Abstract
Background/Aims
Senescent Ccl2−/− mice develop cardinal features of human age-related macular degeneration (AMD). Loss-of-function single-nucleotide polymorphisms within CX3CR1 are associated with AMD.
Methods
We generated Ccl2−/−/Cx3cr1−/− [double-knockout (DKO)] mice and evaluated the eyes using fundoscopy routine histology, immunochemistry, biochemistry and proteomics.
Results
At 6 weeks old, all DKO mice developed AMD-like retinal lesions such as abnormal retinal pigment epithelium cells, drusen, photoreceptor atrophy and choroidal neovascularization, which progressed with age and reversed with high omega-3 long-chain polyunsaturated fatty acid diet. N-retinylidene-N-retinylethanolamine (A2E), a major lipofuscin fluorophore, illustrated by an emission peak at ~600 nm, was significantly higher in DKO retinal pigment epithelium. Decreased ERp29 was found in the retina of DKO mice.
Conclusion
A broad spectrum of AMD pathologies with early onset and high penetrance in these mice implicate certain chemokines, A2E and endoplasmic reticulum proteins in AMD pathogenesis.
Keywords: Age, related macular degeneration, Animal model, CCL2, CX3CR1, Retinal pigment epithelium, N-retinylidene, N-retinylethanolamine, Chaperone, Omega-3 long-chain polyunsaturated fatty acids
Introduction
Age-related macular degeneration (AMD) typically affects people over the age of 50 and involves central visual field loss. Globally, AMD ranks as the third leading cause of visual impairment with a blindness prevalence of 8.7%. In the elderly, however, AMD is the leading cause of worldwide blindness [1]. Conservatively, the World Health Organization estimated that 14 million persons, most of them in developed countries, are blind or are severely visually impaired due to this disease (http://www.who.int/blindness/causes/priority/en/print.html). Roughly 35–40% of the human population >75 years of age has some degree of AMD [2]. The exact pathogenesis of AMD remains unknown. Furthermore, to date, there is no known cure for this disease and the treatment options are limited [3]. Therefore, it is critical to study this disease using suitable animal models [4] including genetically engineered animal models. Although mice have no macula, the use of mouse models can still provide basic physiology and pathology relevant to human AMD.
We have reported that a loss-of-function variation within CX3CR1 (T280M/V249I) is associated with AMD in a case-control study. This association was further demonstrated via molecular pathological analyses. CX3CR1 polymorphisms are associated with an increased risk of AMD development [5]. Decreased CX3CR1 expression is detected in AMD eyes, in particular, within the macular region [5, 6]. Interestingly, Cx3cr1-deficient mice have recently been reported to develop AMD-like features including ‘drusen’ formation, progressive accumulation of subretinal microglia and photoreceptor degeneration in aged animals [7, 8].
Senescent Ccl2- or Ccr2-deficient mice are reported to develop AMD-like pathology including accumulation of lipofuscin in the retinal pigment epithelium (RPE) drusen, photoreceptor atrophy and choroidal neovascularization [9]. Complement and IgG deposits are also found in the RPE and choroid in these mice. Impaired macrophage function may contribute to AMD pathology in this model.
The hypothesis that deficiencies in both Cx3cr1 and Ccl2 might have a synergistic effect resulting in a phenotype displaying typical AMD features with early onset and high penetrance has led us to generate a Ccl2/Cx3cr1 double-knockout (DKO) [10]. This article highlights the pathological features of our DKO mice.
Generation of DKO Mice
Cx3cr1−/− mice on the C57BL/6 background (kindly provided by Dr. Philip Murphy, NIAID/NIH) were cross-bred with Ccl2−/− C57BL/6 mice (kindly provided by Drs. Bao Lu and Barrett J. Rollins of Children’s Hospital, Harvard Medical School) to generate Ccl2+/−/Cx3cr1+/− mice. The heterozygous mice were then intercrossed to generate various combinations of the 4 alleles including the Ccl2−/−/Cx3cr1−/− mice. Among the 400 F2 pups analyzed, 12 were Ccl2−/−/Cx3cr1−/−, indicating an abnormal Mendelian segregation (1 in 16 expected) [10]. Funduscopy was performed on these Ccl2−/−/Cx3cr1−/− mice. The 2 Ccl2−/−/Cx3cr1−/− mice with the most retinal drusen-like lesions were selected as the breeding pair to generate the DKO strain.
