Abstract
Changes in the organization of the musculoskeletal system have accounted for many evolutionary adaptations in the vertebrate body plan. The musculoskeletal system develops from two mesodermal populations: somitic mesoderm gives rise to the axial skeleton and all of the skeletal muscle of the body, and lateral plate mesoderm gives rise to the appendicular skeleton. The recognition of embryonic domains resulting from the dynamics of morphogenesis has inspired new terminology based on developmental criteria. Two mesodermal domains are defined, primaxial and abaxial. The primaxial domain includes musculoskeletal structures comprising just somitic cells. The abaxial domain contains somitic myoblasts in connective tissue derived from lateral plate mesoderm, as well as lateral plate-derived skeletal structures. The boundary between these two domains is the lateral somitic frontier. Recent studies have described the developmental relationship between these two domains in the chick. In the present study, we describe the labelling pattern in the body of the Prx1/Cre/Z/AP compound transgenic mouse. The enhancer employed in this transgenic leads to reporter expression in the postcranial, somatic lateral plate mesoderm. The boundary between labelled and unlabelled cell populations is described at embryonic day (E)13.5 and E15.5. We argue that the distribution of labelled cells is consistent with the somatic lateral plate lineage, and therefore provides an estimate of the position of the lateral somitic frontier. The role of the frontier in both development and evolution is discussed.
Keywords: abaxial, embryonic patterning, frontier, lateral plate, mesoderm, mouse embryo, primaxial, somites
Introduction
Developmental biology is steadily increasing our understanding of the molecular genetic players that contribute to the successful development of the embryo. In numerous instances, details of genetic regulatory networks are mapped out from master regulator genes right down to a particular kinase. The outcome of these pathways is largely context dependent, allowing the same ‘circuits’ to be used at multiple times and locations to different morphogenetic ends (Davidson & Erwin, 2006). Because the contextual environment is the embryo itself, clear knowledge of how cell populations interact and behave is essential to understanding the implications of gene expression during development.
Here we describe the pattern of transgene labelling throughout the body in the Prx1Cre/Z/AP mouse. Transgene activation in this mouse results in two distinct populations in the body wall defined by a clear boundary between labelled and unlabelled cells. This construct provides a unique opportunity to visualize the dynamics of mesodermal populations over developmental time, providing a view of the environment of tissue interaction during morphogenesis.
The musculoskeletal system of vertebrates allows for an astonishing complexity of movement, and has played a crucial role in their evolutionary success. The axial system consists of vertebrae, vertebral ribs and associated muscles; the appendicular system consists of muscles and bones of the limbs and their supporting girdles. The origin of paired appendages was a key innovation at the base of all gnathostomes, and the subsequent evolution of the limbs in tetrapods facilitated the colonization of terrestrial habitats. The evolutionary diversification of paired appendages required integration between the primitive axial and the derived, appendicular musculoskeletal systems.
Two populations of embryonic mesoderm contribute to the vertebrate musculoskeletal system: the somitic mesoderm, and the lateral plate (LP) mesoderm (reviewed in Winslow et al. 2007). The axial system is composed entirely of somitic mesoderm. The appendicular and ventrolateral body wall muscles also originate from the somitic mesoderm, but they differentiate in the context of connective tissue and bones formed by the LP mesoderm. During development, somitic myoblasts migrate into the LP tissue and form all of the musculature of the limbs and the ventral body wall (reviewed in Christ et al. 2007; Bothe et al. 2007).
Extensive fate mapping studies, primarily in chick, have generated a detailed picture of how somite cells migrate, mature and finally differentiate (reviewed in Christ et al. 2007; Bothe et al. 2007). The somite begins as an epithelial ball of cells. Subsequently, cells from the ventromedial half of the somite de-epithelialize and form sclerotome, which gives rise to the vertebrae and dorsal parts of the ribs. The remaining epithelial sheet, called the dermomyotome, gives rise to dermis of the dorsal body wall and all skeletal muscle in the body. The dorsomedial lip of the dermomyotome is referred to as the epaxial dermomyotome, as cells from this site are fated to become the epaxial, axial muscles (Ordahl & Le Douarin, 1992; Christ & Ordahl, 1995). The ventrolateral lip is referred to as the hypaxial dermomyotome, as cells from this region are fated to become hypaxial muscles of the limbs and ventral body wall. In certain cases somitic cells in the hypaxial somite population contribute to cartilage rather than muscle, for instance in the avian scapula and sternal ribs (Fell, 1939; Chevallier et al. 1977; Nowicki et al. 2003).
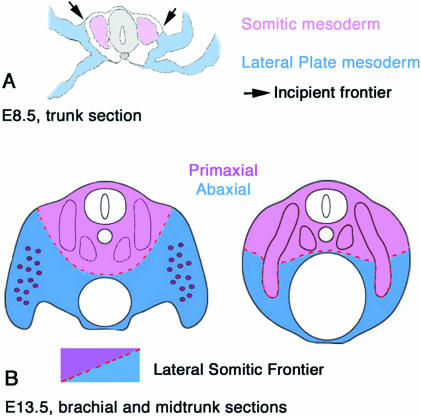
The lateral somitic frontier defines primaxial and abaxial domains
The interface between somitic cell populations and cells of the LP mesoderm was first mapped in the chick using quail–chick chimeras (Nowicki et al. 2003). The recognition of embryonic boundaries resulting from the processes of morphogenesis has necessitated new terminology based on developmental criteria (Fig. 1). All skeletal muscle comes from the somites, but, as noted above, somitic myoblasts differentiate in two distinct environments. Some myoblasts differentiate in an environment composed completely of somitic cells. These myoblasts form the muscles most closely associated with the vertebrae. We have defined the region made up entirely of somitic cells as the ‘primaxial’ domain, which includes but is not limited to the epaxial muscles. At trunk levels, the thoracic body wall is formed as the ventrolateral lip of the dermomyotome expands and displaces the LP to a more ventral and lateral position. The somite cells that contribute to the vertebral ribs and most of the intercostal muscles remain within a wholly somitic environment and do not mix with mesenchyme of the LP. Thus, the hypaxial intercostal muscles are primaxial. At other levels along the anterior–posterior (A–P) axis, most notably at limb levels, the myoblasts behave very differently. Myoblasts at the ventrolateral lip of the dermomyotome delaminate from the epithelium and migrate as a mesenchyme into the limb bud to form intrinsic limb muscles (migrating muscle precursors, reviewed by Dietrich, 1999). The limb bud itself is formed from the LP mesoderm. The migrating muscle precursors will differentiate in an environment of LP connective tissue. The region composed of LP connective tissue and myoblasts from the somites is defined as the ‘abaxial’ domain. The border between these two domains is the ‘lateral somitic frontier’ (LSF) (Fig. 1).
Fig. 1.
Schematic illustrations of the vertebrate mesoderm and the definitions and dispositions of the primaxial and abaxial domains separated by the lateral somitic frontier. (A) Cross-section of the midtrunk region of an embryonic day 9 mouse embryo. The paraxial somites (so) are pink, somatic LP mesoderm is blue (lp). For simplicity the intermediate mesoderm is not defined. (B) Cross-section at approximately embryonic day 11. Primaxial domain is pink, abaxial domain is blue, and includes somitic myoblasts that are illustrated in purple.
