Abstract
This paper begins by reviewing the fossil evidence for human evolution. It presents summaries of each of the taxa recognized in a relatively speciose hominin taxonomy. These taxa are grouped in grades, namely possible and probable hominins, archaic hominins, megadont archaic hominins, transitional hominins, pre-modern Homo and anatomically modern Homo. The second part of this contribution considers some of the controversies that surround hominin taxonomy and systematics. The first is the vexed question of how you tell an early hominin from an early panin, or from taxa belonging to an extinct clade closely related to the Pan-Homo clade. Secondly, we consider how many species should be recognized within the hominin fossil record, and review the philosophies and methods used to identify taxa within the hominin fossil record. Thirdly, we examine how relationships within the hominin clade are investigated, including descriptions of the methods used to break down an integrated structure into tractable analytical units, and then how cladograms are generated and compared. We then review the internal structure of the hominin clade, including the problem of how many subclades should be recognized within the hominin clade, and we examine the reliability of hominin cladistic hypotheses. The last part of the paper reviews the concepts of a genus, including the criteria that should be used for recognizing genera within the hominin clade.
Keywords: cladistic analysis, evolution, hominin, human, systematics, taxonomy
Introduction
The hominin fossil record consists of all the fossil taxa that are more closely related to modern humans than they are to any other living taxon. It is these extinct taxa plus modern humans that make up the hominin clade; the equivalent clade containing modern chimpanzees and bonobos (hereafter called chimps/bonobos) is the panin clade.
This contribution focuses on the task of reviewing taxonomic and systematic hypotheses about the hominin clade. The first section presents two hominin taxonomies, one a relatively speciose interpretation, the other a taxonomic hypothesis that resolves the hominin fossil record into fewer, more inclusive, species. The second section considers some (but by no means all) of the controversies that surround hominin taxonomy and systematics. For example, how do you tell an early hominin from an early panin, or an early hominin from the members of an extinct clade closely related to the Pan-Homo clade? How many hominin taxa should be recognized within the hominin fossil record, and what are the criteria for a genus?
Taxonomic hypotheses
We present two taxonomic hypotheses, one speciose (Fig. 1 and Table 1), the other less so (Table 1), in which hominin species are grouped into five informal grades. The concept of a grade is synonymous with the notion of an ‘adaptive zone’ (referred to below in connection with the criteria for a genus). The term grade was introduced by Julian Huxley (Huxley, 1958), but the concept it applies to is similar to what Sewall Wright (1932) referred to as an ‘adaptive plateau’. Unlike the concept of a clade, which refers to the process of evolutionary history, a grade is a category based solely on the outcome of evolutionary history. Taxa in the same grade, adaptive zone or adaptive plateau eat the same sorts of foods, and share the same posture and mode(s) of locomotion; no store is set by how they came by those behaviours.
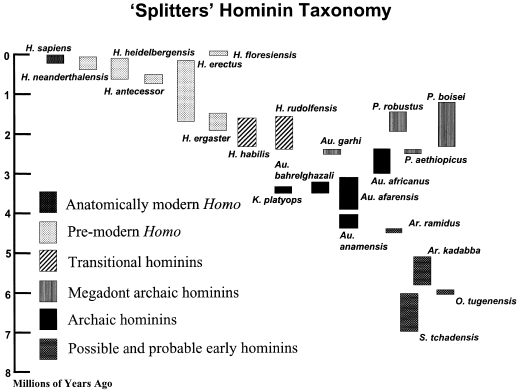
Fig. 1.
Taxa recognized in a typical speciose hominin taxonomy. Note that the height of the columns reflects current ideas about the earliest (called the first appearance datum, or FAD) and the most recent (called the last appearance datum, or LAD) fossil evidence of any particular hominin taxon. However, the time between the FAD and the LAD is likely to be represent the minimum time span of a taxon, as it is highly unlikely that the fossil record of a taxon, and particularly the relatively sparse fossil records of early hominin taxa, include the earliest and most recent fossil evidence of a taxon.
Table 1.
Species recognized in typical ‘splitters’ and ‘lumpers’ lists of hominin taxa
| Splitting taxonomy | Lumping taxonomy |
|---|---|
| S. tchadensis | Ar. ramidus s.l. |
| O. tugenensis | |
| Ar. ramidus s.s. | |
| Ar. kadabba | |
| Au. anamensis | Au. afarensis s.l. |
| Au. afarensis s.s. | |
| K. platyops | |
| Au. bahrelghazali | |
| Au. africanus | Au. africanus |
| Au. garhi | |
| P. aethiopicus | P. boisei s.l. |
| P. boisei s.s. | |
| P. robustus | P. robustus |
| H. habilis s.s. | H. habilis s.l. |
| H. rudolfensis | |
| H. ergaster | H. erectus s.l. |
| H. erectus s.s. | |
| H. floresiensis | |
| H. antecessor | |
| H. heidelbergensis | |
| H. neanderthalensis | |
| H. sapiens s.s. | H. sapiens s.l. |
Grades are as difficult, if not more difficult, to define as species. For example, in the hominin clade just how different do two diets or two locomotor strategies have to be before the taxa concerned are considered to belong to different grades? This is inevitably a subjective judgment, but even subjectivity about grades has some utility as the recognition of grades helps to break the hominin fossil record up into heuristically useful subsets. So, until we can be sure we have access to reliable information about the relationships among taxa, the grade concept helps to sort hominin taxa into broad functional categories, albeit in some cases frustratingly ‘fuzzy’ ones. The grades we use in this review are ‘Possible and probable hominins’; ‘Archaic hominins’; ‘Megadont archaic hominins’; ‘Pre-modern Homo’ and ‘Anatomically modern Homo.’ Within the grades hominin species are reviewed in temporal order, starting with the oldest taxon. The format for each taxon entry is the same and more details about the taxa can be found in the references cited (Table 2).
Table 2.
Hominin species in a speciose taxonomy sorted into six grade groupings
| Grade | Species included in a splitting taxonomy |
|---|---|
| Possible and probable hominins | S. tchadensis |
| O. tugenensis | |
| Ar. ramidus s.s. | |
| Ar. kadabba | |
| Archaic hominins | Au. anamensis |
| Au. afarensis s.s. | |
| K. platyops | |
| Au. bahrelghazali | |
| Au. africanus | |
| Au. garhi | |
| Megadont archaic hominins | P. aethiopicus |
| P. boisei s.s. | |
| P. robustus | |
| Transitional hominins | H. habilis |
| H. rudolfensis | |
| Pre-modern Homo | H. ergaster s.s. |
| H. erectus | |
| H. floresiensis | |
| H. antecessor | |
| H. heidelbergensis | |
| H. neanderthalensis | |
| Anatomically modern Homo | H. sapiens s.s. |
Splitting (speciose) hominin taxonomy
Possible and probable hominins
This group includes one taxon, Ardipithecus ramidus s.s., which is almost certainly a member of the hominin clade, and three taxa, Orrorin tugenensis, Sahelanthropus tchadensis and Ardipithecus kadabba, which may belong to the hominin clade.
Taxon name: Sahelanthropus tchadensisBrunet et al. 2002
Temporal range: c. 7–6 Ma.How dated?: Biochronological dating by matching fossil evidence found in the same layers as the hominins with absolutely dated fossil sites in East Africa (Vignaud et al. 2002).
Initial discovery: TM266-01-060-1 – an adult cranium, Anthracotheriid Unit, Toros-Menalla, Chad, 2001 (Brunet et al. 2002).
Type specimen: See above.
Source(s) of the evidence: Known from localities in Toros-Menalla, Chad, Central Africa.
Nature of the evidence: A plastically deformed cranium, mandibles and some teeth; no postcranial evidence.
Characteristics and inferred behaviour: A chimp/bonobo-sized animal displaying a novel combination of primitive and derived features. Much about the base and vault of the cranium is chimp/bonobo-like, but the relatively anterior placement of the foramen magnum is hominin-like. The supraorbital torus, lack of a muzzle, small, apically worn, canines, low, rounded, molar cusps, relatively thick tooth enamel and relatively thick mandibular corpus (Brunet et al. 2002) suggest that S. tchadensis does not belong in the Pan clade. It is either a primitive hominin, or it belongs to a separate clade of hominin-like apes.
Taxon name: Orrorin tugenensisSenut et al. 2001
Temporal range: c. 5.7–6.6 Ma.
How dated?: Fossils found in sediments that lie between a 6.6 Ma volcanic trachyte below, and an absolutely dated 5.7 Ma volcanic sill above.
Initial discovery: KNM LU 335 – left mandibular molar tooth crown, ‘thick, pink sandy and gritty horizon’, middle Member A, Lukeino Formation, Tugen Hills, Baringo, Kenya, 1974 (Pickford, 1975).
Type specimen: BAR 1000’00 – fragmentary mandible, Kapsomin, Lukeino Formation, Tugen Hills, Baringo, Kenya, 2000 (Senut et al. 2001).
Source(s) of the evidence: The relevant remains come from four localities in the Lukeino Formation, Tugen Hills, Kenya.
Nature of the evidence: The 13 specimens include three femoral fragments.
Characteristics and inferred behaviour: The femoral morphology has been interpreted (Pickford et al. 2002; Richmond & Jungers, in press) as suggesting that O. tugenensis is an obligate biped, but other researchers interpret the radiographs and CT scans of the femoral neck as indicating a mix of bipedal and non-bipedal locomotion (Galik et al. 2004; Ohman et al. 2005). Otherwise, the discoverers admit that much of the critical dental morphology is ‘ape-like’ (Senut et al. 2001, p. 6). O. tugenensis may prove to be a hominin, but it is equally and perhaps more likely that it belongs to another part of the adaptive radiation that included the common ancestor of panins and hominins.
Taxon name: Ardipithecus kadabbaHaile-Selassie, Suwa, and White 2004
Temporal range: 5.8–> 5.2 Ma.
How dated?: Fossils bracketed by dated tuff horizons, with the fossil evidence younger than the Ladina Basaltic Tuff (LABT) and older than the Kuseralee Member of the Sagantole Formation of the Central Awash Complex.
Initial discovery: ALA-VP-2/10 – right mandibular corpus with M3, with associated dentition, Alayla Vertebrate Paleontology Locality Two, Aatu horizon, Asa Koma Member, Adu Asa Formation, Middle Awash (Western Margin), Ethiopia, 1999 (Haile-Selassie, 2001).
Type specimen: As above.
Source(s) of the evidence: Central Awash Complex and the Western Margin, Middle Awash, Ethiopia.
Nature of the evidence: Eleven specimens, six postcranial and five dental, recovered in 1997, plus six more teeth, including an upper canine and a P3, recovered in 2002.
Characteristics and inferred behaviour: The main differences between Ar. kadabba and Ar. ramidus s.s. are that the apical crests of the upper canine crown of the former are longer, and that the P3 crown outline of Ar. kadabba is more asymmetrical than that of Ar. ramidus s.s. The morphology of the postcranial evidence is generally ape-like. Haile-Selassie et al. (2004) suggest that there is a morphocline in upper canine morphology, with Ar. kadabba exhibiting the most ape-like morphology, and Ar. ramidus s.s. and Australopithecus afarensis interpreted as becoming progressively more like the lower and more asymmetric crowns of later hominins (see fig. 1D in Haile-Selassie et al. 2004). The proximal foot phalanx (AME-VP-1/71) combines an ape-like curvature with a proximal joint surface that is like that of Au. afarensis (Haile-Selassie, 2001). The ape-like dental morphology suggests that the case for Ar. kadabba being a primitive hominin is substantially weaker than the case that can be made for Ar. ramidus s.s.(see below).
Taxon name: Ardipithecus ramidus s.s.(White et al. 1994) White, Suwa and Asfaw 1995
Temporal range: c. 4.5–4.3 Ma*. *[NB. The As Duma localities are in three blocks of sediment (GWM-3, -5 and -10) belonging to the Sagantole Formation. The age of this site complex is estimated from laser fusion 40Ar/39Ar ages and from palaeomagnetic data to be 4.51–4.32 Ma, but GWM-5 could be as young as 3.7 Ma.]
How dated?: Absolutely dated layers of volcanic ash above and below the fossil-bearing sediments.
Initial discovery: ARA-VP-1/1 – right M3, Aramis, Middle Awash, Ethiopia, 1993 (White et al. 1994). [NB. If a mandible, KNM-LT 329, from Lothagam, Kenya, proves to belong to the hypodigm then it would be the initial discovery.]
Type specimen: ARA-VP-6/1 – associated upper and lower dentition, Aramis, Middle Awash, Ethiopia, 1993 (White et al. 1994).
Source(s) of the evidence: The initial evidence for this taxon was a collection of c. 4.5-Myr-old fossils recovered from a site called Aramis in the Middle Awash region of Ethiopia but perhaps also Lothagam and Tabarin, Kenya.
Nature of the evidence: The published evidence consists of isolated teeth, a piece of the base of the cranium and fragments of mandibles and long bones.
Characteristics and inferred behaviour: The remains attributed to Ar. ramidus s.s. share some features in common with living species of Pan, others that are shared with the African apes in general, and, crucially, several dental and cranial features that are shared only with later hominins such as Au. afarensis. Thus, the discoverers have suggested that the material belongs to a hominin species. They initially allocated the new species to Australopithecus (White et al. 1994), but they subsequently assigned it to a new genus, Ardipithecus(White et al. 1995), which the authors suggest is significantly more primitive than Australopithecus. Judging from the size of the shoulder joint Ar. ramidus s.s. weighed about 40 kg. Its chewing teeth were relatively small and the position of the foramen magnum suggests that the posture and gait of Ar. ramidus s.s. were respectively more upright and bipedal than is the case in living apes. The thin enamel covering on the teeth suggests that the diet of Ar. ramidus s.s. may have been closer to that of chimps/bonobos than to later archaic hominins.
Archaic hominins
This group includes all the remaining hominin taxa not conventionally included in Homo and Paranthropus. It subsumes two genera, Australopithecus and Kenyanthropus. As it is used in this and many other taxonomies Australopithecus is almost certainly not a single clade, but until sample sizes increase and methods of data capture and analysis are improved to the point that researchers can be sure they have generated a reliable hominin phylogeny there is little point in revising the generic terminology, but students and researchers should do as we have done, and seek a way of referring to this material that does not imply they form a natural group.
Taxon name: Australopithecus anamensisLeakey et al. 1995
Temporal range: c. 4.5–3.9 Ma.
How dated?: Mainly from absolutely dated layers of ash above and below the sediments bearing the hominin fossils.
