Abstract
Incisional hernias represent one of the most common complications after laparotomy. Specific pre-operative risk factors have not yet been identified. Recent studies indicate that changes in extracellular matrix components such as collagen I and collagen III may be involved in hernia development. In the present study we have evaluated the significance of fibrillin-1 in hernia development as one of the main components of the extracellular matrix. Tissue samples from non-scar skin and muscle fascia of 12 patients with incisional hernias as well as from the respective scar tissues were obtained. Corresponding tissue samples of 10 patients with normal postoperative wound healing served as controls. Distribution of fibrillin-1 was evaluated immunohistochemically. Differences in fibrillin-1 distribution in the non-scar tissues of muscle fascia have been found in patients with incisional hernia, compared to those without hernia. In scar regions of both patient groups, slight differences in the pattern of fibrillin-1 were observed. A tendency to a differential deposition of fibrillin-1 in skin samples, although hardly quantifiable, was observed as well. Our results suggest that fibrillin-1 is a relevant factor contributing to tissue stability. Disturbances in its deposition, even before scar formation, may be an important factor to the development of incisional hernias.
Keywords: extracellular matrix, fibrillin-1, incisional hernias, muscle fascia, scar, skin
Introduction
Incisional hernias after laparotomy occur in up to 18.7% of the operations and represent one of the most common complications (Höer et al. 2002a), which in some cases can have severe or even lethal consequences for the patients (Santora & Roslyn, 1993; Krug et al. 1995; Leber et al. 1998). Moreover, the financial aspect of this problem is a significant burden on health care systems. For example, in Germany, 700 000 abdominal operations with an estimated incidence of 15% incisional hernias, a re-operation for about 30% of the patients, and average costs for postoperative care of 4.100 €, contribute a yearly burden of 128 million € for the health care system (Höer et al. 2002b). In this context, the possibility to estimate the risk for incisional hernias with high confidence at the time of the first operation would be very useful. Such a pre-operative risk assessment would enable surgeons prophylactically to implant a mesh in high risk patients (Langer et al. 2005) during the first operation and thus reduce the surgical trauma imposed by a second operation. On the one hand, it is generally known that male patients over the age of 45, or patients with a body mass index above 25 have an increased incidence of incisional hernia after laparotomy (Anthony et al. 2000; Höer et al. 2002b). Nevertheless, pre-operative factors which specifically account for a higher risk of incisional hernia after laparotomy have not yet been identified. Also, very little is known about the molecular mechanisms responsible for the formations of scars which eventually favour the development of incisional hernias.
In recent years increasing attention has been paid to components of the extracellular matrix. It has been in fact proposed that alterations in the collagen III/I protein ratio, as well as similar changes in the procollagen III/I mRNA ratio in the skin may favour development of incisional hernias (Klinge et al. 2000, 2001; Si et al. 2002; Rosch et al. 2003). In the present study we analyzed the potential role of the extracellular matrix glycoprotein, fibrillin-1, in the development of incisional hernias. Fibrillin-1 is one of the main constituents of connective tissue microfibrils (Sakai et al. 1986, 1991; Keene et al. 1991a; Reinhardt et al. 1996; Baldock et al. 2001) and it is widely distributed during development and in adult tissues (Sakai et al. 1986; Keene et al. 1991a,b, 1997; King & Blankenship, 1997; Dingemans et al. 2000; Quondamatteo et al. 2002). Mutation of this gene leads to Marfan syndrome (Dietz et al. 1991; Silverman et al. 1995; Robinson & Godfrey, 2000), and fibrillin-1 knock-out mouse shows a phenotype which resembles its clinical manifestations (Pereira et al. 1997). The clinical manifestations of Marfan syndrome as well as those caused by fibrillin deletion in mouse share a reduced stability of the connective tissue (De Paepe et al. 1996; Pereira et al. 1997; Robinson & Godfrey, 2000).
As it is conceivable that fibrillin-1 has a pivotal role in connective tissue stability (Dietz et al. 1991; Pereira et al. 1997; Robinson & Godfrey, 2000), we analyzed here whether alterations in the distribution of fibrillin-1 correlate with the occurrence of incisional hernias. For this purpose, we investigated the distribution of fibrillin-1 in skin and muscle fascia of patients with incisional hernias, compared to corresponding tissue samples of patients without hernias in scar and non-scar regions. Analysis of scar tissue was firstly aimed to elucidate whether aberrant deposition of fibrillin-1 correlates with less stable scars. Secondly, the study of non-scar regions should answer the question of whether specific alterations in fibrillin-1 distribution indicate a predisposition for hernia development. As skin biopsies are relatively easy to perform, we analyzed the potential to develop an objective, reproducible, reliable and simple pre-operative test based on fibrillin-1 distribution patterns to evaluate the risk for hernia development.
Materials and methods
Tissues samples
The tissue samples were taken from 22 patients (9 female and 13 male) between 35 and 85 years of age who had undergone repeated laparotomy (Table 1 and Table 2). The work was carried out according to the guidelines and with the approval of the local ethics committee. Twelve of these patients (3 female and 9 male) had developed an incisional hernia after a former abdominal operation and were then relaparotomized for hernia reduction (patients with hernia, n = 12). Ten of these patients (6 female and 4 male) had not developed an incisional hernia after a previous abdominal operation but were relaparotomized for other medical indications (patients without hernia, n = 10) and were used as controls. From each of the patients, scar tissue was taken from skin and from muscle fascia. Non-scar skin and muscle fascia samples were taken from the edges of the scar which had to be resected together with the scar to allow a successful postoperative wound healing. None of the patients was known to have any generalized connective tissue disease.
Table 1.
