Abstract
Using the scleral search coil technique to monitor eye movements, we recorded short-latency ocular following responses to displacement steps of large random-dot patterns. On half of the trials, the luminance of the dots and background were reversed during the step, a procedure that is known to reverse the direction of the perceived motion (“reverse phi”). Steps without luminance reversal induced small but consistent ocular following in the direction of the steps at ultra-short latency (<80 ms). Steps with luminance reversal induced small but consistent tracking at the same latency but in the direction opposite to the actual displacement. Tuning curves describing the dependence of initial ocular following on the amplitude of the displacement had a form approximating the derivative of a Gaussian and were well fit by Gabor functions, the cosine term being phase shifted ~180º by the luminance reversal. This result is consistent with the idea that the initial ocular following is mediated, at least in part, by first-order (luminance) motion-energy detectors.
Keywords: apparent motion, ocular tracking, reversed phi motion, motion detectors
1. Introduction
This paper is concerned with the machine-like ocular following responses that are elicited at ultra-short latencies by sudden motion of a large textured pattern (humans: <80 ms; monkeys: <60 ms): see Miles (1998) for recent review. There is considerable psychophysical evidence that motion can be sensed not only from the spatio-temporal distribution of luminance (first-order motion) but also from derived characteristics such as contrast, binocular disparity, spatial frequency, and flicker (second-order motion), or from yet other salient features (third-order motion): see Lu and Sperling (2001) for recent review. It is generally supposed that it takes longer to sense higher-order attributes than first-order ones and there is some psychophysical evidence for this. For example, in the study of Derrington et al (1993), the exposure time required to reliably discriminate the direction of contrast-defined (second-order) motion—about 200 ms—was an order of magnitude longer than for luminance-defined (first-order) motion. This would lead one to expect that responses elicited with latencies appreciably less than 100 ms—such as the initial ocular following under study in the present paper—result mainly from the processing of first-order motion. It is known that second-order motion defined solely by disparity (Archer, Miller & Helveston, 1987; Fox, Lehmkuhle & Leguire, 1978) or flicker (Harris & Smith, 2000) can elicit tracking eye movements but, when defined solely by contrast, does so only weakly, if at all (Harris & Smith, 1992).1 To investigate this issue further, we have now recorded the initial ocular following responses elicited by single step displacements of a large random-dot pattern rather than the continuous ramp displacements employed in all previous studies of these tracking eye movements. When two identical patterns are presented one after another with a small spatial separation between them (single step displacement), apparent motion is perceived in the direction of the image displacement: phi motion (Wertheimer, 1912). However, if the second pattern is a photographic negative of the first (i.e., there is reversal of the luminance polarity during the step—see the cartoons in Figure 1), the perceived apparent motion is in the opposite direction to the image displacement: reversed phi (Anstis, 1970; Anstis & Rogers, 1975).2 A number of models have been proposed that can explain such motion percepts and, essentially, all have a linear spatiotemporal filter and perform a motion-energy computation: see Lu and Sperling (2001) for review. Indeed, reversed phi is regarded as the hallmark of a motion-energy mechanism. However, in the classical reversed-phi stimuli only the first-order (luminance) motion energy is reversed—the second-order (contrast) motion energy remains in the forward direction—and, for this reason, they are now referred to as first-order reversed-phi stimuli (Lu & Sperling, 1999).3 We now report that step-wise displacements of large random-dot patterns elicit ocular following at short latency and that reversing the luminance polarity during the steps—a first-order reversed-phi stimulus—results in a reversal in the direction of these tracking responses, consistent with the idea that these earliest tracking responses result, at least in part, from the operation of a first-order (luminance) motion-energy detection mechanism.
Figure 1.
