflict of Interest Statement
None of the authors has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the paper entitled “Molecular Mechanisms of FIV Infection”.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bach JM, Hurtrel M, Chakrabarti L, Ganiere JP, Montagnier L, Hurtrel B. Early stages of feline immunodeficiency virus infection in lymph nodes and spleen. AIDS Res Hum Retrovir. 1994;10:1731–8. doi: 10.1089/aid.1994.10.1731. [DOI] [PubMed] [Google Scholar]
- Bachmann MH, Mathiason-Dubard C, Learn GH, Rodrigo AG, Sodora DL, Mazzetti P, Hoover EA, Mullins JI. Genetic diversity of feline immunodeficiency virus: dual infection, recombination, and distinct evolutionary rates among envelope sequence clades. J Virol. 1997;71:4241–53. doi: 10.1128/jvi.71.6.4241-4253.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe AM, Dua N, Faith TG, Moore PF, Pedersen NC, Dandekar S. Primary stage of feline immunodeficiency virus infection: viral dissemination and cellular targets. J Virol. 1994;68:3080–91. doi: 10.1128/jvi.68.5.3080-3091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhamdan M, Benichou S, Rey F, Navarro JM, Agostini I, Spire B, Camonis J, Slupphaug G, Vigne R, Benarous R, Spire J. Human immunodeficiency virus type 1 Vpr protein binds to the uracil DNA glycosylase DNA repair enzyme. J Virol. 1996;70:697–704. doi: 10.1128/jvi.70.2.697-704.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brik A, Alexandratos J, Lin YC, Elder JH, Olson AJ, Wlodawer A, Goodsell DS, Wong CH. 1,2,3-triazole as a peptide surrogate in the rapid synthesis of HIV-1 protease inhibitors. Chembiochem. 2005;6:1167–9. doi: 10.1002/cbic.200500101. [DOI] [PubMed] [Google Scholar]
- Brown WC, Bissey L, Logan KS, Pedersen NC, Elder JH, Collisson EW. Feline immunodeficiency virus infects both CD4+ and CD8+ T lymphocytes. J Virol. 1991;65:3359–64. doi: 10.1128/jvi.65.6.3359-3364.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner D, Pedersen NC. Infection of peritoneal macrophages in vitro and in vivo with feline immunodeficiency virus. J Virol. 1989;63:5483–8. doi: 10.1128/jvi.63.12.5483-5488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callanan JJ, Racz P, Thompson H, Jarrett O. Morphologic characterization of the lymph node changes in feline immunodeficiency virus infection as an animal model of AIDS. S. Karger, Basel; Switzerland: 1993. [Google Scholar]
- Chatterji U, de Parseval A, Elder JH. Feline immunodeficiency virus OrfA is distinct from other lentivirus transactivators. J Virol. 2002;76:9624–34. doi: 10.1128/JVI.76.19.9624-9634.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Vogan EM, Gong H, Skehel JJ, Wiley DC. Structure of an unliganded simian immunodeficiency virus gp120 core. Nature. 2005;433:834–41. doi: 10.1038/nature03327. [DOI] [PubMed] [Google Scholar]
- Cohen OJ, Fauci AS. N Engl J Med. 1998;339(5):341–343. doi: 10.1056/NEJM199807303390511. [DOI] [PubMed] [Google Scholar]
- Collier AC, Coombs RW, Schoenfeld DA, Bassett RL, Timpone J, Baruch A, Jones M, Facey K, Whitacre C, McAuliffe VJ, Friedman HM, Merigan TC, Reichman RC, Hooper C, Corey L. N Engl J Med. 1996;334(16):1011–1017. doi: 10.1056/NEJM199604183341602. [DOI] [PubMed] [Google Scholar]
- Condra JH, Schleif WA, Blahy OM, Gabryelski LJ, Graham DJ, Quintero JC, Rhodes A, Robbins HL, Roth E, Shivaprakash M. Nature. 1995;374(6522):569–571. doi: 10.1038/374569a0. [DOI] [PubMed] [Google Scholar]
- Conticello SG, Harris RS, Neuberger MS. The Vif protein of HIV triggers degradation of the human antiretroviral DNA deaminase APOBEC3G. Curr Biol. 2003;13:2009–13. doi: 10.1016/j.cub.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Delwart EL, Gordon CJ. Tracking changes in HIV-1 envelope quasispecies using DNA heteroduplex analysis. Methods. 1997;12:348–54. doi: 10.1006/meth.1997.0489. [DOI] [PubMed] [Google Scholar]
