Abstract
This review has three main aims: (1) to make specific predictions about the habitat of the hypothetical last common ancestor of the chimpanzee/bonobo–human clade; (2) to outline the major trends in environments between 8–6 Ma and the late Pleistocene; and (3) to pinpoint when, and in some cases where, human ancestors evolved to cope with the wide range of habitats they presently tolerate. Several lines of evidence indicate that arboreal environments, particularly woodlands, were important habitats for late Miocene hominids and hominins, and therefore possibly for the last common ancestor of the chimpanzee/bonobo–human clade. However, as there is no clear candidate for this last common ancestor, and because the sampling of fossils and past environments is inevitably patchy, this prediction remains a working hypothesis at best. Nonetheless, as a primate, it is expected that the last common ancestor was ecologically dependent on trees in some form. Understanding past environments is important, as palaeoenvironmental reconstructions provide the context for human morphological and behavioural evolution. Indeed, the impact of climate on the evolutionary history of our species has long been debated. Since the mid-Miocene, the Earth has been experiencing a general cooling trend accompanied by aridification, which intensified during the later Pliocene and Pleistocene. Numerous climatic fluctuations, as well as local, regional and continental geography that influenced weather patterns and vegetation, created hominin environments that were dynamic in space and time. Behavioural flexibility and cultural complexity were crucial aspects of hominin expansion into diverse environments during the Pleistocene, but the ability to exploit varied and varying habitats was established much earlier in human evolutionary history. The development of increasingly complex tool technology facilitated re-expansion into tropical forests. These environments are difficult for obligate bipeds to negotiate, but their exploitation was accomplished by archaic and/or anatomically modern humans independently in Africa and south-east Asia. Complex social behaviour and material culture also allowed modern humans to reach some of the most hostile regions of the globe, above the Arctic Circle, by the late Pleistocene. This, with colonization of the Americas and Australasia, established Homo sapiens as a truly cosmopolitan species.
Keywords: climate change, habitats, Miocene, morphological adaptation, palaeoenvironment, Pleistocene, Pliocene
Introduction
Palaeoenvironmental reconstructions provide the context for human evolution and behaviour. Creating a picture of human evolutionary history that is as complete as possible requires an understanding of the animals and plants that lived alongside hominins, a knowledge of the ways hominins exploited their environments, and investigation of whether and how climate change shaped human behaviour and evolution. For these reasons, the impact of climate and the environment on human evolution has long been debated. Some theories have drawn attention to how macroevolutionary processes, such as speciation and extinction, might respond to climate change (e.g. Vrba, 1995). Others have stressed the links between the cooling trend that began in the Miocene, the aridification associated with it, and hominin adaptation to open environments, particularly with respect to the evolution of bipedalism (e.g. Wheeler, 1991). These studies are complemented by an interest in prehistoric landscape use and hominin palaeoecology (e.g. Blumenschine & Peters, 1998).
Environments comprise living (biotic) and non-living (abiotic) components, so palaeoenvironmental data come from a variety of sources, including fossilized plants, phytoliths and pollen, faunal remains, including insects, birds and small and large mammals, plus geochemical and physical evidence from sediments, including fossilized soils. These data give indications of climate, environment and habitat structure at different scales – global, continental, regional and local. Methods that are global or continental in scale, such as the study of oxygen isotope ratios and aeolian dust from deep-sea cores, give an indication of major climate trends. Methods that are regional in scale rely on material that has probably been transported either during life, after death or throughout the fossilization process, and are commonly used in palaeoanthropology to characterize palaeoenvironments over relatively large areas, usually tens to hundreds of square kilometres. Studies of the flora (often represented by pollen) and fauna of a region fall into this category, and can give useful information about the habitats sampled in the wider area as well as the dietary preferences of a range of organisms. Local, site-specific methods include the study of palaeosols and sediments at a particular fossil locality. These give information about a restricted area and tend to be particular to the site from which they were recovered.
The literature on Neogene climate and environments encompasses reconstructions at all the scales mentioned above. Thus, much of the information presented here will necessarily be of an ‘overview’ nature, and cannot claim to be exhaustive. It will also focus almost exclusively on the broad environmental context of human evolution in the Old World rather than in Australasia and the Neotropics, as human occupation of Australasia probably only dates back to the late Pleistocene (around 50 000 years ago) and colonization of the Americas is even more recent. In addition, discussion of hominin palaeoecology (the interaction between the different actors in an ecosystem) and use of the landscape will be kept to a minimum, in order to focus more on morphological responses to the environment. With these caveats in mind, this review has three main aims. The first is to make specific predictions about the habitat of the hypothetical last common ancestor (LCA) of the chimpanzee/bonobo–human clade. The second is to outline the major trends in environments between 8–6 Ma and the late Pleistocene. As humans adapt morphologically as well as physiologically, behaviourally and culturally to their environments, environmental influences on human and primate morphology will be discussed. The third element of this review will be to pinpoint when, and in some cases where, human ancestors evolved to cope with the wide range of habitats they presently tolerate.
The habitat of the last common ancestor
Modern humans, as discussed in more detail below, exploit a large variety of environments and are globally distributed. Modern chimpanzees and bonobos, by contrast, are confined to parts of West, central and East Africa (below the Sahara), and inhabit a much more restricted range of environments. These usually comprise tropical forest (both primary and secondary), but chimpanzees also exploit woodland and forest fringe habitats in parts of their range (Sponheimer et al. 2006). It is commonly inferred from molecular data (calibrated with the fossil record) that the LCA of the chimpanzee/bonobo–human clade existed between 6 and 8 Ma (Steiper & Young, 2006), although some estimates have put the split considerably later (Patterson et al. 2006). The recent discoveries of Sahelanthropus tchadensis from Chad, dated to c. 7 Ma (Brunet et al. 2002, 2005), the 6 Ma Orrorin tugenensis from Kenya (Senut et al. 2001) and Ardipithecus kadabba from horizons dated to between 5.54 and 5.77 Ma at the Middle Awash in Ethiopia (Haile-Selassie, 2001) have shed some light on the environments, biogeography and adaptive range of hominids (i.e. the clade that includes the extant African great apes and modern humans) from the terminal Miocene. Nevertheless, the terrestrial fossil record of Africa in the Late Miocene remains very poor (Jablonski & Kelley, 1997), although this period is crucial for the understanding of how environmental factors might have influenced the early evolution of the hominin clade.
One central issue when debating the possible or probable habitats and adaptations of the earliest hominins (the informal term for the tribe Hominini, the clade whose only extant representative is Homo sapiens) is whether Sahelanthropus, Orrorin and Ardipithecus are good candidates for the LCA. These taxa are found within the chronological window given by molecular estimates of the divergence of the human and chimpanzee lineages, assuming that these estimates (and the dates of the fossils themselves) are accurate. However, as discussed by Cobb (this issue) and others (Wood & Richmond, 2000; Andrews & Harrison, 2005), the identification of stem hominins is fraught with difficulties. None of the contenders mentioned above unequivocably demonstrates features that align them most closely to the human clade, nor are any of the Miocene fossil apes obviously linked to the hominin lineage.
Bipedalism is one trait – or more precisely behaviour – that is used to define the Hominini. Some workers advocate caution when interpreting the evidence for bipedalism in Sahelanthropus, Orrorin and Ardipithecus(Andrews & Harrison, 2005). Further, it is questionable whether bipedalism can be used as a defining feature of the Hominini, and identifying stem hominins on the basis of bipedality might be misleading (Wood & Richmond, 2000; Andrews & Harrison, 2005; O’Higgins & Elton, 2007). As reviewed by Crompton (this issue), bipedalism is seen in modern apes and there is evidence for bipedal locomotion, possibily arboreal, in Miocene apes. It is also probable that there was significant diversity in the modes of bipedalism used by Plio-Pleistocene hominins (Richmond et al. 2002; Harcourt-Smith & Aiello, 2004), thus raising the possibility that bipedality might have evolved independently in various hominin lineages (Wood & Richmond, 2000; O’Higgins & Elton, 2007). Identification of stem hominins might be reliant, therefore, on a small number of dental traits, particularly in the canines, which are not necessarily truly diagnostic (Andrews & Harrison, 2005).
Even if the actual LCA cannot be pinpointed, reconstructions of the habitats and environments of Miocene taxa can provide important contextual information, especially if considered alongside palaeobiological data, including locomotion and diet, from the fossils themselves (sensuAndrews & Humphrey, 1999). It might also be possible to draw up parameters for the likely environment of a particular animal by examining the environments and palaeobiologies of closely related preceding and succeeding species. Therefore, although it is beyond the scope of this paper to review thoroughly the evolutionary history of Miocene apes, their palaeoenvironments will be outlined, as will those of the Plio-Pleistocene hominins. A parsimonious biogeographical view would predict that as the current evidence for early hominin evolution comes entirely from Africa (Andrews & Harrison, 2005), and that the chimpanzee, the closest living relative of humans, also lives exclusively on that continent, the LCA is also likely to be found there. However, work on other mammalian lineages, including primates, indicates complex patterns of dispersal into as well as out of Africa during the Miocene (Jablonski, 1998; Begun et al. 2003; Begun, 2005; Andrews & Kelley, 2007; Folinsbee & Brooks, 2007). Indeed, it has been suggested that the common ancestor of apes and humans might have moved into Africa from Eurasia in the late Miocene (Begun et al. 2003; Begun, 2005). Thus, it might be appropriate to consider Eurasian as well as African Miocene environments when discussing earliest hominin origins, although as Andrews (2007) points out, the apes currently known from the fossil record may have been ecologically unsuited to widespread, extra-continental dispersal.
Mid and late Miocene African catarrhines are associated with a variety of habitats, over time as well as in different places (Kingston et al. 1994; Andrews, 1996; Andrews & Humphrey, 1999; Scott et al. 1999; Jacobs, 2004; Andrews & Kelley, 2007). Early Miocene sites such as Mfwangano Island and Songhor, sampling several catarrhine genera including Proconsul and Rangwapithecus, probably had environments containing evergreen, multi-canopied forest (Andrews, 1996; Andrews & Humphrey, 1999; Andrews & Kelley, 2007). The early Miocene catarrhines radiated extensively into such arboreal habitats, filling frugivorous, folivorous, above-branch and mixed arboreal/terrestrial niches (Andrews & Humphrey, 1999). The slightly more recent (c. 18 Ma) Hiwegi Formation on Rusinga Island, by contrast, appears to have single-canopied, disturbed woodland, known best from a rich floral assemblage (Andrews & Kelley, 2007). Unfortunately, it has no directly associated ape fossils although several taxa are known from Rusinga Island deposits of a similar age (Andrews & Kelley, 2007).
It has been argued that there may have been less habitat variability in the middle Miocene compared with the early Miocene (Andrews & Humphrey, 1999), but Fort Ternan, an important mid Miocene hominoid locality in western Kenya, appears to have been environmentally diverse (Scott et al. 1999; see also summary in Jacobs, 2004). Although renowned for its grass flora (Dugas & Retallack, 1993), the Fort Ternan ape fauna are likely to have inhabited seasonal woodland habitats (Pickford, 1987; Andrews, 1996; Andrews & Humphrey, 1999). Kenyapithecus, identified from Fort Ternan, has thick tooth enamel argued to be associated with frugivory in a relatively dry, seasonal habitat (Andrews & Humphrey, 1999) and also has some terrestrial adaptations that are consistent with this type of environment (Benefit & McCrossin, 1995; Andrews & Humphrey, 1999; see also Crompton et al. in this issue). Kenyapithecus has also been identified from middle Miocene Paşalar in Turkey, reconstructed as being single-canopied, seasonal subtropical woodland or forest (Andrews & Kelley, 2007). Another middle Miocene ape from Eurasia, Pierolapithecus catalaunicus, recovered from Barranc de Can Vila 1 in Spain and dated to 12.5–13 Ma, shows an unusual mix of adaptations (including a broad, shallow thorax indicative of orthogrady and short phalanges with palmigrade traits, most often seen in monkeys) that may suggest climbing and above-branch quadrupedalism (Moyà-Solà et al. 2004; see also Crompton et al. this issue). The presence of flying squirrels in the faunal assemblage (Moyà-Solà et al. 2004) combined with the skeletal evidence from Pierolapithecus itself indicates a degree of tree cover.