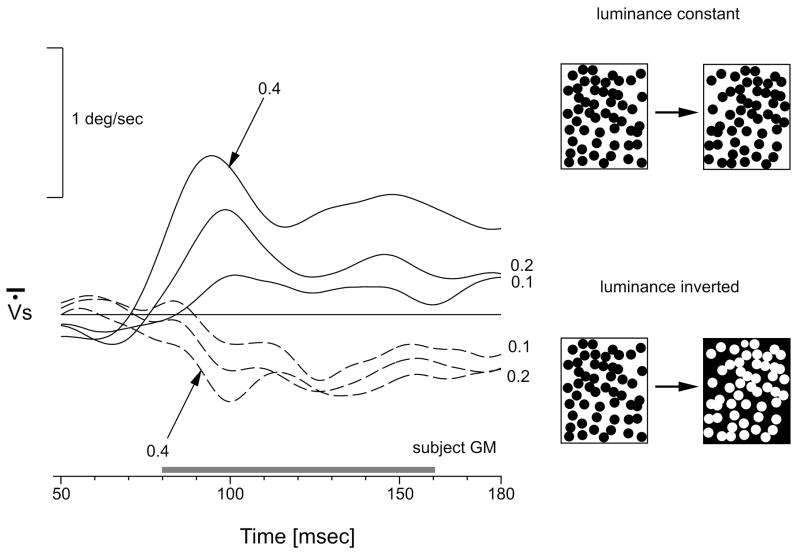
Mean version velocity profiles elicited by rightward stepwise displacements of a high-density random dot pattern. Continuous lines: luminance polarity remained constant across the step (upper right cartoon). Broken lines: luminance polarity was reversed during the step (lower right cartoon). Numbers indicate the amplitude (in degrees) of the image displacement. Horizontal grey bar indicates the time-window over which response amplitude is quantified. Upward displacements represent rightward movements. Traces are each means of ~180 trials. Subject, GSM.
2. Methods
Most of the methods have been described previously (Gellman, Carl & Miles, 1990; Masson, Busettini, Yang & Miles, 2001) and, except where there are substantive differences, only an outline will be given here.
2.1. Subjects
The subjects were the three authors and one additional subject (JKM) who was completely unaware of the purpose of the experiment. All subjects were experienced in eye movement recording and had no known oculomotor or visual problems other than refractive errors that were corrected with spectacles (FAM, JKM & GSM).
2.2. Eye movement recordings
The horizontal and vertical positions of both eyes were recorded with the scleral search coil technique (Collewijn, Van Der Mark & Jansen, 1975; Fuchs & Robinson, 1966). Coils were placed in each eye following application of 1–2 drops of anaesthetic (Proparacaine HCl) and wearing time ranged up to 100min. The AC voltages induced in the scleral search coils were led off to phase-locked amplifiers that provide separate DC voltage outputs proportional to the horizontal and vertical positions of the two eyes with corner frequencies (−3dB) at 1 kHz (CNC Engineering). The outputs from the coils were calibrated at the beginning of each recording session by having the subject fixate small target lights located at known eccentricities along the horizontal and vertical meridia. Peak-to-peak voltage noise levels were equivalent to an eye movement of ~1min of arc. The presentation of the stimuli and the acquisition, on-line display and storage of the data were controlled by a PC using the REX software package (Hays, Richmond & Optican, 1982).
2.3. Behavioral paradigm and visual display
The subject was seated in an acrylic chair with his/her head stabilized by means of a chin support and forehead rest. He/she faced a translucent tangent screen (distance: 33.3cm; subtense: 80° horizontal × 50° vertical) onto which photographic images were back-projected. Two kinds of random-dot patterns were used: one consisted of white dots on a black ground and the other was the same except that the dots were black and the background was white. The dots (diameter ~2°) were randomly distributed and covered 50% of the image space, which always filled the screen. Luminance was 3.2 cd/m2 in the light areas and 0.032 cd/m2 in the dark areas (Spectra Pritchard photometer), values comparable with those in our previous studies of human ocular following using velocity steps. The two random-dot images were generated by two separate slide projectors. The horizontal and vertical positions of the images on the screen were controlled by X-Y pairs of mirror galvanometers (General Scanning Inc., M3-S with vector tuning) located in each light path and driven by the DAC outputs of a PC at a rate of 1 kHz with a resolution of 12 bits (optical range ~50°). Images were turned on and off by means of shutter galvanometers (General Scanning Inc, CX-660) located in each projector path between the light source and the collimator to eliminate moving edges.
Horizontal step stimuli (13 amplitudes ranging from 0.1° to 4.8°, with equal probability of being leftward or rightward) were applied 50 ms after 10° leftward centering saccades to take advantage of post-saccadic enhancement (Gellman et al., 1990; Kawano & Miles, 1986). We also included two types of control trials with zero-amplitude steps, i.e., transient blanking of the images with and without luminance reversal. Saccades were guided by target spots projected onto the random dot pattern, the second (central) spot being extinguished during the saccade. The screen was always blanked briefly (maximum duration, ~14 ms) during the step, using the shutter galvanometers. The image was present on the screen for 200 ms after the step, at which time the shutters were used to blank the screen for 500 ms, ending the trial. Data were collected over several sessions until each of the (54) stimuli had been repeated at least 180 times (random order of presentation).