Summary of the aberrant/unusual localization and/or deposition forms of fibrillin-1 in patients without and with hernias
| Patients without hernias | Patients with hernias | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient number (right) Aberrant deposition of fibrillin-1 (below) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 |
| Non-scar fascia | ||||||||||||||||||||||
| Disarray of fibrillin-1 positive fibrils | Y | Y | ||||||||||||||||||||
| Smooth transitions of fibrillin-1 fibrils | Y | Y | Y | Y | Y | Y | ||||||||||||||||
| Reduced immunoreactivity | Y | Y | Y | Y | Y | Y | Y | Y | Y | |||||||||||||
| Fascial scar tissue | ||||||||||||||||||||||
| Fibrillin-1 positive clots | Y | Y | Y | Y | Y | Y | Y* | Y | ||||||||||||||
| Fibrilllin-1 positive fibril bundles | Y | Y | Y | Y | Y | Y | Y | Y | ||||||||||||||
| Abrupt directional change | Y | Y | Y | Y | ||||||||||||||||||
| Disarrayed distribution | Y | Y | Y | |||||||||||||||||||
| Reduced fibrillar immunoreactivity | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |||||
| Non-scar skin | ||||||||||||||||||||||
| Reduced immunoreactivity papillar layer | Y | Y | Y | Y | Y | Y** | Y | Y** | Y | Y | Y | Y | ||||||||||
| Reduced immunoreactivity reticular layer | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||||||||||||
| Age of scar (years) | 2 | < 1 | 6 | 4 | < 1 | 13 | 4 | < 1 | 41 | 2 | 2 | 18 | < 1 | 1 | 14 | 5 | 2 | 4 | < 1 | 2 | 6 | < 1 |
| Number of laparotomies due to incisional hernia repair*** | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 2 | 1 | 3 | 1 | 2 | 1 | 1 | 1 | |
Y = feature present;
= intense and broadly expanded clots;
= particularly strong reduction in subepithelial fibrillar structures;
= refers to the number of laparotomies due to incisional hernia repair at the timepoint of the removal of the samples examined in this study, including the laparotomy in which the samples examined were removed.
Table 2.
Indications for primary laparotomies
| Patient number | Indication |
|---|---|
| 1 | anterior rectal resection |
| 2 | ileal resection |
| 3 | anterior rectal resection |
| 4 | sigma resection |
| 5 | sigma resection |
| 6 | gastrectomy |
| 7 | anterior rectal resection |
| 8 | anterior rectal resection |
| 9 | cholecystectomy |
| 10 | gastrectomy |
| 11 | sigma resection |
| 12 | hemicolectomy |
| 13 | colectomy |
| 14 | appendectomy |
| 15 | billroth 1 resection |
| 16 | fundoplication |
| 17 | splenectomy |
| 18 | splenectomy |
| 19 | cholecystectomy |
| 20 | explorative laparotomy |
| 21 | cholecystectomy |
| 22 | epigastric hernia |
Immunohistochemical detection of fibrillin-1
Tissue samples were fixed in 4% phosphate-buffered formaldehyde and embedded in paraffin. In addition to routine haematoxylin-eosin staining, the indirect immunoperoxidase-diaminobenzidine method (Quondamatteo et al. 1999, 2002) was used in paraffin sections. Briefly, tissue sections were deparaffinized and rehydrated in a descending ethanol series and then rinsed for 10 min in 0.05 m Tris-buffered saline (TBS, pH 7.4). Intrinsic peroxidase activity was blocked by incubation with hydrogen peroxide-methanol (1 : 100) for 45 min in the dark. After rinsing with TBS, sections were pretreated with pepsin (0.4% in 0.1 m HCl, pH 2.0) for 6 min at room temperature. Sections were incubated with the primary antibody, mAb 69, raised in mouse and directed to fibrillin-1 (Maddox et al. 1989) in a humid chamber (1 : 100 in 0.05 m TBS) for 2 h at room temperature. Immunoreactions were detected by means of the indirect immunoperoxidase-diaminobenzidine method using peroxidase conjugate rabbit-anti-mouse immunoglobulins (Dako, Hamburg, Germany) 1 : 100 diluted in 0.05 m TBS for 30 min at room temperature. Color reactions were developed with diaminobenzidine solution. Cell nuclei were counterstained with haematoxylin for 5 s at room temperature followed by a 20-min rinse with water. Subsequently, the sections were dehydrated in an ascending ethanol series, cleared in xylene and cover slipped. Negative controls were performed by replacing the primary antibody with buffer.
Morphometric evaluation of the immunohistochemical reactions
To evaluate significant differences in the density of fibrillin-1 positive structures in non-scar skin of patients with and without incisional hernias, counting was carried out by three independent observers using a standardized template to quantify the amount of fibrils intersecting cross-sections of a grid. Computer-based statistical evaluation was used, which included a repeated-measures analysis of variance (split-plot-plot design) (Kirk, 1982). In this design, gender and healing success after laparotomy are the whole plot factors, and observer (n = 3) is the subplot factor. Tissue section and images are hierarchically nested under the patients who are nested under both the whole-plot factors. The significance level was set to 5%.
Results
The occurrence of aberrant/unusual localization and/or deposition forms of fibrillin-1 in tissues of patients without and with hernias is summarized in Table 1.
Fibrillin-1 in non-scar regions of the muscle fascia
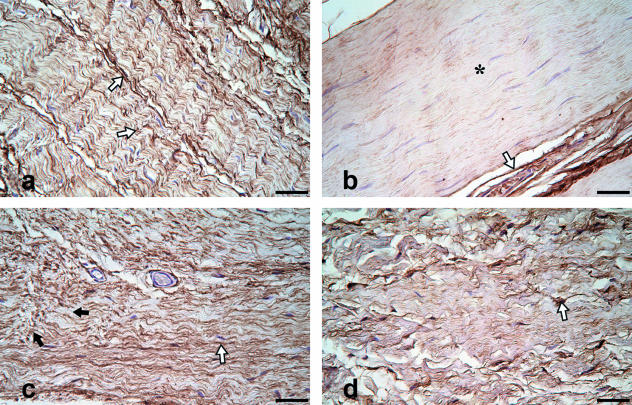
In the muscle fascia of control patients, i.e. without incisional hernia, outside of the scar regions, regular fibril structures immunoreactive for fibrillin-1 were localized aligned in parallel between the collagen fiber bundles (Fig. 1a). Immunostaining was strong and constant in eight of 10 cases. Continuously ordered and densely packed fibrillar structures were visible. Samples of the remaining two patients showed a markedly decreased and/or inconstant immunoreactivity in some regions (Fig. 1b), although maintaining a regular morphology.
Fig. 1.