- Demirov DG, Orenstein JM, Freed EO. The late domain of human immunodeficiency virus type 1 p6 promotes virus release in a cell type-dependent manner. J Virol. 2002;76:105–17. doi: 10.1128/JVI.76.1.105-117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Parseval A, Grant CK, Sastry KJ, Elder JH. Sequential CD134- CXCR4 interactions in feline immunodeficiency virus (FIV): soluble CD134 activates FIV Env for CXCR4-dependent entry and reveals a cryptic neutralization epitope. J Virol. 2006;80:3088–91. doi: 10.1128/JVI.80.6.3088-3091.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Parseval A, Bobardt MD, Elder JH, David G, Zolla-Pazner S. A Highly Conserved Arginine in Gp120 Governs HIV-1 Binding to Both CCR5 and Syndecans Via a Sulfated Motif. J Biol Chem. 2005;280:39493–504. doi: 10.1074/jbc.M504233200. [DOI] [PubMed] [Google Scholar]
- de Parseval A, Chatterji U, Sun P, Elder JH. Feline immunodeficiency virus targets activated CD4+T cells by using CD134 as a binding receptor. Proc Natl Acad Sci USA. 2004;101:13044–9. doi: 10.1073/pnas.0404006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Parseval A, Chatterji U, Morris G, Sun P, Olson AJ. Structural mapping of CD134 residues critical for interaction with feline immunodeficiency virus. Nat Struct Mol Biol. 2005;12:60–6. doi: 10.1038/nsmb872. [DOI] [PubMed] [Google Scholar]
- de Parseval A, Elder JH. Binding of recombinant feline immunodeficiency virus surface glycoprotein to feline cells: role of CXCR4, cell-surface heparans, and an unidentified non-CXCR4 receptor. J Virol. 2001;75:4528–39. doi: 10.1128/JVI.75.10.4528-4539.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Parseval A, Elder JH. Demonstration that orf2 encodes the feline immunodeficiency virus transactivating (Tat) protein and characterization of a unique gene product with partial rev activity. J Virol. 1999;73:608. doi: 10.1128/jvi.73.1.608-617.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rozieres S, Mathiason CK, Rolston MR, Chatterji U, Hoover EA. Characterization of a highly pathogenic molecular clone of feline immunodeficiency virus clade C. J Virol. 2004;78:8971–82. doi: 10.1128/JVI.78.17.8971-8982.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn BM, Gustchina A, Wlodawer A, Kay J. Methods Enzymol. 1994;241:254–278. doi: 10.1016/0076-6879(94)41068-2. [DOI] [PubMed] [Google Scholar]
- Dunn BM, Pennington MW, Frase DC, Nash K. Biopolymers. 1999;51(1):69–77. doi: 10.1002/(SICI)1097-0282(1999)51:1<69::AID-BIP8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Elder JH, Lerner DL, Hasselkus-Light CS, Fontenot DJ, Hunter E. Distinct subsets of retroviruses encode dUTPase. J Virol. 1992;66:1791–4. doi: 10.1128/jvi.66.3.1791-1794.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder JH, Schnolzer M, Hasselkus-Light CS, Henson M, Lerner DA, Phillips TR, Wagaman PC, Kent SB. Identification of proteolytic processing sites within the Gag and Pol polyproteins of feline immunodeficiency virus. J Virol. 1993;67:1869–76. doi: 10.1128/jvi.67.4.1869-1876.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English RV, Nelson P, Johnson CM, Nasisse M, Tompkins WA. Development of clinical disease in cats experimentally infected with feline immunodeficiency virus. J Infect Dis. 1994;170:543–52. doi: 10.1093/infdis/170.3.543. [DOI] [PubMed] [Google Scholar]
- Erickson J, Neidhart DJ, VanDrie J, Kempf DJ, Wang XC, Norbeck DW, Plattner JJ, Rittenhouse JW, Turon M, Wideburg N. Science. 1990;249(4968):527–533. doi: 10.1126/science.2200122. [DOI] [PubMed] [Google Scholar]
- Freed EO. Viral late domains. J Virol. 2002;76:4679–87. doi: 10.1128/JVI.76.10.4679-4687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrus JE, von Schwedler UK, Pornillos OW, Morham SG, Zavitz KH. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell. 2001;107:55–65. doi: 10.1016/s0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Gemeniano MC, Sawai ET, Sparger EE. Feline immunodeficiency virus Orf-A localizes to the nucleus and induces cell cycle arrest. Virology. 2004;325:167–74. doi: 10.1016/j.virol.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Gemeniano MC, Sawai ET, Leutenegger CM, Sparger EE. Feline immunodeficiency virus ORF-A is required for virus particle formation and virus infectivity. J Virol. 