The arboreal component of Eurasian ape environments appears to persist into the late Miocene. Dryopithecus was probably exclusively arboreal and inhabited subtropical forest habitats (Andrews & Harrison, 2005; see also Crompton et al. this issue). The insular Oreopithecus may have combined arboreality with terrestrial bipedalism (Kohler & Moya-Sola, 1997; Rook et al. 1999) but it is equally possible that its bipedality was above-branch (Harrison, 1991; see also Crompton et al. this issue). Its palaeoenvironment has been variously interpreted as being seasonal warm-temperate forest (Harrison & Rook, 1997) and bushland/woodland (Köhler & Moyà-Solà, 2003). Other than the putative stem hominins, apes are rare from the Late Miocene in Africa (with their fossil records being much better in Eurasia). One of the few known post-10 Ma species, Samburupithecus kiptalami(dated to c. 9.5 Ma), lacks detailed palaeoecological context but it has been argued to exist in a woodland habitat in a region that also included grassland (Tsujikawa, 2005). Another, Chororapithecus abyssinicus (dated to 10–10.5 Ma), may have inhabited a forested lake-margin environment, although this has yet to be properly determined, and is complicated by the presence of a probably open-adapted Hipparion assemblage at closely related localities (Suwa et al. 2007).
The palaeoenvironment of the possible hominin S. tchadensis Toros-Menalla 266 locality in Chad has been reconstructed from sedimentological, floral and faunal data (Vignaud et al. 2002). There is evidence for extensive aquatic environments, including more than ten taxa of freshwater fish, some of which were likely to have measured over 1 m in length (Vignaud et al. 2002). Liana-like papilionoid plants show that gallery forest might have existed, and sediments suggest sandy desert in the vicinity (Vignaud et al. 2002). The apparently mosaic nature of the environment (Vignaud et al. 2002) has led to comparisons between Toros-Menalla and the modern Okavango Delta (Brunet et al. 2005), although this and other late Miocene hominin sites still require rigorous palaeoecological reconstruction (Andrews & Bamford, 2008).
Interpretation of the Toros-Menalla fauna is far from straightforward, as simple analogy with modern taxa can conceal complexities in past habitats. It was argued that the colobine monkeys recovered from the site represent gallery forest (Vignaud et al. 2002) but as discussed below the presence of colobines does not always indicate such habitats (Elton, 2001). The hypsodont dentition of bovids from Toros-Menalla has been interpreted as showing proximity of open grassland (Vignaud et al. 2002). There is a strong relationship between hypsodonty and grassland in modern animals, but it cannot be automatically argued that hypsodonty equates to open environments. Grass forms part of many environments, including woodland, and animals can be grazers without necessarily inhabiting open areas. In the Plio-Pleistocene pig Kolpochoerus limnetes, for example, hypsodonty increases over time with no concomitant alteration of postcranial adaptations to cursoriality and hence open environments (Bishop, 1999). Thus, examining different parts of the skeleton might help to refine reconstructions of past ecological niches. Unfortunately, thorough ecomorphological analysis of S. tchadensis itself is difficult due to the paucity of material assigned to the species. Although it is best known from a relatively complete cranium with a number of isolated dental and gnathic fragments, the lack of postcranial fossils restricts palaeobiological inference. Its cranial base and position of the foramen magnum indicate that it was orthograde (Brunet et al. 2002) but it is not possible to determine whether it was bipedal (Andrews & Harrison, 2005) or indeed whether it exploited arboreal or terrestrial substrates. Its molar teeth have reasonably thick enamel but their relatively small size help to distinguish S. tchadensis from later hominins (Andrews & Harrison, 2005). Based on the palaeobiologies of other Miocene ape species with similar sized molars (Andrews & Harrison, 2005) it is possible that the diet of S. tchadensis included soft fruits, which implies a degree of reliance on trees.
Orrorin tugenensis shares a similar pattern of relatively small molar teeth with thick enamel (Andrews & Harrison, 2005). Its forelimb shows adaptations to arboreality whereas its hindlimb, specifically the femur, has traits commonly associated with bipedalism (Senut et al. 2001). Although further functional analyses and more data are required to ascertain this (Andrews & Harrison, 2005; Crompton et al. this issue), the adaptations of O. tugenensis strongly suggest that it exploited arboreal environments at least some of the time (Senut et al. 2001). Several catarrhine species have been recovered from Tugen Hills localities, from the mid Miocene onwards (Pickford & Kunimatsu, 2005). Stable carbon isotope analysis has indicated that a mix of environments, including tropical (C4) grasses as well as C3 vegetation (that could include trees, shrubs and non-tropical grasses) were present in the succession from c. 15.5 Ma (Kingston et al. 1994). Fossil plants from late Miocene Tugen Hills horizons (up to 6.8 Ma, slightly less recent than the age given for O. tugenensis) suggest dry forest and woodland, with a fauna that indicates both open and closed habitats (Jacobs, 2004). The palaeoenvironment of the Late Miocene Lukeino Formation, from which specimens attributed to O. tugenensis have been recovered, has been argued to be open woodland, with possible forest fringes at the margins of the lake and associated streams (Pickford & Senut, 2001). Again, the presence of colobine monkeys has been used to suggest a more closed, forest habitat (Pickford & Senut, 2001). The vast majority of modern primates are ecologically dependent on trees, for food, shelter or both. However, they are also associated with a wide range of habitats, today and in the past. Based on morphological adaptations of the postcranial skeleton, some Plio-Pleistocene monkeys, for example, do not have the same habitat and locomotor preferences as their closest modern relatives (Elton, 2000, 2001, 2002, 2006). Material attributed to the primate family Galagidae was also found in the Lukeino Formation (Pickford & Senut, 2001). This lends support to the notion that there were arboreal environments in the vicinity but does not necessarily confirm the presence of forest, as although modern galagines are confined to arboreal substrates, they are found in a wide range of habitats, from closed-canopy forest to bushland and thickets in savanna areas (Novak, 1999). Thus, although the evidence from primate fossils (including those assigned to hominids and early hominins) very probably indicates a habitat that includes trees, further extrapolation to the type of habitat – without studying postcranial adaptations or other independent lines of evidence – could potentially be misleading. Furthermore, the general caveat that the adaptations of modern animals do not necessarily correspond to those of their extinct relatives has been shown to be applicable to many groups of mammals, including bovids (Kappelman, 1988; Plummer & Bishop, 1994) and suids (Bishop, 1994, 1999).
The importance of arboreal environments in the early stages of the hominin radiation is reinforced by data from the Late Miocene horizons of the Middle Awash, Ethiopia (WoldeGabriel et al. 2001), from which the probable hominin Ar. kadabba, dated to 5.2–5.8 Ma, was recovered (Haile-Selassie, 2001). Geological evidence points to a relatively wet environment during the formation of some of the Late Miocene sediments from the Middle Awash, with fluvial lacustrine sediments as well as the presence of deposits which indicate that explosive volcanic eruptions occurred when magma came into contact with surface or subsurface water (WoldeGabriel et al. 2001). At the Asa Koma and Digba Dora hominin sites, carbon and oxygen stable isotope ratios suggest open woodland (where the tree canopy was not continuous), with 20–45% grassland, in a cool, high-altitude or humid habitat (WoldeGabriel et al. 2001). Faunal remains may indicate closed or wet habitats along with open woodlands (WoldeGabriel et al. 2001), although further analyses are necessary. The younger Kuseralee Member, further east, which has yielded only a single hominin specimen, may have had a warmer, lower altitude or drier grassy woodland habitat (WoldeGabriel et al. 2001). Ar. kadabba is argued to be a biped but has features in its postcranium that are associated with powerful forelimb pronation and phalangeal flexion (see Crompton et al. this issue) and hence a degree of arboreality.
The palaeoenvironmental reconstructions of hominin sites and localities in the late Miocene, including Toros-Menalla (Vignaud et al. 2002), the Lukeino Formation (Pickford & Senut, 2001) and the Middle Awash (WoldeGabriel et al. 2001), as well as many in the Pliocene and early Pleistocene of Africa (e.g. Plummer & Bishop, 1994; Kappelman et al. 1997; Reed, 1997; Elton, 2000; Kingston & Harrison, 2007) indicate mosaic habitats. A mosaic habitat (or habitat mosaic) is one in which there are a range of different habitat types, scattered across and interspersed within a given area. In some, if not all, cases from the past, these could be accurate representations of regional habitats, as a great deal of variation in habitat can be present in a relatively small geographical area (Andrews 2006), as is seen, for example, in modern Maputaland in southern Africa (Van Wyk, 1994) or the Okavango Delta (Ramberg et al. 2006). However, taphonomic processes are likely to have altered the assemblages upon which the reconstructions are based, resulting in ‘time’- and ‘space’-averaged reconstructions that lead to a mosaic environmental signal in the absence of a true mosaic habitat. The possibility of time and space averaging should always be considered when faced with palaeoenvironmental reconstructions indicating mosaics. In these cases, statistical techniques such as ordination and rarefaction may yield valuable information about assemblage biases (Andrews, 2006). Modern evidence also shows that although habitats can be highly variable in a small area, they can also vary over even a short period of time. At Amboseli in Kenya the habitat altered dramatically from the early 1960s to the late 1990s, with decreases in woodland and increases in areas of permanent open water, both of which have had an impact upon the structure of the mammal and bird communities (Altmann et al. 2002). The shifts in habitat at Amboseli may be related, among other things, to the impact of a rising water table and damage from elephant populations (Western & van Praet, 1973; Young & Lindsay, 1988). Past habitats were also likely to have been dynamic in this way, but small changes may not be routinely detected in fossil-bearing horizons that were deposited over tens of thousands of years.
Taking taphonomic processes into consideration, significant tree coverage, either as woodland or forest, was likely to have been an important component of hominid and hominin environments from the Miocene to the Pleistocene (Andrews, 1989, 2006; Plummer & Bishop, 1994; Reed, 1997; Fernandez-Jalvo et al. 1998; Kappelman et al. 1997; Andrews & Humphrey, 1999; Elton, 2000; Pickford & Senut, 2001; WoldeGabriel et al. 2001; Vignaud et al. 2002; Andrews & Harrison, 2005; Kingston & Harrison, 2007; Andrews & Bamford, 2008). It cannot be denied that climatic cooling, increasing aridity and the development of C4 grasslands intensified during the late Miocene (Zachos et al. 2001; Jacobs, 2004), and provided the backdrop for the early stages of human evolution. However, there appears to have been no sudden switch in the late Miocene of Africa from closed to open environments, despite general global and continental trends. For example, woodland and forest habitats have been identified at the Plio-Pleistocene sites of Laetoli (Andrews, 1989, 2006; Kovarovic et al. 2002; Kingston & Harrison, 2007; Andrews & Bamford, 2008), Olduvai Bed I (Plummer & Bishop, 1994; Fernandez-Jalvo et al. 1998) and Koobi Fora Upper Burgi and KBS members (Reed, 1997; Kappelman et al. 1997; Elton, 2000, 2006), although early Pleistocene environments in southern Africa may have been more open (Andrews & Humphrey, 1999; Elton, 2007). Given that woodland and forest was a favoured habitat for many ape species before the emergence of the Hominini (Andrews & Humphrey, 1999; Andrews & Harrison, 2005), and that arboreality cannot be precluded from the adaptations of the putative stem hominins Sahelanthropus, Orrorin and Ardipithecus, it is possible to infer that the LCA exploited arboreal environments in one form or another. This is reinforced by Pliocene and Pleistocene reconstructions of wooded and forest hominin habitats that suggest ongoing occupation of arboreal environments. It is also supported by the strong ecological relationship between primates and trees that implies dependence on arboreal niches for the majority of extinct and extant primates. Indeed, it is possible to argue that, within the apes at least, this relationship was only really broken by later species and populations of Homo. Of course, until a LCA for the human–chimpanzee/bonobo clade is identified with a degree of certainty or palaeontologists can be sure that they have sampled all palaeoenvironments that might be associated with it, it is impossible to test environmental predictions or to determine whether there was a preference for woodland or forest. Nonetheless, it is possible to use existing evidence to formulate hypotheses which can then be challenged or explored further when new data come to light or new techniques developed.