(a–b) Non-scar muscle fascia, fibrillin-1 immunohistochemistry, no incisional hernias. Fibrillin-1 positive fibrillar structures are visible in a parallel wavy order. Patients without hernias (a, b) show a distinct antibody labelling of these structures in most cases (1a; open arrows). There are few exceptions, where the reactivity for fibrillin-1 seems to be generally reduced in the fascial tissue (b; asterisk), whereas a strong reaction surrounding blood vessels as positive control (b; open arrow) is observed. (c–d) Non-scar muscle fascia, fibrillin-1 immunohistochemistry, incisional hernias. Some patients with incisional hernias (c, d) reveal morphological characteristics like fluid transitions from tight to loosely arranged fibrillin-1 positive fibrillar structures (c; black arrows), or a loosely organized connective tissue (d) containing disarrayed fibrillin-1 positive fibrillar structures (d; open arrow). Bar: 50 µm.
Aberrant distribution of fibrillin-1 was observed in non-scar regions of six of 12 patients with incisional hernias. Fibrillin-1 fibrils showed divergent directions and smooth transitions with no clear demarcation from parallel, unidirectionally ordered to multidirectional and loosely arranged fibrils (Fig. 1c). In two of these six patients, additionally, loose deposition of disarrayed fibrillin-1 positive structures was observed. Parallel arrangement of fibrillin-1 containing fibers was found only sporadically (Fig. 1d). Such aberrant patterns were not present in any of the samples taken from the non-scar regions of muscle fascia of patients without incisional hernias (Table 1). Additionally, the number of patients with reduced fibrillin-1 reactivity was higher in the group with hernias (7/12 vs. 2/10).
Fibrillin-1 in the scar tissue of the muscle fascia
Although the pattern of fibrillin-1 distribution in scar regions of the muscle fascia of patients without incisional hernias partly resembled the normal appearance (Fig. 2a), alterations compared to the corresponding non-scar regions occurred. In five of 10 patients, fibrillin-1 positive fibril bundles (Fig. 2b) and/or amorphous ‘clots’ (Fig. 2c) were observed. In the tissue of another patient, abrupt directional changes of immunoreactive fibrillar structures such as depicted in Fig. 2d were seen. Directional changes of fibrillin-1 positive structures also co-existed with clots and thicker bundles in one case (see Table 1 for details). Such unusual deposition of fibrillin-1 in fascial scar tissue as described above was never found in the fascial non-scar tissue of patients without hernia. Additionally, in scar tissue, the immunoreactivity of the fibrillar structures in the group without hernia as well as in half of the cases of the group with hernia was generally reduced with respect to the intensity and/or the constancy of the immunostaining (see Table 1 for details).
Fig. 2.
(a–d) Scar tissue of muscle fascia, fibrillin-1 immunohistochemistry, no incisional hernias. Regular arrangement of fibrillin-1 positive fibrils in few cases (a, open arrows). Characteristic irregularities of the dense connective tissue in the patient group without incisional hernias: Bundles of fibrillin-1 positive structures (b; open arrow) next to areas not labelled by the antibody (asterisk). Disseminated, extensive clots of fibrillin-1 positive material (c; black arrow head). ‘Patch-like’ multidirectional organisation of the tight connective tissue containing fibrillin-1 positive fibrils (d; open arrow heads). (e–h) Scar tissue of muscle fascia, fibrillin-1 immunohistochemistry, incisional hernias. The structural characteristics of the tight connective tissue in the patient group with incisional hernias largely resembled that of the patient group without incisional hernias, but were observed more frequently. Thick bundles of fibrillin-1 positive fibrils (e; open arrow), disseminated, extensive clots of fibrillin-1 positive material (f; black arrow head), ‘patch-like’ multidirectional organization of the tight connective tissue containing fibrillin-1 positive fibrils (g; open arrowheads), disarrayed fibrillar structures reactive for fibrillin-1 (h, open arrows). Bar: 50 µm.
In scar regions of the muscle fascia of patients with incisional hernias, abnormal deposition of fibrillin-1 in the form of thicker fibril bundles (Fig. 2e) and/or clots (Fig. 2f) occurred in eight of 12 patients (See Table 1 for details). In one of these cases, clots were extremely large. Additionally, in three of these eight patients, abrupt orientation changes (Fig. 2g) of immunoreactive fibrils and/or a disarrayed distribution (Fig. 2h) of fibrillin-1 positive structures were seen (Table 1).
Fibrillin-1 in non-scar skin samples
In the non-scar skin sections of patients without incisional hernias we found subepithelial fibrillin-1 positive fibrillar structures showing strong immunoreactivity in five of 10 patients. In the dermal papillae, starting from the basement membrane zone, fibrillin-1 positive fibrils were typically directed to the deeper regions of the papillar layer at the boundary to the reticular layer and interwoven in a mesh-like network (Fig. 3a). In comparison with this, the tissue samples taken from the remaining five patients showed subepithelial fibrillin-1 positive fibrillar structures in the dermal papillae with decreased immunoreactivity, but with a typical ‘fibril’-like shape extending to the deeper regions of the papillar layer. In such cases, fine fibrillin-1 positive structures were also present in the regions adjacent to the reticular layer (Fig. 3b). In the reticular layer, elongated fibrillin-1 positive fibrillar structures accompanied the collagen fibril bundles. The skin sections of seven of 10 patients showed a dense distribution of fibrillin-1 positive structures with strong immunoreactivity (Fig. 3c). In the remaining three patients, fibrillin-1 positive structures had a more rarefied appearance (Fig. 3d) (see also Table 1 for details). Immunoreactivity for fibrillin-1 was consistently observed in blood vessels.
Fig. 3.
(a–d) Non-scar skin, fibrillin-1 immunohistochemistry, no incisional hernias. Patients without incisional hernias show a distinct immunohistochemical reaction against fibrillin-1 in the papillar layer (a, open arrows). In some cases a more rarefied fibrillar network was visible (b, open arrow), however continuously running to the reticular layer, where a strong immunoreactivity was observed (c, open arrows). Three of 10 patients show an inconstant fibrillin-1 distribution in the reticular layer (d, asterisk). (e–h) Non-scar skin, fibrillin-1 immunohistochemistry, incisional hernias. In the skin of patients with incisional hernias, the subepidermal fibrillar network (e, f) shows a reduction of structural complexity (open arrows). Two patients nearly completely lack fibrillin-1 positive fibrillar structures in the deep regions of the papillar layer (f, asterisk). In the reticular layer, interindividual differences are visible (g, h open arrows) with seven of 12 patients showing an inconstant fibrillin-1 distribution (h, asterisk). e = epithelium, P = papillae, r = reticular layer. In each of the eight images (a–h), the inlay shows fibrillin-1 deposition at higher magnification in the papillar layer (a, b, e, f) and in the reticular layer (c, d, g, h). Bar: 50 µm.