Primaxial and abaxial terminology is not intended to replace the terms epaxial and hypaxial. As mentioned above, the designations of epaxial and hypaxial for the respective parts of the dermomyotome are consistent with the existing fate maps of the respective muscle groups. However, the classical terms are based on the adult innervation of muscles by either the dorsal (epaxial) or the ventral (hypaxial) rami of the spinal nerves, and do not describe the dynamics of cell behaviours during development. The intercostal and ventral vertebral muscles, for instance, are hypaxial by innervation, but group with the epaxial muscles with respect to the developmental environment (primaxial) in which they form. The key distinction and defining criteria between abaxial and primaxial domains and structures is the lineage of the investing connective tissue: primaxial connective tissue arises from somite cells; abaxial connective tissue arises from cells of the LP mesoderm.
Mapping the LSF in the avian system using quail–chick chimeras is limited by the size of isotopic grafts. Furthermore, the current absence of any permanent LP lineage markers makes visualizing this boundary outside of the experimental context impossible.
The Prx1Cre mouse utilizes an enhancer that appears to be restricted to the postcranial somatic LP mesoderm (see below). We provide a description of the boundary between labelled and unlabelled cell populations revealed by the Prx1/Cre-mediated reporter at stages where body muscles have become defined and are readily identified [embryonic day (E)13.5 and E15.5]. We argue that the pattern of reporter gene labelling can be used as a proxy for the LSF. This interpretation seeks to illuminate the embryonic associations between mesodermal cell populations and suggests an embryonic context for studies of gene regulation and the interpretation of both experimental and spontaneous mutant phenotypes in mammals.
Materials and methods
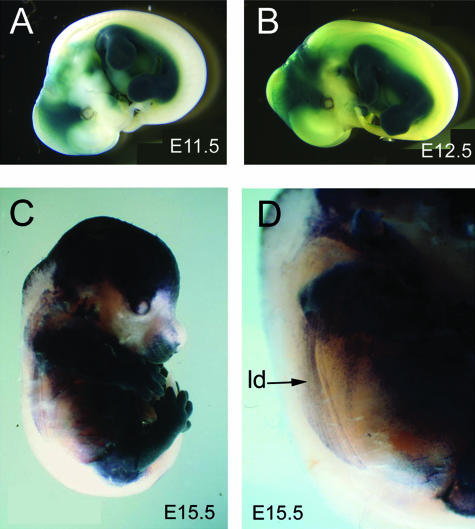
Two lines of transgenic mice were used in our investigation: Prx1Cre and Z/AP. The Prx1-Cre transgenic contains regulatory sequences of the prx1 gene that drive Cre recombinase expression initially in the limb mesenchyme and subsequently in the LP of the trunk (Martin & Olson, 2000; Logan et al. 2002). The Z/AP Cre+/– reporter line contains a floxed beta-gal (LacZ) and polyA signal cassette upstream of sequences coding for human placental alkaline phosphatase (AP) (Lobe et al. 1999). In embryos that carry both transgenes, AP activity will be present in all cells where Cre has been expressed and has catalysed a recombination event removing the β-gal/polyA cassette (Logan et al. 2002). This also occurs in some cell populations in the head and anterior cervical region; therefore, we limit our discussion to more caudal regions where we believe the LP is labelled exclusively (Fig. 2).
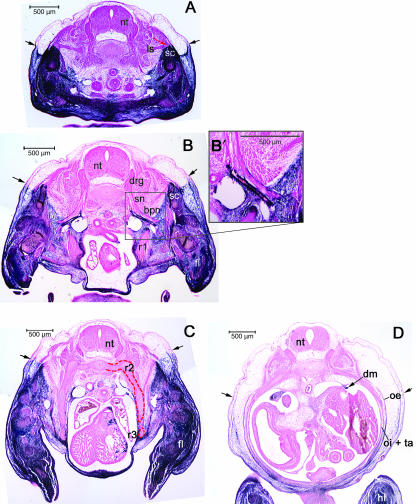
Fig. 2.
Lateral view of whole mount Prx1Cre:Z/AP embryos stained for alkaline phosphatase (A–E). Labelled domain is blue/black. Unlabelled domain is white/yellow. (A) E10.5, (B) E11.5, (C) E12.5, (D) E15.5, (E) magnified view of E15.5. Note the labelled streaks in (E) are labelled connective tissue investing the unlabelled muscle cells in the latissimus dorsi (ld).
Prx1Cre males were crossed with Z/AP females in timed breedings to generate double transgenics. The date of fertilization was determined by the presence of a vaginal plug. The first morning a plug was seen was designated day 0.5, and embryo stages were counted from this point. Embryos were collected at E11.5–E15.5 and staged according to Kaufman (1992). After fixation in 4% paraformaldehyde (PFA) embryos were dehydrated into MeOH and stored at –20 °C. Whole mount AP staining included heat inactivation of endogenous AP by 30–60 min of incubation in phosphate buffered saline with 0.1% Tween (PBT) at 70 °C. Transgenic AP was visualized by incubating in NTMT (100 mm NaCl, 100 mm Tris-HCl, pH 9.5, 50 mm MgCl2, 0.1% Tween) containing 250 µg mL−1 Nitro BT (NBT) and 130 µg/mL 5-bromo-4-chloro-3-indolyl phosphate (BCIP). Embryos were then postfixed in 4% PFA and stored at 4 °C in PBT. To generate histological sections, embryos fixed in 4% PFA were embedded in paraplast and sectioned at 12 µm. The slides were stained using the same reagents listed above, counter stained in eosin and cover slipped with Permount.
Results
This study focuses on the development of the postcranial musculoskeletal system. Bones, muscles and nerves of different body regions were examined with respect to the boundary between AP-labelled and unlabelled tissues (referred to below as the ‘label boundary’).
We begin with a general description of the label boundary in the E15.5 embryo followed by details of the brachial, thoracic, abdominal/lumbar and pelvic regions. Within each region, the relationship of muscles, bones and nerves to the label boundary is described. Anatomical elements are described as unlabelled, labelled or in several cases a combination of the two. An element or portion of an element is considered labelled if its investing connective tissue is labelled by AP. This includes unlabelled chondrocytes invested in labelled periosteum.
After describing the position of the label boundary relative to well-delineated anatomical elements at E15.5, we describe specific regions and elements in an E13.5 embryo. Although anatomical resolution is more difficult at E13.5, these data in combination with the later stage reveal the active spatiotemporal nature of the label boundary as the embryo develops.
General description: day 15.5
In whole mount preparations, the position of the label boundary is visible superficially, although its topography at deeper levels within the body is obscured (Fig. 2). The dorsum of the body and the tail are the only unlabelled regions visible in whole mounts. The limbs and ventrolateral body wall are labelled. As mentioned above, the head and anterior cervical region shows AP labelling of multiple tissues that are clearly not associated with the trunk LP. Because of the potential for ambiguity in the cervical region, we have limited our observations to the brachial region and posterior.
Superficially, the label boundary runs laterally along the trunk of the embryo at a fairly consistent dorsoventral level (Fig. 2). Portions of superficial body wall muscles are visible through the skin. The labelled streaks in Fig. 2D represent connective tissue investing the unlabelled muscle cells in the latissimus dorsi.