2003;77:8819–30. doi: 10.1128/JVI.77.16.8819-8830.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross I, Hohenberg H, Wilk T, Wiegers K, Grattinger M, Muller B, Fuller S, Krausslich HG. EMBO J. 2000;19(1):103–113. doi: 10.1093/emboj/19.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, McMahon D, Richman DD, Valentine FT, Jonas L, Meibohm A, Emini EA, Chodakewitz JA. N Engl J Med. 1997;337(11):734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- He M, Gani M, Livnah O, Stura EA, Beale D. Sequence, specificity and crystallization of an oestrone-3-glucuronide antibody (3910) Immunology. 1997;90:632–9. doi: 10.1046/j.1365-2567.1997.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtrel B, Chakrabarti L, Hurtrel M, Bach JM, Ganiere JP. Early events in lymph nodes during infection with SIV and FIV. Res Virol. 1994;145:221–7. doi: 10.1016/s0923-2516(07)80026-4. [DOI] [PubMed] [Google Scholar]
- Jacobsen H, Hanggi M, Ott M, Duncan IB, Owen S, Andreoni M, Vella S, Mous J. J Infect Dis. 1996;173(6):1379–1387. doi: 10.1093/infdis/173.6.1379. [DOI] [PubMed] [Google Scholar]
- Johnson VA, Brun-Vezinet F, Clotet B, Conway B, Kuritzkes DR, Pillay D, Schapiro JM, Telenti A, Richman DD. Top HIV Med. 2005;13(4):125–131. [PubMed] [Google Scholar]
- Joshi A, Garg H, Tompkins MB, Tompkins WA. Preferential feline immunodeficiency virus (FIV) infection of CD4+ CD25+ T-regulatory cells correlates both with surface expression of CXCR4 and activation of FIV long terminal repeat binding cellular transcriptional factors. J Virol. 2005;79:4965–76. doi: 10.1128/JVI.79.8.4965-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz RA, Skalka AM. Annu. Rev Biochem. 1994;63:133–173. doi: 10.1146/annurev.bi.63.070194.001025. [DOI] [PubMed] [Google Scholar]
- Kirk O, Katzenstein TL, Gerstoft J, Mathiesen L, Nielsen H, Pedersen C, Lundgren JD. AIDS. 1999;13(1):F9–16. doi: 10.1097/00002030-199901140-00002. [DOI] [PubMed] [Google Scholar]
- Kozal M. AIDS Patient Care STDS. 2004;18(4):199–208. doi: 10.1089/108729104323038874. [DOI] [PubMed] [Google Scholar]
- Kutilek VD, Sheeter DA, Elder JH, Torbett BE. Curr Drug Targets Infect Disord. 2003;3(4):295–309. doi: 10.2174/1568005033481079. [DOI] [PubMed] [Google Scholar]
- Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA. HIV- 1 evades antibody-mediated neutralization through conformational masking of receptor- binding sites. Nature. 2002;420:678–82. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- Labrijn AF, Poignard P, Raja A, Zwick MB, Delgado K. Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J Virol. 2003;77:10557–65. doi: 10.1128/JVI.77.19.10557-10565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laco GS, Fitzgerald MC, Morris GM, Olson AJ, Kent SB, Elder JH. J Virol. 1997;71(7):5505–5511. doi: 10.1128/jvi.71.7.5505-5511.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laco GS, Schalk-Hihi C, Lubkowski J, Morris G, Zdanov A. Crystal structures of the inactive D30N mutant of feline immunodeficiency virus protease complexed with a substrate and an inhibitor. Biochemistry. 1997;36:10696–708. doi: 10.1021/bi9707436. [DOI] [PubMed] [Google Scholar]
- Lafrado L, Podell M, Krakowka S, Hayes K, Hanlon M, Hanlon M, Mathes LE. FIV: a model for retrovirus-induced pathogenesis. AIDS Res Rev. 1993;3:115–150. [Google Scholar]
- Lawrence J, Schapiro J, Winters M, Montoya J, Zolopa A, Pesano R, Efron B, Winslow D, Merigan TC. J Infect Dis. 1999;179(6):1356–1364. doi: 10.1086/314751. [DOI] [PubMed] [Google Scholar]
- Lee T, Laco GS, Torbett BE, Fox HS, Lerner DL. Analysis of the S3 and S3′ subsite specificities of feline immunodeficiency virus (FIV) protease: Development of a broad-based protease inhibitor efficacious against FIV, SIV, and HIV in vitro and ex vivo. Proc Natl Acad Sci USA. 1998;95:939–44. doi: 10.1073/pnas.95.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Le VD, Lim D, Lin YC, Morris G, Wong AL, Olson AJ, Elder JH, Wong CH. J. Am Chem Soc. 1999;121:1145–1155. [Google Scholar]