In the group with incisional hernias, the subepithelial network showed a general trend to a reduced structural complexity, and to more irregular and disarrayed structures (Fig. 3e) having in part a fragmented appearance. Five of 12 patients showed a reduced immunoreactivity. In two further patients with hernias, in addition to a strong reduction of the fibrillin-1 positive fibers below the basement membrane zone, fibrillin-1 was lacking completely in the deep region of the papillar layer (Fig. 3f, Table 1). In the reticular layer, a high density of fibrillin-1 positive structures was recognizable in five of 12 cases (Fig. 3g). In the remaining seven patients a generally rarefied fibrillin-1 positive network was seen (Fig. 3h). In contrast to this, in the group of patients without incisional hernias, a similar rarefaction of the fibrillin-1-immunoreactivity was found in only three of 10 cases (Table 1).
Statistical evaluation of fiber density in the subepithelial fibrillin-1 positive network of non-scar skin
The observations described above effectively describe a tendency, and do not provide sufficient objectivity for a pre-operative clinical assessment of the patients. Therefore, on this basis, we intended to establish an objective and highly reproducible method to quantify the density of fibrillin-1 in the skin.
For this purpose, we measured the spatial density of fibrillin-1 immunoreactive subepidermal networks. No significant difference in fibre density was detected between the two examined groups (P = 0.55). Also, the gender of the patients did not show a significant impact on the measured density values (P = 0.20). In contrast to that, the influence of the observers was significant (P < 0.0001).
Fibrillin-1 in the scar tissue of the skin
In scar regions of the skin of patients without incisional hernias, fibrillin-1 positive fibers were found. The pattern of immunoreactivity revealed a distinctive interindividual variability, however, mostly resembling the corresponding non-scar regions of the respective patients. The reactivity varied from strong in four patients (Fig. 4a) to a thinned out distribution (Fig. 4b). In the reticular layer of three patients from the group without incisional hernias, characteristic fibrillin-1 positive fibers were found, appearing more densely packed and markedly disarrayed compared with the corresponding non-scar tissues (Fig. 4c). Loosely scattered fibril components (Fig. 4d) resembling the normal condition (see Fig. 3d for comparison) were also present. Moreover, a wide range of intermediate conditions were observed.
Fig. 4.
(a–d) Scar tissue of the skin, fibrillin-1 immunohistochemistry, no incisional hernias. Similarly to non-scar regions, scarred skin of patients without incisional hernias (a–d) shows interindividual differences in morphology and distribution of fibrillin-1 positive structures in the papillar layer (a, b; open arrows). Fibrillin-1 positive fibrils in the reticular layer of some patients without hernias have a comparatively dense and disorganized appearance (a, c; ‘+’), whereas others reveal a scattered and rarefied distribution (d; asterisk) of these structures (b, d; open arrows). (e–h) Scar tissue of the skin, fibrillin-1 immunohistochemistry, incisional hernias. A discontinuous and structurally less constant fibrillin-1 positive fibrillar network in scarred skin of patients with incisional hernias (e, f; open arrows) is visible in comparison with patients without herniation. The subepidermal fibrillar network is nearly completely missing in two cases (f; asterisk). In the reticular layer, the density range of fibrillin-1 positive fibrillar structures varied from condensed and disarrayed (g; ‘+’) to thin-filamentous and loosely distributed (h, open arrow). e = epithelium, P = papillae, r = reticular layer. In each of the eight images (a–h), the inlay shows fibrillin-1 deposition at higher magnification in the papillar layer (a, b, e, f) and in the reticular layer (c, d, g, h). Bar: 50 µm.
In the scar tissue of most of the patients with incisional hernias the fibrillin-1 positive subepithelial structures morphologically largely resembled those of the corresponding non-scar tissues (Fig. 4e,f) both in the papillar and in the reticular layer (Fig. 4g, see Fig. 4c for comparison). In three patients, a distinctive decrease in the density of fibrillar fibrillin-1 structures was visible when compared with non-scar regions; in one patient filiform fibrils stained for fibrillin-1 in an abnormally condensed dermal connective tissue were observed (Fig. 4h).
Discussion
Fibrillin-1 in muscle fascia
Fascial layers are primarily responsible for the stability of the abdominal wall. Therefore, we first aimed to answer the question as to whether an altered distribution of fibrillin-1 in the muscle fascia occurs in patients with hernia compared to those without hernia.
Fibrillin-1 in scar regions of the muscle fascia
Scar samples of muscle fascia generally presented a reduction in fibrillar reactivity and some alterations in fibrillin distribution with presence of clots, thicker bundles and abrupt directional changes when compared to reference tissue (i.e. non-scar fascia of patients without hernia). Studies of human fascial scar tissue revealed a distinct accumulation of heparan sulfate proteoglycans (Kozma et al. 2000). This, in turn, can inhibit the aggregation of the fibrillin-1 network in vitro (Tiedemann et al. 2001) and would for example explain why the majority of scar samples presented reduced reactivity for fibrillin-1. Unusual distribution of fibrillin-1 in fascial scar tissue as described above was never found in the fascial non-scar tissue of patients without hernia. This indicates that these features develop during the wound healing process and that they are typical of scars.