Stressing the importance of wooded or forested environments in the early stages of human evolution calls into question traditional interpretations of the relationship between human origins and the environment (Kingston & Harrison, 2007), including the hypothesized links between bipedalism and open savanna landscapes. This issue, much debated since the discovery of the well-preserved Australopithecus afarensis AL 288-1 skeleton, which shows adaptations to arboreality (see, for example, Stern & Susman, 1983), is discussed at length elsewhere (Crompton et al. this issue), so will not be repeated here. However, it is worth noting that the links between early hominin evolution and woodland habitats were made at least two decades ago (e.g. Andrews, 1989). The conclusions drawn in this paper are not novel, therefore, but rather reinforce the growing appreciation of the importance of varied and arboreal environments in human evolutionary history.
Another common assumption, that early hominins became open habitat specialists whilst panins remained in forested habitats, is also muddied by the likelihood that late Miocene hominids as well as hominins exploited woodland habitats. The fossil record of the modern chimpanzee, Pan, is extremely sparse, with the species currently known only from dental material recovered from the Middle Pleistocene Kapthurin Formation in Kenya (McBrearty & Jablonski, 2005). The palaeoenvironment of this area, which has also yielded archaic Homo fossils (that may have been sympatric with Pan), is reconstructed as being locally wooded near a lake (McBrearty & Jablonski, 2005). This suggests that chimpanzees, prehistorically and historically, may have inhabited a range of habitats alongside modern humans (McBrearty & Jablonski, 2005), and that only relatively recent modern human population pressure has forced chimpanzees into a more restricted range of largely forested habitats.
Major trends in Old World environments between 6–8 Ma and the late Pleistocene
Two main, but not exclusive, forces interacted to alter hominin environments in the late Miocene, Pliocene and Pleistocene. One was hominin dispersal, first through Africa, and then out of the tropics into temperate and eventually Arctic zones. The other was global climate change. Since the mid-Miocene climatic optimum, some 16 million years ago, the Earth has been experiencing a general cooling trend (Zachos et al. 2001). This was not a steady progression, as O18 ratios from deep-sea cores give evidence for periodic warming, with marked temperature increases occurring in the late Miocene (7–9 Ma) and early Pliocene (Kennett, 1995; Zachos et al. 2001). Cooling intensified during the later Pliocene and Pleistocene. Important climatic transition events have been noted at c. 2.5 Ma, when there was the initial build up of ice sheets (Marlow et al. 2000), at the Pliocene–Pleistocene boundary (c. 1.8 Ma), and at c. 1.0–0.8 Ma, linked to the intensification of global ice volumes, the formation of much larger ice sheets, and a shift from 41-kyr to 100-kyr cycles in climate variability (DeMenocal, 2004), although this shift may have occurred earlier, at c. 1.5 Ma (Rutherford & D’Hondt, 2000). The 1 Ma event heralded the start of the rapid Middle to Late Pleistocene glacial–interglacial fluctuations that were likely to have had profound effects on hominin evolution and biogeography, especially in temperate regions.
With climatic cooling comes aridification and concomitant changes in vegetative structure. Since the Miocene, one of the most important trends in vegetation has been the development of extensive C4 ecosystems. Grass pollen is evident in the Palaeocene fossil record (64 Ma), and tropical grassland ecosystems began to expand in Africa during the mid Miocene, with significant grass cover emerging after 8 Ma (Dugas & Retallack, 1993; Kingston et al. 1994; Jacobs, 2004). Palaeosol data show that tropical grasses became increasingly common in African hominin habitats after 1.7 Ma (Sikes, 1999), and after the mid-Pleistocene transition there was an appreciable shift from closed, C3 pathway vegetation towards more open, C4 pathway grasses (Cerling & Hay, 1988). Also around 0.95 Ma, aeolian dust records demonstrate an increase in dust production, suggesting the beginning of the modern extent of the Sahara (Larrasoaña et al. 2003).
The trend towards open environments also occurred in Eurasia. In the Pliocene warm period, c. 3 Ma, higher latitudes experienced the greatest relative warming (Dowsett et al. 1999). The presence of Asian warm temperate tree species as far north as Reuver in Holland (Zagwijn, 1998) and arboreal vegetation in Iceland (Denton, 1999) indicates that Europe was more densely forested than it is today. Forests began to fragment at 2.5 Ma (Agusti & Antón, 2002), and Italian fossil sites show a slow transition from warm-humid forest animals to temperate grass and woodland faunas that began at c. 2.6 Ma (Sardella et al. 1998). Although the trend noted by Sardella et al. (1998) appears unrelated to global climatic fluctuations, the onset of the more extreme climatic fluctuations of the later Pleistocene brought considerable changes to European environments, with ice sheet incursion and contraction episodically shifting the major vegetational biomes, including those such as tundra and boreal forest that were difficult for archaic hominins to exploit. One response to this, discussed further below, was to retreat into milder, temperate habitats during glacials, with populations re-expanding out of these refugia during interglacials (Finlayson, 2004).
Global trends towards drier conditions are evident in Australasian–Asian vegetation (Hope et al. 2004). On the basis of palaeosol evidence from the Siwaliks, the tropical grasslands that are today characteristic of the Indo-Gangetic region of South Asia became established in the late Miocene (Quade & Cerling, 1995). In northern China, micromammal assemblages suggest that prior to 2.5 Ma, grasslands with broadleaved and conifer forests were present, which were then replaced after the transition by open grasslands that persisted well into the Pleistocene (Jin et al. 1999). In central and eastern Asia, the transitional cooling event at 2.5 Ma is also seen as the beginning of the loess accumulations, which indicate aridity (Dodonov & Baiguzina, 1995), and the development of widespread grasslands in these regions (Yang & Ding, 2006). Although habitats responded in a cyclical manner to climatic fluctuations, shifts in the precipitation regime linked to the larger glacial cycles led to increasing proportions of C4 grasses in Asia after c. 0.85 Ma (Yang & Ding, 2006). Changes to the intensity of the annual monsoon plus other frequent events like El Niño probably had a major impact on Asian vegetation (Quade & Cerling, 1995; Hope et al. 2004), as did glacial–interglacial cycles (Hope et al. 2004). During the last glacial maximum (LGM), pollen records show that grasslands encroached on some tropical lowland forests in Thailand and Sulawesi but the main tropical forest belt remained relatively unchanged (Hope et al. 2004). At higher latitudes and altitudes, vegetation responded as might be predicted, with a lowered treeline and southwards movement of steppe, grassland, temperate forest and woodland (Hope et al. 2004).
The relative importance of grassy and forest habitats in human evolution is discussed elsewhere in this review, but it is important to note that a dichotomy between closed and open habitats at any one point in human evolutionary history was unlikely. Wooded or even forested environments are likely to have been significant during at least the earliest stages of human evolution (Andrews, 1989; Fernandez-Jalvo et al. 1998; Andrews & Harrison, 2005; Kingston & Harrison, 2007; Andrews & Bamford, 2008), and mosaic habitats are reconstructed for many Pliocene and Pleistocene sites in Africa, although C4 ecosystems dominate some key Pleistocene palaeontological and archaeological sites such as Kanjera and Olorgesailie (Plummer et al. 1999; Sikes et al. 1999). Even though taphonomic factors such as time averaging might well have affected palaeoenvironmental reconstructions for some sites, fossil lake levels periodically record increasing moisture in East Africa during the Plio-Pleistocene (Trauth et al. 2005). This demonstrates that although there was a general trend towards cooler and drier environments, it was not an uninterrupted progression. Throughout the Pleistocene, Eurasian hominins also exploited numerous and variable habitats (Zhu et al. 2001; Barker et al. 2007), with cyclical environmental change being highly probable (Dodonov & Baiguzina, 1995).
Notwithstanding their general importance, global climatic patterns caused by orbital forcing are not the only agents of environmental change, and it has been pointed out that the East Africa palaeosol record, which gives a good indication of highly localized conditions, does not necessarily reflect major global events such as the 2.5 Ma transition (Sikes, 1999). It has also been argued that global climatic change did not have a significant impact within Equatorial Africa (O’Brien & Peters, 1999), although recent evidence from the Baringo Basin implicates orbital forcing in late Pliocene environmental change (Kingston et al. 2007). Nonetheless, at continental, regional and local levels, a range of factors, including topography, orography (the average height of land) and sea surface temperatures also act on weather patterns, ecosystems, vegetation and animal communities. Tectonic activity, which influences topography and orography, can therefore have a profound effect on environments. The Messinian Event, which caused desiccation of the Mediterranean at 5.96–5.33 Ma, may have been the result of crustal uplift (Duggen et al. 2003), although it is far from certain whether it had any widespread effect on African environments (Partridge et al. 1995a). The uplift of the Tibetan Plateau, by contrast, which accelerated in the Miocene, may have caused an intensification of the Indian Ocean monsoon (Molnar et al. 1993), significantly influencing vegetative environments in Asia (Hope et al. 2004). Much of what is known about the earliest period of human evolution comes from the East African rift valley, a region that was highly tectonically active during the Pliocene and Pleistocene. Such activity, as well as potentially prompting vicariance events, probably contributed significantly to the development of hominin environments in East Africa (Partridge et al. 1995b). At a more localized level, vegetation patterns and hence spatial distributions of habitat would have been shaped partly by soils and geology (Andrews & Bamford, 2008).
It is becoming increasingly clear from palaeoenvironmental reconstructions and hominin biogeography that even early hominins could exploit numerous habitats. The types of habitats associated with late Miocene to early Pleistocene hominins in Africa range from forest or woodland (e.g. at the Middle Awash: WoldeGabriel et al. 2001) to more open environments, such as those found at Swartkrans in southern Africa (Andrews & Humphrey, 1999; Elton, 2001, 2007). Dispersal within the continent, although presumably driven in part by habitat corridors, potentially exposed early hominins to a range of previously unknown ecosystems. With the onset of extra-African dispersal, the opportunities for a behaviourally flexible, intelligent, large-bodied primate would have widened further. The development and refinement of material culture expanded the ‘niches’ that were available, resulting in the eventual colonization by late Pleistocene humans of the most challenging environments, including Arctic areas and dense tropical rainforest. Thus, attempting to pinpoint a typical hominin habitat or even a general pattern of hominin habitat use over time might be, at best, misleading. In addition, changes in habitat preferences or shifts in the way they are exploited do not necessarily imply the presence of a strong external climatic pressure – Miocene apes, for example, may have diversified into a number of different environments well before significant Miocene forest shrinkage (Andrews & Humphrey, 1999). Nonetheless, there was significant climate change from the late Miocene to the Pleistocene, leading to a general Old World trend towards more open habitats. Hominin species such as Paranthropus boisei and Homo erectus, which endured in the fossil record for upwards of one million years, as well as high-latitude later hominins such as H. neanderthalensis that were subject to rapid climatic fluctuations, would have needed to respond to environmental changes behaviourally, culturally and even physiologically and morphologically in order to survive.
