Abstract
In order to increase current knowledge regarding statherin secretion into the oral cavity, ultrastructural localization of this peptide was investigated in human salivary glands by using a post-embedding immunogold staining technique. Statherin reactivity was found inside the granules of serous cells of parotid and submandibular glands. In parotid granules immunostaining was preferentially present in the less electron-dense region, whereas in submandibular serous granules the reactivity was uniform and the dense core always stained. By contrast, none or weak reactivity was observed in serous cells of major sublingual glands. These findings reveal for the first time the subcellular localization of statherin by electron transmission microscopy and confirm that of the three major types of salivary glands, the parotid and submandibular glands are the greatest source of salivary statherin. Moreover, they suggest that more than one packaging mechanism may be involved in the storage of statherin within serous granules of salivary glands.
Keywords: human salivary glands, immunogold, secretory granules, statherin
Introduction
Saliva is crucial for the maintenance of oral health (Humphrey & Williamson, 2001) in that it contains a complex mixture of molecules possessing a multitude of functions, such as lubrication of the oral mucosa, defence from infections, prevention of tooth demineralization and promotion of tooth remineralization.
Salivary components include two small phosphoproteins, statherin and histatin, which seem to share a common ancestral gene responsible for their sequence. Both statherin and histatin contribute to form the acquired enamel pellicle, which enhances the function of saliva as a protective layer on the mucosal surfaces and teeth (Schupbach et al. 2001). A strong correlation between high salivary concentrations of phosphopeptides and the absence of dental caries was demonstrated (Sreebny, 2000; Vitorino et al. 2005). However, the mechanisms by which they act to maintain oral health seem to be quite different. Whereas histatin acts by entering bacterial or fungal cells and altering directly their metabolism, the most well-known activities of statherin are, by contrast, related to microbial adhesion events associated with dental plaque formation (Douglas et al. 1991) and bacterial colonization (Gibbons & Hay, 1988). Moreover, statherin is the only salivary protein that inhibits both primary and secondary calcium phosphate precipitation (Hay & Schlesinger, 1977; Moreno et al. 1979; Schwartz et al. 1992). Through its influence on the transport of calcium and phosphate, statherin can also affect salivary gland secretion (Bennick et al. 1981). Interestingly, a reduction of statherin levels was observed in saliva of patients with precancerous and cancerous lesions (Contucci et al. 2005).
Statherin mRNA was detected in human and rhesus parotid and submandibular glands, in human bronchus, in rhesus lacrimal glands, and in macaque taste-bud tissues containing serous cells of von Ebner's glands (Sabatini et al. 1989; Azen et al. 1990). Until now, direct evidence for the presence of statherin in human salivary glands has not been provided, except for data obtained in human von Ebner's gland by immunohistochemistry at the light microscopy level (Azen et al. 1990).
The aim of the present study was to show the subcellular localization of statherin in human salivary glands at the electron transmission microscopy (TEM) level applying a post-embedding immunogold (IGS) technique.
Materials and methods
Samples of normal parotid, submandibular and major sublingual glands were obtained from 15 consenting male and female patients, aged 40–60 years, undergoing surgery at the Otorhinolaryngology Clinic, University of Cagliari, Italy. All procedures were approved by the respective institutional committees on human experimentation at the ASL 8 (azienda sanitaria locale 8), Cagliari. For TEM studies the glands were cut into small pieces and fixed for 2 h in a mixture of 3% paraformaldehyde and 1% glutaraldehyde in 0.1 m cacodylate buffer (pH 7.2). The samples were then rinsed in 0.1 m cacodylate buffer supplemented with 3.5% sucrose, dehydrated, and embedded in Epon Resin (Glycide Ether 100, Merk, Darmstadt, Germany). Ultrathin sections collected on nickel grids were treated with 1% bovine serum albumin (BSA) and 5% normal rabbit serum (NRS) in phosphate-buffered saline (PBS) solution to block non-specific binding. The sections were incubated overnight at 4 °C with a goat polyclonal antibody specific for statherin (Santa Cruz Biotechnology), diluted 1 : 50 in 1% BSA and 5% NRS. After rinsing, the grids were incubated for 1 h at room temperature with the secondary antiserum, a rabbit anti-goat IgG labelled with 10-nm gold particles (Sigma) diluted 1 : 50 in BSA–PBS. The grids were washed with PBS and distilled water, stained with uranyl acetate and bismuth subnitrate (Riva, 1974), and finally observed and photographed in a JEOL 100S transmission electron microscope. Sections incubated with medium devoid of primary antibody or containing non-immune goat serum were used as controls.