- Lerner DL, Wagaman PC, Phillips TR, Prospero-Garcia O, Henriksen SJ. Increased mutation frequency of feline immunodeficiency virus lacking functional deoxyuridine-triphosphatase. Proc Natl Acad Sci USA. 1995;92:7480–4. doi: 10.1073/pnas.92.16.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Beck Z, Lee T, Le VD, Morris GM, Olson AJ, Wong CH, Elder JH. Alteration of Substrate Inhibitor Specificity of Feline Immunodeficiency Virus Protease. J Virol. 2000;74(10):4710–4720. doi: 10.1128/jvi.74.10.4710-4720.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Beck Z, Morris GM, Olson AJ, Elder JH. Structural Basis for Distinctions between Substrate and Inhibitor Specificities for Feline Immunodeficiency Virus and Human Immunodeficiency Virus Proteases. J Virol. 2003;77(12):6589–6600. doi: 10.1128/JVI.77.12.6589-6600.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Brik A, de Parseval A, Tam K, Torbett BE, Wong CH, Elder JH. Altered Gag Polyprotein Cleavage Specificity of Feline Immunodeficiency Virus/Human Immunodeficiency Virus Mutant Proteases as Demonstrated in a Cell-Based Expression System. J Virol. 2006;80(16):7832–7843. doi: 10.1128/JVI.00374-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusso P, Earl PL, Sironi F, Santoro F, Ripamonti C. Cryptic nature of a conserved, CD4-inducible V3 loop neutralization epitope in the native envelope glycoprotein oligomer of CCR5-restricted, but not CXCR4-using, primary human immunodeficiency virus type 1 strains. J Virol. 2005;79:6957–68. doi: 10.1128/JVI.79.11.6957-6968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangeat B, Turelli P, Caron G, Friedli M, Perrin L. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424:99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- Manrique ML, Rauddi ML, Gonzalez SA, Affranchino JL. Functional domains in the feline immunodeficiency virus nucleocapsid protein. Virology. 2004;327:83–92. doi: 10.1016/j.virol.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Mansky LM, Preveral S, Selig L, Benarous R, Benichou S. The interaction of vpr with uracil DNA glycosylase modulates the human immunodeficiency virus type 1 In vivo mutation rate. J Virol. 2000;74:7039–47. doi: 10.1128/jvi.74.15.7039-7047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin M, Rose KM, Kozak SL, Kabat D. HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nat Med. 2003;9:1398–403. doi: 10.1038/nm946. [DOI] [PubMed] [Google Scholar]
- Martin-Serrano J, Zang T, Bieniasz PD. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat Med. 2001;7:1313–9. doi: 10.1038/nm1201-1313. [DOI] [PubMed] [Google Scholar]
- Mehle A, Strack B, Ancuta P, Zhang C, McPike M. Vif overcomes the innate antiviral activity of APOBEC3G by promoting its degradation in the ubiquitin- proteasome pathway. J Biol Chem. 2004;279:7792–8. doi: 10.1074/jbc.M313093200. [DOI] [PubMed] [Google Scholar]
- Miller M, Schneider J, Sathyanarayana BK, Toth MV, Marshall GR, Clawson L, Selk L, Kent SB, Wlodawer A. Science. 1989;246(4934):1149–1152. doi: 10.1126/science.2686029. [DOI] [PubMed] [Google Scholar]
- Molla A, Korneyeva M, Gao Q, Vasavanonda S, Schipper PJ, Mo HM, Markowitz M, Chernyavskiy T, Niu P, Lyons N, Hsu A, Granneman GR, Ho DD, Boucher CA, Leonard JM, Norbeck DW, Kempf DJ. Nat Med. 1996;2(7):760–766. doi: 10.1038/nm0796-760. [DOI] [PubMed] [Google Scholar]
- Moore ML, Bryan WM, Fakhoury SA, Magaard VW, Huffman WF, Dayton BD, Meek TD, Hyland L, Dreyer GB, Metcalf BW. Biochem Biophys Res Commun. 1989;159(2):420–425. doi: 10.1016/0006-291x(89)90008-9. [DOI] [PubMed] [Google Scholar]