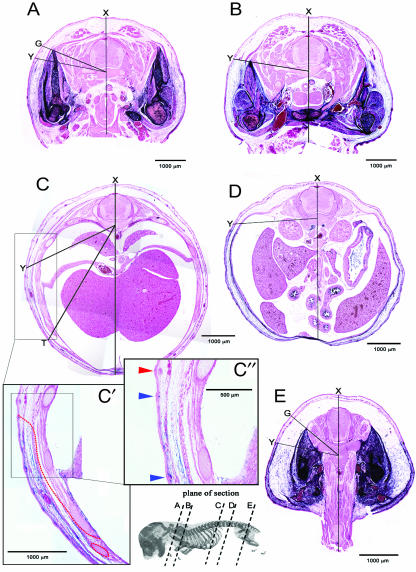
The label boundary in sectioned material is obvious between the exclusively eosin-stained tissue (pink) and the tissue where AP staining overlaps with eosin stain (Fig. 3). The point at which the label boundary contacts the surface ectoderm (point ‘Y’Fig. 3), approximates the original boundary between LP and somitic mesoderm before somite dispersal (Fig. 1A). The profile of the label boundary in transverse section varies considerably along the A–P axis. Figure 3 illustrates this variation using lines to show the extent of angular displacement from the saggital midline (marked dorsally by point ‘X’). No attempt has been made to quantify these angles, as they are superimposed on different planes of section due to the natural curvature of the A–P axis. However, they are internally consistent in individual sections, and demonstrate the variable degree of topography generated at different A–P levels. At limb levels a substantial portion of cross-sectional area is labelled, and at thoracic and lumbar regions, the unlabelled area dominates and the labelled tissues are compressed in the body wall.
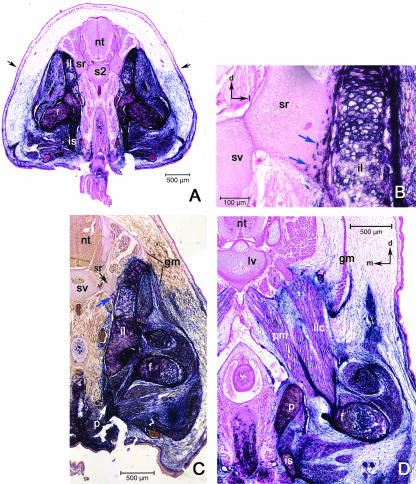
Fig. 3.
A series of transverse sections along the A–P axis of E15.5 Prx1cre:Z/AP embryos. Lines are used to illustrate differences in the expansion of the body wall and variation in the cross-sectional profile of the label boundary. Note the variation in topography at different levels. ‘X’ marks the dorsal point of the saggital midline. ‘Y’ marks the superficial label boundary, which approximates to the original boundary between LP and somitic mesoderm. Angle ‘XY’ marks the extent of angular displacement of the label boundary from the saggital midline. In (A,E) the labelled domain is expanded around the limb girdles (points ‘G’) dorsal to the superficial label boundary. (A) Brachial level; the dorsalmost extent of the labelled domain in the pectoral girdle is marked by point ‘G’. (B) Brachial-to-thoracic transition. (C) Thoracic level; the unlabelled thoracic wall comprising ribs and intercostal muscles has displaced the frontier to point ‘T’. (C′) Higher magnifications of the flank region in (C) with the label boundary marked with a red dashed line. (C″) Further magnification of the area of the boundary. Note the three hair follicles, two developing in the labelled domain (blue arrowhead) and one developing in the unlabelled domain (red arrowhead). (D) Lumbar region. (E) Sacral level; the dorsalmost extent of the labelled domain in the pelvic girdle is marked by point ‘G’.
In the thoracic region, the labelled domain is further enlarged ventrally as the clavicle meets the anterior sternum. The label boundary outlines the scapular complex and crosses the midline deep to the sternum (Fig. 3B). Moving posteriorly, the ribcage expands and the labelled zone is gradually reduced to a thin layer between the surface ectoderm and the expanding rib cage (Fig. 3C). Although the distal intercostal muscles are labelled, the ribs themselves are not and as a result the label boundary zigzags along the ventral midline of the thorax (see below).
In the lumbar region the unlabelled domain becomes a thin band due to a reduction in space between surface ectoderm and coelom. The label boundary lies at the interface between the abdominal wall and the coelomic space (Fig. 3D).
In the sacral region, the presence of the posterior limb enlarges the labelled domain, similar to the situation in the brachial region (Fig. 3E). The labelled domain makes up more than half of the cross-sectional area in the sacral region. The label boundary approximates a straight line inward from the surface until it meets the pelvic girdle. From this point to the midline, the deep (dorsomedial) border of the pelvic girdle forms the label boundary (see Fig. 7A). Posterior to the sacral region, the tail is completely unlabelled and lacks a boundary.
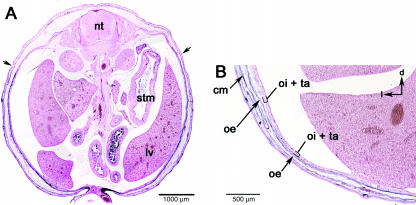
Fig. 7.
Sections at the lumbar level. (A) The LSF has a simple profile from its superfical postion (black arrows) directly through the body wall to the peritoneum. (B) Higher magnification of the abdominal wall showing the labelled connective tissue associated with the three body wall muscles: obliquus externus (oe), obliquus internus (oi) and transversus abdominis (ta). Black arrows in upper right indicate dorsal (d) and lateral (l).
Regional descriptions at day 15.5: brachial region
The pectoral girdle dominates the brachial region, and serves to translate muscular force from the axial skeleton to the appendage, and vice versa. The scapula is the major element in the pectoral girdle of mammals and includes an acromial process for the articulation of the clavicle, and the glenoid fossa for the head of the humerus. The scapula is labelled except for a portion of the vertebral border of the scapular blade, which is unlabelled (Fig. 4B,C). The vertebral border is the attachment site for the levator scapulae, serratus anterior and rhomboideus major muscles (Fig. 4B,C). All of these muscles arise from axial elements, either the neural arches of the vertebrae or the superficial surface of the ribs. The levator scapulae and rhomboideus major are entirely unlabelled. The serratus anterior may include some AP staining (Fig. 4C), but it is not clear whether this labelling is cellular or not.
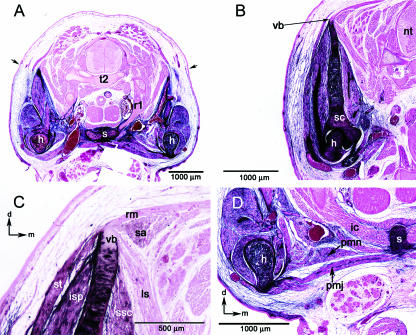
Fig. 4.
General views of the brachial level. (A) Section through the 2nd thoracic vertebra (t2); black arrows indicate the superficial label boundary. (B) Section through the blade of the scapula (sc) and vertebral border (vb). (C) Higher magnification view of the vertebral border of the scapula. Note the unlabelled muscles rhomboideus major (rm), levator scapulae (ls), and seratus anterior (sa) attaching to the unlabelled vertebral border. (D) View of sternum (s), intercostals (ic) and the pectoral muscles; pectoralis major (pmj), pectoralis minor (pmn). Black arrows left upper and lower corners orientate dorsal (d) and medial (m). Abbreviations: (t2) second thoracic vertebrae; (nt) neural tube; (h) humerus; (r1) first rib; (s) sternum; (vb) vertebral border; (st) spinotrapezius; (isp) infraspinatus; (ssc); subscapularis; (ic) intercostal muscles.