In particular, clots, with their composition as amorphous, non-orientated structures, do not seem to be suitable to fulfil stabilizing tasks. In contrast, as other aberrant forms of fibrillin-1 deposition, e.g. thicker fibrillin-1 positive bundles, they may reflect a pathological aggregation of fibrillin-1 in the tissue which could possibly lead to disarray and eventually negatively influence scar stability. However, in addition to a structural role in scars, it is conceivable that fibrillin-1 molecules may play important cell biological roles in scar formation. Fibrillin-1 may act as a reservoir for transforming growth factor beta 1 (TGF-β1) via latent TGF-β binding protein 1 (LTBP-1) which binds to the microfibrillar structures and fixes TGF-β1 (Raghunath et al. 1998; Isogai et al. 2003). Recent studies revealed an important role for fibrillin-1 in the release and activation of TGF-β1 (Kaartinen & Warburton, 2003; Neptune et al. 2003; Zhou et al. 2005). TGF-β1 coordinates important cellular interactions like inflammation, reepithelialization, angiogenesis and production of matrix components, e.g. type I collagen or fibrillin-1 itself (O’Kane & Ferguson, 1997; Zhou et al. 1997; Sterzel et al. 2000), and it also accelerates wound healing and fibrillogenesis of microfibrils containing fibrillin-1 in vitro (Mustoe et al. 1987; Kissin et al. 2002). In this context, the regulation of type I collagen deposition could also be relevant. A decrease of type I/III collagen or procollagen ratio may in fact be favourable for incisional hernias (Klinge et al. 2000; Klinge et al. 2001; Si et al. 2002; Rosch et al. 2003). In addition to TGF-β1 (Zhou et al. 1997), which shows functional interactions with fibrillin-1 (Raghunath et al. 1998; Sterzel et al. 2000; Isogai et al. 2003; Kaartinen & Warburton, 2003; Neptune et al. 2003) other factors which are not known to functionally interact with fibrillin-1, such as interleukin (IL) β, interferon (IFN)-γ or tumour necrosis factor (TNF)-α may take part in the complex regulation of type I collagen production (Ghosh, 2002). Furthermore, disturbances in some other extracellular components such as tenascin or in matrix related proteins, e.g. the collagen receptor DDR-2 or in MMP-1, have been implicated as favourable factors for the development of incisional hernias (Klinge et al. 2001; Rosch et al. 2003).
In this complex scenario of factors which regulate scar formation and possibly also scar stability, our results show the presence of fibrillin-1 as one of the regular scar components of the muscle fascia. Its deposition can present patterns which, like clots and thicker bundles, diverge from the typical fibrillar deposition and may, therefore, reflect aberrant aggregation. It is conceivable that this may be detrimental to scar stability. However, clots and/or thicker bundles of fibrillin-1 also occur in fascial scar tissue of patients without hernia, although to a generally lesser extent. This suggests that disturbances in the fibrillin-1 microfibrillar network of the fascial scar tissue may only partially be responsible for a tendency to develop postoperative hernias, probably as part of a more complex system which contributes in toto to formation of stable scars.
Fibrillin-1 in non-scar regions of the muscle fascia
In non-scar regions of six of 12 patients with incisional hernias, aberrant distribution of fibrillin-1 was observed. Here, divergent directions of fibrillin positive structures, smooth transitions with no clear demarcation from parallel, unidirectionally ordered to multidirectional and loosely arranged fibrils, and, loose deposition of disarrayed fibrillin-1 positive structures were observed. It is reasonable to assume that such a disarrayed pattern of fibrillin-1 reactivity in the non-scar muscle fascia (i.e. ‘normal tissue’) of patients with incisional hernia would reflect a weakening of connective tissue which consecutively may lead to insufficient scar formation during wound healing. In this context, it is interesting to note that various types of hernias appear in several fibrillin-1 associated syndromes such as neonatal and classical Marfan syndrome (Parida et al. 1997; Jacobs et al. 2002; Yetman et al. 2003) cutis laxa (Bonneau et al. 1991; Damkier et al. 1991) or Shprintzen-Goldberg syndrome (Sood et al. 1996). Our observations provide evidence that not only defects in collagen deposition in scars (Klinge et al. 2000) but also an altered fibrillin-1 distribution in the tissue, even before scar formation, is a potential factor favouring the development of incisional hernias.
Fibrillin-1 in skin
The skin is a tissue which can relatively easily be accessed for biopsies. For this reason, its examination could be a valuable parameter to investigate more general defects in connective tissue in the context of certain diseases, in this case, incisional hernias.
In non-scar samples of the skin from the group with incisional hernias, a general trend to a reduced structural complexity and to more irregular and disarrayed structures in the subepithelial network was observed compared with the group without hernias.
Such tendency to a reduced structural complexity of the subepithelial fibrillin-1 network could be suggestive of a defect in the deposition and aggregation of fibrillin-1 molecules in the tissue. Although the cellular source of fibrillin-1 has not been demonstrated in vivo, it is known that keratinocytes and dermal fibroblasts are able to produce fibrillin-1 in culture (Haynes et al. 1997; Dzamba et al. 2001). Cultured keratinocytes, in contrast to dermal fibroblasts, produce fibrillin-1 mainly in a non-fibrillar form. Once extracellular, it is assembled to microfibrils in the presence of co-cultured fibroblasts (Dzamba et al. 2001). Therefore, subepidermal microfibril formation appears to be a complex process involving cooperation of several cell types. Possible disturbances in such cellular interactions may therefore favour a defective aggregation and disturbed polymerization of fibrillin-1 monomers which may eventually lead to less stable microfibrillar polymers. Unstable fibrillin-1-polymers are found for example in scleroderma and are also more susceptible of proteolysis (Wallis et al. 2001; Kodera et al. 2002). Microfibrils containing fibrillin-1 may act as a scaffold for the development of elastic fibers which provide considerable support for tissue stability and elasticity (Rosenbloom et al. 1993). Pathological changes in the elastic fiber network result in severe diseases, e.g. cutis laxa, supravalvular aortic stenosis and Williams-Beuren syndrome. These disorders are associated with a structural weakening of the connective tissue, so hernias and diverticulosis are frequently observed (Williams et al. 1961; Eliashar et al. 1998; Tassabehji et al. 1998; Zhang et al. 1999; Milewicz et al. 2000). Interestingly, similar to our observations, changes in fibrillin deposition with reduced structural complexity of the subepithelial fibrillin-1 positive network were observed in the skin of patients with hypertrophic scars and keloids (Amadeu et al. 2004) as well as in patients with acquired cutis laxa (Lebwohl et al. 1994). Changes in the microfibrillar apparatus may per se have consequences for the stability of connective tissue, as shown for example in cutis laxa (Lebwohl et al. 1994), progressive systemic sclerosis (Tan et al. 1999; Saito et al. 2000; Tan et al. 2001; Wallis et al. 2001) and cutaneous manifestations of Marfan syndrome (Pyeritz, 2000; Collod-Beroud & Boileau, 2002). According to our results, the qualitative comparison of cutaneous fibrillin deposition effectively points only to a tendency to a less structured network in patients with hernia compared with patients without hernia, and this does not provide sufficient objectivity for a pre-operative clinical assessment of the patients. To objectively quantify possible changes between the two groups, we measured spatial density of fibrillin-1 subepidermal networks. These measurements, which did not take into account morphological alteration of fibrillin-1 positive structures, did not reveal a significant difference in the density between the two groups examined. Thus, this test, only based on fibrillin-1, is not suitable for a reliable, pre-operative, prognostic risk assessment for postoperative hernias and this issue still remains open.