Initial discovery: KNM-KP 271 – left distal humerus – Naringangoro Hill, Kanapoi, Kenya, 1965 (Patterson & Howells, 1967).
Type specimen: KNM-KP 29281 – an adult mandible with complete dentition and a temporal fragment that probably belongs to the same individual, Kanapoi, Kenya, 1994.
Source(s) of the evidence: Allia Bay and Kanapoi, Kenya.
Nature of the evidence: The evidence consists of jaws, teeth and postcranial elements from the upper and lower limbs.
Characteristics and inferred behaviour: The main differences between Au. anamensis and Au. afarensis s.s. relate to details of the dentition. In some respects the teeth of Au. anamensis are more primitive than those of Au. afarensis s.s.(for example, the asymmetry of the premolar crowns and the relatively simple crowns of the deciduous first mandibular molars), but in others (for example, the low cross-sectional profiles and bulging sides of the molar crowns) they show some similarities to Paranthropus (see below). The upper limb remains are similar to those of Au. afarensis s.s., but a tibia attributed to Au. anamensis has features associated with bipedality.
Taxon name: Australopithecus afarensis s.s.Johanson et al. 1978
Temporal range: c. 4–3 Ma.
How dated?: Mainly from absolutely dated layers of ash above and below the sediments bearing the hominin fossils.
Initial discovery: Garusi 1 – right maxillary fragment, Laetolil Beds, Laetoli, Tanzania, 1939 (Kohl-Larsen, 1943).
Type specimen: LH 4 – adult mandible, Laetolil Beds, Laetoli, Tanzania, 1974.
Source(s) of the evidence: Laetoli, Tanzania; White Sands, Hadar, Maka, Belohdelie and Fejej, Ethiopia; Allia Bay and West Turkana, Kenya.
Nature of the evidence: Au. afarensis s.s. is the earliest hominin to have a comprehensive fossil record including a skull, fragmented skulls, many lower jaws and sufficient limb bones to be able to estimate stature and body mass. The collection includes a specimen, AL-288, that preserves just less than half of the skeleton of an adult female.
Characteristics and inferred behaviour: Most body mass estimates range from c. 30 to 45 kg and the endocranial volume of Au. afarensis s.s. is estimated to be between 400 and 550 cm3. This is larger than the average endocranial volume of a chimpanzee, but if the estimates of the body size of Au. afarensis s.s. are approximately correct then relative to estimated body mass the brain of Au. afarensis is not substantially larger than that of Pan. It has smaller incisors than those of extant chimps/bonobos, but its premolars and molars are relatively larger than those of chimps/bonobos. The hind limbs of AL-288 are substantially shorter than those of a modern human of similar stature. The appearance of the pelvis and the relatively short lower limb suggest that although Au. afarensis s.s. was capable of bipedal walking it was not adapted for long-range bipedalism. This indirect evidence for the locomotion of Au. afarensis s.s. is complemented by the discovery at Laetoli of several trails of fossil footprints. These provide very graphic direct evidence that a contemporary hominin, presumably Au. afarensis s.s., was capable of bipedal locomotion. The upper limb, especially the hand and the shoulder girdle, retains morphology that most likely reflects a significant element of arboreal locomotion. The size of the footprints, the length of the stride and stature estimates based on the length of the limb bones suggest that the standing height of adult individuals in this early hominin species was between 1.0 and 1.5 m. Most researchers interpret the fossil evidence for Au. afarensis s.s. as consistent with a substantial level of sexual dimorphism, but athough a recent study argues that sexual dimorphism in this taxon is relatively poorly developed (Reno et al. 2003), others retain their support for this taxon showing a substantial level of sexual dimorphism (Gordon et al. 2008).
Taxon name: Kenyanthropus platyopsLeakey et al. 2001
Temporal range: c. 3.5–3.3 Ma.
How dated?: Mainly from absolutely dated layers of ash above and below the sediments bearing the hominin fossils.
Initial discovery: KNM-WT 38350 – left maxilla fragment, Lomekwi Member – 17 m above the Tulu Bor Tuff, Lomekwi, West Turkana, Kenya, 1998 (Leakey et al. 2001).
Type specimen: KNM-WT 40000 – a relatively complete cranium that is criss-crossed by matrix-filled cracks, Kataboi Member – 8 m below the Tulu Bor Tuff and 12 m above the Lokochot Tuff, Lomekwi, West Turkana, Kenya, 1999 (Leakey et al. 2001).
Source(s) of the evidence: West Turkana and perhaps Allia Bay, Kenya.
Nature of the evidence: The initial report lists the type cranium and the paratype maxilla plus 34 specimens – three mandible fragments, a maxilla fragment and isolated teeth – some of which may also belong to the hypodigm, but at this stage the researchers are reserving their judgment about the taxonomy of many of these remains (Leakey et al. 2001). Some of them have only recently been referred to Au. afarensis s.s.(Brown et al. 2001).
Characteristics and inferred behaviour: The main reasons Leakey et al. (2001) did not assign this material to Au. afarensis s.s. are its reduced subnasal prognathism, anteriorly situated zygomatic root, flat and vertically orientated malar region, relatively small but thick-enamelled molars and the unusually small M1 compared with the size of the P4 and M3. Some of the morphology of the new genus including the shape of the face is Paranthropus-like yet it lacks the postcanine megadontia that characterizes Paranthropus. The authors note the face of the new material resembles that of Homo rudolfensis (see below), but they rightly point out that the postcanine teeth of the latter are substantially larger than those of KNM-WT 40000. K. platyops apparently displays a hitherto unique combination of facial and dental morphology. White et al. (2003) have taken the view that the new taxon is not justified because the cranium could be a distorted Au. afarensis s.s. cranium, but even if this explanation is correct for the cranium it would not explain the small size of the crowns of the postcanine teeth.
Taxon name: Australopithecus bahrelghazaliBrunet et al. 1996
Temporal range: c. 3.5–3.0 Ma.
How dated?: Relative dating based on matching mammalian fossils found in the caves with fossils from absolutely dated sites in East Africa.
Initial discovery: KT 12/H1 – anterior portion of an adult mandible, Koro Toro, Chad, 1995 (Brunet et al. 1996).
Type specimen: See above.
Source(s) of the evidence: Koro Toro, Chad.
Nature of the evidence: Published evidence is restricted to a fragment of the mandible and an isolated tooth.
Characteristics and inferred behaviour: Its discoverers claim that its thicker enamel distinguishes the Chad remains from Ar. ramidus s.s., and that its smaller and more vertical mandibular symphysis and more complex mandibular premolar roots distinguish it from Au. afarensis s.s. Otherwise there is too little evidence to infer any behaviour. It is most likely a regional variant of Au. afarensis s.s.
Taxon name: Australopithecus africanusDart 1925
Temporal range: c. 3*–2.4† Ma. [NB. *It remains to be seen whether the associated skeleton StW 573 from Mb2 and 12 hominin fossils recovered from the Jacovec Cavern since 1995 (Partridge et al. 2003) belong to the Au. africanus hypodigm, †and some researchers have advanced reasons for Sterkfontein Mb4 being as young as 2.1 Ma.]
How dated?: Mostly relative dating based on matching mammalian fossils found in the caves with fossils from absolutely dated sites in East Africa. Samples of quartz grains from Mb2 and the Jacovec Cavern have been dated to c. 4.0–4.2 Ma using ratios of the radionuclides 29Ae and 10Be (Partridge et al. 2003).
Initial discovery: Taung 1 – a juvenile skull with partial endocast, Taung (formerly Taungs) now in South Africa, 1924.
Type specimen: See above.
Source(s) of the evidence: Most of the evidence comes from two caves, Sterkfontein and Makapansgat, with other evidence coming from Taung and Gladysvale.
Nature of the evidence: This is one of the better fossil records of an early hominin taxon. The cranium, mandible and the dentition are well sampled. The postcranium and particularly the axial skeleton is less well represented in the sample, but there is at least one specimen of each of the long bones. However, many of the fossils have been crushed and deformed by rocks falling on the bones before they were fully fossilized.
Characteristics and inferred behaviour: The picture emerging from morphological and functional analyses suggests that although Au. africanus was capable of walking bipedally it was probably more arboreal than other archaic hominin taxa. It had relatively large chewing teeth and apart from the reduced canines the skull is relatively ape-like. Its mean endocranial volume is c. 460 cm3. The Sterkfontein evidence suggests that males and females of Au. africanus differed substantially in body size, but probably not to the degree they did in Au. afarensis s.s. (see above).
Megadont archaic hominins
This group includes hominin taxa conventionally included in the genus Paranthropus and one Australopithecus species, Australopithecus garhi. The genus Paranthropus was reintroduced when cladistic analyses suggested that three of the species listed in this section formed a clade. Two genera, Zinjanthropus and Paraustralopithecus, are subsumed within the genus Paranthropus. Some individuals assigned to other pre-Homo hominin taxa have teeth as big (or slightly bigger) than the taxa referred to here. We use the term ‘megadont’ to refer to the absolute size of the postcanine tooth crowns, but stress that the presumed adaptations to mastication in this group encompass much more than postcanine tooth enlargement.
Taxon name: Paranthropus aethiopicus(Arambourg & Coppens, 1968) Chamberlain & Wood 1985
Temporal range: c. 2.5–2.3 Ma.
How dated?: Mainly from absolutely dated layers of ash above and below the sediments bearing the hominin fossils.
Initial discovery: Omo 18.18 (or 18.1967.18) – an edentulous adult mandible, Locality 18, Section 7, Member C, Shungura Formation, Omo Region, Ethiopia, 1967.
Type specimen: See above.
Source(s) of the evidence: Shungura Formation, Omo region, Ethiopia; West Turkana, Kenya; Melema, Malawi.
Nature of the evidence: The hypodigm includes a well-preserved adult cranium from West Turkana (KNM-WT 17000) together with mandibles (for example, the juvenile mandible KNM-WT 16005) and isolated teeth from the Shungura Formation, and some also assign Omo 338y-6 to this taxon. No postcranial fossils have been assigned to this taxon.
Characteristics and inferred behaviour: Similar to Paranthropus boisei (see below) except that the face is more prognathic, the cranial base is less flexed, the incisors are larger and the postcanine teeth are not so large or morphologically specialized, but note there is only one relatively complete P. aethiopicus cranium, and note the warnings of Smith (2005) about making taxonomic inferences based on small samples. The only source of endocranial volume data is KNM-ER WT 17000. When P. aethiopicus was introduced in 1968 it was the only megadont hominin in this time range. With the discovery of Au. garhi (see below) it is apparent that robust mandibles with long premolar and molar tooth rows are being associated with what are claimed to be two distinct forms of cranial morphology.
Taxonomic note: If it transpires that Omo 18.18 belongs to the same hypodigm as the BOU-VP-12/130 cranium then P. aethiopicus would have priority, and the P. aethiopicus hypodigm would then include the fossils presently assigned to Au. garhi.
Taxon name: Australopithecus garhiAsfaw et al. 1999
Temporal range: c. 2.5 Ma.
How dated?: From absolutely dated layers of ash above and below the sediments bearing the hominin fossils.
Initial discovery: ARA-VP-12/130 – cranial fragments, Aramis, Middle Awash, Ethiopia, 1997.
Type specimen: BOU*-VP-12/130 – a cranium from the Hata Member, Bouri, Middle Awash, Ethiopia, 1997. [*The prefix ‘ARA’ was erroneously used in the text of Asfaw et al. (1999).]
Source(s) of the evidence: Bouri, Middle Awash, Ethiopia.
Nature of the evidence: A fragmented cranium and two partial mandibles.
Characteristics and inferred behaviour: Australopithecus garhi combines a primitive cranium with large-crowned post-canine teeth. However, unlike Paranthropus (see above) the incisors and canines are large and the enamel lacks the extreme thickness seen in the latter taxon. A partial skeleton combining a long femur with a long forearm was found nearby, but is not associated with the type cranium (Asfaw et al. 1999) and these fossils have not been formerly assigned to Au. garhi.
Taxonomic note: The mandibular morphology of Au. garhi is in some respects like that of P. aethiopicus. If it is demonstrated that the type specimen of P. aethiopicus, Omo 18.18, belongs to the same hypodigm as the mandibles that appear to match the Au. garhi cranium, then P. aethiopicus would have priority as the name for the hypodigm presently attributed to Au. garhi.
Taxon name: Paranthropus boisei s.s.(Leakey, 1959) Robinson 1960
Temporal range: c. 2.3–1.4 Ma.
How dated?: Mainly from absolutely dated layers of ash above and below the sediments bearing the hominin fossils.
Initial discovery: OH 3 – deciduous mandibular canine and molar, BK, Lower Bed II, Olduvai Gorge, Tanzania, 1955 (Leakey, 1958).
Type specimen: OH 5 – subadult cranium, FLK, Bed I, Olduvai Gorge, Tanzania, 1959 (Leakey, 1959).
Source(s) of the evidence: Olduvai and Peninj, Tanzania; Omo Shungura Formation and Konso, Ethiopia; Koobi Fora, Chesowanja and West Turkana, Kenya.
Nature of the evidence: Paranthropus boisei s.s. has a comprehensive craniodental fossil record. There are several skulls (the one from Konso being remarkably complete and well preserved), several well-preserved crania, and many mandibles and isolated teeth. There is evidence of both large and small-bodied individuals, and the range of the size difference suggests a substantial degree of sexual dimorphism.
Characteristics and inferred behaviour: Paranthropus boisei s.s. is the only hominin to combine a massive, wide, flat, face, massive premolars and molars, small anterior teeth, and a modest endocranial volume (c. 480 cm3). The face of P. boisei s.s. is larger and wider than that of P. robustus, yet their brain volumes are similar. The mandible of P. boisei s.s. has a larger and wider body or corpus than any other hominin (see P. aethiopicus above). The tooth crowns apparently grow at a faster rate than has been recorded for any other early hominin. There is, unfortunately, no postcranial evidence that can with certainty be attributed to P. boisei s.s., but some of the postcranial fossils from Bed I at Olduvai Gorge currently attributed to Homo habilis s.s. may belong to P. boisei s.s. The fossil record of P. boisei s.s. extends across about one million years of time, during which there is little evidence of any substantial change in the size or shape of the components of the cranium, mandible and dentition.
Taxon name: Paranthropus robustusBroom 1938
Temporal range: c. 2.0–1.5 Ma.
How dated?: Relative dating based on matching mammalian fossils found in the caves with fossils from absolutely dated sites in East Africa.
Initial discovery: TM 1517 – an adult, presumably male, cranium and associated skeleton, ‘Phase II Breccia’, now Mb 3, Kromdraai B, South Africa, 1938.