Results
Immunogold staining experiments showed that statherin was reactive in serous cells of human parotid and submandibular glands, primarily in the secretory granules.
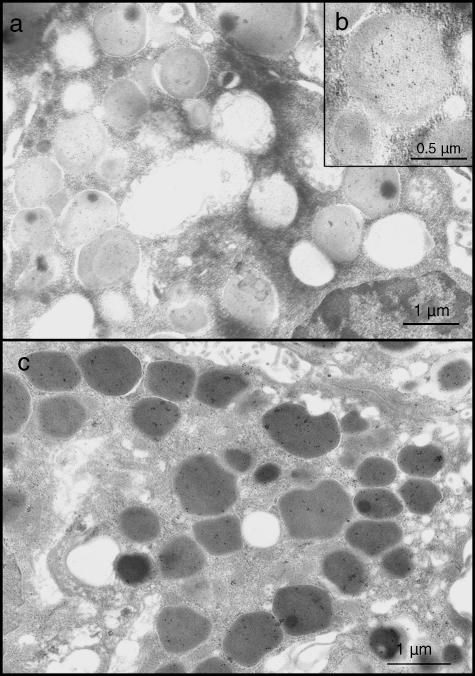
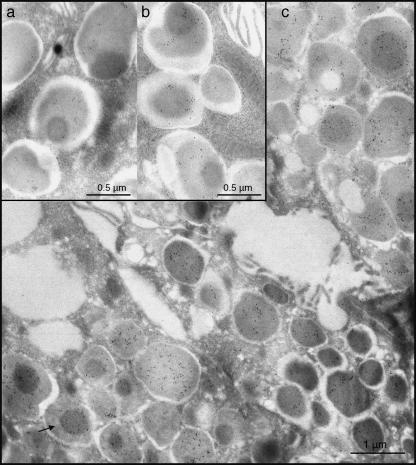
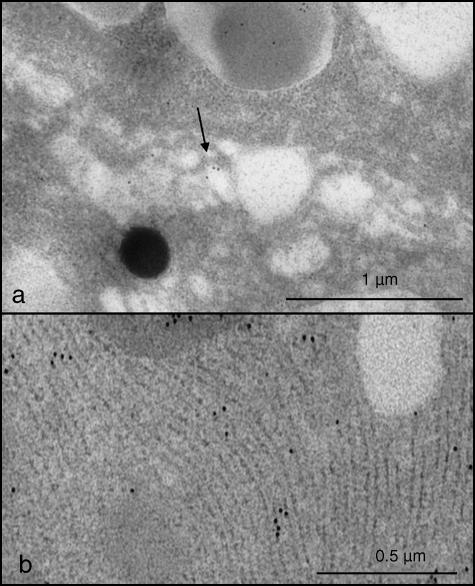
The intragranular distribution of the gold particles was different in the two glands. In granules of the six parotid glands examined, a strong reactivity was usually observed in the pale portions, while those of medium density appeared labelled less frequently (Fig. 1a,b). The spherules were punctuated only exceptionally. In granules of the four submandibular glands examined, statherin labelling usually appeared uniformly distributed (Fig. 1c), although in some instances the dense core displayed an intense reactivity, whereas the remaining area of the granules was unstained (Fig. 2a–c). In both parotid and submandibular glands, a weak reactivity was often detected in Golgi cisternae and in rough endoplasmic reticulum (Fig. 3a,b), while all other organelles were always negative.
Fig. 1.
Granules of acinar parotid cells; immunogold labelling is preferentially present in the pale portion (a,b). Submandibular serous cells. Immunoreactivity is found throughout the granule content (c).
Fig. 2.
Submandibular granules (a–c). Almost all granules show a uniform reactivity. Occasionally (arrow), they show a strongly reactive dense core.
Fig. 3.
Portions of a serous cell of the submandibular gland. Golgi apparatus (a, arrow) and rough endoplasmic reticulum (b) are decorated by gold particles.