Conclusions
The present study shows for the first time the pattern of fibrillin-1 in human skin and muscle fascia in correlation with a clinical history of incisional hernias, both in scarred and in non-scarred tissue. We also show for the first time that fibrillin-1 is a regular component of human muscle fascia and of postoperative scars in this tissue. Our results indicate fibrillin-1 as one of the relevant factors for the stabilization of the connective tissue in the context studied.
Fibrillin-1 polymorphism is not only associated to Marfan syndrome but also to other related microfibrillopathies (Robinson & Godfrey, 2000). Likewise, some ‘minor’ defects in fibrillin genetic and/or biology, may contribute to disturbing its deposition even before scar formation. Taken together, our results and these considerations indicate the possibility that a complex picture of connective tissue defects present prior to scar formation, which also include disturbed fibrillin-1 deposition, could represent an important factor favouring development of incisional hernias. In this light, future investigations directed towards the genetics and/or expression of fibrillin-1 and other connective tissue components may potentially improve the understanding of the mechanisms which lead to incisional hernias.
Acknowledgments
We wish to dedicate this paper to the memory of our mentor Prof. Rainer Herken, who passed away on 3 November 2005. Without his steady encouragement and precious support this project would not have been possible.
We thank Prof. Peter Dockery of the Dept. of Anatomy of the NUI Galway for critical review of the manuscript.
We would also like to thank Berti Manshausen, Christina Zelent and Rod Dungan for their technical assistance as well as Cyrilla Maelicke and Dolores Tierney for editing the manuscript. This work was supported by the Canadian Institutes of Health Research (MOP-68836) and the Canadian Marfan Association (to DPR).
References
- Amadeu TP, Braune AS, Porto LC, Desmouliere A, Costa AM. Fibrillin-1 and elastin are differentially expressed in hypertrophic scars and keloids. Wound Repair Regen. 2004;12:169–174. doi: 10.1111/j.1067-1927.2004.012209.x. [DOI] [PubMed] [Google Scholar]
- Anthony T, Bergen PC, Kim LT, et al. Factors affecting recurrence following incisional herniorrhaphy. World J Surg. 2000;24:95–100. doi: 10.1007/s002689910018. discussion 101. [DOI] [PubMed] [Google Scholar]
- Baldock C, Koster AJ, Ziese U, et al. The supramolecular organization of fibrillin-rich microfibrils. J Cell Biol. 2001;152:1045–1056. doi: 10.1083/jcb.152.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau D, Huret JL, Godeau G, et al. Recurrent ctb(7)(q31.3) and possible laminin involvement in a neonatal cutis laxa with a Marfan phenotype. Hum Genet. 1991;87:317–319. doi: 10.1007/BF00200911. [DOI] [PubMed] [Google Scholar]
- Collod-Beroud G, Boileau C. Marfan syndrome in the third Millennium. Eur J Hum Genet. 2002;10:673–681. doi: 10.1038/sj.ejhg.5200876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damkier A, Brandrup F, Starklint H. Cutis laxa: autosomal dominant inheritance in five generations. Clin Genet. 1991;39:321–329. doi: 10.1111/j.1399-0004.1991.tb03038.x. [DOI] [PubMed] [Google Scholar]
- De Paepe A, Devereux RB, Dietz HC, Hennekam RC, Pyeritz RE. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet. 1996;62:417–426. doi: 10.1002/(SICI)1096-8628(19960424)62:4<417::AID-AJMG15>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Dietz HC, Cutting GR, Pyeritz RE, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- Dingemans KP, Teeling P, Lagendijk JH, Becker AE. Extracellular matrix of the human aortic media: an ultrastructural histochemical and immunohistochemical study of the adult aortic media. Anat Rec. 2000;258:1–14. doi: 10.1002/(SICI)1097-0185(20000101)258:1<1::AID-AR1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Dzamba BJ, Keene DR, Isogai Z, et al. Assembly of epithelial cell fibrillins. J Invest Dermatol. 2001;117:1612–1620. doi: 10.1046/j.0022-202x.2001.01588.x. [DOI] [PubMed] [Google Scholar]
- Eliashar R, Sichel JY, Biron A, Dano I. Multiple gastrointestinal complications in Marfan syndrome. Postgrad Med J. 1998;74:495–497. doi: 10.1136/pgmj.74.874.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AK. Factors involved in the regulation of type I collagen gene expression: implication in fibrosis. Exp Biol Med (Maywood) 2002;227:301–314. doi: 10.1177/153537020222700502. [DOI] [PubMed] [Google Scholar]
- Haynes SL, Shuttleworth CA, Kielty CM. Keratinocytes express fibrillin and assemble microfibrils: implications for dermal matrix organization. Br J Dermatol. 1997;137:17–23. [PubMed] [Google Scholar]
- Höer J, Lawong G, Klinge U, Schumpelick V. Einflussfaktoren der Narbenhernienentstehung. Retrospektive Untersuchung an 2983 laparotomierten Patienten über einen Zeitraum von 10 Jahren. Chirurg. 2002a;73:474–480. doi: 10.1007/s00104-002-0425-5. [DOI] [PubMed] [Google Scholar]
- Höer J, Stumpf M, Rosch R, Klinge U, Schumpelick V. Prophylaxe der Narbenhernie. Chirurg. 2002b;73:881–887. doi: 10.1007/s00104-002-0539-9. [DOI] [PubMed] [Google Scholar]