2.4. Data analysis
Voltage signals separately encoding the horizontal and vertical positions of both eyes were low-pass filtered (Bessel, 6 poles, DC-180Hz) and digitized (16 bits resolution; sampling rate: 1kHz). After linearization, the horizontal and vertical position signals were smoothed with a cubic spline function of weight 107 selected by means of a cross-validation procedure (Busettini, Miles & Schwarz, 1991). Horizontal version position was computed by averaging the horizontal positions of the left and right eyes and this was then used to determine the horizontal version velocity by two-point backward differentiation. For each stimulus, we computed the mean changes in version velocity over time and estimated the magnitude of the associated ocular following response by measuring the mean change in horizontal version position over the 80-ms time-window starting 80ms after stimulus onset. Small post-saccadic drifts were effectively removed by subtracting the mean responses to the zero-amplitude steps (transient blanking only) and all data shown have been so adjusted. Tuning curves describing the dependence of these response measures on the step size were fitted with a Gabor function using a least-squares regression procedure.
3. Results
3.1. Position steps without luminance reversal
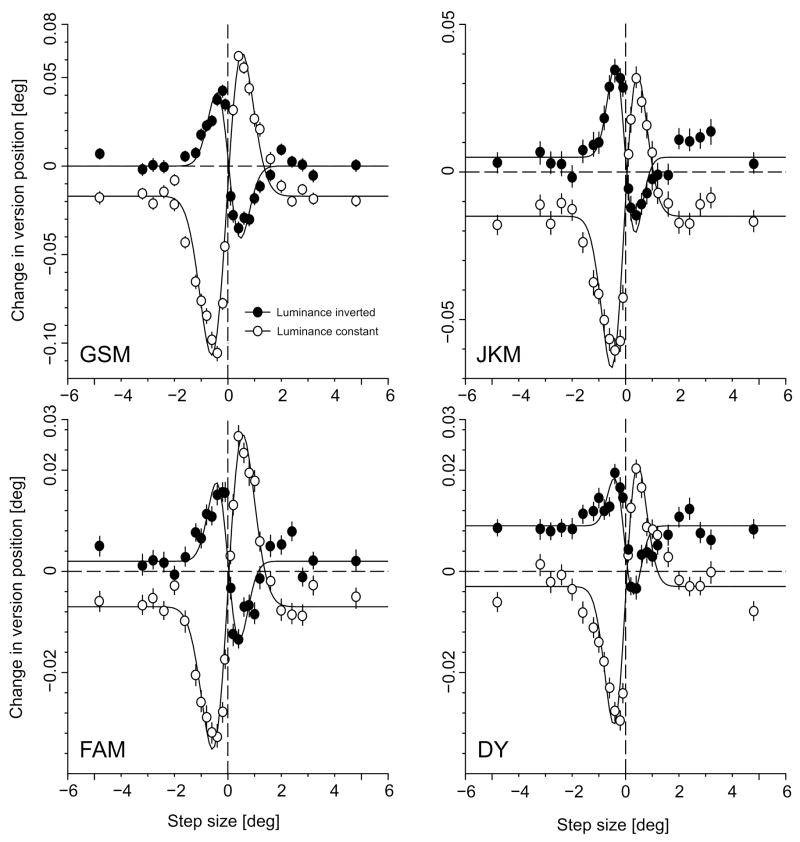
Step displacements of a large, random dot pattern elicited consistent ocular following responses at ultra-short latencies (<80 ms) in all four subjects tested. Figure 1 (continuous lines) shows sample mean eye velocity profiles over time in response to rightward steps ranging from 0.1° to 0.4° for one subject (GSM). Responses were always very small, especially compared with our previously published responses to velocity steps (Busettini, Masson & Miles, 1996; Busettini, Miles, Schwarz & Carl, 1994; Masson et al., 2001; Masson, Rybarzyck, Castet & Mestre, 2000), and generally showed an initial transient peak with a subsequent decline back to a non-zero asymptote that was sustained throughout the acquisition time window (180ms) despite the transient (pulsatile) nature of the (motion) stimulus. The quantitative dependence of the tracking response on the size of the step is illustrated in Fig. 2 (open symbols), which plots the change in version position (over the time window, 80–160ms) as a function of the amplitude of the step (in degrees) for all four subjects. These response measures generally peak with steps of 0.2°–0.4°, and decline thereafter, reaching an asymptote with steps of ~2°. The continuous lines in Fig. 2 are best-fit Gabor functions, which provide a reasonably good representation of the data, r2 values ranging from 0.95 to 0.98: see Table 1 for a complete listing.
Figure 2.