The scapula is also associated with a number of muscles that exert force between the scapula and the shoulder joint/proximal limb. These muscles are all labelled, and form a substantial portion of the brachial region. They include: the infraspinatus, subscapularis and supraspinatus, which all take their origin from the scapula, and attach to the head of the humerus (Fig. 4C). The pectoralis major and pectoralis minor muscles arise from the sternebrae and manubrium of the sternum and insert on the proximal humerus. The pectoralis major and minor are completely labelled (Fig. 4D).
The dorsolateral superficial muscles of the brachial/thoracic region are unique in that they have both labelled and unlabelled portions. Most superficially, the cutaneous maximus muscle takes its origin on the deltoid ridge of the humerus and inserts on the subcutaneous fascia of the dorsal trunk (Fig. 5B). At its origin this muscle is a thick cord that then thins out into a broad sheet of fibres encompassing almost the entire trunk. The cutaneous maximus is labelled at its origin, and unlabelled at its insertion on the skin. The position of the label boundary within this muscle is continuous with the label boundary in the surrounding mesenchyme (Fig. 5B).
Fig. 5.
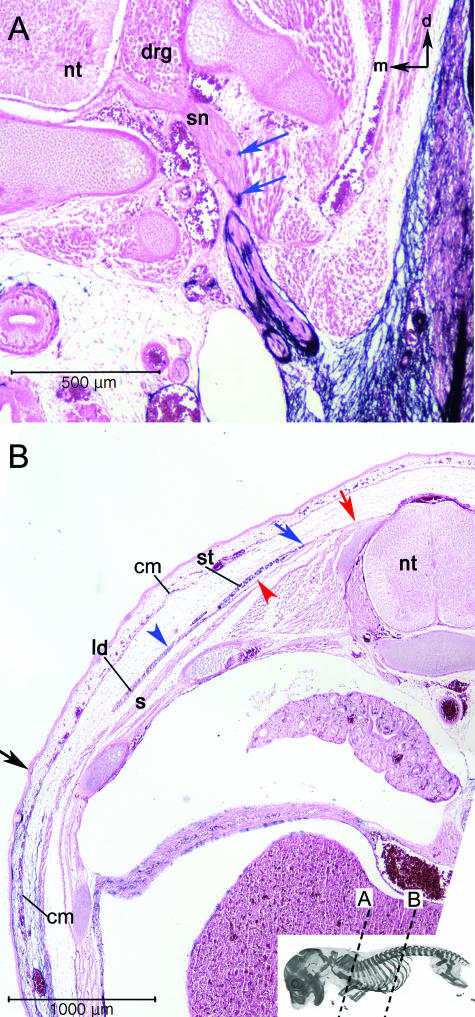
Extreme discontinuities in the label boundary. (A) AP-labelled cells (blue arrows) are associated with spinal nerves (sn) of the brachial plexus close to the point where they emerge from the vertebral column. This is more proximal than the rest of the label boundary. Black arrows in upper right orientate dorsal (d) and medial (m). (B) The dorsolateral superficial muscles of the brachial/thoracic region also cause discontinuities in the label boundary. The cutaneous maxiums (cm) is unlabelled at its insertion on the subcutaneous fascia of the dorsal trunk although it is labelled at its origin on the humerus. The frontier in the cm is continuous with the label boundary in the surrounding mesenchyme (black arrow = superficial label boundary). The blue arrowhead and arrow indicate the labelled region the spinotrapezius (st) and the latissimus dorsi (ld), respectively. These muscles are unlabelled at their origins on the vertebrae and thoracolumbar fascia (red arrow and arrowhead) and labelled at their insertion on the scapula and humerus, respectively. The label boundary in these mucles is far removed from the major par of the boundary, which angles ventrally through the body wall from its superficial boundary (black arrow). The red arrowhead indicates the unlabelled region of the muscle. The underlying serratus dorsalis (s) contains no blue label. Abbreviations: neural tube (nt); spinal nerves (sn); spinotrapezius (st); latissimus dorsi (ld); serratus dorsalis (s).
Lying deep to the cutaneous maximus is the spinotrapezius muscle, which takes its origin on the thoracic and lumbar vertebrae and fascia and inserts on the spine of the scapula. The spinotrapezius serves to retract the anterior appendage relative to the axial skeleton. All of the myofibres of the muscle are invested in labelled connective tissue; only the tendinous fascia near its origin is unlabelled (Fig. 5B). In contrast to the cutaneous maximus, the position of the label boundary in the spinotrapezius does not match the label boundary in surrounding tissue. The most proximal labelled portion of the spinotrapezius is largely surrounded by unlabelled tissue.
Deep to the spinotrapezius lies the latissimus dorsi, a broad, sheet-like muscle that takes an extensive origin on the posterior thoracic vertebrae and anterior lumbar fascia and inserts via a tendon on the medial shaft of the humerus. No AP staining is seen in the tendinous connective tissue at the origin, but appears distally around the muscle fibres. The label boundary traverses the latissimus muscle high on the side of the body, and so is discontinuous with the label boundary in the surrounding tissue as seen with the spinotrapezius (Fig. 5B).
The brachial plexus includes the ventral rami of the last four cervical spinal nerves and some portion of the first two thoracic spinal nerves. After leaving the dorsal root ganglion (DRG), these nerves come together into a plexus near the axila before dividing again to innervate muscles of the brachial region. All spinal nerves, including those of the brachial plexus, are unlabelled at their origin where the investing connective tissue (the peri- and epineurium) is somitic. Brachial plexus nerves cross into labelled mesenchyme shortly after they emerge from the DRG (Fig. 5A). Like the latissimus dorsi and the spinotrapezius muscles, the position of the label boundary within the perineurium of the brachial plexus nerves does not match that of surrounding tissue. The proximal, labelled portions of plexus nerves are surrounded by unlabelled tissue.
Thoracic region day 15.5
Subjacent to the ectoderm in the thoracic region, the label boundary dives at a steep angle ventromedially through the mesenchyme where it is likely to represent the cryptic boundary between somitic and LP-derived dermis. When it reaches the external surface of the ribcage, the label boundary follows the ribs and intercostal muscles ventrally. This implies a differential expansion of the unlabelled domain in the thoracic region, which displaces the label boundary ventrally. The longitudinal path of the label boundary at the ventral midline is jagged because it traverses the intercostal muscles before they reach the sternum, at approximately 80% of their proximal–distal tragectory, but follows the ribs all the way to their union with the sternum (Figs 1 and 6B, see below). In the abdominal/lumbar region both the superficial aspect of the label boundary and its deep contact with the peritoneum are high along the body wall where the angle XY approaches 90°. This angle is most acute in pectoral and pelvic regions (Fig. 3A,E), and most obtuse in the thoracic region (Fig. 3C).
Fig. 6.
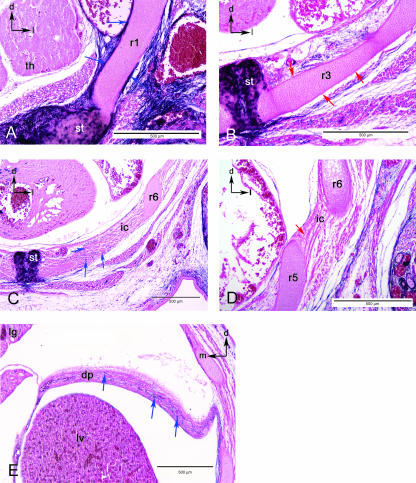
Sections in the thoracic region. (A) The chondrocytes of the first rib (r1) are somite derived, yet the periosteum is labelled (blue arrows) as is the associated connective tissue and the sternum (st). Black arrows in upper left indicate dorsal (d) and lateral (l). (B) View of the third rib (r3), where the periosteum is unlabelled (red arrows) although the surrounding connective tissue is labelled blue. Black arrows in upper left indicate dorsal (d) and lateral (l). (C) View of the intercostals muscle (ic) at the level of the sixth rib (r6). Distal portion of the intercostal muscles are associated with blue-labelled cells (blue arrows). (D) Proximal portion of the intercostal muscles (ic) do not include blue cells (red arrow). (E). Section through the diaphram (d) showing the inclusion of blue-labelled cells (blue arrows). Black arrows in upper corners orientate dorsal (d), lateral (l) or medial (m). Abbreviations: intercostals muscle (ic); liver (lv); lung (lg); thymic rudiment (th).