Characterization of DKO Mice
DKO mice appear normal although slightly underweight and smaller in size. They are less fertile, averaging 4 pups per litter as compared to 8 pups in normal C57BL/6 mice. Loss of hair and pigmentation are observed in some DKO. Most importantly, all DKO mice show multiple small retinal lesions [10]. These lesions are similar to human drusen and can be observed as early as at 6 weeks of age. The round or dome-shaped, yellowish deposits are observed mostly within the deep retina subretinal space (fig. 1). Most lesions will become larger and confluent (by 4–6 months) and some progress to flattened or scar-like atrophic areas with aging (>6 months).
Fig. 1.
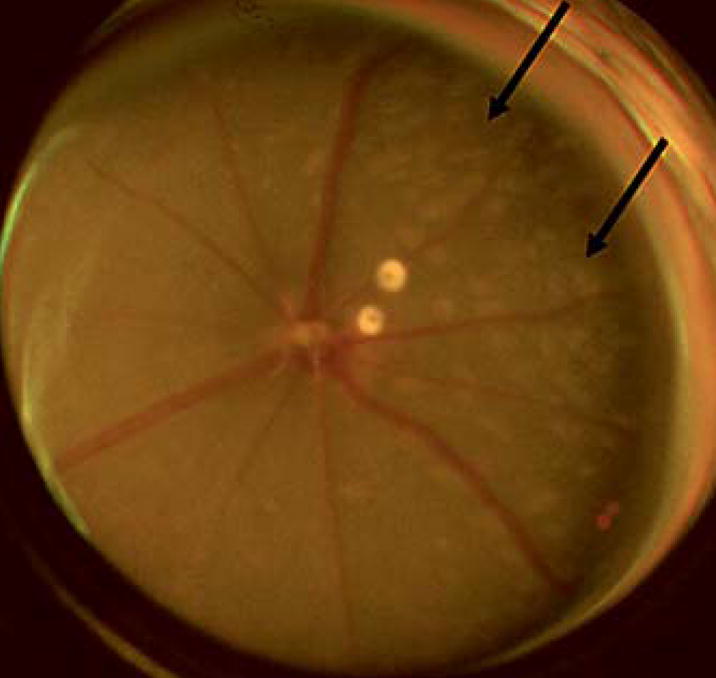
Funduscopy showing multiple drusen-like lesions (arrows) in a DKO mouse eye.
The most prominent histopathology noted is the presence of abnormal RPE cells (fig. 2). Focal or diffuse RPE hypopigmentation, depigmentation, vacuolization and atrophy resulting from loss of melanosomes and increased lipofuscin are frequently observed [10]. In some rare cases, focal RPE hyperpigmentation is also observed. An abnormal thickened Bruch membrane further characterized by irregular deposits also develops. In general, these drusen appear to be much smaller as compared to classical drusen in AMD patients. Spontaneous choroidal neovascularization develops in approximately 15% of DKO mice. These choroidal neovascularizations are surrounded by little or no areas of hemorrhage and are often tiny and fragile, thus they are difficult to identify. Photoreceptor abnormalities are usually in small foci of the outer nuclear layer. Photoreceptor atrophy is found in older DKO mice.
Fig. 2.