Initial ocular following responses to step displacements: dependence on the magnitude and direction of the step (four subjects: GSM, FAM, DY, JKM). Ordinate: Change in version position (mean ±SE) over the 80-160ms time window (in degrees). Abscissa: Image displacement (in degrees). Open symbols: luminance polarity remained constant across the step. Closed symbols: luminance polarity was reversed during the step. Continuous lines are best-fitting Gabor functions. Positive values represent rightward version responses and step displacements.
Table 1.
Best-fit parameters of Gabor functions
| Subject | A | σ | D | f | ϕ | B | R | r2 |
|---|---|---|---|---|---|---|---|---|
| Luminance constant | ||||||||
| G.S.M | 0.535 | 0.58 | −0.055 | 0.073 | 269 | −0.017 | 0.168 | 0.98 |
| F.A.M | 0.145 | 0.58 | 0.020 | 0.099 | 272 | −0.007 | 0.062 | 0.98 |
| D.Y | 0.295 | 0.45 | −0.005 | 0.051 | 270 | −0.003 | 0.048 | 0.95 |
| J.K.M. | 0.540 | 0.49 | −0.050 | 0.049 | 269 | −0.015 | 0.100 | 0.97 |
| Luminance reversed | ||||||||
| G.S.M | 0.295 | 0.45 | 0.030 | 0.081 | 90 | 0.000 | 0.081 | 0.95 |
| F.A.M | 0.270 | 0.43 | 0.000 | 0.035 | 90 | 0.002 | 0.031 | 0.91 |
| D.Y | 0.120 | 0.36 | −0.030 | 0.069 | 92 | 0.009 | 0.023 | 0.86 |
| J.K.M. | 0.255 | 0.38 | −0.035 | 0.077 | 89 | 0.005 | 0.057 | 0.93 |
where s is the size of the stimulus step, A is a gain factor, σ is the Gaussian width, f and ϕ are the spatial frequency and phase of the cosine term, S is the displacement, and B is an offset parameter to allow for non-zero asymptotes. R is the peak-to-peak amplitude derived from the best-fit Gabor functions. All units are in degrees except for f, which is in cycles per degree. r2 provides a measure of the goodness of fit, indicating the proportion of the step-induced responses accounted for by the Gabor function.
3.2. Position steps with luminance reversal
Reversing the luminance polarity during the step resulted in reversal of the ocular following responses: rightward (leftward) steps induced leftward (rightward) tracking responses. This is apparent from the mean eye velocity profliles in Fig. 1 (dashed traces) and from the response measures plotted in Fig. 2 (closed symbols). Gabor functions once more provided a reasonably good representation of the data, though slightly worse than for the data without luminance reversal (r2 values ranging from 0.86 to 0.95: see Table 1). The cosine terms for the best-fit Gabor functions with and without reversal of luminance polarity differ by ~180° (actual values for the four subjects: 179°, 182°, 178°, 180°): see Table 1. It is also clear that, in all cases, the responses with reversal of luminance polarity were even smaller than the (already small) responses to steps without the reversal in luminance. This is apparent from the peak-to-peak amplitudes of the best-fit Gabor functions (R in Table 1), those for the data obtained with luminance reversal being about half those without (actual ratios for the four subjects: 0.48, 0.50, 0.48, 0.57). In addition, the Gaussian width of the Gabor functions (s in Table 1) was significantly smaller with the luminance reversal (one-tail t-test, p<0.05), on average by 23%.
4. Discussion
In the present study, we found that small step-displacements of a large random-dot pattern elicited ocular following responses at ultra-short latencies. These eye movements were always in the direction of both the stimulus displacement and the perceived (phi) motion (Sato, 1989), and we assume that they are generated by the same mechanisms that produce the ocular following elicited at similarly short latencies by velocity steps (Busettini et al., 1994; Gellman et al., 1990; Masson et al., 2001; Miles, Kawano & Optican, 1986). However, the responses to velocity steps are generally much more sustained and achieve much higher velocities, presumably in large part because the motion stimulus with velocity steps is sustained throughout the 200-ms duration of the step whereas that with position steps is restricted to the initial transient (pulse).
Reversing the luminance of the pattern during the steps also resulted in ocular following but in the reverse direction, i.e., in the direction opposite to the actual displacement of the pattern but still in the direction of the perceived motion: reversed phi (Anstis, 1970; Anstis & Rogers, 1975; Sato, 1989; Smith & Ledgeway, 2001). The spatial tuning characteristics evident in Fig. 2 are in rough quantitative agreement with the perceived direction of motion in visual psychophysical experiments using comparable stimuli. For example, in the recent study of Smith and Ledgeway (2001), when random-dot kinematograms with large texture elements (1.25°, compared with 2° in our experiments) were subject to step displacements, forward phi was reliably reported with steps up to 1.7–1.8° and reverse phi with steps up to 1.3–1.4°, values that are roughly comparable with the effective stimulus ranges in our experiments. Based on the Gaussian widths of the best-fit Gabor functions, the effective stimulus ranges in our experiments were on average 23% smaller with the reverse-phi stimuli than with the forward-phi stimuli, which is comparable with the differences in the data of Smith and Ledgeway (2001).