Type specimen: See above.
Source(s) of the evidence: Kromdraai, Swartkrans, Gondolin, Drimolen, and Cooper's caves, all situated in the Blauuwbank Valley, near Johannesburg, South Africa.
Nature of the evidence: The fossil record is similar to, but less numerous, than that of Au. africanus. The dentition is very well represented, some of the cranial remains are well preserved, but most of the mandibles are crushed or distorted. The postcranial skeleton is not well represented. Research at Drimolen was only initiated in 1992 yet already more than 80 hominin specimens have been recovered and it promises to be a rich source of evidence about P. robustus.
Characteristics and inferred behaviour: The brain, face and chewing teeth of P. robustus are larger than those of Au. africanus, yet the incisor teeth are smaller. What little is known about the postcranial skeleton of P. robustus suggests that the morphology of the pelvis and the hip joint is much like that of Au. africanus. It was most likely capable of bipedal walking, but most researchers subscribe to the view that it was not an obligate biped (but see Susman, 1988). It has been suggested that the thumb of P. robustus would have been capable of the type of grip necessary for stone tool manufacture, but this claim is not accepted by all researchers.
Transitional hominins
This group contains the earliest members of the genus Homo. Some researchers have suggested that these taxa (H. habilis s.s. and H. rudolfensis) may not belong in the Homo clade, but until we can generate sound phylogenetic hypotheses about these taxa and the archaic hominins it is not clear what their new generic attribution should be. For the purposes of this review these two taxa are retained within Homo. The crania within this grade subsume a wide range of absolute and relative brain size (see below).
Taxon name: Homo habilis s.s.Leakey et al. 1964
Temporal range: c. 2.4–1.4 Ma.
How dated?: Absolute dates from layers of volcanic ash and basalt above and below the fossil horizons.
Initial discovery: OH 4 – fragmented mandible, MK, Bed I, Olduvai Gorge, Tanzania, 1959.
Type specimen: OH 7 – partial skull cap and hand bones, FLKNN, Bed I, Olduvai Gorge, Tanzania, 1960.
Source(s) of the evidence: Olduvai Gorge, Tanzania; Koobi Fora and perhaps Chemeron, Kenya; Omo (Shungura) and Hadar, Ethiopia, East Africa; perhaps also Sterkfontein, Swartkrans, and Drimolen, South Africa.
Nature of the evidence: Mostly cranial and dental evidence with only a few postcranial bones that can with some confidence be assigned to H. habilis s.s.
Characteristics and inferred behaviour: The endocranial volume of H. habilis s.s. ranges from c. 500 cm3 to c. 700 cm3, but most commentators opt for an upper limit closer to 600 cm3. All the crania are wider at the base than across the vault, but the face is broadest in its upper part. The only postcranial fossils that can with confidence be assigned to H. habilis s.s. are the postcranial bones associated with the type specimen, OH 7, and the associated skeleton, OH 62. Isolated postcranial bones from Olduvai Gorge (for example, OH 10) could belong to P. boisei s.s. If OH 62 is representative of H. habilis s.s. the skeletal evidence suggests that its limb proportions and locomotion were archaic hominin-like. The curved proximal phalanges and well-developed muscle markings on the phalanges of OH 7 indicate that the hand of H. habilis s.s. was capable of the type of powerful grasping associated with arboreal activities. The inference that H. habilis s.s. was capable of spoken language was based on links between endocranial morphology, on the one hand, and language comprehension and production, on the other, that are no longer valid.
Taxon name: Homo rudolfensis(Alexeev, 1986) sensuWood 1992
Temporal range: c. 2.4–1.6 Ma.
How dated?: Mainly absolute dates for volcanic ash layers above and below the fossil horizons.
Initial discovery: KNM-ER 819, Area 1, Okote Member, Koobi Fora Formation, Koobi Fora, Kenya, 1971.
Type specimen: Lectotype: KNM-ER 1470, Area 131, Upper Burgi Member, Koobi Fora Formation, Koobi Fora, Kenya, 1972 (Leakey, 1973).
Source(s) of the evidence: Koobi Fora, and perhaps Chemeron, Kenya; Uraha, Malawi.
Nature of the evidence: Several incomplete crania, two relatively well-preserved mandibles and several isolated teeth.
Characteristics and inferred behaviour: Homo rudolfensis and H. habilis s.s. show different mixtures of primitive and derived, or specialized, features. For example, although the absolute size of the brain case is greater in H. rudolfensis, its face is widest in its mid-part whereas the face of H. habilis s.s. is widest superiorly. Despite the absolute size of its brain (c. 725 cc) when it is related to estimates of body mass the brain of H. rudolfensis is not substantially larger than those of the archaic hominins. The more primitive face of H. rudolfensis (although the polarity is difficult to determine, so it may actually be derived in some aspects) is combined with a robust mandible and mandibular postcanine teeth with larger, broader, crowns and more complex premolar root systems than those of H. habilis s.s. At present no postcranial remains can be reliably linked with H. rudolfensis. The mandible and postcanine teeth are larger than one would predict for a generalized hominoid of the same estimated body mass, suggesting that its dietary niche made similar mechanical demands to those of the archaic hominins.
Pre-modern Homo
This group includes two Pleistocene Homo taxa that exhibit modern human-like body proportions, and they are thought to be the first Homo taxa for which obligate bipedalism is strongly supported, but they possessed only medium-sized brains. It includes the recently discovered Homo floresiensis, which is most reasonably interpreted as a member of a population of a Homo erectus, or Homo erectus-like, taxon that has undergone endemic dwarfing on the island of Flores, Indonesia. It also includes later taxa attributed to Homo such as Homo antecessor, Homo heidelbergensis and Homo neanderthalensis.
Taxon name: Homo ergasterGroves & Mazák 1975
Temporal range: c. 1.9–1.5 Ma.
How dated?: Mainly absolute dates for volcanic ash layers above and below the fossil horizons.
Initial discovery: KNM-ER 730 – corpus of an adult mandible with worn teeth, Area 103, KBS Member, Koobi Fora, Kenya, 1970.
Type specimen: KNM-ER 992 – well-preserved adult mandible, Area 3, Okote Member, Koobi Fora Formation, Koobi Fora, Kenya, 1971.
Source(s) of the evidence: Koobi Fora and West Turkana, Kenya; possibly Dmanisi, Georgia.
Nature of the evidence: Cranial, mandibular and dental evidence and an associated skeleton of a juvenile male individual from Nariokotome, West Turkana.
Characteristics and inferred behaviour: Two sets of features are claimed to distinguish H. ergaster from H. erectus s.s. The first comprises features for which H. ergaster is more primitive than H. erectus s.s., with the most compelling evidence coming from details of the mandibular premolars. The second set comprises features of the vault and base of the cranium for which H. ergaster is less specialized, or derived, than H. erectus s.s. Overall H. ergaster s.s. is the first hominin to combine modern human-sized chewing teeth with a postcranial skeleton (for example, long legs, large femoral head) apparently committed to long-range bipedalism. It lacks morphological features associated with arboreal locomotion. The small chewing teeth of H. ergaster imply that it was either eating different food than the archaic hominins, or that it was consuming the same food, but was preparing it extra-orally. This preparation could have involved the use of stone tools, or cooking, or a combination of the two. Although its dentition and postcranial skeleton are much more like later Homo than the archaic hominins, the absolute endocranial capacity of H. ergaster(mean = c. 760 mL) does not reach the levels seen in later Homo, and when scaled to body mass it shows relatively little advance over the levels seen in the archaic and transitional hominins.
Taxon name: Homo erectus s.s. (Dubois 1892) Weidenreich 1940
Temporal range: c. 1.8 Ma–c. 30 ka.
How dated?: A mixture of biochronology and a few absolute dates that are mostly tenuously linked with the fossiliferous horizons.
Initial discovery: Kedung Brubus 1 – mandible fragment, Kedung Brubus, Java (now Indonesia), 1890.
Type specimen: Trinil 2 – adult calotte, Trinil, Ngawi, Java (now Indonesia), 1891.
Source(s) of the evidence: Sites in Indonesia (e.g. Trinil, Sangiran, Sambungmachan), China (e.g. Zhoukoudian, Lantian) and Africa (e.g. Olduvai Gorge, Melka Kunture, Thomas Quarry).
Nature of the evidence: Mainly cranial with some postcranial evidence, but little or no evidence of the hand or foot.
Characteristics and inferred behaviour: The crania belonging to H. erectus s.s. have a low vault, a substantial more-or-less continuous torus above the orbits and the occipital region is sharply angulated. The inner and outer tables of the cranial vault are thick. The body of the mandible is less robust than that of the archaic hominins and in this respect it resembles Homo sapiens except that the symphyseal region lacks the well-marked chin that is a feature of later Homo and modern humans. The tooth crowns are generally larger and the premolar roots more complicated than those of modern humans. The cortical bone of the postcranial skeleton is thicker than is the case in modern humans. The limb bones are modern human-like in their proportions and have robust shafts, but the shafts of the long bones of the lower limb are flattened from front to back (femur) and side to side (tibia) relative to those of modern humans. All the dental and cranial evidence points to a modern human-like diet for H. erectus s.s. and the postcranial elements are consistent with a habitually upright posture and obligate, long-range bipedalism. There is no fossil evidence relevant to assessing the manual dexterity of H. erectus s.s., but if H. erectus s.s. manufactured Acheulean artefacts then dexterity would be implicit.
Taxon name: Homo floresiensisBrown et al. 2004
Temporal range: c. 95–12 ka.
How dated?: Radiocarbon, luminescence, uranium-series and electron spin resonance (ESR) dates on associated sediments and faunal specimens and dated horizons above and below skeletal material (Morwood et al. 2004).
Initial discovery: LB1 – associated partial adult skeleton.
Type specimen: See above.
Source(s) of the evidence: Presently, only known from Liang Bua, a cave 500 m above sea level and 25 km from the north coast of Flores. The cave is in a limestone hill on the southern edge of the Wae Racang valley.
Nature of the evidence: A partial adult skeleton (LB1) with some components still articulated, an isolated left P3 (LB2), and a left radius. The partial skeleton preserves the skull, and other components include the right pelvic bone, femur and tibia.
Characteristics and inferred behaviour: This hominin displays a unique combination of H. ergaster-like cranial and dental morphology, a hitherto unknown suite of pelvic and femoral features, archaic hominin-like carpal bones, a small brain (c. 380 cm3), small body mass (25–30 kg) and small stature (1 m).
Taxonomic note: The researchers responsible for the find decided, despite the small brain size, nonetheless to recognize its morphological affinities with Homo and refer LB1 to a new species within the genus Homo. The shape of the LB1 cranium as judged by six external linear dimensions is unlike that of any modern human comparative sample, even when it is scaled to the same overall size as LB1. The fossil hominin taxon closest in shape to LB1 is early African H. erectus, or H. ergaster.
Taxon name: Homo antecessorBermúdez de Castro et al. 1997
Temporal range: c. 780–500 ka.
How dated?: Biochronology.
Initial discovery: ATD6–1 – left mandibular canine, Level 6, Gran Dolina, Spain, 1994.
Type specimen: ATD6–5 – mandible and associated teeth, Level 6, Gran Dolina, Spain, 1994.
Source(s) of the evidence: Gran Dolina, Atapuerca, Spain and perhaps also Ceprano, Italy.
Nature of the evidence: The partial cranium of a juvenile, parts of mandibles and maxillae and isolated teeth.
Characteristics and inferred behaviour: Researchers who found the remains claim the combination of a modern human-like facial morphology with the large and relatively primitive crowns and roots of the teeth is not seen in H. heidelbergensis(see below). The Gran Dolina remains also show no sign of any derived H. neanderthalensis traits. Its discoverers suggest H. antecessor is the last common ancestor of Neanderthals and H. sapiens.
Taxon name: Homo heidelbergensisSchoetensack 1908
Temporal range: c. 600–100 ka.
How dated?: Mostly biochronological with some uranium series and ESR absolute dates.
Initial discovery: Mauer 1 – adult mandible, Mauer, Heidelberg, Germany, 1907.
Type specimen: As above.
Source(s) of the evidence: Sites in Europe (e.g. Mauer, Petralona); Near East (e.g. Zuttiyeh); Africa (e.g. Kabwe, Bodo); China (e.g. Dali, Jinniushan, Xujiayao, Yunxian) and possibly India (Hathnora).
Nature of the evidence: Many crania but little mandibular and postcranial evidence, unless the Gran Dolina fossils are included.
Characteristics and inferred behaviour: What sets this material apart from H. sapiens and Homo neanderthalensis(see below) is the morphology of the cranium and the robustness of the postcranial skeleton. Some brain cases are as large as those of modern humans, but they are always more robustly built with a thickened occipital region and a projecting face and with large separate ridges above the orbits unlike the more continuous brow ridge of H. erectus s.s. Compared with H. erectus s.s. the parietals are expanded, the occipital is more rounded and the frontal bone is broader. The crania of H. heidelbergensis lack the specialized features of H. neanderthalensis such as the anteriorly projecting midface and the distinctive swelling of the occipital region. H. heidelbergensis is the earliest hominin to have a brain as large as that of anatomically modern Homo and its postcranial skeleton suggests that its robust long bones and large lower limb joints were well suited to long-distance bipedal walking.
Taxonomic note: Researchers who see the African part of this hypodigm as distinctive refer to it Homo rhodesiensis. Others, who claim that the main European component of the H. heidelbergensis hypodigm already shows signs of Homo neanderthalensis autapomorphies, would sink the former into the latter.
Taxon name: Homo neanderthalensisKing 1864
Temporal range: c. 200–28 ka (but if the Sima de los Huesos material is included c. 400–28 ka).
How dated?: A mix of techniques including radiocarbon, uranium series and ESR.
Initial discovery: Engis 1 – a child's cranium, Engis, Belgium, 1829.
Type specimen: Neanderthal 1 – adult calotte and partial skeleton, Feldhofer Cave, Elberfield, Germany, 1856.
Source(s) of the evidence: Fossil evidence for H. neanderthalensis has been found throughout Europe, with the exception of Scandinavia, as well as in the Near East, the Levant and Western Asia.
Nature of the evidence: Many are burials and so all anatomical regions are represented in the fossil record.