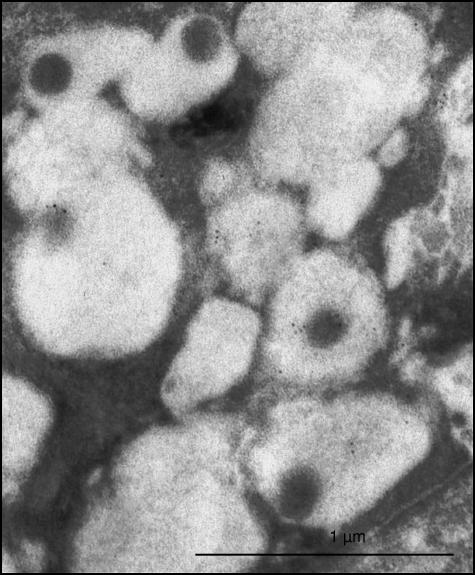
In the five sublingual glands examined, a faint reactivity to statherin was occasionally observed in a small number of serous granules (Fig. 4). The controls of all glands examined were negative.
Fig. 4.
Serous cells of the major sublingual gland. Note that only a limited number of serous granules show a faint immunoreactivity in their matrix.
Discussion
The present study demonstrates for the first time the ultrastructural localization of statherin in human salivary glands. Immunoreactivity was invariably detected in serous cells of human parotid and submandibular glands, but only occasionally in this type of cells in the sublingual glands. No reactivity was observed in mucous and duct cells of these glands.
Results support earlier studies that demonstrated the presence of statherin mRNA in parotid and submandibular gland parenchyma (Sabatini et al. 1989), thus confirming that these glands are the principal source of salivary statherin. By contrast, no previous papers have mentioned the presence of statherin or mRNA statherin in sublingual gland. Thus, the weak or absent reactivity of the sublingual granules testifies that the contribution of this gland, comprising predominantly mucous elements, in the production of statherin is insignificant. It is reasonable to assume therefore that the synthesis of statherin is a property of the serous cells of the parotid and submandibular, whereas the sublingual and minor mucous salivary glands participate only minimally to the production of this peptide.
In parotid and submandibular glands, immunoreactivity was primarily confined to serous granules, where gold particles exhibited a peculiar distribution pattern in the different secretory components. Typical serous granules of human salivary glands display a complex internal substructure owing to the presence of components of various electron densities, possibly reflecting a different chemical composition (Tandler & Erlandson, 1972; Riva & Riva-Testa, 1973; Riva et al. 1974, 1988; Tandler & Phillips, 1993). Immunohistochemical investigations reveal that granules of serous cells of parotid and submandibular glands contain amylase especially concentrated in the electron-dense regions, whereas agglutinin, mucins and blood group antigens are seen in those of lower density (Takano et al. 1991, 1993; Cossu et al. 1992). In contrast, the proline-rich proteins are distributed throughout the granules (Takano et al. 1993). It was supposed that the intrinsic domain of salivary proteins may be responsible for the localization of these proteins into distinct regions of the granules (Castle & Castle, 1993; Takano et al. 1993). In the present experiments, statherin reactivity showed a different intragranular distribution in the two glands, as submandibular granules usually appeared uniformly labelled, whereas, in parotid granules, only the pale component was stained. On the one hand, this difference could be an artefact resulting from a step in tissue processing; on the other hand, it could be related to the presence of different forms and derivatives of statherin in human saliva (Jensen et al. 1991; Inzitari et al. 2006). The primary structure of statherin was characterized by Schlesinger & Hay (1977) and the sequences of statherin variants by Jensen et al. (1991). These variants are the result of unknown internal control mechanisms that are responsible for post-translational modification and alternative splicing of the statherin gene product. The differing distribution of immunoreactivity in the granules of parotid and submandibular glands could be imputed to these statherin variants, which might be arranged into complex structures with calcium or bound to other proteins, occupying diverse electron-dense areas within the granules.
Our immunohistochemical results revealed a distribution pattern of statherin very different to that obtained for histatin (Ahmad et al. 2004). In the experiments of Ahmad et al. (2004), parotid granules stained uniformly for histatin, whereas the submandibular and sublingual serous granules showed heterogeneous patterns of labelling. Although histatin seems to share a common ancestral gene with statherin (Dickinson et al. 1987) and responds similarly to masticatory and gustatory stimulations, there is increasing evidence that their synthesis and packaging undergo different control pathways. For example, statherin secretion is modified to significantly different degrees in response to secretagogues and, in contrast to histatin, does not follow a circadian rhythm (Jensen et al. 1994; Castagnola et al. 2002).