- Isogai Z, Ono RN, Ushiro S, et al. Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J Biol Chem. 2003;278:2750–2757. doi: 10.1074/jbc.M209256200. [DOI] [PubMed] [Google Scholar]
- Jacobs AM, Toudjarska I, Racine A, et al. A recurring FBN1 gene mutation in neonatal Marfan syndrome. Arch Pediatr Adolesc Med. 2002;156:1081–1085. doi: 10.1001/archpedi.156.11.1081. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Warburton D. Fibrillin controls TGF-beta activation. Nat Genet. 2003;33:331–332. doi: 10.1038/ng0303-331. [DOI] [PubMed] [Google Scholar]
- Keene DR, Jordan CD, Reinhardt DP, et al. Fibrillin-1 in human cartilage: developmental expression and formation of special banded fibers. J Histochem Cytochem. 1997;45:1069–1082. doi: 10.1177/002215549704500805. [DOI] [PubMed] [Google Scholar]
- Keene DR, Maddox BK, Kuo HJ, Sakai LY, Glanville RW. Extraction of extendable beaded structures and their identification as fibrillin-containing extracellular matrix microfibrils. J Histochem Cytochem. 1991a;39:441–449. doi: 10.1177/39.4.2005373. [DOI] [PubMed] [Google Scholar]
- Keene DR, Sakai LY, Burgeson RE. Human bone contains type III collagen, type VI collagen, and fibrillin: type III collagen is present on specific fibers that may mediate attachment of tendons, ligaments, and periosteum to calcified bone cortex. J Histochem Cytochem. 1991b;39:59–69. doi: 10.1177/39.1.1983874. [DOI] [PubMed] [Google Scholar]
- King BF, Blankenship TN. Immunohistochemical localization of fibrillin in developing macaque and term human placentas and fetal membranes. Microsc Res Tech. 1997;38:42–51. doi: 10.1002/(SICI)1097-0029(19970701/15)38:1/2<42::AID-JEMT6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the Behavioural Sciences. Pacific Grove, CA: Brooks/Cole Pub. Co; 1982. [Google Scholar]
- Kissin EY, Lemaire R, Korn JH, Lafyatis R. Transforming growth factor beta induces fibroblast fibrillin-1 matrix formation. Arthritis Rheum. 2002;46:3000–3009. doi: 10.1002/art.10621. [DOI] [PubMed] [Google Scholar]
- Klinge U, Si ZY, Zheng H, et al. Abnormal collagen I to III distribution in the skin of patients with incisional hernia. Eur Surg Res. 2000;32:43–48. doi: 10.1159/000008740. [DOI] [PubMed] [Google Scholar]
- Klinge U, Si ZY, Zheng H, et al. Collagen I/III and matrix metalloproteinases (MMP) 1 and 13 in the fascia of patients with incisional hernias. J Invest Surg. 2001;14:47–54. doi: 10.1080/089419301750072202. [DOI] [PubMed] [Google Scholar]
- Kodera T, Tan FK, Sasaki T, Arnett FC, Bona CA. Association of 5′-untranslated region of the Fibrillin-1 gene with Japanese scleroderma. Gene. 2002;297:61–67. doi: 10.1016/s0378-1119(02)00862-4. [DOI] [PubMed] [Google Scholar]
- Kozma EM, Olczyk K, Glowacki A, Bobinski R. An accumulation of proteoglycans in scarred fascia. Mol Cell Biochem. 2000;203:103–112. doi: 10.1023/a:1007012321333. [DOI] [PubMed] [Google Scholar]
- Krug F, Herold A, Wenk H, Bruch HP. [Incisional hernias after laparoscopic interventions] Chirurg. 1995;66:419–423. [PubMed] [Google Scholar]
- Langer C, Schaper A, Liersch T, et al. Prognosis factors in incisional hernia surgery: 25 years of experience. Hernia. 2005;9:16–21. doi: 10.1007/s10029-004-0265-y. [DOI] [PubMed] [Google Scholar]
- Leber GE, Garb JL, Alexander AI, Reed WP. Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg. 1998;133:378–382. doi: 10.1001/archsurg.133.4.378. [DOI] [PubMed] [Google Scholar]
- Lebwohl MG, Schwartz E, Jacobs L, et al. Abnormalities of fibrillin in acquired cutis laxa. J Am Acad Dermatol. 1994;30:950–954. doi: 10.1016/s0190-9622(94)70115-6. [DOI] [PubMed] [Google Scholar]
- Maddox BK, Sakai LY, Keene DR, Glanville RW. Connective tissue microfibrils. Isolation and characterization of three large pepsin-resistant domains of fibrillin. J Biol Chem. 1989;264:21381–21385. [PubMed] [Google Scholar]
- Milewicz DM, Urban Z, Boyd C. Genetic disorders of the elastic fiber system. Matrix Biol. 2000;19:471–480. doi: 10.1016/s0945-053x(00)00099-8. [DOI] [PubMed] [Google Scholar]
- Mustoe TA, Pierce GF, Thomason A, et al. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987;237:1333–1336. doi: 10.1126/science.2442813. [DOI] [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, et al. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- O’Kane S, Ferguson MW. Transforming growth factor beta s and wound healing. Int J Biochem Cell Biol. 1997;29:63–78. doi: 10.1016/s1357-2725(96)00120-3. [DOI] [PubMed] [Google Scholar]
- Parida SK, Kriss VM, Hall BD. Hiatus/paraesophageal hernias in neonatal Marfan syndrome. Am J Med Genet. 1997;72:156–158. doi: 10.1002/(sici)1096-8628(19971017)72:2<156::aid-ajmg6>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Pereira L, Andrikopoulos K, Tian J, et al. Targetting of the gene encoding fibrillin-1 recapitulates the vascular aspect of Marfan syndrome. Nat Genet. 1997;17:218–222. doi: 10.1038/ng1097-218. [DOI] [PubMed] [Google Scholar]
- Pyeritz RE. The Marfan syndrome. Annu Rev Med. 2000;51:481–510. doi: 10.1146/annurev.med.51.1.481. [DOI] [PubMed] [Google Scholar]
- Quondamatteo F, Knittel T, Mehde M, Ramadori G, Herken R. Matrix metalloproteinases in early human liver development. Histochem Cell Biol. 1999;112:277–282. doi: 10.1007/s004180050448. [DOI] [PubMed] [Google Scholar]