Characteristics and inferred behaviour: The distinctive features of the cranium of H. neanderthalensis include thick, double-arched brow ridges, a face that projects anteriorly in the midline, a large nose, laterally projecting and rounded parietal bones and a rounded, posteriorly projecting occipital bone (i.e. an occipital ‘bun’). The endocranial volume of H. neanderthalensis is, on average, larger than that of modern humans. Mandibular and dental features include a retromolar space and distinctively high incidences of non-metrical dental traits. Postcranially Neanderthals were stout with a broad rib cage, a long clavicle, a wide pelvis and limb bones that are generally robust with well-developed muscle insertions. The distal extremities tend to be short compared with most modern H. sapiens, but Neanderthals were evidently obligate bipeds. The generally well-marked muscle attachments and the relative thickness of long bone shafts point to a strenuous lifestyle. The size and wear on the incisors suggest that the Neanderthals regularly used their anterior teeth as ‘tools’ either for food preparation or to grip hide or similar material.
Taxonomic note: The scope of the hypodigm of H. neanderthalensis depends on how inclusively the taxon is defined. For some researchers the taxon is restricted to fossils from Europe and the Near East that used to be referred to as ‘Classic’ Neanderthals. Others interpret the taxon more inclusively and include within the hypodigm fossil evidence that is generally older and less derived [for example, Steinheim, Swanscombe and Atapuerca (Sima de los Huesos)].
Recent developments: Researchers have recovered short fragments of mitochondrial DNA from the humerus of the Neanderthal type specimen (Krings et al. 1997, 1999). The fossil sequence falls well outside the range of variation of a diverse sample of modern humans. Researchers suggest that Neanderthals would have been unlikely to have made any contribution to the modern human gene pool and they estimate this amount of difference points to 550–690 kyr of separation. Subsequently, mtDNA has been recovered at other Neanderthal sites, including from rib fragments of a child's skeleton at Mezmaiskaya (Ovchinnikov et al. 2000) from several individuals from Vindija (Krings et al. 2000). As of November 2007, sequences are known from 13 Neanderthal specimens from sites in Western Europe and the Caucasus. The latest Neanderthal fossils to yield mtDNA are the left femur of the Teshik-Tash Neanderthal from Uzbekistan, and from the femur of the subadult individual from Okladnikov, a site in the Altai Mountains in Western Asia (Krause et al. 2007). The differences among the fossil mtDNA fragments known up until 2002 are similar to the differences between any three randomly selected African modern humans, but the differences between the mtDNA recovered from Neanderthals and the mtDNA of modern humans is substantial and significant (Knight, 2003).
Anatomically modern Homo
This group includes all the fossil evidence that is indistinguishable from the morphology found in all populations of modern humans.
Taxon name: Homo sapiens s.s.Linnaeus 1758
Temporal range: c. 200 ka to the present day.
How dated?: A mix of techniques including radiocarbon, uranium series, ESR, and some 40Ar/39Ar dates.
Initial fossil discovery: With hindsight the first recorded evidence to be recovered was the ‘Red Lady of Paviland’, Wales, 1824.
Type specimen: Linnaeus did not designate a type specimen.
Source(s) of the evidence: Fossil evidence of H. sapiens has been recovered from sites on all continents except Antarctica. The earliest absolutely dated remains are from Kibish in Ethiopia (McDougall et al. 2005).
Nature of the evidence: Many are burials so the fossil evidence is abundant and generally in good condition, but in some regions of the world (for example, West Africa) remains are few and far between.
Characteristics and inferred behaviour: The earliest evidence of anatomically modern human morphology in the fossil record comes from sites in Africa and the Near East. It is also in Africa that there is evidence for a likely morphological precursor of anatomically modern human morphology. This takes the form of crania that are generally more robust and archaic-looking than those of anatomically modern humans yet which are not archaic enough to justify their allocation to H. heidelbergensis or derived enough to be H. neanderthalensis(see above). Specimens in this category include Jebel Irhoud from North Africa; Omo 2, and Laetoli 18 from East Africa, and Florisbad and Cave of Hearths in southern Africa. There is undoubtedly a gradation in morphology that makes it difficult to set the boundary between anatomically modern humans and H. heidelbergensis, but unless at least one other taxon is recognized the variation in the later Homo fossil record is too great to be accommodated in a single taxon.
Taxonomic note: Researchers who wish to make a taxonomic distinction between fossils such as Florisbad, Omo 2 and Laetoli 18 and subrecent and living modern humans refer the earlier African subset to Homo(Africanthropus) helmeiDreyer, 1935. White et al. (2003) also suggest that the Herto crania should be distinguished from modern humans at the subspecific level as H. sapiens idaltu.
Lumping (less speciose) hominin taxonomy
We use the same grades for this taxonomy, but the taxa within them are more inclusive (Table 1).
Possible and probable hominins
Only one genus and species, Ardipithecus ramidus s.l., is recognized in the more inclusive taxonomy. In addition to Ar. ramidus s.s. and Ar. kaddaba this taxon also incorporates the hypodigms of S. tchadensis and O. tugenensis.
Taxon name: Ardipithecus ramidus s.l.(White et al. 1994) White, Suwa and Asfaw 1995
Archaic hominins
In the more lumping taxonomy one monospecific genus, Kenyanthropus, and two Australopithecus species, Au. bahrelghazali and Au. anamensis, are sunk into Au. afarensis s.l. Otherwise the taxa remain the same as in the splitting taxonomy.
Taxon names: Australopithecus afarensis s.l.Johanson et al. 1978 and Australopithecus africanusDart 1925
Megadont archaic hominins
In the more inclusive taxonomy two species, P. aethiopicus and Au. garhi, are sunk into P. boisei s.l. Some would sink all the Paranthropus taxa recognized in the speciose taxonomy into a single species, P. robustus.
Taxon names: Paranthropus boisei s.l.(Leakey, 1959) Robinson 1960 and Paranthropus robustusBroom 1938
Transitional hominins
In the lumping taxonomy H. habilis s.l. subsumes H. rudolfensis and H. habilis s.s.
Taxon name: Homo habilis s.l.Leakey et al. 1964
Pre-modern Homo
In the more inclusive taxonomy H. erectus s.l. subsumes H. erectus s.s., H. ergaster and H. floresiensis.
Taxon name: Homo erectus s.l. (Dubois, 1892) Weidenreich 1940
Anatomically modern Homo
In the lumping taxonomy H. sapiens s.l. subsumes H. antecessor, H. heidelbergensis, H. neanderthalensis and. H. sapiens s.s. An even more conservative taxonomy (for example, Wolpoff et al. 1994; Tobias, 1995) suggests that all taxa within pre-modern Homo, including H. erectus s.l., should be sunk into H. sapiens s.l.
Taxon name: Homo sapiens s.l.Linnaeus 1758
Controversies
The second part of this contribution considers some (but by no means all) of the controversies that surround hominin taxonomy and systematics. The first of the controversies is the vexed question of how you tell an early hominin from an early panin or from taxa belonging to an extinct clade closely related to the Pan-Homo clade? The second is how many species should be recognized within the hominin fossil record? The third controversy is how best to investigate relationships within the hominin clade. What methods should be used to break down an integrated structure such as the cranium into tractable analytical units? How many subclades are there within the hominin clade, and how reliable are hominin cladistic hypotheses? The fourth controversy concerns the concept of a genus. Specifically, what criteria should be used for recognizing genera within the hominin clade?
How to tell an early hominin taxon from a taxon in a closely related clade?
There are many differences between the skeletons of living modern humans and their closest living relatives, common chimpanzees and bonobos. These differences are particularly marked in the brain case, face and base of the cranium, and in the teeth, hand, pelvis, knee and the foot. There are also other important contrasts, such as the rates at which modern humans and chimps/bonobos develop and mature, and the relative lengths of the limbs, but one needs immature specimens to detect the former, and well-preserved associated skeletons to detect the latter. However, scientists searching in 8–5 Ma sediments for fossil evidence of the earliest members of the hominin clade must consider a different question. What were the differences between the first hominins and the first panins? These are likely to have been much more subtle than the differences between contemporary hominins and contemporary panins.
The common ancestor of the hominin and panin twigs was almost certainly not like either a modern human, nor exactly like a living chimp/bonobo. Nonetheless, most researchers agree that the last common ancestor (LCA) of the hominin and panin twigs was probably more likely to have been chimp/bonobo-like than modern human-like. Why? Genetic and morphological evidence suggests that gorillas are the living animals most closely related to the combined chimp/bonobo and modern human twig of the Tree of Life (TOL). Gorillas share more morphology with chimpanzees and bonobos than they do with modern humans (gorilla bones are more likely to be confused with the bones and teeth of a chimpanzee or a bonobo than with the bones and teeth of a modern human). Therefore, the common ancestor of chimpanzees, bonobos and modern humans was probably more like a chimp/bonobo than a modern human.
If this logic is followed, then the skeleton of the LCA of modern humans and chimps/bonobos would most likely show evidence of still being adapted for life in the trees. For example, its fingers would have been curved to enable it to grasp branches, and its limbs would have been adapted to walk both on all fours and on the hind limbs alone. Its face would most likely have been snout-like, not flat, like that of modern humans, and its elongated jaws would have had relatively modestly sized chewing teeth, prominent canines and relatively and absolutely large upper central incisor teeth.
In what ways would the earliest hominins have differed from the LCA of chimps/bonobos and modern humans, and from the earliest panins? Compared with panins they would most likely have had smaller canine teeth, larger chewing teeth and thicker lower jaws. There would also have been some changes in the skull and skeleton linked with more time spent upright, and with a greater dependence on the hind limbs for bipedal walking. These changes would have included, among other things, a forward shift in the foramen magnum, wider hips, habitually more extended knees and a more stable foot.
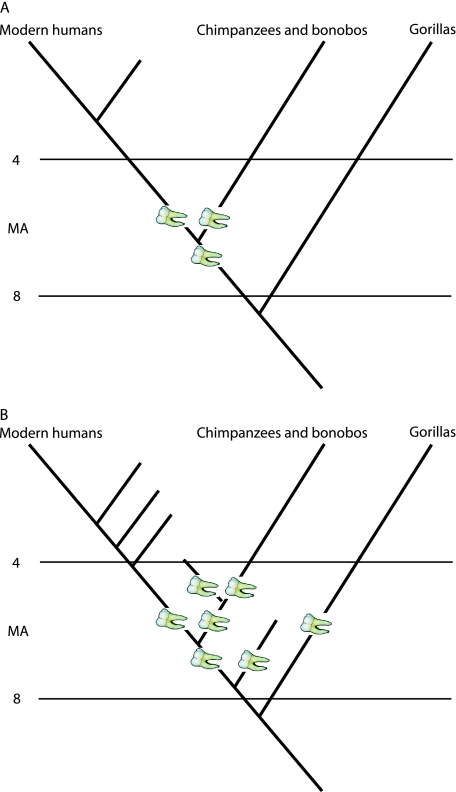
But all this assumes there is no homoplasy (see below) and that the only options for a 8–5 Ma African higher primate are being the LCA of modern humans and chimps/bonobos, a primitive hominin, or a primitive panin. It is, however, perfectly possible that such a creature may belong to an extinct clade that is the sister taxon of the LCA of modern humans and chimps/bonobos, or the sister taxon of the earliest hominins or panins (Fig. 2).
Fig. 2.
Options for an 8–5 Ma African higher primate taxon that is more closely related to Homo and Pan than to Gorilla. Scheme A assumes no homoplasy and the only options within it for such a taxon is that it is the last common ancestor (LCA) of modern humans and chimps/bonobos, a primitive hominin, or a primitive panin. Scheme B takes into account the probability of homoplasy, and in addition to the above options such a taxon could be a member of an extinct clade that is the sister taxon of the LCA of modern humans and chimps/bonobos, or a hitherto unkown clade that is the sister taxon of the earliest hominins or panins.
How many taxa are represented in the hominin clade?
Two questions have to be answered before any hypotheses can be generated about what Simpson referred to as the alpha taxonomy of the hominin clade (i.e. how many species are sampled in the hominin fossil record). First, what species definition should be used? Second, how should that species definition be applied to the hominin fossil evidence?
The definition of any taxonomic category is a vexed issue, but the problem of how to define an extinct species is especially contentious. The species is the least inclusive category in the Linnaean taxonomic system and since the species category was introduced the way it has been defined has been modified to reflect developments in our understanding of the living world. Smith (1994) provides a useful classification of the main contemporary species concepts. He suggests they can be divided into those that focus on the processes involved in the generation and maintenance of species, and those that emphasize the method used to recognize species (Table 1). The species concepts in the former subcategory are called process-related, and the species concepts in the latter subcategory are called pattern-related.
The three main concepts in the process-related category are the biological species concept (BSC), the evolutionary species concept (ESC) and the recognition species concept (RSC). The BSC definition given below is a modified version of Mayr's original definition (Mayr, 1942). It suggests that species are ‘groups of interbreeding natural populations reproductively isolated from other such groups’ (Mayr, 1982). Note that this is a relational definition in the sense that to define one species, reference has to be made to at least one other species. It also stresses mechanisms for maintaining genetic isolation, rather than emphasizing the features that conspecific individuals have in common.
The ESC was an attempt by Simpson to add a temporal dimension to the BSC. According to Simpson an ESC species is ‘an ancestral–descendant sequence of populations evolving separately from others and with its own evolutionary role and tendencies’ (Simpson, 1961). This ancestral–descendant sequence or lineage can be divided into segments called chronospecies. The boundaries of chronospecies can be discontinuities, or gaps, in the fossil record, and nowadays they are interpreted as representing cladogenic events (but this was not part of Simpson's original formulation of the ESC). Alternatively, a lineage can be broken up into segments because the variation within the fossil sample from a particular segment, or time period, respectively, exceeds, or differs, in either, or both, the degree or pattern of the variation observed within closely related, living, reference species.
Instead of emphasizing reproductive isolation the RSC, the third concept in the process-related category, emphasizes the processes that promote interbreeding. An RSC species is ‘the most inclusive population of individual, biparental organisms which shares a common fertilization system’ (Paterson, 1985). Paterson refers to the fertilization system of a species as its specific mate recognition system, or SMRS. The latter is the system used by members of that species to recognize a potential mate. The signal(s) involved may be a distinctive external morphological feature (see below), a characteristic coloration, a distinctive call, or even an odour. Paterson claims that the RSC is, at least potentially, applicable to the fossil record as long as a species’ SMRS fossilizes. This may well be the case in antelopes. The shape of the horns of antelopes is apparently crucial for mate recognition, and although the horns themselves do not fossilize, the bony horn cores do, and these are apparently distinctive enough to be useful for bovid taxonomy.