Thus, our data increase current knowledge regarding the distributions of the different salivary proteins in the parenchyma of salivary glands and provide novel evidence that different mechanisms may be involved in the packaging of salivary proteins within the secretory granule.
Acknowledgments
We wish to thank Mrs Silvana Bernardini, Mr Gabriele Conti and Mr Alessandro Cadau for technical assistance. The investigation was supported by MUR (PRIN 2006) and by Fondazione Banco di Sardegna.
References
- Ahmad M, Piludu M, Oppenheim FG, Helmerhorst EJ, Hand AR. Immunocytochemical localization of histatins in human salivary glands. J Histochem Cytochem. 2004;52:361–370. doi: 10.1177/002215540405200307. [DOI] [PubMed] [Google Scholar]
- Azen AE, Hellekant G, Sabatini LM, Warner TF. mRNAs for PRPs, statherin, and histatins in von Ebner's gland tissues. J Dent Res. 1990;69:1724–1730. doi: 10.1177/00220345900690110401. [DOI] [PubMed] [Google Scholar]
- Bennick A, McLaughlin AC, Grey AA, Madapallimattam G. The location and nature of calcium-binding sites in salivary acidic praline-rich proteins. J Biol Chem. 1981;256:4741–4746. [PubMed] [Google Scholar]
- Castagnola M, Cabras T, Denotti G, et al. Circadian rhythms of histatin 1, histatin 3, histatin 5, statherin and uric acid in whole human saliva secretion. Biol Rhythm Res. 2002;33:213–222. [Google Scholar]
- Castle JD, Castle AM. Sorting and secretion of salivary proteins. Crit Rev Oral Biol Med. 1993;4:393–398. doi: 10.1177/10454411930040031901. [DOI] [PubMed] [Google Scholar]
- Contucci AM, Inzitari R, Agostino S, et al. Statherin levels in saliva of patients with precancerous and cancerous lesions of the oral cavity: a preliminary report. Oral Diseases. 2005;11:95–99. doi: 10.1111/j.1601-0825.2004.01057.x. [DOI] [PubMed] [Google Scholar]
- Cossu M, Floris A, Lantini MS. An immunohistochemical study of the distribution of ABH antigens in human submandibular glands at the light and electron microscopic levels. Eur J Histochem. 1992;36:489–499. [PubMed] [Google Scholar]
- Dickinson DP, Ridall AL, Levine MJ. Human submandibular gland statherin and basic histidine-rich peptide are encoded by highly abundant mRNA's derived from a common ancestral sequenze. Biochem Biophys Res Comm. 1987;149:784–790. doi: 10.1016/0006-291x(87)90436-0. [DOI] [PubMed] [Google Scholar]
- Douglas WH, Reeh ES, Ramasubbu N, Raj PA, Bhandary KK, Levine MJ. Statherin: a major boundary lubricant of human saliva. Biochem Biophys. 1991;180:91–97. doi: 10.1016/s0006-291x(05)81259-8. [DOI] [PubMed] [Google Scholar]
- Gibbons RJ, Hay DI. Human salivary acidic proline-rich proteins and statherin promote the attachment of Actinomyces viscosus LY7 to apatitic surfaces. Infect Immun. 1988;56:439–445. doi: 10.1128/iai.56.2.439-445.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DI, Schlesinger DH. Human salivary statherin: a peptide inhibitor of calcium phosphate precipitation. In: Wassermann RH, Corradino RA, Carafoli E, Kretsinger RM, MacLennon DH, Siegal FL, editors. Calcium Binding Proteins and Calcium Function. North Holland, NY: Elsevier; 1977. pp. 401–408. [Google Scholar]
- Humphrey SP, Williamson RT. A review of saliva: normal composition, flow and fuction. J Prost Dent. 2001;85:162–168. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- Inzitari R, Cabras T, Rossetti DV, et al. Detection in human saliva of different statherin and P-B fragments and derivatives. Proteomics. 2006;6:6370–6379. doi: 10.1002/pmic.200600395. [DOI] [PubMed] [Google Scholar]