For any given position step, the ocular following responses to the reversing-luminance stimuli were invariably weaker than those to the constant-luminance stimuli. Thus, on average, the peak-to-peak amplitudes of the best-fit Gabor functions with luminance-reversal were about half those without reversal. A part of this difference could be due to the fact that, as pointed out by Dosher, Landy and Sperling (1989), the first-order motion energy is slightly smaller for the reversing-luminance stimuli than for the constant stimuli. When applied to our stimuli, the Fourier computation of Dosher and colleagues (1989) indicated that the net Directional Power of the first-order motion was on average 9% (±2%, SD) lower in the reversed condition, based on 200 randomly selected (x,t) slices4. Thus, the difference in motion energy is sufficient to explain only a small proportion (~18%) of the reduction in ocular following when luminance polarity was reversed during the step.
As pointed out in the Introduction, with our luminance-reversing stimulus it is only the first-order (luminance) motion energy that is reversed: the second- and third-order motion energy is in the forward direction (Lu & Sperling, 1999; Nishida, 1993). Thus, the extent to which initial ocular following shows reversal with our luminance-reversing stimulus depends on the relative strengths and efficacy of the first- and higher-order motion-energy components, which in turn depends on the spatiotemporal characteristics of the stimulus (Chubb & Sperling, 1989; Gorea, 1995; Solomon & Sperling, 1995). This means that the reduction in the amplitude of the ocular following responses with luminance reversal that cannot be directly accounted for by the reduced first-order motion energy can be attributed to the competing higher-order motion components. Such competitive effects have also been observed in pursuit initiation (Lindner & Ilg, 2000) and the slow phases of OKN (Harris & Smith, 2000), though here too ocular responses were always dominated by the first-order motion components.
Attempts to generate ocular tracking with pure second-order motion stimuli were not very successful when pure contrast was used (Harris & Smith, 1992), though flicker-defined motion was somewhat more effective under some conditions (Harris & Smith, 2000). A potentially important factor in our studies is that we are dealing with responses that have ultra-short latencies (<100 ms) and second-order (contrast) mechanisms have been shown to be slower than first-order mechanisms (Derrington et al., 1993), though it is also possible that this difference in dynamics is small in our situation, as for instance in the experiments of Masson et al (2000), who observed a latency difference of ~20 ms. This suggests that the very earliest ocular following responses in our experiments (latency<100 ms) might have escaped the effects of the competing higher-order components of motion.
The reversed ocular following responses in the present study probably share the same etiology as the self-sustaining eye movements observed by Spillman, Anstis, Kurtenbach and Howard (1997) when subjects viewed a random-dot pattern undergoing repeated luminance reversals (“counterphase flicker”). Spillman et al state that, “eye movements occasionally began spontaneously, but generally needed to be started by tracking a finger that moved across the surface of the stimulus screen” and attributed them to “positive retinal feedback from the contrast-reversing pattern”. Once initiated, these eye movements were self-sustaining for 3–5 s but thereafter could not be self-initiated and had to be restarted with a moving finger. It is possible that the gradual decay in the nystagmus with prolonged exposure to the stimulus was due to the gradual intervention of second-order mechanisms, reinforcing the idea that the latter have more sluggish dynamics than first-order mechanisms. This would be consistent with the finding of Harris and Smith (1992) that prolonged flicker-defined (second-order) motion of a large pattern can produce reflexive ocular tracking, albeit weak. Also, a single moving object defined by second-order attributes can elicit vigorous smooth pursuit eye movements, although with a longer latency (Butzer, Ilg & Zanker, 1997).