In mice, there are seven ‘true’ ribs connected to the sternum, and six ‘false’ ribs that do not reach the sternum. Ribs 2–13 are unlabelled for their entire length, from their proximal articulation with the vertebrae to their connection to the sternum or free distal end in the body wall. There is a clean boundary between the unlabelled rib chondrocytes and those of the sternum (Fig. 6B). The periosteum of these ribs is unlabelled throughout their length. Rib 1 is unusual in that the periosteum of the anterior, distal portion of the rib cartilage is labelled (Fig. 6A). Although the cartilage cells are unlabelled, the investing tissue of the first rib is labelled.
The intercostal muscles arise along the length of all the ribs, attaching adjacent ribs to one another. The dorsal four fifths of the intercostal muscles is unlabelled (Fig. 6D). However, the distal end of each intercostal muscle (i.e. the portion spanning the distal ends of adjacent ribs) is labelled (Fig. 6C,D). The absolute size of the labelled domain in each intercostal muscle is relatively consistent and independent of the size of the entire muscle. As false ribs do not reach the sternum, their associated intercostal muscles do not extend as far ventrally as those between true ribs, and do not have an unlabelled domain.
The diaphragm attaches ventrally to the last true rib, the false ribs, the posterior sternum and dorsally to the roof of the coelom ventral to the vertebrae. The diaphragm appears to be labelled throughout (Fig. 6E).
Abdominal/lumbar region
There are three broad, sheet-like muscle layers in the abdominal wall. The obliquus externus abdominis is most superficial and by far the largest abdominal muscle, and changes character significantly over its length. It originates on ribs 4–12 and the lumbar fascia. It inserts on the crest of the ilium, the pubis and the fibrous linea alba at the ventral midline where it merges with the rectus abdominis muscle. At its origin the external oblique is superficial to the ribcage and unlabelled (Fig. 7A). The remaining bulk of the muscle is labelled. As the ribcage recedes in the lumbar region, the external oblique gradually merges with the two deeper abdominal muscles (Fig. 7B).
The obliquus internus abdominis and the transversus abdominis lie beneath the external oblique in the lumbar region. Technically, the internal oblique is more superficial, but it is nearly indistinguishable from the transversus and the muscles are described together. They have an extensive posterior origin on the dorso-lumbar fascia, the crest of the ilium and the inguinal (Poupart's) ligament. Both muscles insert on the false ribs anteriorly and the linea alba medially. These muscles are extremely thin and sandwiched between the heavily labelled external oblique and parietal lining of the coelom (Fig. 7A,B). They are labelled except for their insertions on the ribs.
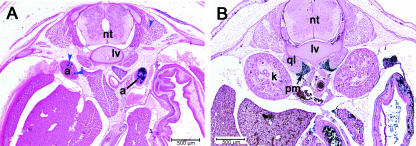
Sections through the anterior lumbar region show the paired adrenal glands (Fig. 8A). They show a strong degree of asymmetrical labelling. Asymmetry of the adrenal glands has been observed in a number of mammals; for instance, there are marked differences in mass and relative proportions of cortex and medulla between left and right adrenals (Trut et al. 2002; Aliab’ev & Paderov, 2004). Supraspinal innervation is also asymmetric, with a predominance on the left side in rodents (Gerendai et al. 2007). We consistently see more labelling in the left adrenal gland. The crus of the diaphragm are visible between the glands at the midline. The plane of section is close to transverse through the muscle fibres and as a result blue labelling is sparse, although present. We do not see any label in the kidneys (Fig. 8B) or the gonads, although there is clear labelling of the müllerian duct at E13.5 (data not shown).
Fig. 8.
Labelled ‘islands’. (A) The adrenal glands (a) show highly asymmetric inclusion of AP-positive cells (blue arrowheads). Labelling is consistently heavier in the left adrenal. (B) View of the deep muscles of the lumbar level. The psoas major (pm) is densely populated by blue-labelled cells close to its origin. The quadratus lumborum (ql) does not include AP-positive cells. Abbreviations: kidney (k); neural tube (nt); stomach (stm); liver (lv); cutaneous muscle (cm); lumbar vertebrae (lv).
The quadratus lumborum originates on the last rib and on the lumbar vertebrate and runs posteriorly to insert on the iliolumbar ligament and iliac crest. The majority of this muscle as it runs along the posterior axis is unlabelled (Fig. 8A,B). The insertion site is diffuse in cross-section at E15.5, although all connective tissue around the iliac crest is labelled.
The psoas major originates on the centrum and transverse processes of all the lumbar vertebrae, more posterior than the quadratus (Fig. 8B). It runs caudally along the dorsal body wall from the diaphragm through the pelvic space, joins with the iliacus and inserts near the head of the femur (Fig. 9D). This muscle is labelled for its entire length, but is largely surrounded by unlabelled structures, including the quadratus. Nerve roots of the lumbar plexus run within the muscle (see Discussion).
Fig. 9.
General view of the sacral level. (A) The bulk of the pelvic girdle appears to dorsally expand the labelled domain above the superficial label boundary (black arrows). (B) Higher magnification of a sacral rib (sr). AP-positive cells, apparently chondroblasts (blue arrows), are included in the cartilage at the distal end of the sacral rib where it meets the ilium (il). (C) The bones of the pelvis are labelled; the gluteus maximus muscle (gm) is associated with labelled connective tissue as it inserts on the proximal femur and at its origin on the ilium, although it is associated with unlabelled connective tissue at its origin from the lumbar fascia. (D) Insertion of the psoas major (pm) and iliacus (ilc) onto the lesser trochanter of the femur (f). Black arrows in upper right corner orientates dorsal (d) and medial (m). Abbreviations: lumbar vertebrae (lv); neural tube (nt); sacral vertebrae (sv); sacral rib (sr); ilium (il); femur (f); ischium (is); psoas major (pm); iliacus (ilc).
Pelvic region
The pelvis connects the vertebral column with the posterior appendage via the sacral ribs. The pelvis is tripartite, comprising the ilium, ischium and pubic bones. The chondrocytes making up these endochondral elements are derived from the LP, and they make up the bulk of the labelled domain in the pelvic region (Fig. 9A,C).
The sacral ribs are lateral extensions of the sacral vertebrae that directly articulate with the ilium of the pelvis. They are unlabelled except for a scattered band of cells along the lateral border that articulates with the ilium. The cells appear to be cartilaginous (Fig. 9B). Along with the 1st rib and the scapula, the sacral ribs are the only bones that straddle the label boundary.
The gluteus maximus is unlabelled near its origin on the last three lumbar and first sacral vertebrae, but its origin on the ilium is labelled. The bulk of the muscle is labelled and it inserts on the proximal femur (Fig. 9C). The label boundary in the gluteus maximus is discontinuous with the label boundary in the surrounding tissue anteriorly, but continuous with the boundary posteriorly.