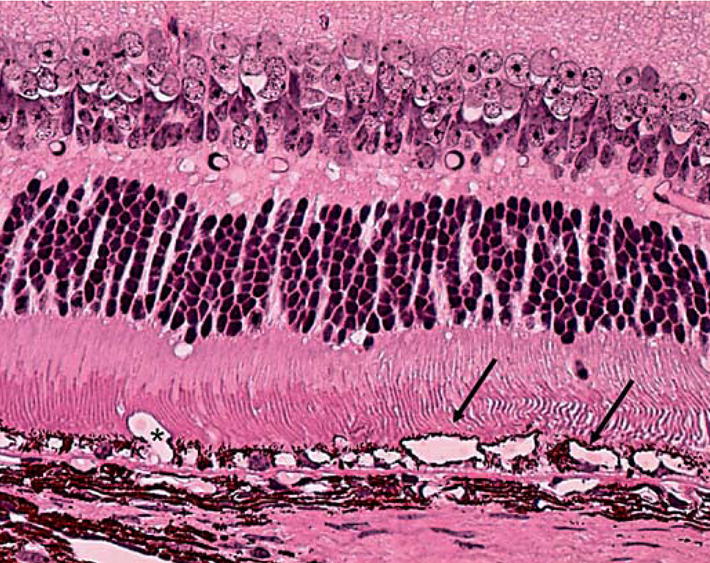
Photomicrograph showing severe RPE degeneration and irregular Bruch’s membrane (arrows = RPE vacuolation; asterisk = ill-defined neovascular lumen). Hematoxylin and eosin. Original magnification ×400.
DKO mice demonstrate aberrant immunological responses. Complement factor deposits and microglial accumulation are detected surrounding, as well as within, the lesions [10]. These mice show decreased response to lipopolysaccharide as compared to C57BL/6 mice. This may be primarily due to the low expression of toll-like receptor 4 observed in the DKO mice [11]. DKO mice exhibit microglial activation impaired macrophage recruitment and function, which may be associated with the development of the observed AMD-like lesions.
A2E (N-retinylidene-N-retinylethanolamine), a major fluorophore of lipofuscin, is a derivative of retinoid metabolism. Lipofuscin is a product of RPE phagocytosis of photoreceptor outer segments and has been shown to increase and accumulate in the RPE of AMD eyes. A 3- to 4-fold increase in A2E is found within the retina and RPE of DKO as compared to C57BL/6 mice [10]. Using spectral fluorescence microscopy, a higher autofluorescence, originating mainly from A2E, is measured in both young and old DKO as compared to C57BL/6 mice (fig. 3a, b). The autofluorescence is associated specifically with the RPE and has a spectral emission peak at ~600–615 nm. The emission spectrum is consistent with that of unbleached RPE cell cultures fed A2E when excited at 458 nm. This likely reflects that more A2E and perhaps its precursors are present in the RPE of the DKO and the spectrum is not dominated by oxidized A2E granules, which have an emission maximum typically between 530 and 560 nm when excited at 458 nm [Majumdar and Bonner, unpublished data].
Fig. 3.
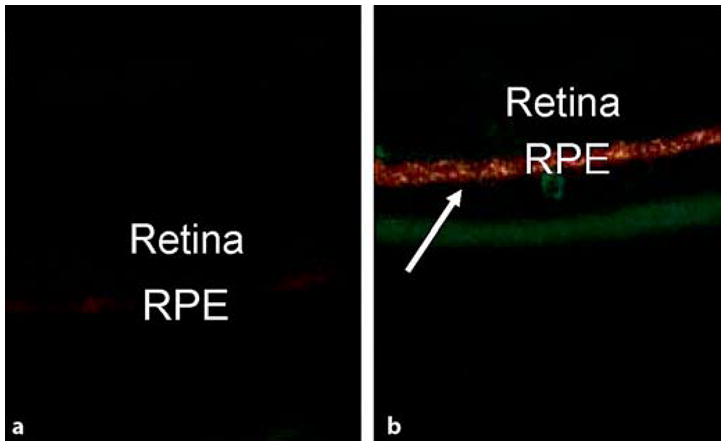
Spectral fluorescent microscopic examinations showing very faint A2E staining (orange-yellow color) in the RPE of a wild-type (a) but strong A2E deposits (arrow) in a DKO mouse (b). Original magnification ×100.
Proteomics of the retina and RPE demonstrate 4 differentially expressed proteins in DKO [10]. One of them is the ERp29 precursor. ERp29 protein is associated with neurodegenerative diseases [12]. ERp29 is a resident endoplasmic reticulum (ER) protein, which functions as a chaperone [13, 14]. Chaperones are proteins that help and guide other proteins as they fold and progress along the productive pathways. Misfolded proteins could aggregate and interfere with intracellular movement. Many chaperones are upregulated under conditions where misfolded proteins accumulate. ERp29 also facilitates the export of membrane or secretory proteins. ERp29 protein is considered to be associated with neurodegenerative diseases [12]. Significantly lower levels of ERp29 (protein and transcript) are found in the eyes, particularly within the retinal lesions, of DKO mice as compared to C57BL/6 mice [10]. This finding supports the reports of decreased ERp29 in the aging retina and AMD maculae [15, 16]. Low ERp29 protein levels may impact chaperone function and lead to the accumulation of incompletely folded aggregates such as lipofuscin or drusen, eventually resulting in the RPE damage observed in DKO mice.