Environmental influences on human and primate morphology
The impact of geography and the environment on morphological variation in animals has long been recognized. Two of the best known associations – that animals living at lower temperatures tend to have larger body sizes overall but with smaller appendages such as limbs or tails – are described by Bergmann's and Allen's rules (Cox & Moore, 2000). These rules have not been extensively tested amongst primates, and studies to date show mixed results. Temperature-related size gradients have been identified in the skulls of Brazilian tufted-eared marmosets (Albrecht, 1982) and pig-tailed and crab-eating macaques in south-east Asia (Albrecht, 1980; Fooden & Albrecht, 1993), as well as in the maxillary sinus volumes of Japanese macaques (Rae et al. 2003). A strict Bergmannian relationship is not always evident, however. At latitudes between 8°S and 13°N, skull length in the crab-eating macaque increases with latitude and decreasing temperature on both sides of the Equator, but above 13°N, skull length decreases (Fooden & Albrecht, 1993). When parts of the body other than the skull are considered, patterns remain indistinct, with tail-length in the rhesus macaque following Allen's Rule above 26°N, but not further south (Fooden & Albrecht, 1999). In living Kenyan vervets from four sites at different latitudes and elevations, clines in tail length and female body mass are detected, but other morphological traits, including male body mass, male and female body length, lower and upper leg lengths, and lower and upper arm lengths, do not follow the pattern predicted by ecogeographic rules (Turner et al. 1997). In a separate study of skull sizes of vervets from across Africa, no strong support was found for Bergmann's rule (Cardini et al. 2007).
Interestingly, the body sizes and shapes of modern humans and their closest relatives show relatively strong relationships with latitude (Ruff, 1994, 2002; Holliday, 1999). Bergmann's rule applies to modern humans with respect to mean body mass (Ruff, 2002), and the positive relationship between temperature and brachial and crural indices indicates that limb proportions in modern humans conform to Allen's rule (Holliday, 1999). Neanderthals and some other high-latitude archaic Homo specimens also have the type of body proportions (i.e. relatively shorter distal limb lengths and larger bi-iliac breadths) that would be predicted from ecogeographic rules (Trinkaus, 1981; Ruff, 1994, 2002). These features appear, at least in part, to have resulted from climatic influences (Ruff, 1994, 2002; Holliday, 1999). This is reinforced by research indicating that limb bone robusticity in humans might be more influenced by temperature than by habitual activity (Pearson, 2000), although others have argued strongly that behaviour affects the skeleton more than does climate (Finlayson, 2004). The relationship between temperature and postcranial morphology might have been established relatively early in hominin evolutionary history: the KNM-WT 15000 H. erectus specimen has been interpreted as having tropical body proportions (Ruff & Walker, 1993). However, it has been argued that this specimen shows signs of skeletal pathology and caution must be exercised when extrapolating from it to other members of the species (Ohman et al. 2003).
Facial shape in modern humans has also been linked to mean temperature, but despite a general association with latitude that indicates some clinal variation, significant effects are only apparent with the inclusion of high-latitude Arctic individuals (Harvati & Weaver, 2006). Taking postcranial and cranial data together, temperature probably helped to determine body size, form and proportions in hominin taxa with highly extensive (almost cosmopolitan) geographical ranges, such as modern and archaic Homo. However, it must also be remembered that some ‘cold-adapted’ hominins, including Neanderthals, were probably only exposed to extreme temperatures for part of their tenure, so their morphology might not be a straightforward response to cold climates (Finlayson, 2004). Recent reviews of research into ecogeographic variation across a wider range of animal taxa suggest that although temperature can have an influential role in determining body size – a common interpretation of Bergmann's rule – other climatic variables, such as rainfall and moisture, are often better correlates (Ashton et al. 2000; Millien et al. 2006). There is thus mounting evidence that traditional interpretations of the mechanisms behind ecogeographic rules are an oversimplification of a complex set of environmental and spatial interactions (Ashton et al. 2000; Millien et al. 2006). In Plio-Pleistocene hominin taxa from Africa, with ranges that did not extend into high-latitude zones, it is therefore possible that environmental variables other than temperature impacted upon morphology.
Mean body size in Malagasy sifakas is positively correlated with annual rainfall and negatively correlated with seasonality, with body size differences attributed to variations in habitat productivity and resource seasonality (Lehman et al. 2005). In Papio baboons, a group of African primates with an extensive geographical range, rainfall is highly correlated with body size (Popp, 1983; Dunbar, 1990; Barrett & Henzi, 1997). Correlations between annual rainfall and skull size and shape are also observed in vervet monkeys, another group of widely dispersed primates from Africa, although altitude and temperature did not contribute significantly (Cardini et al. 2007). However, the same study noted significant longitudinal variation in skull morphology, indicating that both spatial and environmental factors interact to influence size and shape (Cardini et al. 2007). Nonetheless, in modern African primates, rainfall (as a proxy for habitat productivity) consistently emerges as making a significant contribution to intraspecific morphological variation, and thus might also have impacted hominin morphology. The effects of rainfall might be more important than temperature for tropical and subtropical hominin species, as precipitation is a more important component of climatic variation than temperature at low latitudes (deMenocal & Bloemendal, 1995).
When and where anatomically modern humans evolved to cope with the wide range of habitats they presently tolerate
Modern humans live in an array of different environments, with some inhabiting, either year-round or seasonally, regions with highly challenging climatic extremes of precipitation or temperature. They manage to inhabit or exploit in one way or another all the major biomes of the world – freshwater, marine, desert, forest, grassland and tundra. Genetic, physiological, behavioural and cultural adaptations are all important in facilitating this (Harrison, 1993). Disregarding non-permanent settlement such as scientific expeditions, the latitudinal range of modern humans extends from above the Arctic Circle to Tierra del Fuego. Both of these regions have archaeological records that indicate prehistoric human habitation: shell middens in Tierra del Fuego have been dated to 6500 bp (Estevez et al. 2001) and settlement is evident in Arctic Norway as far back as 10 000 bp (Ramstad et al. 2005). The temperatures at which humans can live using ‘traditional’ cultural practices range from well below freezing to over 40 °C. In some desert environments, temperatures fluctuate enormously on a daily basis: in Western Australia, the difference between day and night time temperatures can be as much as 19 °C (Cane, 1987). Modern human ecologies are also remarkably adaptable to differences in precipitation. One of the world's wettest places, Cherrapunja in Meghalaya, India, receives over 11 000 mm of monsoonal rainfall each year, whereas in other inhabited regions of the world, such as the Sahel in Africa, annual rainfall is less than 200 mm. The elevations at which humans live also vary enormously. Although over 30% of the world's population live at or below 100 m above sea level (Cohen & Small, 1998), modern human groups are found at altitudes as high as 3500–4000 m on the Tibetan Plateau, in the Andes and also in the Ethiopian highlands (Beall et al. 2002). Each of these populations have different physiological responses to hypoxia (Beall et al. 2002), showing the environmental adaptation that is possible in modern humans, even without cultural or behavioural intervention.
Palaeontological and archaeological evidence, in combination with palaeoenvironmental reconstruction, can help to shed light on the patterns and timing of the occupation by hominins of such a wide range of habitats. Two of the most interesting topics that relate to this are when (and where) hominins managed to exploit tropical forest habitats successfully, and at what stage hominins expanded out of temperate regions into Arctic and sub-Arctic environments.
Woodland was probably an important habitat for early hominins (Andrews 1989; Fernandez-Jalvo et al. 1998; Andrews & Harrison 2005; Andrews & Bamford 2008). Woodlands of different types (such as acacia and miombo) are a significant component of modern sub-Saharan African landscapes, and were likely to have been so throughout the whole of human evolutionary history (Sikes, 1999; Jacobs, 2004). Based on faunal evidence from Olduvai Bed I, it is likely that some African Plio-Pleistocene woodland was much more species-rich than modern woodland, indicating differences between past and present ecosystems (Fernandez-Jalvo et al. 1998). Woodland trees tend to be smaller than they are in forest, with density of coverage often related to annual precipitation (Sikes, 1999; Jacobs, 2004) or seasonal fluctuations in rainfall (Vincens et al. 2007). There is no formation of continuous canopy, so one of the defining characteristics of woodland is that it has a grassy understorey (Jacobs, 2004). Although the emphasis in hominin palaeoecology is shifting away from considering human evolution in terms of adaptation to savanna living, grassy environments undoubtedly formed an important part of the ecosystems of African Plio-Pleistocene hominins and the other members of their ecological communities, including many species of artiodactyls and perissodactyls, as well as Old World monkeys such as Theropithecus. Modern behavioural studies show that woodlands and grasslands provide vital sources of readily accessible foods for terrestrial primates. Such foods include fruits, young leaves and underground storage organs, as well as invertebrates and vertebrates, exploited by primates like baboons that are eclectic and opportunistic feeders (Altmann, 1998). The majority of primates are dependent on C3 foods, but the use of C4 resources, including tropical grasses and rhizomes as well as fauna that consume tropical grasses, is increasingly being recognized as an important aspect of Old World monkey adaptive flexibility (Codron et al. 2005, 2006; H. J. O’Regan et al. unpublished data). This might also have been the case for early hominins. Although monkeys and humans have different food processing and digestive adaptations [with baboons, for example, able to consume several plants that are poisonous to humans (Peters & O’Brien 1994)], studies of dental microwear (Teaford & Ungar, 2000) and stable carbon isotope ratios (Lee-Thorp et al. 1994; Sponheimer & Lee-Thorp, 1999; Lee-Thorp et al. 2003) indicate that hominins were eclectic feeders, using a range of C3 and C4 foods. The ability to utilize C4 resources, both plant and animal, that were increasingly available during the Plio-Pleistocene was probably crucial to hominin success (Peters & Vogel, 2005), and the exploitation of grassland has been suggested as an important aspect of earliest Homo expansion out of Africa (Dennell & Roebroeks, 2005).
In contrast to woodlands and grasslands, tropical forest is often argued to contain fewer foods that are easily procured and processed by hominins, which might have prevented the exploitation of such habitats by Homo until very recently in human evolutionary history (Bailey & Headland, 1991). Tropical forests are seen as being poor habitats for humans without the benefits of agriculture (Bailey & Headland, 1991; Headland & Bailey, 1991). Fruits are often high in the canopy, other carbohydrates such as wild yam dispersed, and protein can be difficult to find, partly due to the cryptic nature of forest animals (Headland & Bailey, 1991). The archaeological evidence for Holocene forest exploitation without cultivation is very slim, although it does exist, in Asia as well as potentially South America (Bailey & Headland, 1991). Thus, although modern human populations survive very successfully in such environments, they might only have colonized them extensively in the last few millennia (Bailey & Headland, 1991).