General description: day 13.5
The label boundary in the E13.5 embryo is less irregular than that at E15.5. The labelled domain makes up more of the total cross-sectional area relative to the unlabelled domain at E13.5 than at E15.5. Much of the vertebral border of the scapula is unlabelled at E13.5 (Fig. 10A). The vertebral border of the scapula is approximately even with the superficial label boundary at E13.5 (Fig. 10A,B), while at E15.5 the vertebral border is dorsal to the superficial label boundary (compare with Fig. 4). Medial to the scapula, the label boundary runs ventromedially at an approximately 45° angle to the midline. Between the medial edge of the scapular complex and the label boundary is a large portion of labelled mesenchyme (Fig. 10). This mesenchyme has a much greater volume at E13.5 than at E15.5, when the label boundary is at the edge of the scapular complex (compare with Fig. 4).
Fig. 10.
The label boundary in E13.5 mice. (A) At prechondrogenic stages the scapula (sc) has both labelled and unlabelled (red arrow) domains. The unlabelled levator scapulae (ls) can be seen inserting upon the vertebral border (red arrow). The labelled domain rises above the superficial label boundary (black arrows). (B) At deeper brachial levels, the distal tip of the pre-cartilage condensation of the first rib (r1) is completely surrounded by AP-stained mesenchyme. (B′) Higher magnification view of the subclavian artery (sbc) and the nerves of the brachial plexus (bp) that cause a partial discontinuitity in the label boundary while entering the brachial region. (C) Thoracic level on E13.5 showing the unlabelled expansion containing the second and third ribs (r2 and r3, red outline) with labelled intercostals muscle between them (blue arrow) ventrally displacing the label boundary from its superficial location (black arrows). (D) At the lumbar level the obliquus externus (oe), obliquus internus (oi) and transversus abdominis (ta) show staining that increases in intensity in the dorsal-to-ventral direction. The dorsal attachment of the diaphram (dm) to the coelom wall includes many blue cells. Abbreviations: hindlimb (hl); scapula (sc); neural tube (nt); dorsal root ganglion (drg); spinal nerve (sn); forelimb (fl).
Brachial plexus nerves entering the brachial region already cause discontinuities in the label boundary at this stage (Fig. 10B,B′), similar to the situation seen at E15.5 (compare with Fig. 4A). Very little discontinuity, however, is present in the spinotrapezius at E13.5. When the position of the label boundary in the spinotrapezius is measured against the superficial label boundary, the two are clearly closer at E13.5 than at E15.5. This is also true of the latissimus and the cutaneous maximus. Overall, the label boundary in the brachial region appears far more uniform at E13.5 than at E15.5.
In the thoracic region, the ribcage has not joined the sternum in the E13.5 embryo. Large unlabelled buds extend ventrally into the labelled domain, ‘deflecting’ the label boundary ventrally around them. Within these unlabelled extensions, the developing ribs and intercostal muscles are clearly distinguishable (Fig. 10C). The anterodistal portion of rib 1 is completely surrounded by labelled mesenchyme at E13.5 (Fig. 10B). Note that at E15.5, this portion of rib 1 is the only labelled part of the thoracic skeleton.
The labelled domain in the lumbar region forms a thick band around the ventrolateral edge of the body at E13.5 (Fig. 10D). The three layers of abdominal muscle are clearly defined. Superficially, the external oblique is labelled in all but its most dorsal portion. Within the abdominal musculature as a whole, there appears to be a gradient of staining increasing from dorsal-to-ventral and from deep-to-superficial.
Discussion
In the following discussion, we make the assumption that Cre-driven AP is expressed in cells of the somatic LP of the trunk, and therefore all the labelled cells in the trunk belong to the LP lineage. Given this assumption, the unlabelled domain is primaxial–somitic cells differentiating within a somitic connective tissue, and the labelled domain is abaxial–somitic cells differentiating into muscle (and occasionally cartilage) in a connective tissue of LP origin. The label boundary represents the LSF as defined in the introduction and by Burke & Nowicki (2003). Although this interpretation is inevitably circular, we nonetheless argue for the usefulness of the Prx1Cre transgenic in the absence of any other indelible LP markers. The assumption that the label is true to the LP lineage is justified for several reasons. The labelling is fully consistent with what is known about the fate of both somitic and LP cells in the avian trunk, for which there is very thorough mesodermal fate mapping (reviewed by Christ et al. 2007; Winslow et al. 2007). The boundary between labelled and unlabelled cell populations, although dynamic, is consistent through time and the majority of individual bones and muscles are either entirely labelled or unlabelled. Interestingly, the muscles and bones that contain a boundary of labelled and unlabelled cells are structures that bridge the axial and appendicular systems. It is not surprising that these elements include connective tissue cells from both the somitic (axial) and the LP (appendicular) systems (see below). It could be argued that these exceptions to the dichotomy of labelled versus unlabelled structures represent areas where the fidelity of the Cre deleter system no longer accurately reflects cell lineage. In this case some explanation, other than cell lineage, would be needed to account for the general consistency of the labelling. Fidelity of the reporter to the LP lineage is a more parsimonious explanation.
Given our assumption of the fidelity of the Prx1Cre reporter, the ability to visualize cells of the LP provides a novel view of the development of the body wall and exposes several distinct relationships between somitic and LP mesoderm cell lineages. For instance, it reveals the inclusion of somitic cells in the mammalian scapula and LP cells in the diaphragm. Also of interest is the presence of putative LP-derived connective tissue investing spinal nerves very close to their exit from the vertebral canal; the ‘displacement’ of connective tissue in superficial trunk muscles that cross the frontier; the unique identity of the first rib; and a new form of asymmetry in the adrenal glands.
The key defining character of the frontier is the change in the lineage of connective tissue cell populations in primaxial versus abaxial domains. Evidence from embryonic manipulations of chick embryos suggests that the frontier does not simply represent a topography of cell lineage arrangements, but that it also represents a site of patterning information (Burke & Nowicki, 2003). This is dramatically demonstrated in limb regions, where somites from any non-limb level can provide myoblasts to form normal limb muscles when transplanted adjacent to limb-level LP (Chevallier et al. 1977; Christ et al. 1977). The individual somitic cell that migrates into the limb is dependent on signals found in the LP mesoderm to differentiate into endothelium or a particular muscle fibre type (Kardon et al. 2002). A molecular source of muscle patterning in the limbs has been traced to the expression of the Tcf4 transcription factor in the LP cells (Kardon et al. 2003). The patterning role of the LP is not exclusive to the limbs. When somites are transplanted from thoracic to cervical levels in the chick, vertebral ribs (primaxial) form ectopically, but sternal ribs (abaxial) fail to form (Kieny et al. 1972), indicating the primaxial structures are determined autonomously by their somite of origin, while abaxial structures are dependent on the LP environment for normal development. These experiments and similar heterotopic transplants indicate that once somite cells cross the frontier, they come under the influence of patterning intrinsic to the LP (Nowicki & Burke, 2000; Burke & Nowicki, 2003; reviewed in Winslow et al. 2007).