- Morikawa S, Bishop DH. Identification and analysis of the gag-pol ribosomal frameshift site of feline immunodeficiency virus. Virology. 1992;186:389–97. doi: 10.1016/0042-6822(92)90004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulard M, Phogat SK, Shu Y, Labrijn AF, Xiao X. Broadly cross-reactive HIV-1-neutralizing human monoclonal Fab selected for binding to gp120-CD4-CCR5 complexes. Proc Natl Acad Sci USA. 2002;99:6913–8. doi: 10.1073/pnas.102562599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North TW, North GL, Pedersen NC. Feline immunodeficiency virus, a model for reverse transcriptase-targeted chemotherapy for acquired immune deficiency syndrome. Antimicrob Agents Chemother. 1989;33:915–9. doi: 10.1128/aac.33.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotney C, English RV, Housman J, Davidson MG, Nasisse MP. Lymphocyte population changes in cats naturally infected with feline immunodeficiency virus. AIDS. 1990;4:1213–8. doi: 10.1097/00002030-199012000-00005. [DOI] [PubMed] [Google Scholar]
- Obert LA, Hoover EA. Feline immunodeficiency virus clade C mucosal transmission and disease courses. AIDS Res Hum Retrov. 2000;16:677–88. doi: 10.1089/088922200308909. [DOI] [PubMed] [Google Scholar]
- Olmsted RA, Barnes AK, Yamamoto JK, Hirsch VM, Purcell RH. Molecular cloning of feline immunodeficiency virus. Proc Natl Acad Sci USA. 1989;86:2448–52. doi: 10.1073/pnas.86.7.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil LL, Burkhard MJ, Diehl LJ, Hoover EA. Vertical transmission of feline immunodeficiency virus. Semin Vet Med Surg (Small Anim) 1995;10:266–78. [PubMed] [Google Scholar]
- O’Neil LL, Burkhard MJ, Hoover EA. Frequent perinatal transmission of feline immunodeficiency virus by chronically infected cats. J Virol. 1996;70:2894–901. doi: 10.1128/jvi.70.5.2894-2901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- Pancino G, Castelot S, Sonigo P. Differences in feline immunodeficiency virus host cell range correlate with envelope fusogenic properties. Virology. 1995;206:796–806. doi: 10.1006/viro.1995.1002. [DOI] [PubMed] [Google Scholar]
- Pancino G, Fossati I, Chappey C, Castelot S, Hurtrel B. Structure and variations of feline immunodeficiency virus envelope glycoproteins. Virology. 1993;192:659–62. doi: 10.1006/viro.1993.1083. [DOI] [PubMed] [Google Scholar]
- Parodi AL, Femenia F, Moraillon A, Crespeau F, Fontaine JJ. Histopathological changes in lymph nodes of cats experimentally infected with the feline immunodeficiency virus (FIV) J Comp Pathol. 1994;111:165–74. doi: 10.1016/s0021-9975(05)80048-9. [DOI] [PubMed] [Google Scholar]
- Pedersen NC. The Feline Immunodeficiency Virus. In: Levy JA, editor. The Retroviridae. Plenum Press; NewYork: 1993. pp. 181–228. [Google Scholar]
- Pedersen NC, Ho EW, Brown ML, Yamamoto JK. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987;235:790–3. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- Pettit SC, Clemente JC, Jeung JA, Dunn BM, Kaplan AH. J Virol. 2005;79(16):10601–607. doi: 10.1128/JVI.79.16.10601-10607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit SC, Everitt LE, Choudhury S, Dunn BM, Kaplan AH. J Virol. 2004;78(16):8477–8485. doi: 10.1128/JVI.78.16.8477-8485.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit SC, Lindquist JN, Kaplan AH, Swanstrom R. Retrovirology. 2005;2(1):66. doi: 10.1186/1742-4690-2-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit SC, Moody MD, Wehbie RS, Kaplan AH, Nantermet PV, Klein CA, Swanstrom R. J Virol. 1994;68(12):8017–8027. doi: 10.1128/jvi.68.12.8017-8027.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit SC, Sheng N, Tritch R, Erickson-Viitanen S, Swanstrom R. Adv. Exp Med Biol. 1998;436:15–25. doi: 10.1007/978-1-4615-5373-1_2. [DOI] [PubMed] [Google Scholar]
- Pettit SC, Simsic J, Loeb DD, Everitt L, Hutchison CAd, Swanstrom R. J Biol Chem. 1991;266(22):14539–14547. [PubMed] [Google Scholar]
- Phillips TR, Lamont C, Konings DA, Shacklett BL, Hamson CA. Identification of the Rev transactivation and Rev-responsive elements of feline immunodeficiency virus. J Virol. 1992;66:5464–71. doi: 10.1128/jvi.66.9.5464-5471.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TR, Prospero-Garcia O, Puaoi DL, Lerner DL, Fox HS. Neurological abnormalities associated with feline immunodeficiency virus infection. J Gen Virol. 1994;75:979–87. doi: 10.1099/0022-1317-75-5-979. [DOI] [PubMed] [Google Scholar]