Aridification from the Miocene onwards reduced the tropical forest cover of Africa (Jacobs, 2004) but, as is evident from palaeoenvironmental reconstruction and modern vegetative biomes, did not eradicate it altogether. Palaeoenvironmental reconstructions of several African Plio-Pleistocene palaeontological sites indicate the presence of tropical forest within areas inhabited by early hominins (Reed, 1997; WoldeGabriel et al. 2001), and modern rainforests provide a rich habitat for numerous species of primate. Although tropical forests are heterogeneous in space, and therefore probably also in time, it seems unlikely from an ecological point of view that ecosystems which today support large, frugivorous primates such as mandrills, drills, chimpanzees or, in Asia, orangutans and gibbons, could not have supported australopiths (or other hominins with suggested adaptations to arboreality, like Homo floresiensis) in the Pliocene and Pleistocene. Large-bodied extant primates can exhibit considerable ecological flexibility: for example, baboons, usually associated with open habitats, successfully live in forest in some parts of Africa (Rowell, 1966), and chimpanzees, which today are mainly found in forests, also inhabit more open areas in parts of their range (McGrew et al. 1996). Such flexibility was therefore possible in some extinct primates, including early hominins. It is more questionable whether obligate terrestrial bipeds, such as H. ergaster, could have successfully exploited rainforest, given that important food items would have either been in the canopy and not easily accessed, or dispersed and cryptic on the forest floor. Nonetheless, there is increasing archaeological evidence for forest exploitation by archaic Homo in Africa during the Pleistocene (Mercader, 2002). The Middle Stone Age (MSA) Sangoan complex is found across a large part of Africa, associated with a range of open and closed environments, although because of the temporal variability in vegetation during the Pleistocene, further work is needed to link archaeological and palaeoenvironmental evidence in the MSA more precisely (Mercader, 2002). Later Stone Age (LSA) artefacts show much stronger associations with tropical forest habitats, including tools found alongside phytolith (‘plant stone’) sequences indicative of rainforest plants at an 18 000-year-old site in the northern Congo (Mercader, 2002).
In Asia as in Africa, rainforest coverage fluctuated during the Pleistocene (Heaney, 1991), but evidence of rainforest plants have been identified at the important late Pleistocene archaeological site of Niah Cave in Sarawak, Borneo (Barker et al. 2007). Residues on late Pleistocene stone flakes show that they had been used to work lowland rainforest plants, probably bamboo and palm, and the general context of the human fossils and archaeological remains strongly suggests that modern humans colonizing South East Asia 46–34 000 years ago exploited a range of habitats, including rainforest (Barker et al. 2007). Thus, although it is not yet clear whether the earliest members of the genus Homo could survive in such environments, there is mounting evidence for significant rainforest use by archaic and anatomically modern Homo, probably facilitated by technology, in both Africa and Asia during the Pleistocene. This brings full circle the debate about forest use in humans, as prior to the arguments of Headland, Bailey and colleagues, it was commonly assumed that human occupation of rainforests pre-dated the origins of agriculture (Headland & Bailey, 1991).
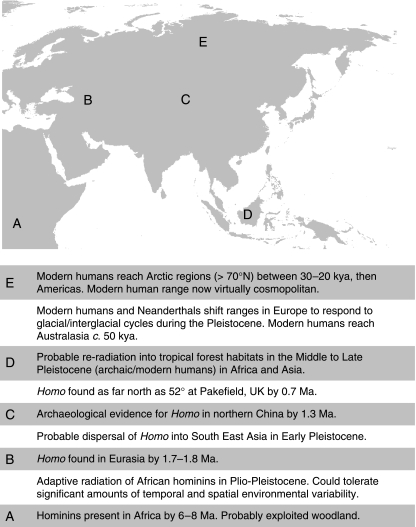
The picture that emerges from palaeoenvironmental reconstructions of palaeontological and archaeological sites throughout the Old World from early in the Pleistocene, if not before, is of hominin ecological and adaptive flexibility. It is clear from the palaeoenvironments inhabited by Pliocene and Pleistocene hominins (Table 1) that the ability to exploit a variety of tropical and subtropical habitats was established relatively early in human evolutionary history, before expansion out of Africa. It has been argued, as part of the ‘Variability Selection Hypothesis’, that key adaptive features of early hominins, including their locomotor behaviours, arose in response to variable environments (Potts, 1998). Pleistocene Homo, as well as other animals that dispersed out of Africa, would have been required to exploit a considerably wider range of environments (Fig. 1). Colonizing much of Europe entailed dispersal into temperate forests (Hughes et al. 2008). The increasing geographical range of hominins also coincided with the onset of the major climatic fluctuations that characterized the Pleistocene, and there is archaeological evidence that prior to 1 Ma, hominins in east Asia shifted their ranges in response to climatic change (Zhu et al. 2001). The Dmanisi fossils demonstrate that by 1.8 Ma, hominins had reached latitudes of 40°N in the Caucasus (Gabunia et al. 2000), and hominins had expanded eastwards into similar latitudes by around 1.3 Ma (Zhu et al. 2001). Some 600 000 years later, hominins were as far north as 52°, at Pakefield in the UK, which at the time had a Mediterranean-type climate (Parfitt et al. 2005).
Table 1.
Major habitats associated with representative Miocene, Pliocene and Pleistocene hominin species
| Hominin species | Region | Tropical woodland | Tropical forest | C4 grassland | Temperate forest, grassland | Boreal forest, tundra | Arctic | |
|---|---|---|---|---|---|---|---|---|
| Mio-Pliocene | S. tchadensis | Central Africa | √ | √ | ? | × | × | × |
| O. tugenensis | East Africa | √ | √ | ? | × | × | × | |
| Ar. kadabba | East Africa | √ | √ | √ | × | × | × | |
| Pliocene | Au. afarensis | East Africa | √ | ? | √ | × | × | × |
| Au. africanus | Southern Africa | √ | √ | √ | × | × | × | |
| Plio-Pleistocene | P. boisei | East Africa | √ | ? | √ | × | × | × |
| P. robustus | Southern Africa | ? | ? | √ | × | × | × | |
| H. erectus | Africa; Central and South-East Asia | √ | ? | √ | √ | × | × | |
| Pleistocene | H. neander- thalensis | Europe | × | × | × | √ | √ | × |
| Pleistocene–Holocene | H. sapiens | Global | √ | √ | √ | √ | √ | √ |
Fig. 1.
Reconstruction of global biomes during the Pliocene and Pleistocene (reproduced with permission; this figure was published in Journal of Human Evolution54, Hughes, J.K., Elton, S. & O’Regan, H.J. Theropithecus and ‘Out of Africa’ dispersals in the Plio-Pleistocene. pp. 43–77. Copyright Elsevier 2008). The data on which these predictions are based were derived from the BIOME4 simulation (Kaplan et al. 2003– see Hughes et al. 2008 for further discussion). These correspond to (a) mid-Pliocene, (b) interglacial and (c) glacial climates. Note the smaller extents of the Sahara, boreal forest, ice and tundra in the Pliocene and the larger extent of warm-temperate forest in Europe at the same time.
The occupation zones and ranges of archaic and anatomically modern humans in Pleistocene Europe altered with glacial and interglacial cycles, with the Balkans and Iberia acting as refuges during glacial times (Finlayson, 2004). On the basis of archaeological evidence, it appears that hominins did not colonize the highest latitudes until late in the Pleistocene (see Fig. 2 for a summary timeline). After around 30 000 years ago, modern humans apparently moved into the Eurasian plains above 55°N (Finlayson, 2004). The earliest occupation of Arctic Siberia (71°N, well above the Arctic Circle) is dated at 27 000 radiocarbon years bp (Pitulko et al. 2004), and there are several archaeological sites documented from slightly lower latitudes shortly after this (Goebel, 1999). After the LGM (19–18 000 years ago), when evidence for human occupation disappears from the archaeological record, the region was re-colonized (Goebels, 1999). It is likely that human range expansion and contraction such as this was an important response to changing climates at high latitudes (Powers & Jordan, 1990; Goebels, 1999), both in the Pleistocene and in the Holocene. In other Arctic regions during the Holocene, there were major shifts in subsistence patterns to further facilitate survival, with populations reducing their dependence on terrestrial resources and increasingly exploiting marine ecosystems (Fitzhugh, 1997).
Fig. 2.
Timeline of major changes in hominin biogeography. The changing extent of hominin geographical ranges and habitat exploitation, from 6–8 Ma to the late Pleistocene.
Exploiting the highest latitude regions requires an array of cultural and behavioural adaptations. Tundra and boreal forest are relatively unproductive habitats, with short growing seasons and a restricted number of plant species. Large game is a key terrestrial resource in such areas, and marine exploitation is also very important. It is probable that high-latitude occupation was only possible through the development of relatively sophisticated material culture, including shelters, weapons to facilitate the hunting of large-bodied prey, and controlled use of fire (Goebels, 1999). Developing specialist technology such as harpoons to hunt marine animals would also have been important. Any hominin taxon dispersing into very high latitudes would have faced latitudinal and seasonal constraints on annual activity patterns, caused by much reduced day length as well as very low temperatures. One behavioural and cultural response to extreme environments is to be seasonally nomadic or transhumant. This strategy has been observed in modern desert populations around the world (Cane, 1987; Meir & Tsoar, 1996), as well as in pastoralists living in seasonally dry grasslands (McCabe, 1990) and those living in regions, like the European Alps, where seasonal snowfalls reduce the availability of pasture (in this case, the transhumance is ‘fixed’ rather than nomadic). The Sami, modern Arctic Circle dwellers, were nomadic pastoralists until the late 19th century, when national governmental pressures in Sweden and Norway made many populations establish permanent settlements (Reader, 1988). Archaeological evidence suggests that nomadism was present in Arctic Circle populations 4000–5000 years ago (Powers & Jordan, 1990), and seasonal movement has been inferred from the earlier Palaeolithic record (Goebels, 1999). Cultural traditions were also vital to the success of Arctic humans in the Holocene, promoting social integrity and, through alterations to technology and material culture, adapting to sometimes rapidly changing environments (Fitzhugh, 1997).
Conclusion
This review had three main aims. The first was to make a prediction about the probable habitat of the LCA. Recent fossil discoveries and associated palaeoenvironmental reconstructions have lent weight to the evidence that woodlands were important habitats for late Miocene hominids and hominins. Similar habitats remained important for hominins in Africa throughout the Plio-Pleistocene. By extension, it might be expected that the LCA also exploited woodlands. However, as there is no clear candidate for the human–chimpanzee/bonobo LCA, and because the sampling of fossils and past environments is inevitably patchy, this prediction remains, at best, a working hypothesis.
The second aim was to outline the major trends in hominin environments in Africa and Eurasia between the time of the LCA, 6–8 Ma, and the late Pleistocene. This is an incredibly complex task, but the global trend was one of cooling, with aridification. Superimposed on this were numerous climatic fluctuations, with attendant changes to vegetation structure and ecosystems. Local, regional and continental geography (including topography, orography and sea surface temperatures) also helped to shape hominin environments, which were highly temporally and spatially variable. Throughout the course of their evolution, hominins have proved to be ecologically flexible in the face of such variation, adapting over time to changing habitats in a particular geographical region or dispersing into new, sometimes immensely challenging, environments. The hominin ability to exploit a range of habitats probably pre-dates the emergence of Homo.
The final aim of this review was to examine when and where humans evolved to cope with the vast range of environments and habitats they currently tolerate, unmatched by few other mammals that are not commensals of Homo. It is clear that the ability to exploit a range of habitats extends at least as far back as the Plio-Pleistocene, first in Africa, then in Asia. With the development of increasingly complex tool technology came the opportunity to re-expand into tropical forests, environments difficult for obligate bipeds to negotiate, and accomplished by archaic and/or anatomically modern humans independently in Africa and south-east Asia. Finally, complex social behaviour and material culture allowed modern humans to reach some of the most hostile regions of the globe, above the Arctic Circle, by the late Pleistocene. This, with colonization of the Americas and Australasia, established Homo sapiens as a truly cosmopolitan species.