It is difficult enough to apply process-related species definitions to living taxa, let alone to the fossil record. So, what is the best way to recognize extinct species? Most paleoanthropologists use one version, or other, of one of the species concepts in the pattern-related subcategory. They are the phenetic species concept (PeSC), the phylogenetic species concept (PySC) and the monophyletic species concept (MSC). They all focus on an organism's phenotype (thus they are sometimes referred to as morphospecies concepts), but they differ because each of the concepts emphasizes a different aspect of the phenotype. The PeSC as interpreted by Sokal & Crovello (1970) gives equal weight to all aspects of the phenotype. It is based on a matrix that records the expression of each phenotypic character for each specimen. Multivariate analysis is then used to detect clusters of individual specimens that share the same, or similar, character expressions. In contrast, the version of the PySC introduced by Cracraft (1983) emphasizes the unique suite of primitive and derived characters that defines each species. According to Nixon & Wheeler (1990) in such a scheme a species is ‘the smallest aggregation of populations diagnosable by a unique combination of character states.’ For the third species concept in the pattern-related subcategory, the MSC, the scope of the morphological evidence is narrower still, for under the MSC definition species are defined according to the unique morphology a species possesses (in cladistic parlance, see below, unique morphologies are known as autapomorphies). The problem with the MSC is that it assumes the observer knows which characters are autapomorphies. But in order to determine which characters are autapomorphic one needs to perform a cladistic analysis, and in order to do that one needs to have operational taxonomic units, and in order to determine what these are one needs an alpha taxonomy (i.e. one needs to be able to recognize species in the fossil record). The MSC is the product of circular reasoning.
Species identification in the hominin fossil record
In practice most researchers involved in hominin taxonomy use one or other version of the PySC. They search for the smallest cluster of individual organisms that is ‘diagnosable’ on the basis of the preserved morphology. Because in the hominin fossil record most preserved morphology is craniodental, diagnoses of early hominin taxa inevitably focus on craniodental morphology. Eldredge (1993) developed a proposal originally made by Ghiselin (1972), and suggested that species can be viewed metaphorically as individuals with a ‘life’ that has a ‘beginning’ (the result of a speciation event), a ‘middle’ (that lasts as long as the species persists) and an ‘end’ (either extinction or participation in another speciation event).
The taxonomic problems facing palaeoanthropologists can be expressed in terms of yet another metaphor. Consider a photographer taking still photographs of the running races at a track and field sports meeting. On one occasion s/he may take several photographs of the same race, one just after the start, two in the middle of the race, and one close to the finishing line. However, on another occasion s/he may take single photographs of four separate races. Each photograph is the equivalent of an individual fossil, and the races the equivalent of species. Without a caption to guide you it would be difficult to tell whether a series of photographs is a comprehensive record of just one running race, or single photographs that provide a record of several running races. In the same way palaeoanthropologists must decide whether a collection of fossils spanning several hundred thousand years is a sample of one, or more than one, hominin taxon. When making these judgments researchers must try not to grossly under-estimate or to extravagantly over-estimate the actual number of species represented in the hominin fossil record.
Another factor palaeoanthropologists must take into account is that they have to work with a fossil record that is confined to remains of the hard tissues (i.e. bones and teeth). We know from living animals that many uncontested species are difficult to distinguish using bones and teeth (e.g. Cercopithecus species). Thus, there are sound reasons to suspect that a hard tissue-bound fossil record is likely to under-estimate the number of species. If a punctuated equilibrium model of evolution is adopted along with a branching, or cladogenetic, interpretation of the fossil record, then researchers will tend to divide the hominin fossil record into a larger rather a smaller number of species (Table 1: left column). Conversely, researchers who favour a phyletic gradualism model that emphasizes morphological continuity instead of morphological discontinuity, and who see species as longer-lived and more prone to substantial changes in morphology through time will inevitably divide the hominin fossil record into fewer, more inclusive, species (Table 1: right column).
In Eldredge's formulation (see above) all species begin when they and their sister taxon (or conceivably, sister taxa) arose from their hypothetical common ancestor. A species may change during the course of its history, but its existence will come to an end when it becomes extinct, or it is the common ancestor of two (or more) daughter taxa. Eldredge acknowledges the reality that the morphological characteristics of either a living (or neontological) species, or of an evolutionary lineage, are never uniformly distributed across its range, and he follows Sewall Wright in being prepared to recognize the existence of distinctive local populations or demes (in the fossil record these are called palaeodemes, or ‘p-demes’). Eldredge suggests that although related demes would share the same SMRS, in some cases their morphological distinctiveness could justify them being regarded as separate species. He also acknowledges that the same logic could be applied to subdivide chronospecies on the basis that cladogenetic events may not always be detectable from the fossil record, and that the number of such events is likely to have been underestimated rather than overestimated. Within the fossil record it may be possible to identify several palaeospecies (sensuCain, 1954) within the equivalent of a neontological BSC/RSC-type of species.
Reticulate evolution
The species concepts considered thus far are all based on a model in which one species splits into two (or more) species, then each of those daughter species either becomes extinct, or undergoes its own furcation, and so on. In this ‘bifurcating hierarchical’ model new species arise in geographically isolated subpopulations by a process called allopatric speciation (which literally means speciation ‘in another place’). These subpopulations gradually develop distinctive combinations of genes, which result eventually in their genetic isolation from the parent population. Proponents of the RSC argue that this occurs when the new species develops a distinctive SMRS.
Speciation is interpreted very differently in so-called reticulate evolution. This interprets speciation as a process whereby a new species can form by the hybridization of two existing species. In this model species are seen as components of a complex network (hence the term reticulation). This model of evolution is close to how some researchers interpret evolution in geographically widespread groups like baboons. There are peaks of morphological distinctiveness in contemporary baboons that are separated by a morphological distance equivalent to distances that in other taxonomic groups are interpreted as species differences. The troughs between these peaks are called hybrid zones, and in these hybrid zones the distinction between baboon groups is much less. Hybrid zones are dynamic, with the nature, location and height of the peaks, and thus the nature of the hybrid zones, liable to change over time (Jolly, 2001).
Hominin taxonomy; putting theory into practice
No two individuals in a species are alike (even monozygotic twins will have minor skeletal differences) so how different does a new fossil have to be from the existing fossil record before a researcher can safely assume it represents a new species? The answer is that the researcher has to make sure the new fossil is not different because of obvious factors such as preservation (deformation, distortion, or inflation or reduction in size due to matrix-filled cracks or erosion, respectively), ontogeny (comparing a young individual with an old individual), sex (comparing a male with a female), or within-species geographical variation (see Wood & Lieberman, 2001, for a brief review of these factors).
Once a researcher has made sure that the above factors can be excluded, then his or her decision about whether a new fossil represents a new species depends on the range of variation s/he is prepared to tolerate within a species. In practical terms, palaeontologists usually use the extent of size and shape variation within closely related living species as the criteria for judging whether the variation within a collection of fossils merits that collection being assigned to more than one species (see Wood et al. 1991 for an example). For hominins the reference taxa would be modern humans, chimpanzees, bonobos, gorillas, and perhaps orangutans. Some researchers make the point that these closely related taxa, for one reason or another, may not be suitable analogues. For example, Jolly (2001) makes the case that because of their being widespread in Africa, baboons are a more suitable analogue than the extant great apes because the latter are impoverished taxa in the sense that, with only a few exceptions, they are confined to forest refugia.
So why do competent researchers subscribe to such different interpretations of how many species should be recognized within the hominin fossil record? It is sometimes difficult to tell whether taxonomic disagreements between palaeoanthropologists are due to genuine differences in the way researchers interpret a particular part of the fossil record, or whether they reflect different ‘world views’ about what a species, or a genus, is. Usually, close textual analysis of systematic wrangles reveals that both reasons play a part. Researchers who favour a more anagenetic (or gradualistic) interpretation of the fossil record tend to stress the importance of continuities in the fossil record and opt for fewer species. They are referred to as lumpers. Researchers who favour a more cladogenetic (or punctuated equilibrium) interpretation of the fossil record tend to stress the importance of discontinuities within the fossil record, and generally opt for more speciose taxonomic hypotheses. The researchers who favour these latter interpretations are referred to as splitters, and their interpretations are called taxic because they stress the importance of taxonomy in their interpretation of evolutionary history.
But, when all is said and done, a taxonomy is a hypothesis; it is not written in stone.
Hominin systematics – clades
The third controversy involving the hominin fossil record is how to investigate and reconstruct relationships within the hominin clade. How many subclades are there within the hominin clade? How reliable are hypotheses about the internal branching structure of the hominin clade?
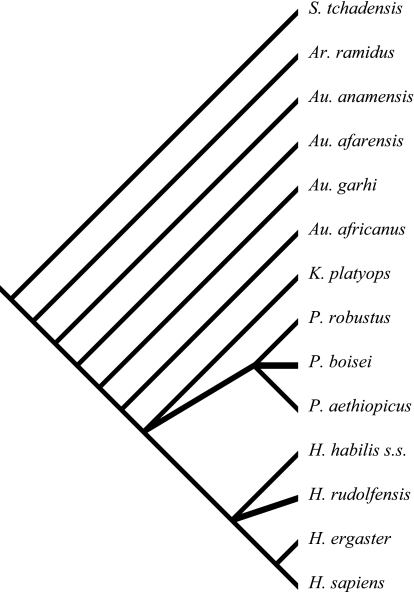
The method that is now almost universally used to reconstruct relationships is called cladistic analysis, and it is usually abbreviated to just cladistics. The logic and the mechanics of cladistic analysis were developed by the German entomologist Willi Hennig in the 1940s. The first German edition of his book entitled (in English) ‘Phylogenetic Systematics’ was published in the 1950s, and Hennig's ideas were introduced to a wider audience when the book was published in English in 1966. In that book Hennig proposed several principles that today form the core of cladistic methodology. These include the expression of evolutionary relationships as hierarchical and genealogical, the importance of synapomorphies (or shared-derived characteristics – see below) as the only true support for evolutionary relationships, an empirical and logical identification of the most likely cladogram based on the largest amount of evidence in the form of congruent synapomorphies (see below), and an emphasis on monophyletic groups, or clades, in taxonomic classifications (see Fig. 3).
Fig. 3.
Hypothesis of the cladistic relationships among fossil hominins and modern humans. This cladogram was determined to be the most parsimonious one in a recent cladistic analysis of fossil hominins (adapted from fig. 10 in Strait & Grine, 2004).
Some of the important concepts used by cladistic theory are set out below. A monophyletic group, or clade, contains all (no more and no less) of the taxa derived from its most recent common ancestor. Sister taxa are two taxa more closely related to each other than to any other taxon; they share a recent common ancestor that is not shared with any other taxon. A plesiomorphy is a primitive character for the group in question, so its possession does not help to sort taxa within that group. For example, the dental formula 2, 1, 2, 3 for the permanent teeth of the Old World higher primates would be a plesiomorphy; it has no valency for sorting taxa within that group. An apomorphy (also called a derived character) is a character peculiar to a subset, or subclade, of the group in question. Small canines in hominins would be an example of an apomorphy within the higher primates. Shared-primitive and shared-derived characters are called, respectively, symplesiomorphies and synapomorphies. It should be understood that the same feature can be both a plesiomorphy and an apomorphy depending on the level of the phylogenetic hierarchy under examination. An autapomorphy is an independently derived character seen in one branch within the group, or evolutionary lineage, which is not shared with any other branch. Extreme molarization of the mandibular premolars in P. boisei is an example of an autapomorphic hypothesis within the hominin clade. Autapomorphies are useless for establishing the pattern of relationships for classification purposes, although they are potentially useful for the diagnosis of a group or species. A cladogram is a branching diagram that reflects the relationships among taxa in a series of dichotomous branches. Outgroup taxa are distantly related to the group under study (which is called the ingroup) and they are used to establish the polarity of character evolution. It is preferable to include several outgroups in a cladistic analysis.
Cladistics rests on the axiom that homology is equal to synapomorphy (Patterson, 1988). Shared characters are only informative if they are shared due to inheritance from the most recent common ancestor. Such characters are called homologous characters. Symplesiomorphies are also homologous, but as they are primitively inherited in the groups being studied, they are not as relevant as synapomorphies for cladistic analysis. Shared characters not inherited from the most recent common ancestor are one type of homoplasy. A second type of homoplasy consists of derived features, or apomorphies, that subsequently reverse to the primitive state. Similar characters should be considered homologous until proven otherwise, or until they have been demonstrated to be homoplasic (also called homoplastic) on a cladogram. The latter is the ultimate test, or confirmation, of homology (de Pinna, 1991).
It should be emphasized that cladistics indicates the relative degree of relationships between taxa, but does not specify any hypothesis about ancestry or descent (i.e. about phylogeny). This is particularly confusing because the alternative name for cladistics is phylogenetic systematics. In a monophyletic group, or clade, in theory a taxon may be ancestral to its sister taxon, sister taxa may share a common ancestor not on the cladogram, or a taxon may have evolved from its sister taxon.
Breaking morphology down into characters
Morphological data must be coded into distinct characters for use in cladistic analysis. Characters must be homologous, which can be a difficult determination, especially if taxa display a range of different morphology for a particular structure. It is important to note here that it is impossible to determine if characters are truly homologous before the cladistic analysis is completed. Only afterwards, with the results in hand, can one make the determination between primary (proposed) and secondary (confirmed) homology (de Pinna, 1991).
Characters can be continuous or discreet (also called discontinuous). Continuous characters generally refer to some aspect of the size or shape of a feature. Discrete characters typically refer to the presence or absence of a feature, or to its shape in cases where there are several well-defined, non-overlapping variants. The reality of morphological features is that often characters labelled as discreet could easily be scored as continuous characters, and in some cases this would more accurately reflect biological reality (Chappill, 1989).
Characters – states and coding methods
Characters have different possibilities, or states; for example, the character ‘tooth root number’ might have the states ‘one’, ‘two’, or ‘three’ roots, along with other characters that describe the shape of any roots, or the character ‘tooth cusp number’ might have the states ‘6 cusps (5 plus C6)’, ‘6 cusps (5 plus C7)’ or ‘7 cusps (5 plus two C6s)’. There are multiple ways to code morphological characters. A good character should be clear, exclusive and defined logically so that other characters do not use the same morphology. Characters can be binary, or they can have three or more states (multistate). Conventional coding identifies different morphologies as different states of each character. The character ‘tooth root number’ mentioned above is an example. Multiple characters or character states can be combined (e.g. State 0: one and conical; State 1: two and molariform) in the composite coding method. According to Hawkins (2000), however, composite coding should not be used unless it is to recognize genetically linked characters as it reduces the information content. Presence/absence, or nominal variable coding, is a method of separating multiple characters or character states into ‘yes/no’ dichotomies. For example, Character 1 might be ‘C6’ with options of present/absent, and Character 2 might be ‘C7’ with the same options. This coding method tends to result in similarities that should properly be coded as different character states for the same character, except in the case that the characters are actually independent (i.e. in the above example, that C6s and C7s were actually determined at different loci, with four possible combinations). It is important to recognize that some characters should logically be transformed in a certain order. Ordered characters are those in which the transition from state to state follows a clear pattern, i.e. 0→1→2, and the cost of moving from state 0 to state 2 is two steps. Unordered characters have no increased cost to move from state 0 to state 2 as compared with moving from state 0 to state 1. Thus, it may be unreasonable to treat 3 roots as having evolved directly from the ‘one root’ or from the ‘two root’ character state in an ordered analysis, although it may make more sense in the case of continuous characters.