The general aspects of the LSF suggested by the E15.5 PrxCre/Z/AP mouse embryo are consistent with findings in avian embryos (Nowicki et al. 2003). The similarity to avians includes the relative increase in volume of the primaxial domain, and its regional variation along the A–P axis. The resulting displacement of the frontier away from the dorsal midline is greatest in the trunk, and least in the limb regions (Fig. 3). Except around the scapula and the ilium, the point where the frontier contacts the surface ectoderm is the dorsal-most position of the frontier (point ‘Y’). This point is probably closest to the original boundary between somites and LP mesoderm, and we refer to it as the incipient frontier. In younger stages this point is visible as a notch in the surface of the mouse (Fig. 1A) and chick (Olivera-Martinez et al. 2000) embryo.
The developmental nature of the thoracic ribs that meet the sternum is different in mice than earlier reports on chick. The rib cage of both mammals and birds is adapted to facilitate respiration by changing the volume of the pleural cavity. The ventral part of mammalian ribs, the sternal ribs, often remains cartilaginous and the sterno-costal joint enables thoracic expansion (Liem et al. 2001). The boundary between the vertebral and sternal portions of the ribs in mammals is an ossification front, rather than a true joint. This is different in many birds, including chickens, where there are definitive sternal ribs that articulate to the vertebral rib as well as the sternum, by movable joints (Nickel et al. 1977). Previous work has indicated the sternal component of the ribs in chick as abaxial (Nowicki et al. 2003). Although the chondrocytes of sternal ribs derive from the somites, chimeras show that the periosteum of some sternal ribs is LP derived, fulfilling our criteria for abaxial elements. In the mouse, with the exception of the first rib, the other ribs are surrounded by unlabelled periosteum for their entire length, and hence primaxial (Fig. 6B). The periosteum of the first rib, however, is densely labelled, and thus is apparently LP derived and abaxial (Fig. 6A). It is interesting to note that the first rib is often uniquely affected in Hox-mutant mice, implying a different molecular patterning routine than the other ribs (reviewed in Wellik, 2007).
Perceived discontinuities in the frontier
There are a number of structures that cause deviations from a continuous, or smooth cross-sectional profile in the LSF as represented by the label boundary. This is seen in the spinal nerve roots of the brachial plexus, and the superficial dorsal trunk muscles: the spinotrapezius and latissimus dorsi. The trapezius and latissimus muscles are unlabelled at their origin on the vertebrae and thoracolumbar fascia, but become labelled a short distance away from the dorsal midline (Fig. 8B), implying they have both primaxial and abaxial components. The quail–chick chimera experiments conducted to date do not provide this degree of detail although it is likely that the situation is similar in the homologous muscles of birds.
Several phenomena could account for the pattern of discontinuities in the frontier. The simplest explanation is a form of differential growth, most easily explained with an analogy. Imagine an anchor line dropped from a pier at high tide. As the tide turns and the water retreats, seaweed floating on the surface clings to the rope as the water drops away. As the primaxial domain expands causing the ‘retreat’ of the frontier, the elements that have established a fixed relationship with the connective tissue (seaweed in our analogy) preserve the original point of contact like a high-water mark. It is likely that expansion of the frontier away from the axis due to growth of the primaxial cell population is initially quite uniform along the A–P axis (the tide going out). The association of LP connective tissue with myoblasts of the latissimus (for instance) relatively close to the primaxial point of the muscle's origin and markedly displaced from the main frontier indicates that those myoblasts crossed the frontier and were specified to participate in this muscle at a stage when the frontier was still relatively close to its incipient position (high tide). Subsequent general growth of the primaxial domain would push the LSF further ventrally, exposing the dorsal part of the abaxial portion of the muscle, which is anchored by its origin on the axial elements (the pier). This scenario would also explain the changes in the label boundary between the whole mount embryo shown in Fig. 2D,E and the sections in Fig. 5B. The AP-labelled connective tissue of the latissimus lies far more dorsal than the rest of the label boundary. The ventral rami of the spinal nerves that contribute to the brachial plexus can also be described by the analogy of seaweed and the retreating tide. The point along the nerve roots where the investing connective tissue is labelled may mark the place where these nerves first encountered the LP and crossed the frontier (Figs 5A, 10B).
Assuming that the label is true to the LP lineage, the only alternative explanation for the discontinuity in the frontier would be retrograde migration of LP connective tissue cells back toward the dorsal midline to invest myoblasts or nerves that remain stationary relative to the axis. This latter possibility seems unlikely given the general expansion of cells away from the dorsal midline. We suggest that the proximal position of the label boundary in certain muscles indicates the specification of the contributing cell populations at very early stages in body wall formation, at the site where the two mesodermal populations meet, the LSF.
The label boundary through the crowded area of the dorsal abdominal wall dramatically demonstrates changes in the orientation of boundaries during development. The juxtaposition of the labelled psoas muscle and the unlabelled quadratus lumborum is a striking example of how final anatomy can obscure embryonic history (Fig. 8B). Most of the nerves of the lumbar plexus run within the psoas muscle, and they are invested in labelled cells. Like the nerves of the brachial plexus, these spinal nerves encounter LP connective tissue almost immediately upon emerging from the vertebral canal, and the majority of their path finding occurs in the abaxial domain.
Conclusions
We have described the labelling pattern of transgene activation in the Prx1Cre transgenic. We make the assumption that this construct provides a marker for cells of the somatic LP lineage, thereby providing a view of mesodermal components that have not previously been visualized or described in the mouse. We take the label boundary to serve as proxy for the LSF, a dramatic but largely cryptic boundary that characterizes the development of the vertebrate body. Similar cryptic boundaries have been revealed in the head and neck of avian and mouse embryos, for example the boundary between neural crest and cranial mesoderm cell lineages (Morriss-Kay & Wilkie, 2005; Evans & Noden, 2006), and neural crest and mesoderm lineages in the cervical and shoulder region (Matsuoka et al. 2005). These boundaries are not visible in adult morphology as they apparently have no adult functional significance. However, during morphogenesis, patterning information for specific cranial muscles is provided by neural crest-derived connective tissue and the coordination of the cell populations at these cryptic boundaries is critical to normal development (reviewed in Noden & Francis-West, 2006).
The frontier is particularly interesting in an evolutionary perspective. The discontinuities in the frontier described above are found in elements that bridge the axial and appendicular systems. The differential growth of the primaxial domain combined with early anchoring of muscle, nerve and connective tissue cell populations in these discontinuous elements provides evidence for specification and global patterning in early embryonic stages. This developmental character (position of the frontier in certain muscles) may be a reflection of the history of the appendicular system itself, when the LP was first recruited to form the paired appendages in an ancestor of the jawed vertebrates. During subsequent evolution of vertebrates with highly divergent locomotor adaptation, the partial independence of embryonic patterning in primaxial and abaxial domains could facilitate dramatic modifications of the body plan (e.g. turtles, Nagashima et al. 2007; limbless squamates, Tsuihiji et al. 2006).
We propose that the developmental boundary called the LSF is the site of patterning decisions along the A–P axis during development, and as such is the site of changes in patterning information that have resulted in evolutionary modifications within the vertebrate body plan.
Acknowledgments
This work was supported in part by funds from the Howard Hughes Medical Institute to support undergraduate initiatives in the life sciences, and by NIHR15HD050282 to A.C.B. We are grateful to R. Shearman, B. Winslow, F. Tulenko and S. Devoto for stimulating discussions of anatomy and of the text.