Acknowledgments
I am grateful to Hannah O’Regan, Laura Bishop, Sam Cobb and John Hughes for discussions about Old World Neogene environments or the contents of this paper, and thank Bernard Wood for his helpful review and editorial suggestions. I am particularly grateful to Peter Andrews, whose thorough and exacting review improved this article significantly. I also thank ASGBI for supporting the symposium at which this paper was first presented, and extend a great deal of gratitude to Edward Fenton and Gillian Morriss-Kay for their immense patience and help during the preparation of this article and volume. This work was funded by a grant from the Leverhulme Trust.
References
- Agusti J, Antón M. Mammoths, Sabretooths and Hominids. New York: Columbia University Press; 2002. [Google Scholar]
- Albrecht GH. Latitudinal, taxonomic, sexual, and insular determinants of size variation in pigtail macaques, Macaca nemestrina. Int J Primatol. 1980;1:141–152. [Google Scholar]
- Albrecht GH. The relationship of size, latitude and habitat in the South American primate Callithrix jacchus. Am J Phys Anthropol. 1982;57:166. [Google Scholar]
- Altmann J, Alberts SC, Altmann SA, Roy SB. Dramatic change in local climate patterns in the Amboseli Basin, Kenya. Afr J Ecol. 2002;40:248–251. [Google Scholar]
- Altmann SA. Foraging for Survival: Yearling Baboons in Africa. Chicago: University of Chicago Press; 1998. [Google Scholar]
- Andrews P. Palaeoecology of Laetoli. J Hum Evol. 1989;18:173–181. [Google Scholar]
- Andrews P. Palaeoecology and hominoid palaeoenvironments. Biol Rev. 1996;71:257–300. [Google Scholar]
- Andrews P, Humphrey L. African Miocene environments and the transition to early hominines. In: Bromage TG, Schrenk F, editors. African Biogeography, Climate Change and Early Hominid Evolution. New York: Oxford University Press; 1999. pp. 282–300. [Google Scholar]
- Andrews P, Harrison T. The last common ancestor of apes and humans. In: Lieberman DE, Smith RJ, Kelley J, editors. Interpreting the Past: Essays on Human, Primate and Mammal Evolution in Honor of David Pilbeam. Boston: Brill Academic Publishers; 2005. pp. 103–121. [Google Scholar]
- Andrews P. Taphonomic effects of faunal impoverishment and faunal mixing. Paleogeog, Palaeoclimatol, Palaeoecol. 2006;241:572–589. [Google Scholar]
- Andrews P. The biogeography of hominid evolution. J Biogeogr. 2007;34:381–382. [Google Scholar]
- Andrews P, Kelley J. Middle Miocene dispersals of apes. Folia Primatol. 2007;78:328–343. doi: 10.1159/000105148. [DOI] [PubMed] [Google Scholar]
- Andrews P, Bamford M. Past and present vegetation ecology of Laetoli, Tanzania. J Hum Evol. 2008;54:78–98. doi: 10.1016/j.jhevol.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Ashton KG, Tracy MC, de Queiroz A. Is Bergmann's rule valid for mammals? Am Nat. 2000;156:390–415. doi: 10.1086/303400. [DOI] [PubMed] [Google Scholar]
- Bailey RC, Headland T. The tropical rainforest: is it a productive habitat for human foragers? Hum Ecol. 1991;19:261–285. [Google Scholar]
- Barker G, Barton H, Bird M, et al. The ‘human revolution’ in lowland tropical Southeast Asia: the antiquity and behaviour of anatomically modern humans at Niah Cave (Sarawak, Borneo) J Hum Evol. 2007;52:243–261. doi: 10.1016/j.jhevol.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Barrett L, Henzi SP. An interpopulation comparison of body weight in chacma baboons. S Afr J Sci. 1997;93:436–438. [Google Scholar]
- Beall CM, Decker MJ, Brittenham GM, Kushner I, Gebremedhin A, Strohl KP. An Ethiopian pattern of human adaptation to high-altitude hypoxia. Proc Natl Acad Sci USA. 2002;99:17215–17218. doi: 10.1073/pnas.252649199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begun DR, Güleç E, Geraads D. Dispersal patterns of Eurasian hominoids: Implications from Turkey. Deinsea. 2003;10:23–39. In Distribution and migration of Tertiary mammals in Eurasia (eds Reumer JWF, Wessels W) [Google Scholar]
- Begun DR. Sivapithecus is east and Dryopithecus is west, and never the twain shall meet. Anthropol Sci. 2005;113:53–64. [Google Scholar]
- Benefit BR, McCrossin ML. Miocene hominoids and hominid origins. Ann Rev Anthropol. 1995;24:237–256. [Google Scholar]
- Bishop LC. Pigs and the ancestors: hominids, suids and environments during the Plio-Pleistocene of East Africa. 1994. PhD dissertation, Yale University. [Google Scholar]
- Bishop LC. Suid paleoecology and habitat preference at African Pliocene and Pleistocene hominid localities. In: Bromage TG, Schrenk F, editors. African Biogeography, Climate Change and Early Hominid Evolution. New York: Oxford University Press; 1999. pp. 216–225. [Google Scholar]
- Blumenschine RJ, Peters CR. Archaeological predictions for hominid land use in the paleo-Olduvai Basin, Tanzania, during lowermost Bed II times. J Hum Evol. 1998;34:565–607. doi: 10.1006/jhev.1998.0216. [DOI] [PubMed] [Google Scholar]
- Brunet M, Guy F, Pilbeam D, et al. A new hominid from the upper Miocene of Chad, central Africa. Nature. 2002;418:145–51. doi: 10.1038/nature00879. [DOI] [PubMed] [Google Scholar]
- Brunet M, Guy F, Pilbeam D, et al. New material of the earliest hominid from the Upper Miocene of Chad. Nature. 2005;434:752–755. doi: 10.1038/nature03392. [DOI] [PubMed] [Google Scholar]
- Cane S. Australian aboriginal subsistence in the western desert. Hum Ecol. 1987;15:391–434. [Google Scholar]
- Cardini A, Jansson A-U, Elton S. A geometric morphometric approach to the study of ecogeographic and clinal variation in vervet monkeys. J Biogeog. 34:1663–1678. [Google Scholar]
- Cerling TE, Hay RL. An isotopic study of paleosol carbonates from Olduvai Gorge. Quatern Res. 1988;25:63–78. [Google Scholar]
- Codron D, Luyt J, Lee-Thorp JA, Sponheimer M, de Ruiter D, Codron J. Utilization of savanna-based resources by Plio-Pleistocene baboons. S Afr J Sci. 2005;101:245–248. [Google Scholar]
- Codron D, Lee-Thorp JA, Sponheimer M, De Ruiter D, Codron J. Inter-and intra-habitat dietary variability of chacma baboons (Papio ursinus) in South African savannas based on fecal δ13C and δ15N. Am J Phys Anthropol. 2006;129:204–214. doi: 10.1002/ajpa.20253. [DOI] [PubMed] [Google Scholar]
- Cohen JE, Small C. Hypsographic demography: the distribution of human population by altitude. Proc Natl Acad Sci USA. 1998;95:14009–14014. doi: 10.1073/pnas.95.24.14009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CB, Moore PD. Biogeography: An Ecological and Evolutionary Approach. 6. Oxford: Blackwell Science; 2000. [Google Scholar]
- DeMenocal PB, Bloemendal J. Plio-Pleistocene climatic variability in subtropical Africa and the palaeoenvironment of hominid evolution: a combined data-model approach. In: Vrba ES, Denton GH, Partridge TC, Burckle LH, editors. Paleoclimate and Evolution, with Emphasis on Human Origins. New Haven, CT: Yale University Press; 1995. pp. 262–288. [Google Scholar]
- DeMenocal PB. African climate change and faunal evolution during the Pliocene-Pleistocene. Earth Planet Sci Lett. 2004;220:3–24. [Google Scholar]
- Dennell R, Roebroeks W. An Asian perspective on early human dispersal from Africa. Nature. 2005;438:1099–1104. doi: 10.1038/nature04259. [DOI] [PubMed] [Google Scholar]
- Denton G. Cenozoic climate change. In: Bromage TG, Schrenk F, editors. African Biogeography, Climate Change and Early Hominid Evolution. New York: Oxford University Press; 1999. pp. 94–114. [Google Scholar]
- Dodonov AE, Baiguzina LL. Loess stratigraphy of Central Asia: Palaeoclimatic and palaeoenvironmental aspects. Quaternary Sci Rev. 1995;14:707–720. [Google Scholar]
- Dowsett HJ, Barron JA, Poore RZ, et al. Middle Pliocene paleoenvironmental reconstruction: PRISM2. US Geol Surv, Reston, Va, Open File Rep. 1999;99–535 [Google Scholar]
- Dugas DP, Retallack GJ. Middle Miocene fossil grasses from Fort Ternan, Kenya. J Paleont. 1993;67:113–128. [Google Scholar]
- Duggen S, Hoernle K, van den Bogaard P, Rupke L, Phipps Morgan J. Deep roots of the Messinian salinity crisis. Nature. 2003;422:602–606. doi: 10.1038/nature01553. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM. Environmental determinants of intraspecific variation in body weight in baboons (Papio spp.) J Zool. 1990;220:157–169. [Google Scholar]
- Elton S. Ecomorphology and evolutionary biology of African Cercopithecoids: providing an ecological context for hominin evolution. 2000. PhD dissertation, University of Cambridge. [Google Scholar]
- Elton S. Locomotor and habitat classification of cercopithecoid postcranial material from Sterkfontein Member 4, Bolt's Farm and Swartkrans Members 1 and 2, South Africa. Palaeont Afr. 2001;37:115–126. [Google Scholar]
- Elton S. A reappraisal of the locomotion and habitat preference of Theropithecus oswaldi. Folia Primatol. 2002;73:252–280. doi: 10.1159/000067457. [DOI] [PubMed] [Google Scholar]
- Elton S. Forty years on and still going strong: the use of the hominin-cercopithecid comparison in palaeoanthropology. J Roy Anthropol Inst. 2006;12:19–38. [Google Scholar]
- Elton S. Environmental correlates of the cercopithecoid radiations. Folia Primatol. 2007;74:344–364. doi: 10.1159/000105149. [DOI] [PubMed] [Google Scholar]
- Estevez J, Pianab E, Schiavinib A, Juan-Muns N. Archaeological analysis of shell middens in the beagle channel, Tierra Del Fuego Island. Int. J. Osteoarchaeol. 2001;11:24–33. [Google Scholar]
- Fernandez-Jalvo Y, Denys C, Andrews P, Williams T, Dauphin Y, Humphrey L. Taphonomy and palaeoecology of Olduvai Bed-I (Pleistocene, Tanzania) J Hum Evol. 1998;34:137–172. doi: 10.1006/jhev.1997.0188. [DOI] [PubMed] [Google Scholar]
- Finlayson C. Neanderthals and Modern Humans: An Ecological and Evolutionary Perspective. Cambridge: Cambridge University Press; 2004. [Google Scholar]
- Fitzhugh WW. Biogeographical archaeology in the Eastern North American Arctic. Hum Ecol. 1997;25:385–418. [Google Scholar]
- Folinsbee KE, Brooks DR. Miocene hominoid biogeography: pulses of dispersal and differentiation. J Biogeogr. 2007;34:383–397. [Google Scholar]