Concerning the neuronal mediation of our response reversals, ocular following has very similar properties in human and non-human primates—see Miles (1998) for recent review—and, based on the data from lesions and single unit recordings in monkeys, the medial superior temporal (MST) area of cortex has been strongly implicated in its initiation (Kawano, Inoue, Takemura, Kodaka & Miles, 2000). Many neurons in the middle temporal (MT) area, which provides major inputs to MST, show directional selectivity for motion and exhibit reversed directionality with luminance-reversing motion stimuli (Livingstone, Pack & Born, 2001), a characteristic also of some simple—but not complex—cells in striate cortex (Livingstone, Tsao & Conway, 2000). Interestingly, there is another type of eye movement—disparity vergence—that shares a number of features with ocular following: 1) It is elicited at ultra-short latency when the appropriate stimuli—this time, disparity steps—are applied to large random-dot patterns (Busettini, FitzGibbon & Miles, 2001; Busettini, Miles & Krauzlis, 1996); 2) it shows response reversal with luminance-reversing stimuli, referred to as “anticorrelated stimuli” (Masson, Busettini & Miles, 1997); 3) it seems to be mediated at least in part by MST (Takemura, Inoue & Kawano, 2000; Takemura, Inoue, Kawano, Quaia & Miles, 1999), whose disparity-selective neurons show response reversals with luminance-reversing stimuli (Takemura, Inoue, Kawano, Quaia & Miles, 2001), as also do the disparity-selective neurons in striate cortex (Cumming & Parker, 1997), many of whose properties have been successfully simulated with an energy model (Fleet, Wagner & Heeger, 1996; Ohzawa, DeAngelis & Freeman, 1990; Qian, 1994). The clear suggestion is that the motion that initiates version and the disparity that initiates vergence are both sensed by first-order energy mechanisms in cortex.
Acknowledgments
GSM is supported by the Fondation pour la Recherche Médicale (France) and by the Ministère de la Recherche (ACI-JC, 2000-5085). We thank Tom Ruffner, Nick Nichols and Lee Jensen for technical assistance, Rich Krauzlis, Ed Fitzgibbon, John McClurkin and Art Hays for software support, Jean Steinberg for secretarial assistance, and Eric Castet for critical reading of an earlier version of the manuscript. A special thanks to Lance Optican for computing the net Directional Power in our stimuli.
Footnotes
Contrast modulated noise, comprising a static random two-dimensional texture carrier modulated by a drifting sinusoid, has been shown to generate tracking eye movements (Benson & Guo, 1999) but it isn’t clear that the active component here was second-order motion: see Smith and Ledgeway (1997) for discussion of the first-order artefacts in such stimuli.
For a demonstation of reversed phi motion see http://www.biols.susx.ac.uk/home/George_Mather/Harley.html
More recently, some second-order reversed-phi stimuli have been devised in which there is a reversal of the second-order (contrast) motion energy (Lu & Sperling, 1999; Nishida, 1993).
For this computation, the upper limits of the window of visibility were set at 10 cpd for the spatial frequency and 40 Hz for the temporal frequency, based on the spatio-temporal characteristics of human ocular following (Gellman et al., 1990). These values are slightly different from those used by Dosher et al (1989).
References
- Anstis SM. Phi movement as a subtraction process. Vision Research. 1970;10:1411–1430. doi: 10.1016/0042-6989(70)90092-1. [DOI] [PubMed] [Google Scholar]
- Anstis SM, Rogers BJ. Illusory reversal of visual depth and movement during changes of contrast. Vision Research. 1975;15:957–961. doi: 10.1016/0042-6989(75)90236-9. [DOI] [PubMed] [Google Scholar]
- Archer SM, Miller KK, Helveston EM. Stereoscopic contours and optokinetic nystagmus in normal and stereoblind subjects. Vision Research. 1987;27:841–844. doi: 10.1016/0042-6989(87)90080-0. [DOI] [PubMed] [Google Scholar]
- Benson PJ, Guo K. Stages in motion processing revealed by the ocular following response. Neuroreport. 1999;10:3803–3807. doi: 10.1097/00001756-199912160-00015. [DOI] [PubMed] [Google Scholar]
- Busettini C, FitzGibbon EJ, Miles FA. Short-latency disparity vergence in humans. Journal of Neurophysiology. 2001;85:1129–1152. doi: 10.1152/jn.2001.85.3.1129. [DOI] [PubMed] [Google Scholar]