- Phillips TR, Prospero-Garcia O, Wheeler DW, Wagaman PC, Lerner DL. Neurologic dysfunctions caused by a molecular clone of Feline Immundeficiency Virus, FIV-PPR. J Neurovirol. 1996;2:388–396. doi: 10.3109/13550289609146904. [DOI] [PubMed] [Google Scholar]
- Phillips TR, Talbott RL, Lamont C, Muir S, Lovelace K. Comparison of two host cell range variants of feline immunodeficiency virus. J Virol. 1990;64:4605–13. doi: 10.1128/jvi.64.10.4605-4613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeschla EM, Looney DJ. CXCR4 is required by a nonprimate lentivirus: heterologous expression of feline immunodeficiency virus in human, rodent, and feline cells. J Virol. 1998;72:6858–66. doi: 10.1128/jvi.72.8.6858-6866.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar L, Szeltner Z, Boros I. Biochemistry. 1994;33(31):9351–9357. doi: 10.1021/bi00197a040. [DOI] [PubMed] [Google Scholar]
- Prasad GS, Stura EA, McRee DE, Laco GS, Hasselkus-Light C. Crystal structure of dUTP pyrophosphatase from feline immunodeficiency virus. Protein Sci. 1996;5:2429–37. doi: 10.1002/pro.5560051205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prospero-Garcia O, Herold N, Phillips TR, Elder JH, Bloom FE. Sleep patterns are disturbed in cats infected with feline immunodeficiency virus. Proc Nat Acad Sci USA. 1994;91:12947–51. doi: 10.1073/pnas.91.26.12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prospero-Garcia O, Herold N, Waters AK, Phillips TR, Elder JH. Intraventricular administration of a FIV-envelope protein induces sleep architecture changes in rats. Brain Res. 1994;659:254–8. doi: 10.1016/0006-8993(94)90888-5. [DOI] [PubMed] [Google Scholar]
- Rhee SY, Gonzales MJ, Kantor R, Betts BJ, Ravela J, Shafer RW. Human Immunodeficiency virus reverse transcripts and protease sequence database. Nucleic Acids Res. 2003;31(1):298–303. doi: 10.1093/nar/gkg100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman DD, Morton SC, Wrin T, Hellmann N, Berry S, Shapiro MF, Bozzette SA. AIDS. 2004;18(10):1393–1401. doi: 10.1097/01.aids.0000131310.52526.c7. [DOI] [PubMed] [Google Scholar]
- Rideout BA, Lowensteine LJ, Hutson CA, Moore PF, Pedersen NC. Characterization of morphologic changes and lymphocyte subset distribution in lymph nodes from cats with naturally acquired feline immunodeficiency virus infection. Vet Pathol. 1992;29:391–9. doi: 10.1177/030098589202900504. [DOI] [PubMed] [Google Scholar]
- Rogers AB, Hoover EA. Maternal-fetal feline immunodeficiency virus transmission: timing and tissue tropisms. J Infect Dis. 1998;178:960–7. doi: 10.1086/515692. [DOI] [PubMed] [Google Scholar]
- Shinazi RF, Larder BA, Mellors JW. Mutations in Retroviral Genes Associated with Drug Resistance. Intl Antiviral News. 1993;5:129–142. [Google Scholar]
- Schnolzer M, Rackwitz HR, Gustchina A, Laco GS, Wlodawer A, Elder JH, Kent SB. Virology. 1996;224(1):268–275. doi: 10.1006/viro.1996.0528. [DOI] [PubMed] [Google Scholar]
- Schrofelbauer B, Yu Q, Zeitlin SG, Landau NR. Human immunodeficiency virus type 1 Vpr induces the degradation of the UNG and SMUG uracil-DNA glycosylases. J Virol. 2005;79:10978–87. doi: 10.1128/JVI.79.17.10978-10987.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shacklett BL, Luciw PA. Analysis of the vif gene of feline immunodeficiency virus. Virology. 1994;204:860–7. doi: 10.1006/viro.1994.1609. [DOI] [PubMed] [Google Scholar]
- Sheehy AM, Gaddis NC, Malim MH. The antiretroviral enzyme APOBEC3G is degraded by theproteasome in response to HIV-1 Vif. Nat Med. 2003;9:1404–7. doi: 10.1038/nm945. [DOI] [PubMed] [Google Scholar]
- Shimojima M, Miyazawa T, Ikeda Y, McMonagle EL, Haining H. Use of CD134 as a primary receptor by the feline immunodeficiency virus. Science. 2004;303:1192–5. doi: 10.1126/science.1092124. [DOI] [PubMed] [Google Scholar]
- Silva AM, Cachau RE, Sham HL, Erickson JW. J Mol Biol. 1996;255(2):321–346. doi: 10.1006/jmbi.1996.0026. [DOI] [PubMed] [Google Scholar]
- Slee DH, Laslo KL, Elder JH, Ollmann IR, Gustchina A, Kervinen K, Zdanov A, Wlodawer A, Wong CH. J. Am Chem Soc. 1995;117:11867–11878. [Google Scholar]