Implications of DKO Mice
Omega-3 fatty acids are long-chain polyunsaturated fatty acids (LCPUFA) that cannot be synthesized de novo by mammals [17]. They have neuroprotective, anti-inflammatory and antiangiogenic effects [18–20]. One of the major components of omega-3 LCPUFA is docosahexaenoic acid, which activates HSP70, a chaperone protein. Omega-3 LCPUFA is metabolized in the ER and stimulates post-ER presecretory proteolysis [21]. Higher intake of omega-3 LCPUFA and fish is associated with a decreased likelihood of developing AMD [22, 23].
In a preliminary experiment, DKO mice were fed with either a high or low in omega-3 LCPUFA starting from preconception. Although similar retinal lesions were observed at 6 weeks of age in both groups, the number of lesions was decreased in the mice that ingested a high omega-3 LCPUFA diet (fig. 4a, b). In contrast, in the mice that ingested a diet deprived of omega-3 LCPUFA, the retinal lesions continued to progress (fig. 4c, d). The likely cause for this observation is the enriched docosahexaenoic acid intake in the mice fed with the high omega-3 LCPUFA diet. Docosahexaenoic acid is shown to promote RPE cell survival by being the precursor of neuroprotectin D1. Neuroprotectin D1 inhibits oxidative-stress-mediated proinflammatory gene induction and apoptosis [24]. Further investigations on these mice are currently underway in our laboratory.
Fig. 4.
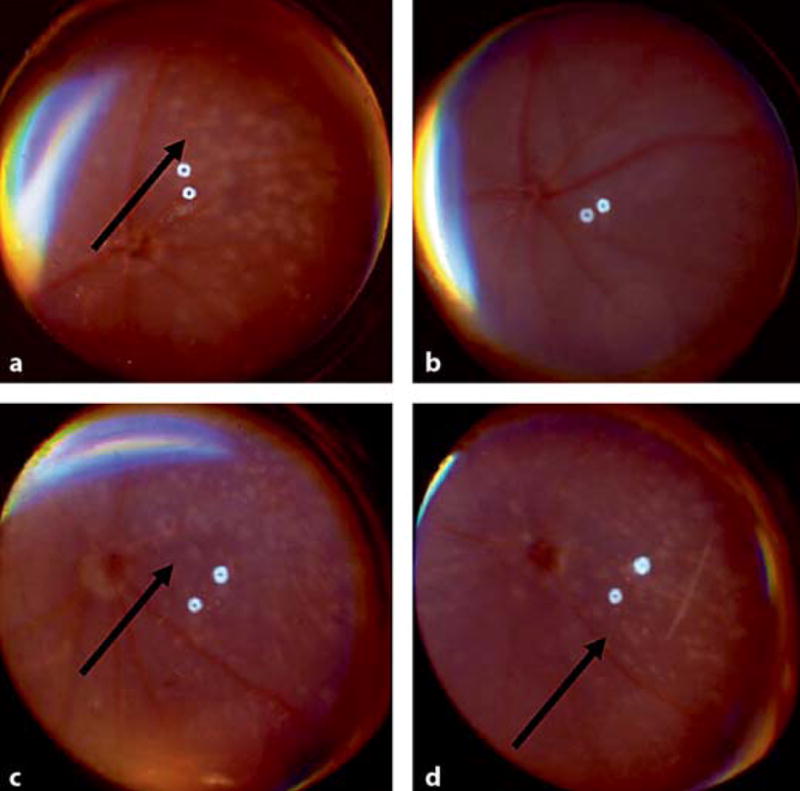
Funduscopic images of 2 DKO mice showing decrease of retinal lesions (arrow) in the mouse fed with high omega-3 LCPUFA (a, b) but increase of retinal lesions (arrows) in the mouse fed with diet deprived of omega-3 LCPUFA (c, d) after 1 month.