- Jensen JL, Lamkin MS, Troxler RF, Oppenheim FG. Multiple forms of statherin in human salivary secretions. Arch Oral Biol. 1991;36:529–534. doi: 10.1016/0003-9969(91)90147-m. [DOI] [PubMed] [Google Scholar]
- Jensen JL, Xu T, Lamkin MS, et al. Physiological regulation of the secretion of Histathin and statherin in human parotid saliva. J Dent Res. 1994;73:1811–1817. doi: 10.1177/00220345940730120401. [DOI] [PubMed] [Google Scholar]
- Moreno EC, Varughese K, Hay DI. Effect of human salivary proteins on the precipitation kinectics of calcium phophate. Calcif Tissue Int. 1979;28:7–16. doi: 10.1007/BF02441212. [DOI] [PubMed] [Google Scholar]
- Riva A, Riva-Testa F. Fine structure of acinar cells of human parotid gland. Anat Rec. 1973;176:149–166. doi: 10.1002/ar.1091760204. [DOI] [PubMed] [Google Scholar]
- Riva A. A simple and rapid staining method for enhancing the contrast of tissues previously treated with uranyl-acetate. J Microsc. 1974;19:105–108. [Google Scholar]
- Riva A, Motta G, Riva-Testa F. Ultrastructural diversity in secretory granules of human major salivary glands. Am J Anat. 1974;139:293–298. doi: 10.1002/aja.1001390210. [DOI] [PubMed] [Google Scholar]
- Riva A, Tandler B, Testa Riva F. Ultrastructural observations on human sublingual gland. Am J Anat. 1988;181:385–392. doi: 10.1002/aja.1001810406. [DOI] [PubMed] [Google Scholar]
- Sabatini LM, Warner TF, Saitho E, Azen EA. Tissue distribution of RNAs for cystatins, histatins, statherin, and proline-rich salivary proteins in humans and macaques. J Dent Res. 1989;68:1138–1145. doi: 10.1177/00220345890680070101. [DOI] [PubMed] [Google Scholar]
- Schlesinger DH, Hay DI. Complete covalent structure of statherin, a tyrosine-rich acidic peptide which inhibits calcium phosphate precipitation from human parotid saliva. J Biol Chem. 1977;252:1689–1695. [PubMed] [Google Scholar]
- Schupbach P, Oppenheim FG, Lendenmann U, Lamkin MS, Yao Y, Guggenheim B. Electron-microscopic demonstration of proline-rich proteins, statherin, and histatins in acquired enamel pellicles in vitro. Eur J Oral Sci. 2001;109:60–68. doi: 10.1034/j.1600-0722.2001.00925.x. [DOI] [PubMed] [Google Scholar]
- Schwartz SS, Hay DI, Schluckebier SK. Inhibition of calcium phosphate precipitation by human salivary statherin: structure–activity relationships. Calcif Tiss. 1992;50:511–517. doi: 10.1007/BF00582164. [DOI] [PubMed] [Google Scholar]
- Sreebny LM. Saliva in health and disease: an appraisal and update. Int Dent J. 2000;50:140–161. doi: 10.1111/j.1875-595x.2000.tb00554.x. [DOI] [PubMed] [Google Scholar]
- Takano K, Bogert M, Malamud D, Lally E, Hand AR. Differential distribution of salivary agglutinin and amylase in the Golgi apparatus and secretory granules of human salivary gland acinar cells. Anat Rec. 1991;230:307–318. doi: 10.1002/ar.1092300303. [DOI] [PubMed] [Google Scholar]
- Takano K, Malamud D, Bennick A, Oppenheim F, Hand AR. Localization of salivary proteins in granules of human parotid and submandibular acinar cells. Crit Rev Oral Biol Med. 1993;4:399–405. doi: 10.1177/10454411930040032001. [DOI] [PubMed] [Google Scholar]
- Tandler B, Erlandson RA. Ultrastructure of the human submaxillary gland. IV. Serous granules. Am J Anat. 1972;135:419–433. doi: 10.1002/aja.1001350308. [DOI] [PubMed] [Google Scholar]
- Tandler B, Phillips CJ. Structure of serous cells in salivary glands. Microsc Res Tech. 1993;26:32–48. doi: 10.1002/jemt.1070260105. [DOI] [PubMed] [Google Scholar]
- Vitorino R, Lobo MJ, Duarte JR, Ferrer-Correia AJ, Domingues PM, Amado FM. The role of salivary peptides in dental caries. Biomed Chromatogr. 2005;19:214–222. doi: 10.1002/bmc.438. [DOI] [PubMed] [Google Scholar]