- Sodora DL, Shpaer EG, Kitchell BE, Dow SW, Hoover EA. Identification of three feline immunodeficiency virus (FIV) env gene subtypes and comparison of the FIV and human immunodeficiency virus type 1 evolutionary patterns. J Virol. 1994;68:2230–8. doi: 10.1128/jvi.68.4.2230-2238.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodora DL, Courcelle J, Brojatsch J, Berson A, Wang YC. Analysis of a feline immunodeficiency virus provirus reveals patterns of gene sequence conservation distinct from human immunodeficiency virus type 1. AIDS Res Hum Retrovir. 1995;11:531–3. doi: 10.1089/aid.1995.11.531. [DOI] [PubMed] [Google Scholar]
- Sparger EE, Luciw PA, Elder JH, Yamamoto JK, Lowenstine LJ. Feline immunodeficiency virus is a lentivirus associated with an AIDS-like disease in cats. AIDS. 1989;3:S43–9. doi: 10.1097/00002030-198901001-00006. [DOI] [PubMed] [Google Scholar]
- Sparger EE, Shacklett BL, Renshaw-Gegg L, Barry PA, Pedersen NC. Regulation of gene expression directed by the long terminal repeat of the feline immunodeficiency virus. Virology. 1992;187:165–77. doi: 10.1016/0042-6822(92)90305-9. [DOI] [PubMed] [Google Scholar]
- Stanfield RL, Ghiara JB, Ollmann Saphire E, Profy AT, Wilson IA. Recurring conformation of the human immunodeficiency virus type 1 gp120 V3 loop. Virology. 2003;315:159–73. doi: 10.1016/s0042-6822(03)00525-7. [DOI] [PubMed] [Google Scholar]
- Staszewski S, Miller V, Sabin C, Carlebach A, Berger AM, Weidmann E, Helm EB, Hill A, Phillips A. AIDS. 1999;13(3):367–373. doi: 10.1097/00002030-199902250-00009. [DOI] [PubMed] [Google Scholar]
- Steagall WK, Robek MD, Perry ST, Fuller FJ, Payne SL. Incorporation of uracil into viral DNA correlates with reduced replication of EIAV in macrophages. Virology. 1995;210:302–13. doi: 10.1006/viro.1995.1347. [DOI] [PubMed] [Google Scholar]
- Stopak K, de Noronha C, Yonemoto W, Greene WC. HIV-1 Vif blocks the antiviral activity of APOBEC3G by impairing both its translation and intracellular stability. Mol Cell. 2003;12:591–601. doi: 10.1016/s1097-2765(03)00353-8. [DOI] [PubMed] [Google Scholar]
- Swain AL, Miller MM, Green J, Rich DH, Schneider J, Kent SB, Wlodawer A. Proc Natl Acad Sci USA. 1990;87(22):8805–8809. doi: 10.1073/pnas.87.22.8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanstrom R, Erona J. Pharmacol Ther. 2000;86(2):145–170. doi: 10.1016/s0163-7258(00)00037-1. [DOI] [PubMed] [Google Scholar]
- Swanstrom R, Wills J. Synthesis, assembly, and processing of viral proteins. In: Coffin J, Hughes S, editors. Retroviruses. Cold Spring Harbor Laboratory Press; NY: 1997. pp. 263–334. [PubMed] [Google Scholar]
- Talbott RL, Sparger EE, Lovelace KM, Fitch WM, Pedersen NC. Nucleotide sequence and genomic organization of feline immunodeficiency virus. Proc Natl Acad Sci USA. 1989;86:5743–7. doi: 10.1073/pnas.86.15.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson FJ, Elder J, Neil JC. Cis- and trans-regulation of feline immunodeficiency virus: identification of functional binding sites in the long terminal repeat. J Gen Virol. 1994;75(Pt 3):545–54. doi: 10.1099/0022-1317-75-3-545. [DOI] [PubMed] [Google Scholar]
- Threadgill DS, Steagall WK, Flaherty MT, Fuller FJ, Perry ST. Characterization of equine infectious anemia virus dUTPase: growth properties of a dUTPase-deficient mutant. J Virol. 1993;67:2592–600. doi: 10.1128/jvi.67.5.2592-2600.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasselli AG, Heinrikson RL. Methods Enzymol. 1994;241:279–301. doi: 10.1016/0076-6879(94)41069-0. [DOI] [PubMed] [Google Scholar]
- Tomonaga K, Norimine J, Shin YS, Fukasawa M, Miyazawa T. Identification of a feline immunodeficiency virus gene which is essential for cell-free virus infectivity. J Virol. 1992;66:6181–5. doi: 10.1128/jvi.66.10.6181-6185.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyosaki T, Miyazawa T, Furuya T, Tomonaga K, Shin YS. Localization of the viral antigen of feline immunodeficiency virus in the lymph nodes of cats at the early stage of infection. Arch Virol. 1993;131:335–47. doi: 10.1007/BF01378636. [DOI] [PubMed] [Google Scholar]