- Quondamatteo F, Reinhardt DP, Charbonneau NL, et al. Fibrillin-1 and fibrillin-2 in human embryonic and early fetal development. Matrix Biol. 2002;21:637–646. doi: 10.1016/s0945-053x(02)00100-2. [DOI] [PubMed] [Google Scholar]
- Raghunath M, Unsold C, Kubitscheck U, et al. The cutaneous microfibrillar apparatus contains latent transforming growth factor-beta binding protein-1 (LTBP-1) and is a repository for latent TGF-beta1. J Invest Dermatol. 1998;111:559–564. doi: 10.1046/j.1523-1747.1998.00339.x. [DOI] [PubMed] [Google Scholar]
- Reinhardt DP, Keene DR, Corson GM, et al. Fibrillin-1: organization in microfibrils and structural properties. J Mol Biol. 1996;258:104–116. doi: 10.1006/jmbi.1996.0237. [DOI] [PubMed] [Google Scholar]
- Robinson PN, Godfrey M. The molecular genetics of Marfan syndrome and related microfibrillopathies. J Med Genet. 2000;37:9–25. doi: 10.1136/jmg.37.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosch R, Junge K, Knops M, et al. Analysis of collagen-interacting proteins in patients with incisional hernias. Langenbecks Arch Surg. 2003;387:427–432. doi: 10.1007/s00423-002-0345-3. [DOI] [PubMed] [Google Scholar]
- Rosenbloom J, Abrams WR, Mecham R. Extracellular matrix 4: the elastic fiber. FASEB J. 1993;7:1208–1218. [PubMed] [Google Scholar]
- Saito S, Nishimura H, Phelps RG, et al. Induction of skin fibrosis in mice expressing a mutated fibrillin-1 gene. Mol Med. 2000;6:825–836. [PMC free article] [PubMed] [Google Scholar]
- Sakai LY, Keene DR, Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol. 1986;103:2499–2509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai LY, Keene DR, Glanville RW, Bachinger HP. Purification and partial characterization of fibrillin, a cysteine-rich structural component of connective tissue microfibrils. J Biol Chem. 1991;266:14763–14770. [PubMed] [Google Scholar]
- Santora TA, Roslyn JJ. Incisional hernia. Surg Clin North Am. 1993;73:557–570. doi: 10.1016/s0039-6109(16)46037-8. [DOI] [PubMed] [Google Scholar]
- Si Z, Bhardwaj R, Rosch R, et al. Impaired balance of type I and type III procollagen mRNA in cultured fibroblasts of patients with incisional hernia. Surgery. 2002;131:324–331. doi: 10.1067/msy.2002.121376. [DOI] [PubMed] [Google Scholar]
- Silverman DI, Burton KJ, Gray J, et al. Life expectancy in the Marfan syndrome. Am J Cardiol. 1995;75:157–160. doi: 10.1016/s0002-9149(00)80066-1. [DOI] [PubMed] [Google Scholar]
- Sood S, Eldadah ZA, Krause WL, McIntosh I, Dietz HC. Mutation in fibrillin-1 and the Marfanoid-craniosynostosis (Shprintzen-Goldberg) syndrome. Nat Genet. 1996;12:209–211. doi: 10.1038/ng0296-209. [DOI] [PubMed] [Google Scholar]
- Sterzel RB, Hartner A, Schlotzer-Schrehardt U, et al. Elastic fiber proteins in the glomerular mesangium in vivo and in cell culture. Kidney Int. 2000;58:1588–1602. doi: 10.1046/j.1523-1755.2000.00320.x. [DOI] [PubMed] [Google Scholar]
- Tan FK, Arnett FC, Antohi S, et al. Autoantibodies to the extracellular matrix microfibrillar protein, fibrillin-1, in patients with scleroderma and other connective tissue diseases. J Immunol. 1999;163:1066–1072. [PubMed] [Google Scholar]
- Tan FK, Wang N, Kuwana M, et al. Association of fibrillin 1 single-nucleotide polymorphism haplotypes with systemic sclerosis in Choctaw and Japanese populations. Arthritis Rheum. 2001;44:893–901. doi: 10.1002/1529-0131(200104)44:4<893::AID-ANR146>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Tassabehji M, Metcalfe K, Hurst J, et al. An elastin gene mutation producing abnormal tropoelastin and abnormal elastic fibres in a patient with autosomal dominant cutis laxa. Hum Mol Genet. 1998;7:1021–1028. doi: 10.1093/hmg/7.6.1021. [DOI] [PubMed] [Google Scholar]
- Tiedemann K, Batge B, Muller PK, Reinhardt DP. Interactions of fibrillin-1 with heparin/heparan sulfate, implications for microfibrillar assembly. J Biol Chem. 2001;276:36035–36042. doi: 10.1074/jbc.M104985200. [DOI] [PubMed] [Google Scholar]
- Wallis DD, Tan FK, Kielty CM, et al. Abnormalities in fibrillin 1-containing microfibrils in dermal fibroblast cultures from patients with systemic sclerosis (scleroderma) Arthritis Rheum. 2001;44:1855–1864. doi: 10.1002/1529-0131(200108)44:8<1855::AID-ART324>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Williams JCP, Barrat-Boyes BG, Lowe JB. Supravalvular aortic stenosis. Circulation. 1961;24:1311–1318. doi: 10.1161/01.cir.24.6.1311. [DOI] [PubMed] [Google Scholar]
- Yetman AT, Greenberg SB, Ghaffar S. Diaphragmatic hernia. Pediatr Cardiol. 2003;24:307–308. doi: 10.1007/s00246-002-2365-8. [DOI] [PubMed] [Google Scholar]
- Zhang MC, He L, Giro M, et al. Cutis laxa arising from frameshift mutations in exon 30 of the elastin gene (ELN) J Biol Chem. 1999;274:981–986. doi: 10.1074/jbc.274.2.981. [DOI] [PubMed] [Google Scholar]
- Zhou LJ, Ono I, Kaneko F. Role of transforming growth factor-beta 1 in fibroblasts derived from normal and hypertrophic scarred skin. Arch Dermatol Res. 1997;289:646–652. doi: 10.1007/s004030050254. [DOI] [PubMed] [Google Scholar]
- Zhou X, Tan FK, Milewicz DM, et al. Autoantibodies to fibrillin-1 activate normal human fibroblasts in culture through the TGF-beta pathway to recapitulate the ‘scleroderma phenotype’. J Immunol. 2005;175:4555–4560. doi: 10.4049/jimmunol.175.7.4555. [DOI] [PubMed] [Google Scholar]