- Fooden J, Albrecht GH. Latitudinal and insular variation of skull size in crab-eating macaques (Primates, Cercopithecidae: Macaca fascicularis. Am J Phys Anthropol. 1993;92:521–538. doi: 10.1002/ajpa.1330920409. [DOI] [PubMed] [Google Scholar]
- Fooden J, Albrecht GH. Tail length evolution in Fascicularis-group macaques (Cercopithecidae: Macaca. Int J Primatol. 1999;20:431–440. [Google Scholar]
- Gabunia L, Vekua A, Swisher CC, et al. Earliest Pleistocene hominid cranial remains from Dmanisi, Republic of Georgia: taxonomy, geological setting, and age. Science. 2000;288:1019–25. doi: 10.1126/science.288.5468.1019. [DOI] [PubMed] [Google Scholar]
- Goebel T. Pleistocene human colonization of Siberia and peopling of the Americas: an ecological approach. Evol Anthropol. 1999;8:208–227. [Google Scholar]
- Haile-Selassie Y. Late Miocene hominids from the Middle Awash, Ethiopia. Nature. 2001;412:178–181. doi: 10.1038/35084063. [DOI] [PubMed] [Google Scholar]
- Harcourt-Smith WEH, Aiello LC. Fossils, feet and the evolution of human bipedal locomotion. J Anat. 2004;204:403–416. doi: 10.1111/j.0021-8782.2004.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison GA. Human Adaptation. Oxford: Oxford University Press; 1993. [Google Scholar]
- Harrison T. The implications of Oreopithecus for the origins of bipedalism. In: Coppens Y, Senut B, editors. Origine(s) de la Bipedie Chez les Hominides. Paris: Cahiers de Paleoanthropologie, CNRS; 1991. pp. 235–244. [Google Scholar]
- Harrison T, Rook L. Enigmatic anthropoid or misunderstood ape? The phylogenetic status of Oreopithecus bambolii reconsidered. In: Begun DR, Ward CV, Rose MD, editors. Function, Phylogeny, and Fossils: Miocene Hominoid Evolution and Adaptations. New York: Plenum Press; 1997. pp. 327–362. [Google Scholar]
- Harvati K, Weaver TD. Human cranial anatomy and the differential preservation of population history and climate signatures. Anat Rec. 2006;228A:1225–1233. doi: 10.1002/ar.a.20395. [DOI] [PubMed] [Google Scholar]
- Headland TN, Bailey RC. Introduction: have hunter-gatherers ever lived in tropical rain forest independently of agriculture? Hum Ecol. 1991;19:115–122. [Google Scholar]
- Heaney LR. A synopsis of climatic and vegetational change in Southeast Asia. Climatic Change. 1991;19:53–61. [Google Scholar]
- Holliday TW. Brachial and crural indices of European Late Upper Palaeolithic and Mesolithic humans. J Hum Evol. 1999;36:549–566. doi: 10.1006/jhev.1998.0289. [DOI] [PubMed] [Google Scholar]
- Hope G, Kershaw AP, van der Kaars S, et al. History of vegetation and habitat change in the Austral-Asian region. Quat Internat. 2004;118–119:103–126. [Google Scholar]
- Hughes JK, Elton S, O’Regan HJ. Theropithecus and ‘Out of Africa’ dispersal in the Plio-Pleistocene. J Hum Evol. 2008;54:43–77. doi: 10.1016/j.jhevol.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Jablonski NG, Kelley J. Did a major immunological event shape the evolutionary histories of apes and Old World monkeys? J Hum Evol. 1997;33:513–520. doi: 10.1006/jhev.1997.0160. [DOI] [PubMed] [Google Scholar]
- Jablonski NG. The evolution of the doucs and snub-nosed monkey and the question of the phyletic unity of the odd-nosed colobines. In: Jablonski NG, editor. The Natural History of the Doucs and Snub-nosed Monkeys. Singapore: World Scientific; 1998. pp. 13–52. [Google Scholar]
- Jacobs BF. Palaeobotanical studies from tropical Africa: relevance to the evolution of forest, woodland and savannah biomes. Phil Trans R Soc Lond. 2004;B359:1573–1583. doi: 10.1098/rstb.2004.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C-Z, Kawamura Y, Taruno H. Pliocene and Early Pleistocene insectivore and rodent faunas from Dajushan, Qipanshan and Haimao in North China and the reconstruction of the faunal succession from the Late Miocene to Middle Pleistocene. J Geosci Osaka City Univ. 1999;41:1–19. [Google Scholar]
- Kaplan JO, Bigelow NH, Prentice IC, et al. Climate change and Arctic ecosystems: 2. Modeling, paleodata-model comparisons, and future projections. J Geophys Res Atmospheres. 2003;108(D19) art. no. 8171. [Google Scholar]
- Kappelman J. Morphology and locomotor adaptations of the bovid femur in relation to habitat. J Morph. 1988;198:119–130. doi: 10.1002/jmor.1051980111. [DOI] [PubMed] [Google Scholar]
- Kappelman J, Plummer T, Bishop L, Duncan A, Appleton S. Bovids as indicators of Plio-Pleistocene palaeoenvironments in East Africa. J Hum Evol. 1997;32:229–256. doi: 10.1006/jhev.1996.0105. [DOI] [PubMed] [Google Scholar]
- Kennett JP. A review of polar climatic evolution during the Neogene, based on the marine sediment record. In: Vrba ES, Denton GH, Partridge TC, Burckle LH, editors. Paleoclimate and Evolution, with Emphasis on Human Origins. New Haven, CT: Yale University Press; 1995. pp. 49–64. [Google Scholar]
- Kingston JD, Hill A, Marino BD. Isotopic evidence for Neogene hominid palaeoenvironments in the Kenya rift valley. Science. 1994;264:955–959. doi: 10.1126/science.264.5161.955. [DOI] [PubMed] [Google Scholar]
- Kingston J, Harrison T. Isotopic dietary reconstructions of Pliocene herbivores at Laetoli: implications for hominin paleoecology. Palaeogeog Palaeoclimatol Palaeoecol. 2007;243:272–306. [Google Scholar]
- Kingston JD, Deino A, Hill A, Edgar R. Astronomically forced climate change in the Kenyan Rift Valley 2.7–2.55 Ma: Implications for the evolution of early hominin ecosystems. J Hum Evol. 2007;53:487–503. doi: 10.1016/j.jhevol.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Kohler M, Moya-Sola S. Ape-like or hominid-like? The positional behavior of Oreopithecus bambolii reconsidered. Proc Natl Acad Sci USA. 1997;94:11747–11750. doi: 10.1073/pnas.94.21.11747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler M, Moyà-Solà S. Understanding the enigmatic ape Oreopithecus bambolii. Cour Forsch-Inst Senckenberg. 2003;243:111–123. [Google Scholar]
- Kovarovic KM, Andrews P, Aiello L. The palaeoecology of the Upper Ndolanya Beds, Laetoli, Tanzania. J Hum Evol. 2002;43:395–418. doi: 10.1006/jhev.2002.0580. [DOI] [PubMed] [Google Scholar]
- Larrasoaña JC, Roberts AP, Rohling EJ, Winklhofer M, Wehausen R. Three million years of monsoon variability over the northern Sahara. Clim Dynam. 2003;21:689–698. [Google Scholar]
- Lee-Thorp JA, van der Merwe NJ, Brain CK. Diet of Australopithecus robustus at Swartkrans from stable carbon isotopic analysis. J Hum Evol. 1994;27:361–372. [Google Scholar]
- Lee-Thorp JA, Sponheimer M, van der Merwe NJ. What do stable isotopes tell us about hominid dietary and ecological niches in the Pliocene ? Int J Osteoarchaeol. 2003;13:104–113. [Google Scholar]
- Lehman SM, Mayor M, Wright PC. Ecogeographic size variation in sifakas: a test of the resource seasonality and resource quality hypotheses. Am J Phys Anthropol. 2005;126:318–328. doi: 10.1002/ajpa.10428. [DOI] [PubMed] [Google Scholar]
- Marlow JR, Lange CB, Wefer G, Rosell-Melé A. Upwelling intensification as part of the Pliocene-Pleistocene climate transition. Science. 2000;290:2288–2291. doi: 10.1126/science.290.5500.2288. [DOI] [PubMed] [Google Scholar]
- McBrearty S, Jablonski NG. First fossil chimpanzee. Nature. 2005;437:105–108. doi: 10.1038/nature04008. [DOI] [PubMed] [Google Scholar]
- McCabe JT. Turkana pastoralism: a case against the Tragedy of the Commons. Hum Ecol. 1990;18:81–103. [Google Scholar]
- McGrew WC, Marchant LF, Nishida T. Great Ape Societies. Cambridge: Cambridge University Press; 1996. [Google Scholar]
- Meir A, Tsoar H. International borders and range ecology: the case of Bedouin transborder grazing. Hum Ecol. 1996;24:39–64. [Google Scholar]
- Mercader J. Forest People: the role of African rainforests in human evolution and dispersal. Evol Anthropol. 2002;11:117–124. [Google Scholar]
- Millien V, Lyons SK, Olson L, Smith FA, Wilson AB, Yom-Tov Y. Ecotypic variation in the context of global climate change: revisiting the rules. Ecol Lett. 2006;9:853–869. doi: 10.1111/j.1461-0248.2006.00928.x. [DOI] [PubMed] [Google Scholar]
- Molnar P, England P, Martinod J. Mantle dynamics, uplift of the Tibetan Plateau, and the Indian monsoon. Rev Geophys. 1993;31:357–396. [Google Scholar]
- Moyà-Solà S, Köhler M, Alba DM, Casanovas-Vilar I, Galindo J. Pierolapithecus catalaunicus, a new Middle Miocene great ape from Spain. Science. 2004;306:1339–1344. doi: 10.1126/science.1103094. [DOI] [PubMed] [Google Scholar]
- Nowak NM. Walker's Mammals of the World. 6. Baltimore: Johns Hopkins University Press; 1999. [Google Scholar]
- O’Brien EM, Peters CR. Landforms, climate, ecogeographic mosaics, and the potential for hominid diversity in Pliocene Africa. In: Bromage TG, Schrenk F, editors. African Biogeography, Climate Change and Early Hominid Evolution. New York: Oxford University Press; 1999. pp. 115–137. [Google Scholar]
- O’Higgins P, Elton S. Walking on trees. Science. 2007;316:1292–1294. doi: 10.1126/science.1143571. [DOI] [PubMed] [Google Scholar]
- Ohman JC, Wood C, Wood B, et al. Stature-at-death of KNM-WT 15000. Hum Evol. 2003;17:129–141. [Google Scholar]
- Parfitt SA, Barendregt RW, Breda M, et al. The earliest record of human activity in northern Europe. Nature. 2005;438:1008–1012. doi: 10.1038/nature04227. [DOI] [PubMed] [Google Scholar]
- Partridge TC, Bond GC, Hartnady CJH, deMenocal PB, Ruddiman WF. Climatic effects of late Neogene tectonism and volcanism. In: Vrba ES, Denton GH, Partridge TC, Burckle LH, editors. Paleoclimate and Evolution, with Emphasis on Human Origins. New Haven, CT: Yale University Press; 1995a. pp. 8–23. [Google Scholar]
- Partridge TC, Wood BA, deMenocal PB. The influence of global climatic change and regional uplift on large-mammal evolution in East and southern Africa. In: Vrba ES, Denton GH, Partridge TC, Burckle LH, editors. Paleoclimate and Evolution, with Emphasis on Human Origins. New Haven, CT: Yale University Press; 1995b. pp. 331–355. [Google Scholar]