- Tozser J, Bagossi P, Weber IT, Louis JM, Copeland TD, Oroszlan S. J Biol Chem. 1997;272(27):16807–16814. doi: 10.1074/jbc.272.27.16807. [DOI] [PubMed] [Google Scholar]
- Tozser J, Gustchina A, Weber IT, Blaha I, Wondrak EM, Oroszlan S. FEBS Lett. 1991;279(2):356–360. doi: 10.1016/0014-5793(91)80186-7. [DOI] [PubMed] [Google Scholar]
- Tozser J, Weber IT, Gustchina A, Blaha I, Copeland TD, Louis JM, Oroszlan S. Biochemistry. 1992;31(20):4793–4800. doi: 10.1021/bi00135a008. [DOI] [PubMed] [Google Scholar]
- Vacca JP. Drug Discov. 1997;2(7) [Google Scholar]
- Vagin A, Teplyakov A. MOLREP: an Automated Program for Molecular Replacement. J Appl Crystallogr. 1997;30:1022–11025. [Google Scholar]
- VerPlank L, Bouamr F, LaGrassa TJ, Agresta B, Kikonyogo A. Tsg101, a homologue of ubiquitin-conjugating (E2) enzymes, binds the L domain in HIV type 1 Pr55(Gag) Proc Natl Acad Sci USA. 2001;98:7724–9. doi: 10.1073/pnas.131059198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. Curr. Top Microbiol Immunol. 1996;214:95–131. doi: 10.1007/978-3-642-80145-7_4. [DOI] [PubMed] [Google Scholar]
- Waters AK, De Parseval AP, Lerner DL, Neil JC, Thompson FJ. Influence of ORF2 on host cell tropism of feline immunodeficiency virus. Virology. 1996;215:10–6. doi: 10.1006/viro.1996.0002. [DOI] [PubMed] [Google Scholar]
- Weber IT, Miller M, Jaskolski M, Leis J, Skalka AM, Wlodawer A. Science. 1989;243(4893):928–931. doi: 10.1126/science.2537531. [DOI] [PubMed] [Google Scholar]
- Wiegers K, Rutter G, Kottler H, Tessmer U, Hohenberg H, Krausslich HG. J Virol. 1998;72(4):2846–2854. doi: 10.1128/jvi.72.4.2846-2854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett BJ, Adema K, Heveker N, Brelot A, Picard L, Alizon M, Turner JD, Hoxie JA, Peiper S, Neil JC, Hosie MJ. The second extracellular loop of CXCR4 determines its function as a receptor for feline immunodeficiency virus. J Virol. 1998;72:6475–81. doi: 10.1128/jvi.72.8.6475-6481.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett BJ, Hosie MJ, Callanan JJ, Neil JC, Jarrett O. Infection with feline immunodeficiency virus is followed by the rapid expansion of a CD8+ lymphocyte subset. Immunology. 1993;78:1–6. [PMC free article] [PubMed] [Google Scholar]
- Wlodawer A, Gustchina A, Reshetnikova L, Lubkowski J, Zdanov A, Hui KY, Angleton EL, Farmerie WG, Goodenow MM, Bhatt D. Nat Struct Biol. 1995;2(6):480–488. doi: 10.1038/nsb0695-480. [DOI] [PubMed] [Google Scholar]
- Woo JC, Dean GA, Pedersen NC, Moore PF. Immunopathologic changes in the thymus during the acute stage of experimentally induced feline immunodeficiency virus infection in juvenile cats. J Virol. 1997;71:8632–41. doi: 10.1128/jvi.71.11.8632-8641.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang SH, Farzan M, Si Z, Madani N, Wang L, Rosenberg E, Robinson J, Sodroski J. Functional mimicry of a human immunodeficiency virus type 1 coreceptor by a neutralizing monoclonal antibody. J Virol. 2005;79:6068–77. doi: 10.1128/JVI.79.10.6068-6077.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto JK, Sparger E, Ho EW, Andersen PR, O’Connor TP, Mandell CP, Lowenstine L, Munn R, Pedersen NC. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am J Vet Res. 1988;49:1246–58. [PubMed] [Google Scholar]
- Young B, Johnson S, Bahktiari M, Shugarts D, Young RK, Allen M, Ramey RR, 2nd, Kuritzkes DR. J Infect Dis. 1998;178(5):1497–1501. doi: 10.1086/314437. [DOI] [PubMed] [Google Scholar]
- Yu X, Yu Y, Liu B, Luo K, Kong W. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science. 2003;302:1056–60. doi: 10.1126/science.1089591. [DOI] [PubMed] [Google Scholar]
- Zhang YM, Imamichi H, Imamichi T, Lane HC, Falloon J, Vasudevachari MB, Salzman NP. J Virol. 1997;71(9):6662–6670. doi: 10.1128/jvi.71.9.6662-6670.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yang B, Pomerantz RJ, Zhang C, Arunachalam SC, Gao L. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature. 2003;424:94–8. doi: 10.1038/nature01707. [DOI] [PMC free article] [PubMed] [Google Scholar]