Conclusion
DKO mice present a board spectrum of clinical, biochemical, immunological and pathological features of human AMD lesions. The spontaneously developing features are highly reproducible. In these mice, the disease onset is earlier than that observed in most genetically engineered AMD mouse models. DKO mice implicate the important roles of certain inflammatory molecules and chaperone proteins in AMD pathogenesis. DKO mice may be used to direct future therapeutic strategies, such as the attenuation of retinal pathology with a diet rich in omega-3 LCPUFA for the treatment of AMD.
Fig. 5.
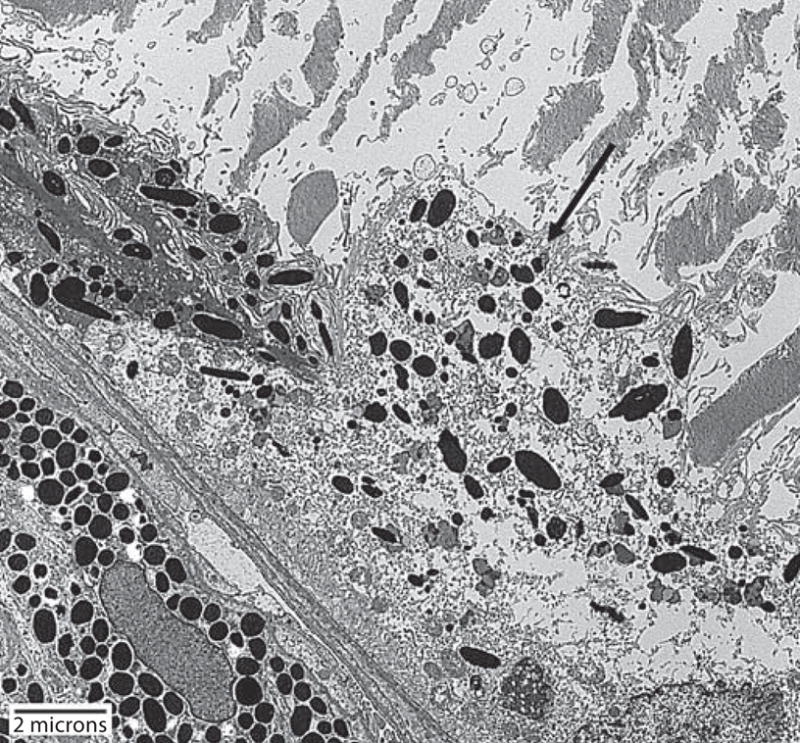
Transmission electron micrograph of a DKO mouse shows severe degeneration of RPE cell (arrow) and disorganized and partial loss of photoreceptor outer segments.
References
- 1.Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 2.Smith W, Assink J, Klein R, Mitchell P, Klaver CC, Klein BE, Hofman A, Jensen S, Wang JJ, de Jong PT. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001;108:697–704. doi: 10.1016/s0161-6420(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 3.Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration – emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38:450–471. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rakoczy PE, Yu MJ, Nusinowitz S, Chang B, Heckenlively JR. Mouse models of age-related macular degeneration. Exp Eye Res. 2006;82:741–752. doi: 10.1016/j.exer.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Tuo J, Smith BC, Bojanowski CM, Meleth AD, Gery I, Csaky KG, Chew EY, Chan CC. The involvement of sequence variation and expression of CX3CR1 in the pathogenesis of age-related macular degeneration. FASEB J. 2004;18:1297–1299. doi: 10.1096/fj.04-1862fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan CC, Tuo J, Bojanowski CM, Csaky KG, Green WR. Detection of CX3CR1 single nucleotide polymorphism and expression on archived eyes with age-related macular degeneration. Histol Histopathol. 2005;20:857–863. doi: 10.14670/hh-20.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Combadiere C, Feumi C, Raoul W, Keller N, Rodero M, Pezard A, Lavalette S, Houssier M, Jonet L, Picard E, Sirinyan M, Deterre P, Ferroukhi T, Cohen SY, Chauvaud D, Jeanny J, Chemtob S, Behar-Cohen F, Sennlaub F. CX3CR1 dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117:2920–2928. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raoul W, Combadiere C, Keller N, Rodero M, Jeanny JC, Behar-Cohen F, Sennlaub F. Retinal degeneration occurs in CX3CR1 knockout animals secondary to subretinal microglia accumulation. Invest Ophthalmol Vis Sci. 2007;48:3023. [Google Scholar]
- 9.Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, Rollins BJ, Ambati BK. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9:1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- 10.Tuo J, Bojanowski CM, Zhou M, Shen D, Ross RJ, Rosenberg KI, Cameron DJ, Yin C, Kowalak JA, Zhuang Z, Zhang K, Chan CC. Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:3827–3836. doi: 10.1167/iovs.07-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen D, Tuo J, Bojanowski CM, Zhou M, Ross RJ, Chan CC. Hyporeactive ocular responses to lipopolysaccharide in ccl2/cx3cr1 double deficient mice. Invest Ophthalmol Vis Sci. 2006;47:4521. [Google Scholar]