- Patterson N, Richter DJ, Gnerre S, Lander ES, Reich D. Genetic evidence for complex speciation of humans and chimpanzees. Nature. 2006;441:1103–1107. doi: 10.1038/nature04789. [DOI] [PubMed] [Google Scholar]
- Pearson OM. Activity, climate and postcranial robusticity: implications for modern human origins and scenarios of adaptive change. Curr Anthropol. 2000;41:569–607. [PubMed] [Google Scholar]
- Peters CR, O’Brien EM. Potential hominid plant foods from woody species in semi-arid versus sub-humid sub-topical Africa. In: Chivers DJ, Langer P, editors. The Digestive System in Mammals: Food, Form and Function. Cambridge: Cambridge University Press; 1994. pp. 166–190. [Google Scholar]
- Peters CR, Vogel JC. Africa's wild C4 plant foods and possible early hominid diets. J Hum Evol. 2005;48:219–236. doi: 10.1016/j.jhevol.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Pickford M. Fort Ternan (Kenya) palaeoecology. J Hum Evol. 1987;16:305–309. [Google Scholar]
- Pickford M, Senut B. The geological and faunal context of Late Miocene hominid remains from Lukeino, Kenya. CR Acad Sci Paris, Sci de la Terre et des plan. 2001;332:145–152. [Google Scholar]
- Pickford M, Kunimatsu Y. Catarrhines from the middle Miocene (ca. 14.5 Ma) of Kipsaraman, Tugen Hills, Kenya. Anthropol Sci. 2005;113:189–224. [Google Scholar]
- Pitulko VV, Nikolsky PA, Girya EYu, et al. The Yana RHS site: humans in the arctic before the Last Glacial Maximum. Science. 2004;303:52–56. doi: 10.1126/science.1085219. [DOI] [PubMed] [Google Scholar]
- Plummer TW, Bishop LC. Hominid palaeoecology at Olduvai Gorge, Tanzania as indicated by antelope remains. J Hum Evol. 1994;27:47–75. [Google Scholar]
- Plummer T, Bishop LC, Ditchfield P, Hicks J. Research on late Pliocene Oldowan sites at Kanjera South, Kenya. J Hum Evol. 1999;36:151–170. doi: 10.1006/jhev.1998.0256. [DOI] [PubMed] [Google Scholar]
- Popp JL. Ecological determinism in the life histories of baboons. Primates. 1983;24:198–210. [Google Scholar]
- Potts R. Variability selection in hominid evolution. Evol Anthropol. 1998;7:81–96. [Google Scholar]
- Powers WR, Jordan RH. Human biogeography and climate change in Siberia and Arctic North America in the Fourth and Fifth Millennia BP. Phil Trans Roy Soc Lond A. 1990;330:665–670. [Google Scholar]
- Quade J, Cerling TE. Expansion of C4 grasses in the Late Miocene of northern Pakistan: evidence from stable isotopes in paleosols. Paleogeog Paleoclimatol Paleoecol. 1995;115:91–116. [Google Scholar]
- Rae TC, Hill RA, Hamada Y, Koppe T. Clinal variation of maxillary sinus volume in Japanese macaques (Macaca fuscata. Am J Primatol. 2003;59:153–158. doi: 10.1002/ajp.10072. [DOI] [PubMed] [Google Scholar]
- Ramberg L, Hancock P, Lindholm M, et al. Species diversity of the Okavango Delta, Botswana. Aquatic Sci. 2006;68:310–337. [Google Scholar]
- Ramstad M, Hesjedal A, Roth Niemi A. The Melkøya project: maritime hunter-fisher island settlements and the use of space through 11 000 years on Melkøya, Arctic Norway. 2005 June 2005 http://www.antiquity.ac.uk/projgall/ramstad/index.html.
- Reader J. Man on Earth. London: Penguin Books; 1988. [Google Scholar]
- Reed KE. Early hominid evolution and ecological change through the African Plio-Pleistocene. J Hum Evol. 1997;32:289–322. doi: 10.1006/jhev.1996.0106. [DOI] [PubMed] [Google Scholar]
- Richmond BG, Aiello L, Wood BA. Early hominin limb proportions. J Hum Evol. 2002;43:529–548. [PubMed] [Google Scholar]
- Rook L, Bondioli L, Kohler M, Moya-Sola S, Macchiarelli R. Oreopithecus was a bipedal ape after all: evidence from the iliac cancellous architecture. Proc Natl Acad Sci USA. 1999;96:8795–8799. doi: 10.1073/pnas.96.15.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell TE. Forest living baboons in Uganda. J Zool, Lond. 1966;149:365–393. [Google Scholar]
- Ruff CB, Walker A. Body size and body shape. In: Walker A, Leakey R, editors. The Nariokotome Homo erectus Skeleton. Cambridge, MA: Harvard University Press; 1993. pp. 234–265. [Google Scholar]
- Ruff CB. Morphological Adaptation to Climate in Modern and Fossil Hominids. Ybk Phys Anthropol. 1994;37:65–107. [Google Scholar]
- Ruff C. Variation in human body size and shape. Annu Rev Anthropol. 2002;31:211–232. [Google Scholar]
- Rutherford S, D’Hondt S. Early onset and tropical forcing of. 100 000-year Pleistocene glacial cycles. Nature. 2000;408:72–75. doi: 10.1038/35040533. [DOI] [PubMed] [Google Scholar]
- Sardella R, Caloi L, Di Stefano G, et al. Mammal faunal turnover in Italy from the Middle Pliocene to the Holocene. Mededelingen Nederlands Instituut voor Toegepaste Geowetenschappen TNO. 1998;60:499–511. [Google Scholar]
- Scott RS, Kappelman J, Kelley J. The palaeoenvironment of Sivapithecus parvada. J Hum Evol. 1999;36:245–274. doi: 10.1006/jhev.1998.0269. [DOI] [PubMed] [Google Scholar]
- Senut B, Pickford M, Gommery D, Mein P, Cheboi K, Coppens Y. First hominid from the Miocene (Lukeino Formation, Kenya) CR Acad Sci. 2001;332:137–144. [Google Scholar]
- Sikes N. Plio-Pleistocene floral context and habitat preferences of sympatric hominid species in East Africa. In: Bromage TG, Schrenk F, editors. African Biogeography, Climate Change and Early Hominid Evolution. New York: Oxford University Press; 1999. pp. 301–315. [Google Scholar]
- Sikes NE, Potts R, Behrensmeyer AK. Early Pleistocene habitat in Member 1 Olorgesailie based on palaeosol stable isotopes. J Hum Evol. 1999;37:721–746. doi: 10.1006/jhev.1999.0343. [DOI] [PubMed] [Google Scholar]
- Sponheimer M, Lee-Thorp JA. Isotopic evidence for the diet of an early hominid, Australopithecus africanus. Science. 1999;283:368–369. doi: 10.1126/science.283.5400.368. [DOI] [PubMed] [Google Scholar]
- Sponheimer M, Loudon JE, Codron D, et al. Do ‘savanna’ chimpanzees consume C4 resources? J Hum Evol. 2006;51:128–133. doi: 10.1016/j.jhevol.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Steiper ME, Young NM. Primate molecular divergence dates. Mol Phylog Evol. 2006;41:384–394. doi: 10.1016/j.ympev.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Stern JT, Susman RL. The locomotor anatomy of Australopithecus afarensis. Am J Phys Anthropol. 1983;60:279–317. doi: 10.1002/ajpa.1330600302. [DOI] [PubMed] [Google Scholar]
- Suwa G, Kono RT, Katoh S, Asfaw B, Beyene Y. A new species of great ape from the late Miocene epoch in Ethiopia. Nature. 2007;448:921–924. doi: 10.1038/nature06113. [DOI] [PubMed] [Google Scholar]
- Teaford MF, Ungar PS. Diet and the evolution of the earliest human ancestors. Proc Natl Acad Sci USA. 2000;97:13506–13511. doi: 10.1073/pnas.260368897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth MH, Maslin MA, Deino A, Strecker MR. Late Cenozoic moisture history of East Africa. Science. 2005;309:2051–2053. doi: 10.1126/science.1112964. [DOI] [PubMed] [Google Scholar]
- Trinkaus E. Neanderthal limb proportions and cold adaptation. In: Stringer CB, editor. Aspects of Human Evolution. London: Taylor & Francis; 1981. pp. 187–224. [Google Scholar]
- Tsujikawa H. The palaeoenvironment of Samburupithecus kiptalami based on its associated fauna. African Study Monographs, Suppl. 2005;32:51–62. [Google Scholar]
- Turner TR, Anapol F, Jolly CJ. Growth, development, and sexual dimorphism in vervet monkeys (Cercopithecus aethiops) at four sites in Kenya. Am J Phys Anthropol. 1997;103:19–35. doi: 10.1002/(SICI)1096-8644(199705)103:1<19::AID-AJPA3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Van Wyk AE. Maputaland-Pondoland region. In: Davis SD, Heywood VH, Hamilton AC, editors. Centres of Plant Diversity A guide and Strategy for Their Conservation Volume 1. Cambridge: IUCN Publications Unit; 1994. pp. 227–235. [Google Scholar]
- Vignaud P, Duringer P, Mackaye HT, et al. Geology and palaeontology of the Upper Miocene Toros-Menalla hominid locality, Chad. Nature. 2002;418:152–155. doi: 10.1038/nature00880. [DOI] [PubMed] [Google Scholar]
- Vincens A, Garcin Y, Buchet G. Influence of rainfall seasonality on African lowland vegetation during the Late Quaternary: pollen evidence from Lake Masoko, Tanzania. J Biogeogr. 2007;34:1274–1288. [Google Scholar]
- Vrba ES. On the connections between paleoclimate and evolution. In: Vrba ES, Denton GH, Partridge TC, Burckle LH, editors. Paleoclimate and Evolution, with Emphasis on Human Origins. New Haven, CT: Yale University Press; 1995. pp. 24–45. [Google Scholar]
- Western D, van Praet C. Cyclical changes in the habitat and climate of an East African ecosystem. Nature. 1973;241:104–106. [Google Scholar]
- Wheeler PE. The thermoregulatory advantages of hominid bipedalism in open equatorial environments: the contribution of increased convective heat loss and cutaneous evaporative cooling. J Hum Evol. 1991;21:107–115. [Google Scholar]
- WoldeGabriel G, Haile-Selassie Y, Renne P, et al. Geology and palaeontology of the Late Miocene Middle Awash valley, Afar rift, Ethiopia. Nature. 2001;412:175–178. doi: 10.1038/35084058. [DOI] [PubMed] [Google Scholar]
- Wood BA, Richmond B. Human evolution: taxonomy and paleobiology. J Anat. 2000;97:19–60. doi: 10.1046/j.1469-7580.2000.19710019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Ding Z. Winter-spring precipitation as the principle control on predominance of C3 plants in Central Asia over the past 1.77 Myr: Evidence from delta13C of loess organic matter in Tajikistan. Palaeogeog Palaeoclimatol Palaeoecol. 2006;235:330–339. [Google Scholar]
- Young TP, Lindsay WK. Role of even-age population structure in the disappearance of Acacia xanthophloea woodlands. Afr J Ecol. 1988;26:69–72. [Google Scholar]
- Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292:686–693. doi: 10.1126/science.1059412. [DOI] [PubMed] [Google Scholar]
- Zagwijn WH. Borders and boundaries: a century of stratigraphical research in the Tegelen-Reuver area of Limburg (the Netherlands) Mededelingen Nederlands Instituut voor Toegepaste Geowetenschappen TNO. 1998;60:19–34. [Google Scholar]
- Zhu RX, Hoffman KA, Potts R, et al. Earliest presence of humans in northeast Asia. Nature. 2001;413:413–417. doi: 10.1038/35096551. [DOI] [PubMed] [Google Scholar]