- Busettini C, Masson GS, Miles FA. A role for stereoscopic depth cues in the rapid visual stabilization of the eyes. Nature. 1996;380:342–345. doi: 10.1038/380342a0. [DOI] [PubMed] [Google Scholar]
- Busettini C, Miles FA, Krauzlis RJ. Short-latency disparity vergence responses and their dependence on a prior saccadic eye movement. Journal of Neurophysiology. 1996;75:1392–1410. doi: 10.1152/jn.1996.75.4.1392. [DOI] [PubMed] [Google Scholar]
- Busettini C, Miles FA, Schwarz U. Ocular responses to translation and their dependence on viewing distance. II. Motion of the scene. Journal of Neurophysiology. 1991;66:865–878. doi: 10.1152/jn.1991.66.3.865. [DOI] [PubMed] [Google Scholar]
- Busettini C, Miles FA, Schwarz U, Carl JR. Human ocular responses to translation of the observer and of the scene: dependence on viewing distance. Experimental Brain Research. 1994;100:484–494. doi: 10.1007/BF02738407. [DOI] [PubMed] [Google Scholar]
- Butzer F, Ilg UJ, Zanker JM. Smooth-pursuit eye movements elicited by first-order and second-order motion. Experimental Brain Research. 1997;115:61–70. doi: 10.1007/pl00005686. [DOI] [PubMed] [Google Scholar]
- Chubb C, Sperling G. Two motion perception mechanisms revealed through distance-driven reversal of apparent motion. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:2985–2989. doi: 10.1073/pnas.86.8.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collewijn H, Van Der Mark F, Jansen TC. Precise recording of human eye movements. Vision Research. 1975;15:447–450. doi: 10.1016/0042-6989(75)90098-x. [DOI] [PubMed] [Google Scholar]
- Cumming BG, Parker AJ. Responses of primary visual cortical neurons to binocular disparity without depth perception. Nature. 1997;389:280–283. doi: 10.1038/38487. [DOI] [PubMed] [Google Scholar]
- Derrington AM, Badcock DR, Henning GB. Discriminating the direction of second-order motion at short stimulus durations. Vision Research. 1993;33:1785–1794. doi: 10.1016/0042-6989(93)90169-w. [DOI] [PubMed] [Google Scholar]
- Dosher BA, Landy MS, Sperling G. Kinetic depth effect and optic flow. I. 3D shape from Fourier motion. Vision Research. 1989;29:1789–1813. doi: 10.1016/0042-6989(89)90161-2. [DOI] [PubMed] [Google Scholar]
- Fleet DJ, Wagner H, Heeger DJ. Neural encoding of binocular disparity: energy models, position shifts and phase shifts. Vision Research. 1996;36:1839–1857. doi: 10.1016/0042-6989(95)00313-4. [DOI] [PubMed] [Google Scholar]
- Fox R, Lehmkuhle S, Leguire LE. Stereoscopic contours induce optokinetic nystagmus. Vision Research. 1978;18:1189–1192. doi: 10.1016/0042-6989(78)90103-7. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. Journal of Applied Physiology. 1966;21:1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Gellman RS, Carl JR, Miles FA. Short latency ocular-following responses in man. Visual Neuroscience. 1990;5:107–122. doi: 10.1017/s0952523800000158. [DOI] [PubMed] [Google Scholar]
- Gorea A. Spatiotemporal characterization of a Fourier and non-Fourier motion system. Vision Research. 1995;35:907–914. doi: 10.1016/0042-6989(94)00193-p. [DOI] [PubMed] [Google Scholar]
- Harris LR, Smith AT. Motion defined exclusively by second-order characteristics does not evoke optokinetic nystagmus. Visual Neuroscience. 1992;9:565–570. doi: 10.1017/s0952523800001802. [DOI] [PubMed] [Google Scholar]
- Harris LR, Smith AT. Interactions between first- and second-order motion revealed by optokinetic nystagmus. Experimental Brain Research. 2000;130:67–72. doi: 10.1007/s002219900232. [DOI] [PubMed] [Google Scholar]
- Hays AV, Richmond BJ, Optican LM. A UNIX-based multiple process system for real-time data acquisition and control. WESCON Conference Proceedings. 1982;2(1):1–10. [Google Scholar]
- Kawano K, Inoue Y, Takemura A, Kodaka Y, Miles FA. The role of MST neurons during ocular tracking in 3D space. International Review of Neurobiology. 2000;44:49–63. doi: 10.1016/s0074-7742(08)60737-0. [DOI] [PubMed] [Google Scholar]
- Kawano K, Miles FA. Short-latency ocular following responses of monkey. II. Dependence on a prior saccadic eye movement. Journal of Neurophysiology. 1986;56:1355–1380. doi: 10.1152/jn.1986.56.5.1355. [DOI] [PubMed] [Google Scholar]