- 12.Willis D, Li KW, Zheng JQ, Chang JH, Smit A, Kelly T, Merianda TT, Sylvester J, van Minnen J, Twiss JL. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J Neurosci. 2005;25:778–791. doi: 10.1523/JNEUROSCI.4235-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mkrtchian S, Fang C, Hellman U, Ingelman-Sundberg M. A stress-inducible rat liver endoplasmic reticulum protein, erp29. Eur J Biochem. 1998;251:304–313. doi: 10.1046/j.1432-1327.1998.2510304.x. [DOI] [PubMed] [Google Scholar]
- 14.Sargsyan E, Baryshev M, Szekely L, Sharipo A, Mkrtchian S. Identification of erp29, an endoplasmic reticulum lumenal protein, as a new member of the thyroglobulin folding complex. J Biol Chem. 2002;277:17009–17015. doi: 10.1074/jbc.M200539200. [DOI] [PubMed] [Google Scholar]
- 15.Li D, Sun F, Wang K. Protein profile of aging and its retardation by caloric restriction in neural retina. Biochem Biophys Res Commun. 2004;318:253–258. doi: 10.1016/j.bbrc.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 16.Ethen CM, Reilly C, Feng X, Olsen TW, Ferrington DA. The proteome of central and peripheral retina with progression of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47:2280–2290. doi: 10.1167/iovs.05-1395. [DOI] [PubMed] [Google Scholar]
- 17.Salem NJ. Omega-3 fatty acids: molecular and biochemical aspects. In: Spiller G, Scala J, editors. New Protective Roles of Selected Nutrients in Human Nutrition. New York: Liss; 1989. pp. 109–228. [Google Scholar]
- 18.Sterescu AE, Rousseau-Harsany E, Farrell C, Powell J, David M, Dubois J. The potential efficacy of omega-3 fatty acids as anti-angiogenic agents in benign vascular tumors of infancy. Med Hypotheses. 2006;66:1121–1124. doi: 10.1016/j.mehy.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg RJ, Katz J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. 2007;129:210–223. doi: 10.1016/j.pain.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Bazan NG. Neuroprotectin D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. 2005;15:159–166. doi: 10.1111/j.1750-3639.2005.tb00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narayanan NK, Narayanan BA, Bosland M, Condon MS, Nargi D. Docosahexaenoic acid in combination with celecoxib modulates hsp70 and p53 proteins in prostate cancer cells. Int J Cancer. 2006;119:1586–1598. doi: 10.1002/ijc.22031. [DOI] [PubMed] [Google Scholar]
- 22.Sangiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005;24:87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Sangiovanni JP, Chew EY, Clemons TE, Davis MD, Ferris FL, 3rd, Gensler GR, Kurinij N, Lindblad AS, Milton RC, Seddon JM, Sperduto RD. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS Report No 20. Arch Ophthalmol. 2007;125:671–679. doi: 10.1001/archopht.125.5.671. [DOI] [PubMed] [Google Scholar]
- 24.Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29:263–271. doi: 10.1016/j.tins.2006.03.005. [DOI] [PubMed] [Google Scholar]