When several characters concern different and potentially independent aspects of the same structure, such as a tooth, it is essential there be a way of differentiating between a short conical root in a taxon as a derived character, and the inapplicability of root number, size and shape as a character in the case of another taxon. For example, if there are five characters that relate to aspects of root morphology, and a specific taxon never had a root on that tooth, then this taxon must be coded as inapplicable (commonly noted as ‘–’), rather than an additional character state of ‘no root’ because the latter will generate similarities among rootless taxa that do not exist in reality. The use of ‘?’, also called reductive coding, to code unknown or missing characters is a common practice, and results in the computer program evaluating the taxa in question with all possible character states for the best fit. This technique is commonly used in the analysis of fossils with missing data, and at this time it is probably the best way to code missing characters given the state of the computer programs available for analysis (Strong & Lipscomb, 1999).
Quantitative (or continuous) characters avoid some of the difficulty involved in devising schemes for scoring the different manifestations of discrete characters, particularly the arbitrariness encountered when characters based on similar morphology are given different states due to a lack of precision in coding. They do, however, have their own share of problems, namely how continuous distributions of morphology are to be divided into separate character states. There are several methods for doing this. Characters can be divided into equal segments, such that 0–1 inch is state one, 1–2 inches state two, etc. States can also be assigned on the basis of the gaps between species means. Unweighted methods, such as simple gap-coding, recognize different character states if there is a clear gap in mean value that is greater than the pooled within-group standard deviation; weighted methods recognize the absolute size of the gap between taxa, so that taxa with values 0–1 and 1–2 are more closely related than the next taxon, with a value of 12–13 (Chappill, 1989). Statistically more complicated methods also exist, such as generalized gap-coding (Archie, 1985) and finite mixture coding (Strait et al. 1996), that try to identify populations within the data. Quantitative characters have been criticized on the grounds that they do not measure homology, but as mentioned above, many discrete characters are poorly coded continuous characters in disguise, and several analyses (e.g. Wiens, 2001) have documented the usefulness of continuous characters in phylogenetic studies. Fully addressing character coding and theory is beyond the scope of this analysis; therefore, the more popular methods will be briefly mentioned, but the reader is encouraged to follow up on these topics.
Aside from direct methods for coding characters, models of character evolution are also relevant. Traditional parsimony methods use unweighted characters, where each character contributes equally to the construction of a cladogram. Within this system, however, it is possible to analyse characters as either ordered or unordered (see above). Several models of character state change, or optimization, exist. Wagner optimization assumes the characters are ordered, and has no penalty for character reversal (Farris, 1970). Fitch optimization also allows reversals, but the characters are unordered (Fitch, 1971). Dollo optimization is based on Dollo's ‘Law of Phylogenetic Irreversibility’, a model of evolution that posits that specialized forms are unlikely to evolve multiple times, so that homoplasy in complex structures must be interpreted as secondary loss from a more derived form, rather than the independent origin of a complex feature (Le Quesne, 1974). Camin–Sokal optimization is also predicated on a model of evolution, but in this case it is that once a complex structure evolves, it is unlikely to be lost (Camin & Sokal, 1965). Thus, Camin–Sokal optimization assumes that homoplasy in the data has an independent origin. It should be emphasized that the common approach of unweighted, unordered characters makes an assumption about the process of evolution, in much the same way that Dollo optimization, or other models, do.
Cladistic hypotheses and cladogram construction
Since the initial impact Hennigian cladistics had on the field of taxonomy in the 1960s, several major developments have occurred in the methods of interpreting and testing phylogenetic hypotheses. Compatibility methods like clique analysis analyse the fit of each character to a given tree to see if that character can be mapped onto the tree with no homoplasy (Felsenstein, 2004). Distance matrix methods calculate pairwise distances between all taxa to generate a tree that best predicts the observed differences (Felsenstein, 2004). They include methods like Least Squares, Generalized Least Squares and UPGMA, and can be used with various models of evolution. These methods are not commonly used in hominin systematics, so will not be further discussed. The following section focuses on parsimony and maximum-likelihood methods, the two most commonly used methods in systematics.
Traditional cladistic methods use the concept of scientific parsimony to construct and evaluate cladograms (Table 3). These methods are designed to create phylogenetic hypotheses that are empirically testable, which have few necessary theoretical assumptions and that lead to specific conclusions with a high information content in a ‘bold’ sense sensu Popper (Kluge, 2001). Parsimony cladograms can be constructed using either Hennig Argumentation or the Wagner method. The former considers the effect of each character on the taxa separately, while the latter joins taxa together one at a time based on the effect of all the characters, and results in the formation of a Wagner network, or unrooted tree. In both cases, the shortest tree with the fewest number of steps minimizes the assumptions of homoplasy necessary to explain the evolution of the taxa in question (Kluge & Farris, 1969).
Table 3.
Methods for cladogram construction
| Categories | Individual methods |
|---|---|
| Parsimony cladistics | Hennig argumentation |
| Wagner method (heuristic, exhaustive) | |
| Distance matrix | UPGMA |
| Least squares | |
| Generalized least squares | |
| Neighbour joining | |
| Statistical methods | Maximum likelihood |
| Bayesian inference (Markov chain Monte Carlo) |
The Wagner method forms the basis of most computerized parsimony analyses in use today. These analyses are performed using either exhaustive or heuristic search options. The former analyse every possible network, from every possible starting point, to identify the most parsimonious tree, and they are very time consuming. Branch and bound analyses start with a Wagner network and set the resulting length as the maximum number of steps allowable. Additional networks are created from different start points, and they are discarded if at any point they exceed the maximum allowable tree length. This method is faster than an exhaustive search, but may still be too time-consuming for large data sets. Heuristic methods save time by analysing subsets of all possible trees. Branch swapping methods check different tree topologies at various levels. These methods include nearest neighbour interchange, which rearranges sister groups at a local level, and more comprehensive methods, such as subtree pruning and regrafting, in which part of the cladogram is removed and reattached at different locations, and tree bisection and reconnection, in which a subset of the cladogram is re-rooted and then reattached to each branch in turn. These branch swapping methods use different algorithms to test various combinations in an attempt to identify the shortest and most parsimonious tree. While branch swapping methods are considerably faster than an exhaustive search, the possibility exists that they will neglect the most parsimonious tree.
Maximum-likelihood methods utilize a model for evolutionary change to predict the likelihood of a tree as the probability of the data (character matrix) given that tree (Felsenstein, 2004). This statistical approach requires the assumption that the characters studied are independent and are drawn at random from a set of all possible characters. These assumptions are usually violated in the case of cladistic inference (Felsenstein, 2004). Some argue that maximum-likelihood methods are equivalent to parsimony-based cladistics, and this argument is frequently used to justify their use in cladistics (Farris, 1973). This is only true, however, when the probability of evolutionary change over time is assumed to be very small (Felsenstein, 1973). Bayesian inference is similar to maximum-likelihood methods in that it requires a model of evolution, but it also uses additional statistics to compute directly the probability of the phylogenetic hypothesis (cladogram) given the data (making it comparable with parsimony-generated hypotheses, although evaluated in terms of probability and not tree length). In this sense it is the opposite of maximum-likelihood methods (Felsenstein, 2004). Bayesian inference adds a prior probability statistic that measures the confidence in a hypothesis prior to the addition of evidence (in this case, the character matrix). The result is the calculation of a posterior probability, which measures the confidence in the hypothesis as the effect of the data on the prior probability. Calculating the posterior probability requires the calculation of the probability of observing the data under all mutually exclusive hypotheses of relationships. This is often impracticable, so Markov chain Monte Carlo methods are commonly used in its stead. In these latter methods, the posterior distribution is estimated by randomly sampling from the possible hypotheses until a degree of stability is reached, providing a degree of certainty. Brownian motion, although typically used in studies of molecular evolution, has also been applied to quantitative morphological characters. It allows for the probability of branching patterns to be estimated based on a model of neutral, essentially random evolution (Felsenstein, 2004).
Evaluating cladograms
The primary method for evaluating traditional cladograms (Table 4) is to measure tree length. The shortest tree is the most parsimonious, has the highest information content and therefore minimizes the number of ad hoc assumptions of homoplasy required to explain the topology. Numerical indices have been devised to evaluate and compare parsimony cladograms, but three are commonly in use. The Consistency Index (CI) is calculated as the minimum number of steps (character state changes) necessary to explain the distribution of character states in the taxa being analysed, divided by the observed number of steps in a cladogram. This index measures the extent of homoplasy in the data. The Retention Index (RI) is calculated as the maximum number of steps possible on a tree minus the observed number of steps, divided by the maximum number of steps possible on a tree minus the minimum number of steps calculated from the data. It is a measure of the amount of expected synapomorphy from the data retained as homologies on the cladogram. The RI contains additional information about homoplasy compared with the CI, and is not inversely related to tree length, as is the CI; however, both indices have their uses (Farris, 1989). The third commonly used measure of the quality and information content of a cladogram, the Rescaled Consistency Index (RCI), is the product of the RI and the CI.
Table 4.
Methods for cladogram evaluation
| Categories | Methods |
|---|---|
| Numerical indicators | Consistency Index (CI) |
| Retention Index (RI) | |
| Rescaled Consistency Index (RCI) | |
| Tree length | |
| Resampling | Bootstrap |
| Jackknife | |
| Bremer support | |
| Consensus | Strict |
| Majority rules | |
| Adams | |
| Combinable component |
More specific methods for evaluating cladograms include methods that measure the support for a particular tree topology. In re-sampling methods, numerous replications are made, using strategies that exclude either taxa or characters (jackknife) one at a time, or take a random sample from the data (bootstrap), and the consensus of these replications is used to generate a probability value for individual branches (Felsenstein, 2004). Bremer support (also called the decay index) measures the stability of each node or branching point on the cladogram by calculating how many additional steps (character changes) would be necessary before a clade collapses (Bremer, 1988). The difference between the observed number of steps and the steps at which the clade collapses is the Bremer support value.
Even if the above methods are used there is still the problem of how to choose between equally parsimonious cladograms with different branching patterns, or topologies. Consensus methods are used to evaluate different but equally parsimonious cladograms (Kluge & Wolf, 1993). A strict consensus contains only those branches found in every equally parsimonious cladogram, with the remaining branches reduced to unresolved polytomies (branches with more than two stems). The majority rules consensus contains the topology for the branches that are found most frequently in the equally parsimonious cladograms. Combinable component consensus includes branches that are compatible with all of the cladograms, but which are not necessarily supported by each individual cladogram (i.e. a specific branching pattern found in half of the cladograms that was shown as an unresolved polytomy in the others). Lastly, Adams consensus trees isolate highly mobile branches to the node in common between all of the equally parsimonious trees, revealing the stable aspects of the topology. This method is particularly useful when one or more taxa shift considerably within the most parsimonious trees, which prevents a consensus from being produced by another method, but there is a degree of stability in most of the major clades.
Consensus trees are likely to contain the correct topology, but they are liable to be less precise than the original trees they incorporate. This imprecision is viewed as a liability by some authors who view the specificity and testability of a cladogram as a hypothesis of phylogeny as one of the primary advantages of cladistic methods (Kluge & Wolf, 1993). Additionally, consensus trees have a longer length, and thus more indication of homoplasy, than any of their component trees.
Homoplasy in the hominin clade
There are many aspects of hard tissue morphology that might represent homoplasy, or convergent evolution, or evolutionary reversals in the hominin clade. The genus Paranthropus is based primarily on craniofacial characters that suggest an adaptation to feeding on hard or tough objects. These features include postcanine megadontia, thick enamel, and changes to the zygomatic and other cranial bones that result in an improved mechanical advantage for chewing on the postcanine tooth crowns. If these adaptations of the megadont archaic hominins were inherited from a recent common ancestor then a separate Paranthropus genus is justified; however, if they occurred independently in the Au. aethiopicus and P. boisei lineage in East Africa, and in the Au. africanus and P. robustus lineage in southern Africa, then a separate genus would not be justified.
Postcranial adaptation is another possible source of homoplasy, but thus far most hominin cladistic analyses have focused exclusively on the craniodental evidence. It is generally assumed that bipedal locomotion, and the morphological changes it entails, arose only once during the course of hominin evolution, but it is possible that bipedality arose more than once in the hominin clade (Wood, 2000). For example, there is some evidence that there is more than one pattern of limb proportions in archaic hominins (Green et al. 2007).
Internal structure of the hominin clade
The topology of the hominin clade has changed substantially over the last several decades as new fossils have been discovered and new species and genera named for them. Space limitations preclude us from presenting an exhaustive review of the results of hominin cladistic analyses, so this section merely summarizes the current consensus on the topology of the hominin clade, and points to the areas of agreement and disagreement. It should be emphasized that differences in the taxa and characters included mean that the analyses reviewed here are not strictly comparable. Nonetheless, areas of agreement in the results of studies that are based on slightly different data sets suggest that some aspects of our understanding of the topology of the hominin clade are likely to be substantially accurate.
Chamberlain & Wood (1987) noted that up to 30% of characters used in phylogenetic studies of hominins (frequently craniodental characters) might be homoplasic. It is often the case that relatively few character state changes separate the most parsimonious cladogram from cladograms with a substantially different topology (e.g. Strait et al. 1997). Thus, we recommend that the results of hominin cladistic analyses be interpreted with care, for the vagaries of taphonomy mean that some aspects of morphology are consistently better represented in the hominin fossil record than others. The most parsimonious cladogram may not be more accurate than cladograms that are only a little less parsimonious.
Possible and probable hominins
The relationships of taxa such as S. tchadensis, O. tugenensis, Ar. kadabba and Ar. ramidus are unclear and because data are limited these taxa are only rarely included in hominin cladistic analyses. These are taxa that most likely occupy either a basal position on the hominin clade immediately above the root, or they may belong to one, or more, extinct clades closely related to the hominin clade.