- Lindner A, Ilg UJ. Initiation of smooth-pursuit eye movements to first-order and second-order motion stimuli. Experimental Brain Research. 2000;133:450–456. doi: 10.1007/s002210000459. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Pack CC, Born RT. Two-dimensional substructure of MT receptive fields. Neuron. 2001;30:781–793. doi: 10.1016/s0896-6273(01)00313-0. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Tsao DY, Conway BR. What happens if it changes contrast when it moves? Society for Neuroscience Abstracts. 2000;26:447. [Google Scholar]
- Lu Z, Sperling G. Three-systems theory of human visual motion perception: review and update. Journal of the Optical Society of America A. Optics and Image Science. 2001;18:2331–2370. doi: 10.1364/josaa.18.002331. [DOI] [PubMed] [Google Scholar]
- Lu Z-L, Sperling G. Second-order reversed phi. Perception and Psychophysics. 1999;61:1075–1088. doi: 10.3758/bf03207615. [DOI] [PubMed] [Google Scholar]
- Masson GS, Busettini C, Miles FA. Vergence eye movements in response to binocular disparity without depth perception. Nature. 1997;389:283–286. doi: 10.1038/38496. [DOI] [PubMed] [Google Scholar]
- Masson GS, Busettini C, Yang DS, Miles FA. Short-latency ocular following in humans: sensitivity to binocular disparity. Vision Research. 2001;41:3371–3387. doi: 10.1016/s0042-6989(01)00029-3. [DOI] [PubMed] [Google Scholar]
- Masson GS, Rybarzyck Y, Castet E, Mestre DR. Temporal dynamics of motion integration for the initiation of tracking eye movements at ultra-short latencies. Visual Neuroscience. 2000;17:753–767. doi: 10.1017/s0952523800175091. [DOI] [PubMed] [Google Scholar]
- Miles FA. The neural processing of 3-D visual information: Evidence from eye movements. European Journal of Neuroscience. 1998;10:811–822. doi: 10.1046/j.1460-9568.1998.00112.x. [DOI] [PubMed] [Google Scholar]
- Miles FA, Kawano K, Optican LM. Short-latency ocular following responses of monkey. I. Dependence on temporospatial properties of the visual input. Journal of Neurophysiology. 1986;56:1321–1354. doi: 10.1152/jn.1986.56.5.1321. [DOI] [PubMed] [Google Scholar]
- Nishida S. Spatiotemporal properties of motion perception for random-check contrast modulations. Vision Research. 1993;33:633–645. doi: 10.1016/0042-6989(93)90184-x. [DOI] [PubMed] [Google Scholar]
- Ohzawa I, DeAngelis GC, Freeman RD. Stereoscopic depth discrimination in the visual cortex: neurons ideally suited as disparity detectors. Science. 1990;249:1037–1041. doi: 10.1126/science.2396096. [DOI] [PubMed] [Google Scholar]
- Qian N. Computing stereo disparity and motion with known binocular properties. Neural Computation. 1994;6:390–404. [Google Scholar]
- Sato T. Reversed apparent motion with random dot patterns. Vision Research. 1989;29:1749–1758. doi: 10.1016/0042-6989(89)90157-0. [DOI] [PubMed] [Google Scholar]
- Smith AT, Ledgeway T. Separate detection of moving luminance and contrast modulations: fact or artifact? Vision Research. 1997;37:45–62. doi: 10.1016/s0042-6989(96)00147-2. [DOI] [PubMed] [Google Scholar]
- Smith AT, Ledgeway T. Motion detection in human vision: a unifying approach based on energy and features. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2001;268:1889–1899. doi: 10.1098/rspb.2001.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon JA, Sperling G. 1st- and 2nd-order motion and texture resolution in central and peripheral vision. Vision Research. 1995;35:59–64. doi: 10.1016/0042-6989(94)e0077-x. [DOI] [PubMed] [Google Scholar]
- Spillmann L, Anstis S, Kurtenbach A, Howard I. Reversed visual motion and self-sustaining eye oscillations. Perception. 1997;26:823–830. doi: 10.1068/p260823. [DOI] [PubMed] [Google Scholar]
- Takemura A, Inoue Y, Kawano K. The role of MST neurons in short-latency visual tracking eye movements. Society for Neuroscience Abstracts. 2000;26:1715. [Google Scholar]
- Takemura A, Inoue Y, Kawano K, Quaia C, Miles FA. Evidence that disparity-sensitive cells in medial superior temporal area contribute to short-latency vergence eye movements. Society for Neuroscience Abstracts. 1999;25:1400. [Google Scholar]
- Takemura A, Inoue Y, Kawano K, Quaia C, Miles FA. Single-unit activity in cortical area mst associated with disparity-vergence eye movements: evidence for population coding. Journal of Neurophysiology. 2001;85(5):2245–2266. doi: 10.1152/jn.2001.85.5.2245. [DOI] [PubMed] [Google Scholar]
- Wertheimer M. Experimentelle Studien über das Sehen von Bewegung. Zeitschrift Psychologische. 1912:161–265. [Google Scholar]