References
- Aliab’ev FB, Paderov IUM. Age-related structural asymmetry of human adrenal glands. Morfologiia. 2004;125:61–64. [PubMed] [Google Scholar]
- Bothe I, Ahmed MU, Winterbottom FL, von Scheven G, Dietrich S. Extrinsic versus intrinsic cues in avian paraxial mesoderm patterning and differentiation. Dev Dyn. 2007;236:2397–2409. doi: 10.1002/dvdy.21241. [DOI] [PubMed] [Google Scholar]
- Burke AC, Nowicki JL. A new view of patterning domains in the vertebrate mesoderm. Developmental Cell. 2003;4:159–165. doi: 10.1016/s1534-5807(03)00033-9. [DOI] [PubMed] [Google Scholar]
- Chevallier A, Kieny M, Mauguer A. Limb-somite relationship: origin of the limb musculature. J Embr Exper Morph. 1977;41:245–258. [PubMed] [Google Scholar]
- Christ B, Jacob HJ, Jacob M. Experimental analysis of the origin of the wing musculature in avian embryos. Anat Embryol. 1977;150:171–186. doi: 10.1007/BF00316649. [DOI] [PubMed] [Google Scholar]
- Christ B, Ordahl CP. Early stages of chick somite development. Anat Embry. 1995;191:381–396. doi: 10.1007/BF00304424. [DOI] [PubMed] [Google Scholar]
- Christ B, Huang R, Scaal M. Amniote somite derivatives. Dev Dyn. 1977;236:2382–2396. doi: 10.1002/dvdy.21189. [DOI] [PubMed] [Google Scholar]
- Davidson EH, Erwin DH. Gene regulatory networks and the evolution of animal body plans. Science. 2006;311:796–800. doi: 10.1126/science.1113832. [DOI] [PubMed] [Google Scholar]
- Dietrich S. Regulation of hypaxial muscle development. Cell Tissue Res. 1999;296:175–182. doi: 10.1007/s004410051278. [DOI] [PubMed] [Google Scholar]
- Evans DJ, Noden DM. Spatial relations between avian craniofacial neural crest and paraxial mesoderm cells. Dev Dyn. 2006;235:1310–1325. doi: 10.1002/dvdy.20663. [DOI] [PubMed] [Google Scholar]
- Fell HB. On the origin and developmental mechanics of the avian sternum. Philos Trans R Soc Lond [Biol] 1939;229:407–464. [Google Scholar]
- Gerendai I, Wiesel O, Boldogkoi Z, Toth IE. The supraspinal innervation of the left adrenal is more intense than that of the right one. Ideggyogy Sz. 2007;60:159–161. [PubMed] [Google Scholar]
- Kardon G, Campbell JK, Tabin CJ. Local extrinsic signals determine muscle and endothelial cell fate and patterning in the vertebrate limb. Developmental Cell. 2002;3:533–545. doi: 10.1016/s1534-5807(02)00291-5. [DOI] [PubMed] [Google Scholar]
- Kardon G, Harfe BD, Tabin CJ. A Tcf4-positive mesodermal population provides a prepattern for vertebrate limb muscle patterning. Developmental Cell. 2003;5:937–944. doi: 10.1016/s1534-5807(03)00360-5. [DOI] [PubMed] [Google Scholar]
- Kaufman MH. The Atlas of Mouse Development. New York: Academic Press; 1992. [Google Scholar]
- Kieny M, Mauger A, Sengel P. Early regionalization of the somitic mesoderm as studied by the development of the axial skeleton of the chick embryo. JEEM. 1972;28:142–161. doi: 10.1016/0012-1606(72)90133-9. [DOI] [PubMed] [Google Scholar]
- Liem KF, Bemis WE, Walker WF, Grande L. Functional Anatomy of the Vertebrates. 3. Thompson, USA: Brooks/Cole; 2001. [Google Scholar]
- Lobe CG, Koop KE, Kreppner W, Lomeli H, Gertsenstein M, Nagy A. Z/AP, a double reporter for Cre-mediated recombination. Dev Biol. 1999;208:281–292. doi: 10.1006/dbio.1999.9209. [DOI] [PubMed] [Google Scholar]
- Logan M, Martin JF, Nagy A, Lobe C, Olsen EN, Tabin CJ. Expression of Cre recombinase in the developing mouse limb bud driven by a Prx1 enhancer. Genesis. 2002;33:77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- Martin JF, Olson EN. Identification of a prx1 limb enhancer. Genesis. 2000;26:225–229. [PubMed] [Google Scholar]
- Matsuoka T, Ahlberg PE, Kessaris N, et al. Neural crest origins of the neck and shoulder. Nature. 2005;436:347–355. doi: 10.1038/nature03837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morriss-Kay GM, Wilkie AO. Growth of the normal skull vault and its alteration in craniosynostosis: insights from human genetics and experimental studies. J Anat. 2005;207:637–653. doi: 10.1111/j.1469-7580.2005.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima H, Kuraku S, Uchida K, Ohya YK, Narita Y, Kuratani S. On the carapacial ridge in turtle embryos: its developmental origin, function and the chelonian body plan. Development. 2007;134:2219–2226. doi: 10.1242/dev.002618. [DOI] [PubMed] [Google Scholar]
- Nickel R, Schummer A, Seferle E, Siller WG, Wight PAL. Anatomy of the Domestic Birds. New York, Berlin: Springer-Verlag; 1977. [Google Scholar]
- Noden DM, Francis-West P. The differentiation and morphogenesis of craniofacial muscles. Dev Dyn. 2006;235:1194–1218. doi: 10.1002/dvdy.20697. [DOI] [PubMed] [Google Scholar]
- Nowicki JL, Burke AC. Hox genes and morphological identity: axial versus lateral patterning in the vertebrate mesoderm. Development. 2000;127:4265–4275. doi: 10.1242/dev.127.19.4265. [DOI] [PubMed] [Google Scholar]
- Nowicki JL, Takimoto R, Burke AC. The lateral somitic frontier: dorso-ventral aspects of anterio-posterior regionalization in avian embryos. Mechanic Deve. 2003;120:227–240. doi: 10.1016/s0925-4773(02)00415-x. [DOI] [PubMed] [Google Scholar]
- Olivera-Martinez I, Coltey M, Dhouailly D, Pourquie O. Mediolateral somitic origin of ribs and dermis determined by quail-chick chimeras. Development. 2000;127:4611–4617. doi: 10.1242/dev.127.21.4611. [DOI] [PubMed] [Google Scholar]
- Ordahl CP, Le Douarin NM. Two myogenic lineages within the developing somite. Development. 1992;114:339–353. doi: 10.1242/dev.114.2.339. [DOI] [PubMed] [Google Scholar]
- Trut LN, Prasolova LA, Kharlamova AV, Plyusnina IZ. Directional left-sided asymmetry of adrenals in experimentally domesticated animals. Bull Exp Biol Med. 2002;133:506–509. doi: 10.1023/a:1019886426571. [DOI] [PubMed] [Google Scholar]
- Tsuihiji T, Kearney M, Rieppel O. The first report of a pectoral girdle muscle in snakes, with comments on the cervico-dorsal boundary. COPEIA. 2006;2:206–215. [Google Scholar]
- Wellik D. Hox patterning of the vertebrate axial skeleton. Dev Dyn. 2007;236:2454–2463. doi: 10.1002/dvdy.21286. [DOI] [PubMed] [Google Scholar]
- Winslow BB, Takimoto-Kimura R, Burke AC. Global patterning of the vertebrate mesoderm. Dev Dyn. 2007;236:2371–2381. doi: 10.1002/dvdy.21254. [DOI] [PubMed] [Google Scholar]