Archaic hominins
Cladistic analyses of the hominin clade tend to show Australopithecus species not as a monophyletic group, but as a series of offshoots from the branch leading to Homo. A number of analyses have concluded that Au. afarensis is the sister of all later hominins (e.g. Skelton et al. 1986; Chamberlain & Wood, 1987; Skelton & McHenry, 1992; Strait et al. 1997; Kimbel et al. 2004; Strait & Grine, 2004). Until more information is available for the taxa in the ‘Possible and probable hominin’ grade Au. afarensis will probably continue to be viewed as the most primitive of the hominin taxa with hypodigms large enough to have the potential to provide reliable evidence about phylogenetic relationships. Australopithecus africanus is generally the sister-taxon to a clade that includes some combination of species typically attributed to Homo and/or Paranthropus (discussed below).
Megadont archaic hominins
Chamberlain & Wood (1987) identified the monophyletic group comprising P. boisei and P. robustus as the sister taxon of Au. africanus, but they did not include Au. aethiopicus in their analysis. Wood (1988) noted that 11 out of 18 major cladistic studies prior to 1987 identify P. robustus and P. boisei as sister species, and the other seven did not differentiate between them. The composition of the Paranthropus clade in more recent cladistic analyses varies, but the results of subsequent analyses mostly differ in the location and relationships of Au. africanus and Au. aethiopicus. Such is the case in the analyses conducted by Strait et al. (1997) and Skelton & McHenry (1998). Whereas the former analysis supports Paranthropus monophyly, with P. aethiopicus as the stem taxon of the clade, the Skelton & McHenry (1998) analysis supports a sister group relationship between a monophyletic group comprising P. robustus and P. boisei, and Homo. In this latter analysis P. aethiopicus is the sister taxon of a monophyletic group containing Homo, the megadont archaic hominins, and Au. africanus. Strait & Grine (2004) found the three ‘robust’ taxa consistently formed an independent clade, and in the same year, Kimbel et al. (2004) also found consistent support for a ‘robust’ australopith clade. What is clear is that the balance of the present cladistic evidence is in favour of Paranthropus monophyly. If one is sanguine that hard tissue morphology is capable of recovering phylogenetic relationships established on the basis of independent genetic evidence (e.g. Strait & Grine, 2004), then Paranthropus monophyly must be the hypothesis of choice. But if one is more skeptical about its ability to do so (e.g. Collard & Wood, 2000), then what many researchers interpret as overwhelming evidence for Paranthropus monophyly looks less compelling.
Homo
A Homo clade, including at the minimum H. sapiens and H. erectus as sister-groups, with H. habilis s.l. one step removed, is supported by the results of a number of cladistic analyses (e.g. Skelton & McHenry, 1992; Wood, 1994; Strait & Grine, 2004). Only a few analyses have focused on the relationships of the taxa in the pre-modern Homo grade. The taxa within the transitional hominin grade retain a substantial number of primitive character states, but they are generally located within the Homo clade, usually at its base. In some earlier analyses (e.g. Chamberlain & Wood, 1987) the transitional hominins are linked to the clade that includes P. robustus and P. boisei. See Wood (in press) for an evaluation of current thinking about the relationships of early Homo.
What qualifies a group of taxa to be a genus?
The fourth taxonomic controversy we discuss in this review refers to the genus category. The genus has received comparatively little attention from evolutionary biologists, despite Simpson's statement that ‘it frequently appears that the genus is a more usable and reliable unit for classification than the species’ (Simpson, 1961: 199).
At the present time there are two main competing definitions of a genus. The first, associated with Ernst Mayr and the ‘Evolutionary Systematic’ school of classification, suggests that ‘a genus consists of one species, or a group of species of common ancestry, which differ in a pronounced manner from other groups of species and are separated from them by a decided morphological gap’ (Mayr, 1950: 110). Mayr went on to state that the genus ‘has a very distinct biological meaning’. Species united in a genus occupy an ecological situation that is different from that occupied by the species of another genus, or, to use the terminology of Sewall Wright, they occupy a ‘different adaptive plateau’ (Mayr, 1950: 110). Thus, a genus is interpreted as a group of species of common ancestry that are both adaptively coherent and morphologically distinctive. But it is implicit that ‘common ancestry’ subsumes both monophyletic and paraphyletic groups. Thus, in a ‘Mayrian’ genus ‘common ancestry’ is not synonymous with the component species all being more closely related to one another than to any other species.
The second definition of the genus is associated with the ‘Phylogenetic Systematic’ or ‘Cladistic’ school of classification, and it can be traced back to the work of Willi Hennig (1950). In this cladistic or ‘Hennigian’ sense a genus is defined as a group of species that are more closely related to one another than they are to species assigned to another genus (Stevens, 1985). In this definition a genus can only be monophyletic (i.e. only and all the members of a clade): it cannot be paraphyletic (i.e. contain only part of a monophyletic group). But this definition makes no stipulations about adaptive coherence or about morphological distinctiveness (see above).
Wood & Collard (1999a: 201) proposed that a genus should be defined as ‘a species, or monophylum, whose members occupy a single adaptive zone.’ It is important to note that contrary to Leakey et al. (2001) this definition does not require the adaptive zone to be unique, or even distinctive. It just requires the adaptive zone to be consistent and coherent across the species taxa in the putative genus. Thus, for a species to be included in an existing genus Wood & Collard (1999a) suggest the following criteria. First, the species should belong to the same monophyletic group as the type species of that genus. Second, the adaptive strategy of the species should be closer to the adaptive strategy of the type species of the genus in which it is included, than it is to the type species of any other genus. The operative word is ‘closer’; the adaptive strategy of the species under consideration does not have to be identical to that of the type species of its genus.
Conclusions
The authors of the other papers delivered in the symposium from which the papers in this issue of the Journal of Anatomy are drawn were each asked to take an organ (e.g. the brain), or an important function (e.g. locomotion), or an anatomical region (e.g. the face), and make predictions about its state in the last common ancestor of the Pan-Homo clade, and then trace its evolutionary history within the hominin clade. To do this the contributors need a reliable taxonomy and a sound assessment of the relationships among the taxa within the hominin clade. This paper presents two consensus hominin taxonomies and summarizes our present state of knowledge about the relationships among the taxa within the hominin clade.
It was our intention to provide the consumers of taxonomy and systematics with some insight into the challenges that face those whose research focuses on those topics. As in any research field the practitioners of hominin taxonomy and systematics do not always see eye to eye. But it is relatively rare for the differences to be about the nature of the data. Most of the different interpretations are due to the researchers involved having different analytical philosophies. This is difficult to appreciate unless each one of us is willing to familiarize ourselves with the principles underlying each method, and with the methods themselves. Although this may be desirable, it is not likely to occur soon. In the meantime, we hope that these relatively simple explanations of the background to some of the main controversies will enable readers to apply a healthy dose of skepticism to pronouncements about the taxonomy and systematics of the hominin clade.
Acknowledgments
We are grateful to the Anatomical Society of Great Britain and Ireland for their invitation to take part in this symposium, and to David Strait and Rui Diogo for their constructive and important comments on previous versions of the manuscript. The participation of B.W. was supported by George Washington University's Academic Excellence initiative, the George Washington University VPAA, by the George Washington University Professorship in Human Origins and by the ASGBI. N.L. is supported by a George Washington University Academic Excellence Graduate Fellowship.
References
- Alexeev V. The Origin of the Human Race. Moscow: Progress Publishers; 1986. [Google Scholar]
- Arambourg C, Coppens Y. Decouverte d’un australopithecien nouveau dans les Gisements de L’Omo (Ethiopie) South Afr J Sci. 1968;64:58–59. [Google Scholar]
- Archie JW. Methods for coding variable morphological features for numerical taxonomic analysis. Syst Zool. 1985;34:326–345. [Google Scholar]
- Asfaw B, White T, Lovejoy O, Latimer B, Simpson S, Suwa G. Australopithecus garhi: a new species of early hominid from Ethiopia. Science. 1999;284:629–635. doi: 10.1126/science.284.5414.629. [DOI] [PubMed] [Google Scholar]
- Bermúdez de Castro JM, Arsuaga JL, Carbonell E, et al. A hominid from the Lower Pleistocene of Atapuerca, Spain: possible ancestor to Neandertals and modern humans. Science. 1997;276:1392–1395. doi: 10.1126/science.276.5317.1392. [DOI] [PubMed] [Google Scholar]
- Bremer K. The limits of amino-acid sequence data in Angiosperm phylogenetic reconstruction. Evolution. 1988;42:795–803. doi: 10.1111/j.1558-5646.1988.tb02497.x. [DOI] [PubMed] [Google Scholar]
- Broom R. The Pleistocene anthropoid apes of South Africa. Nature. 1938;142:377–379. [Google Scholar]
- Brown B, Brown FH, Walker A. New hominids from the Lake Turkana Basin, Kenya. J Hum Evol. 2001;41:29–44. doi: 10.1006/jhev.2001.0476. [DOI] [PubMed] [Google Scholar]
- Brown P, Sutikna T, Morwood MJ, et al. A new small-bodied hominin from the Late Pleistocene of Flores, Indonesia. Nature. 2004;431:1055–1061. doi: 10.1038/nature02999. [DOI] [PubMed] [Google Scholar]
- Brunet M, Beauvilain A, Coppens Y, Heintz E, Moutaye AHE, Pilbeam D. Australopithecus bahrelghazali, une nouvelle espece d’Hominide ancien de la region de Koro Toro (Tchad) CR Acad Sci. 1996;322:907–913. [Google Scholar]
- Brunet M, Guy F, Pilbeam D, et al. A new hominid from the Upper Miocene of Chad, Central Africa. Nature. 2002;418:145–151. doi: 10.1038/nature00879. [DOI] [PubMed] [Google Scholar]
- Cain AJ. Animal Species and Evolution. Princeton, NJ: Princeton University Press; 1954. [Google Scholar]
- Camin JH, Sokal RR. A method for deducing branching sequences in phylogeny. Evolution. 1965;19:311–326. [Google Scholar]
- Chamberlain AT, Wood BA. A reappraisal of the variation in hominid mandibular corpus dimensions. Am J Phys Anthropol. 1985;66:399–403. [Google Scholar]
- Chamberlain AT, Wood BA. Early hominid phylogeny. J Hum Evol. 1987;16:119–133. [Google Scholar]
- Chappil JA. Quantitative characters in phylogenetic analysis. Cladistics. 1989;5:217–234. doi: 10.1111/j.1096-0031.1989.tb00487.x. [DOI] [PubMed] [Google Scholar]
- Collard MC, Wood BA. How reliable are human phylogenetic hypotheses? Proc Natl Acad Sci USA. 2000;97:5003–5006. doi: 10.1073/pnas.97.9.5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cracraft J. Species concepts and speciation analysis. In: Johnson RF, editor. Current Ornithology. New York: Plenum Press; 1983. pp. 159–187. [Google Scholar]
- Dart RA. Australopithecus africanus: the man-ape of South Africa. Nature. 1925;115:195–199. [Google Scholar]
- De Pinna M. Concepts and tests of homology in the cladistic paradigm. Cladistics. 1991;7:367–394. [Google Scholar]
- Dreyer TF. A human skull from Florisbad, Orange Free State, with a note on the Endocranial Cast (by C. U. Ariëns-Kappers) Proc Acad Sci Amst. 1935;38:119–128. [Google Scholar]
- Dubois E. Palaeontologische andrezoekingen op Java. Versl Mijnw Batavia. 1892;3:10–14. [Google Scholar]
- Eldredge N. What, if anything, is a species? In: Kimbel WH, Martin LB, editors. Species, Species Concepts, and Primate Evolution. New York: Plenum Press; 1993. pp. 3–20. [Google Scholar]
- Farris JS. Methods for computing Wagner trees. Syst Zool. 1970;19:83–92. [Google Scholar]
- Farris JS. On the use of the parsimony criterion for inferring evolutionary trees. Syst Zool. 1973;22:250–256. [Google Scholar]
- Farris JS. The retention index and homoplasy excess. Syst Zool. 1989;38:406–407. [Google Scholar]
- Felsenstein J. Maximum likelihood and minimum-steps methods for estimating evolutionary trees from data on discrete characters. Syst Zool. 1973;22:240–249. [Google Scholar]
- Felsenstein J. Inferring Phylogenies. Sunderland, MA: Sinauer Associeates, Inc; 2004. [Google Scholar]
- Fitch WM. Toward defining the course of evolution: minimal change for a specific tree topology. Syst Zool. 1971;20:406–416. [Google Scholar]
- Galik K, Senut B, Pickford M, et al. External and internal morphology of the BAR 1002’00 Orrorin tugenensis femur. Science. 2004;305:1450–1453. doi: 10.1126/science.1098807. [DOI] [PubMed] [Google Scholar]
- Ghiselin MT. Models in phylogeny. In: Schopf TJM, editor. Models in Paleobiology. Cooper: Freeman; 1972. pp. 130–145. [Google Scholar]
- Gordon AD, Green DJ, Richmond BG. Strong postcranial size dimorphism in Australopithecus afarensis: results from two new multivariate resampling methods for multivariate data sets with missing data. Am Phys Anthropol. 2008;135:311–328. doi: 10.1002/ajpa.20745. [DOI] [PubMed] [Google Scholar]
- Green DJ, Gordon AD, Richmond BG. Limb-size proportions in Australopithecus afarensis and Australopithecus africanus. J Hum Evol. 2007;52:187–200. doi: 10.1016/j.jhevol.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Groves CP, Mazák V. An approach to the taxonomy of the Hominidae: gracile Villafranchian hominids of Africa. Casopis pro mineralogii a geologii. 1975;20:225–247. [Google Scholar]
- Haile-Selassie Y. Late Miocene hominids from the Middle Awash, Ethiopia. Nature. 2001;412:178–181. doi: 10.1038/35084063. [DOI] [PubMed] [Google Scholar]
- Haile-Selassie Y, Asfaw B, White TD. Hominid cranial remains from Upper Pleistocene deposits at Aduma, Middle Awash, Ethiopia. Am J Phys Anthropol. 2004;123:1–10. doi: 10.1002/ajpa.10330. [DOI] [PubMed] [Google Scholar]
- Hawkins JA. A survey of primary homology assessment: different botanists perceive and define characters in different ways. In: Scotland R, Pennington RT, editors. Homology and Systematics. London: Taylor and Francis; 2000. pp. 22–53. [Google Scholar]
- Hennig W. Grundzüge einer Theorie der Phylogenetischen Systematik. Berlin: Deutscher Zentralverlag; 1950. [Google Scholar]
- Hennig W. Phylogenetic Systematics. Chicago: University of Illinois Press; 1966. [Google Scholar]
- Huxley JS. Evolutionary process and taxonomy with special reference to grades. Upps Univ Arssks. 1958;6:21–38. [Google Scholar]
- Johanson DC, White TD, Coppens Y. A new species of the genus Australopithecus(Primates: Hominidae) from the Pliocene of East Africa. Kirtlandia. 1978;28:1–14. [Google Scholar]
- Jolly CJ. A proper study for Mankind: analogies from the Papionin monkeys and their implications for human evolution. Yearbook Phys Anthropol. 2001;44:177–204. doi: 10.1002/ajpa.10021. [DOI] [PubMed] [Google Scholar]
- Kimbel W, Rak Y, Johanson DC. The Skull of Australopithecus afarensis. New York: Oxford University Press; 2004. [Google Scholar]
- King W. The reputed fossil man of the Neanderthal. Q J Sci. 1864;1:88–97. [Google Scholar]
- Kluge AG, Farris JS. Quantitative phyletics and the evolution of anurans. Syst Zool. 1969;18:1–32. [Google Scholar]
- Kluge AG, Wolf AJ. Cladistics: what's in a word? Cladistics. 1993;9:183–199. doi: 10.1111/j.1096-0031.1993.tb00217.x. [DOI] [PubMed] [Google Scholar]
- Kluge AG. Parsimony with and without scientific justification. Cladistics. 2001;17:199–210. doi: 10.1111/j.1096-0031.2001.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Knight A. The phylogenetic relationship of Neandertal and modern human mitochondrial DNAs based on informative nucleotide sites. J Hum Evol. 2003;44:627–632. doi: 10.1016/s0047-2484(03)00044-7. [DOI] [PubMed] [Google Scholar]
- Kohl-Larsen L. Auf den Spuren des Vormenschen: forschungen, Fahrten und Erlebnisse in Deutsch-Ostafrika (Deutshe Afrika-expedition 1934–1936 und 1937–1939. Stuttgart: Strecker und Schröder; 1943. [Google Scholar]
- Krause J, Orlando L, Serre D, et al. Neanderthals in central Asia and Siberia. Nature. 2007;449:902–904. doi: 10.1038/nature06193. [DOI] [PubMed] [Google Scholar]
- Krings M, Stone A, Schmitz RW, Krainitzki H, Stoneking M, Pääbo S. Neandertal DNA sequences and the origin of modern humans. Cell. 1997;90:19–30. doi: 10.1016/s0092-8674(00)80310-4. [DOI] [PubMed] [Google Scholar]
- Krings M, Geisert H, Schmitz RW, Krainitzk H, Pääbo S. DNA sequence of the mitochondrial hypervariable region II from the Neandertal type specimen. Proc Natl Acad Sci USA. 1999;96:5581–5585. doi: 10.1073/pnas.96.10.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krings M, Capelli C, Tschentscher F, et al. A view of Neandertal genetic diversity. Nat Genet. 2000;26:144–146. doi: 10.1038/79855. [DOI] [PubMed] [Google Scholar]
- Le Quesne WJ. The uniquely evolved character concept and its cladistic application. Syst Zool. 1974;23:513–517. [Google Scholar]
- Leakey LSB. Recent discoveries at Olduvai Gorge, Tanganyika. Nature. 1958;181:1099–1103. doi: 10.1038/1811099a0. [DOI] [PubMed] [Google Scholar]
- Leakey LSB. A new fossil skull from Olduvai. Nature. 1959;184:491–493. [Google Scholar]
- Leakey LSB, Tobias PV, Napier JR. A new species of the genus Homo from Olduvai Gorge. Nature. 1964;202:7–9. doi: 10.1038/202007a0. [DOI] [PubMed] [Google Scholar]
- Leakey MG, Feibel CS, McDougall I, Walker A. New four-million year old hominid species from Kanapoi and Allia Bay, Kenya. Nature. 1995;376:565–571. doi: 10.1038/376565a0. [DOI] [PubMed] [Google Scholar]
- Leakey MG, Spoor F, Brown FH, et al. New hominin genus from eastern Africa shows diverse middle Pliocene lineages. Nature. 2001;410:433–440. doi: 10.1038/35068500. [DOI] [PubMed] [Google Scholar]
- Leakey REF. Evidence for an advanced Plio-Pleistocene hominid from East Rudolf, Kenya. Nature. 1973;242:447–450. doi: 10.1038/242447a0. [DOI] [PubMed] [Google Scholar]
- Linnaeus C. Systema Naturae. Stockholm: Laurentii Salvii; 1758. [Google Scholar]
- Mayr E. Systematics and the Origin of Species. New York: Columbia University Press; 1942. [Google Scholar]
- Mayr E. Taxonomic categories in fossil hominids. C.S.H. Symp Quat Biol. 1950;15:109–118. doi: 10.1101/sqb.1950.015.01.013. [DOI] [PubMed] [Google Scholar]
- Mayr E. The Growth of Biological Thought: Diversity, Evolution and Inheritance. Cambridge, MA: Harvard University Press; 1982. [Google Scholar]
- McDougall I, Brown FH, Fleagle JG. Stratigraphic placement and age of modern humans from Kibish, Ethiopia. Nature. 2005;433:733–736. doi: 10.1038/nature03258. [DOI] [PubMed] [Google Scholar]
- Morwood MJ, Soejono RP, Roberts RG, et al. Archaeology and age of a new hominin from Flores in eastern Indonesia. Nature. 2004;431:1097–1091. doi: 10.1038/nature02956. [DOI] [PubMed] [Google Scholar]
- Nixon KC, Wheeler QD. An amplification of the phylogenetic species concept. Cladistics. 1990;6:211–233. [Google Scholar]
- Ohman JC, Lovejoy CO, White T. Questions about Orrorin tugenensis. Science. 2005;307:845. doi: 10.1126/science.307.5711.845b. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov IV, Gotherstrom A, Romanova GP, Khritonov VM, Liden K, Goodwin W. Molecular analysis of Neanderthal DNA from the northern Caucasus. Nature. 2000;404:490–493. doi: 10.1038/35006625. [DOI] [PubMed] [Google Scholar]
- Partridge TC, Granger DE, Caffee MW, Clarke RJ. Lower Pliocene hominid remains from Sterkfontein. Science. 2003;300:607–612. doi: 10.1126/science.1081651. [DOI] [PubMed] [Google Scholar]
- Paterson HEH. The recognition concept of species. In: Vrba E, editor. Trans. Mus. Monographs, No. 4. Pretoria: Transvaal Museum; 1985. pp. 21–29. Species and Speciation. [Google Scholar]
- Patterson B, Howells WW. Hominid humeral fragment from early Pleistocene of northwest Kenya. Science. 1967;156:64–66. doi: 10.1126/science.156.3771.64. [DOI] [PubMed] [Google Scholar]
- Patterson C. Homology in classical and molecular biology. Mol Biol Evol. 1988;5:603–625. doi: 10.1093/oxfordjournals.molbev.a040523. [DOI] [PubMed] [Google Scholar]
- Pickford M. Late Miocene sediments and fossils from the Northern Kenya Rift valley. Nature. 1975;256:279–284. [Google Scholar]
- Pickford M, Senut B, Gommery D, Treil J. Bipedalism in Orrorin tugenesis revealed by its femora. C.R. Academy of Science, Éditions Scientifiques et Médicales Elsevier SAS, Paris. 2002;1:1–13. [Google Scholar]
- Reno PL, Meindl RS, McCollum MA, Lovejoy CO. Sexual dimorphism in Australopithecus afarensis was similar to that of modern humans. Proc Natl Acad Sci USA. 2003;100:9404–9409. doi: 10.1073/pnas.1133180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond BG, Jungers WL. Orrorin tugenensis femoral morphology and the evolution of hominin bipedalism. Science. 2008;319:1662–1665. doi: 10.1126/science.1154197. [DOI] [PubMed] [Google Scholar]
- Robinson JT. The affinities of the new Olduvai australopithecine. Nature. 1960;186:456–458. [Google Scholar]
- Schoetensack O. Der Unterkiefer des Homo heidelbergensis aus den Sanden von Mauer bei Heidelberg. Leipzig: W. Engelmann; 1908. [Google Scholar]
- Senut B, Pickford M, Gommery D, Mein P, Cheboi K, Coppens Y. First hominid from the Miocene (Lukeino Formation, Kenya) CR Acad Sci, Paris. 2001;332:137–144. [Google Scholar]
- Simpson GG. Principles of Animal Taxonomy. New York: Columbia University Press/Oxford University Press; 1961. [DOI] [PubMed] [Google Scholar]
- Skelton RR, McHenry HM, Drawhorn GM. Phylogenetic analysis of early hominids. Curr Anthropol. 1986;27:21–43. [Google Scholar]
- Skelton RR, McHenry HM. Evolutionary relationships among early hominids. J Hum Evol. 1992;23:309–349. [Google Scholar]
- Skelton RR, McHenry HM. Trait list bias and a reappraisal of early hominid phylogeny. J Hum Evol. 1998;34:109–113. doi: 10.1006/jhev.1997.0195. [DOI] [PubMed] [Google Scholar]
- Smith AB. Systematics and the Fossil Record: Documenting Evolutionary Patterns. Oxford: Blackwell Publishing; 1994. [Google Scholar]
- Smith RJ. Species recognition in paleoanthropology; implications of small sample sizes. In: Lieberman DE, Smith RJ, Kelley J, editors. Interpreting the Past: Essays on Human, Primate, and Mammal Evolution in honor of David Pilbeam. Boston: Brill Academic Publishers; 2005. pp. 207–219. [Google Scholar]
- Sokal RR, Crovello TJ. The biological species concept: A critical evaluation. Am Nat. 1970;104:127–153. [Google Scholar]
- Stevens PF. The genus concept in practice-but for what practice? Kew Bull. 1985;40:457–465. [Google Scholar]
- Strait DS, Moniz MA, Strait PT. Finite mixture coding: a new approach to coding continuous characters. Syst Biol. 1996;45:67–78. [Google Scholar]
- Strait DS, Grine FE, Moniz MA. A reappraisal of early hominid phylogeny. J Hum Evol. 1997;32:17–82. doi: 10.1006/jhev.1996.0097. [DOI] [PubMed] [Google Scholar]
- Strait DS, Grine FE. Inferring hominoid and early hominid phylogeny using craniodental characters: the role of fossil taxa. J Hum Evol. 2004;47:399–452. doi: 10.1016/j.jhevol.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Strong EE, Lipscomb D. Character coding and inapplicable data. Cladistics. 1999;15:363–371. doi: 10.1111/j.1096-0031.1999.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Susman RL. New postcranial remains from Swartkrans and their bearing on the functional morphology and behavior of Paranthropus robustus. In: Grine FE, editor. Evolutionary History of the ‘Robust’ Australopithecines. New York: Aldine de Gruyter; 1988. pp. 149–172. [Google Scholar]
- Tobias PV. The place of Homo erectus in nature with a critique of the cladistic approach. In: Bower JRF, Sartono S, editors. Human Evolution in its Ecological Context. Leiden: Pithecanthropus Centennial Foundation; 1995. pp. 31–41. [Google Scholar]
- Vignaud P, Duringer P, Mackaye HT, et al. Geology and paleontology of the Upper Miocene Toros-Menalla hominid locality, Chad. Nature. 2002;418:152–155. doi: 10.1038/nature00880. [DOI] [PubMed] [Google Scholar]
- Weidenreich F. Some problems dealing with ancient man. Am Anthropol. 1940;42:375–383. [Google Scholar]
- White T, Asfaw B, DeGusta D, et al. Pleistocene Homo sapiens from Middle Awash, Ethiopia. Nature. 2003;423:742–747. doi: 10.1038/nature01669. [DOI] [PubMed] [Google Scholar]
- White TD, Suwa G, Asfaw B. Australopithecus ramidus, a new species of early hominid from Aramis, Ethiopia. Nature. 1994;371:306–312. doi: 10.1038/371306a0. [DOI] [PubMed] [Google Scholar]
- White TD, Suwa G, Asfaw B. Australopithecus ramidus, a new species of early hominid from Aramis, Ethiopia – a corrigendum. Nature. 1995;375:88. doi: 10.1038/375088a0. [DOI] [PubMed] [Google Scholar]
- Wiens JJ. Character analysis in morphological phylogenetics: problems and solutions. Syst Biol. 2001;50:689–699. doi: 10.1080/106351501753328811. [DOI] [PubMed] [Google Scholar]
- Wolpoff MH, Thorne AG, Jelinek J, Yinyun Z. The case for sinking Homo erectus: 100 years of Pithecanthropus is enough! Courier Forschungs-Institut Senckenberg. 1994;171:341–361. [Google Scholar]
- Wood BA. Are ‘robust’ australopithecines a monophyletic group? In: Grine FE, editor. Evolutionary History of the ‘Robust’ Australopithecines. New York: Aldine de Gruyter; 1988. pp. 269–284. [Google Scholar]
- Wood BA, Li Y, Willoughby C. Intraspecific variation and sexual dimorphism in cranial and dental variables among higher primates and their bearing on the hominid fossil record. J Anat. 1991;174:185–205. [PMC free article] [PubMed] [Google Scholar]
- Wood BA. Origin and evolution of the genus Homo. Nature. 1992;355:783–790. doi: 10.1038/355783a0. [DOI] [PubMed] [Google Scholar]
- Wood B. Taxonomy and evolutionary relationships of Homo erectus. Courier Forschungs-Institut Senckenberg. 1994;171:159–165. [Google Scholar]
- Wood BA, Collard M. The changing face of genus Homo. Evol Anthropol. 1999a;8:195–207. [Google Scholar]
- Wood B. Investigating human evolutionary history. J Anat. 2000;197:1–17. doi: 10.1046/j.1469-7580.2000.19710003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood B, Lieberman DE. Craniodental variation in Paranthropus boisei: a developmental and functional perspective. Am J Phys Anthropol. 2001;116:13–25. doi: 10.1002/ajpa.1097. [DOI] [PubMed] [Google Scholar]
- Wood BA. The changing boundary of the genus Homo. In: Grine FE, Fleagle JG, Leakey RE, editors. The First Humans: Origins of the Genus Homo. New York: Springer; in press. [Google Scholar]
- Wright S. The roles of mutation, inbreeding, crossbreeding and selection in evolution. Proceedings of the Sixth International Congress Genetics. 1932;1:356–366. [Google Scholar]