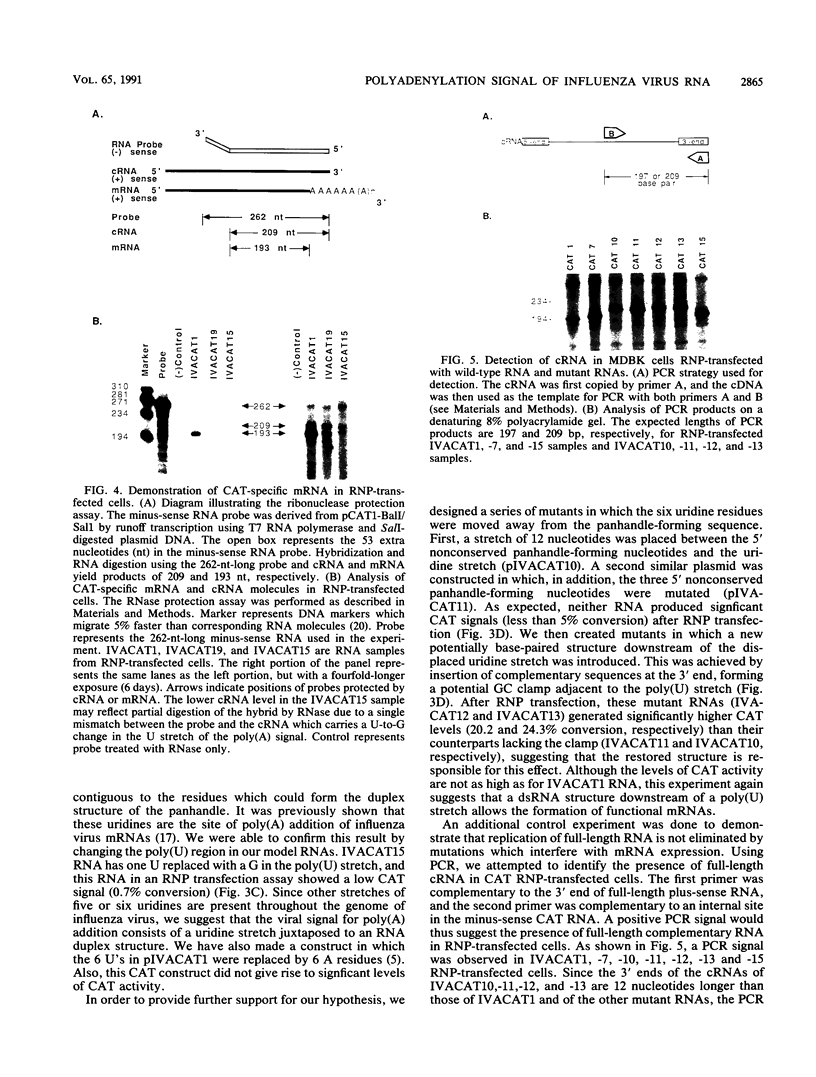
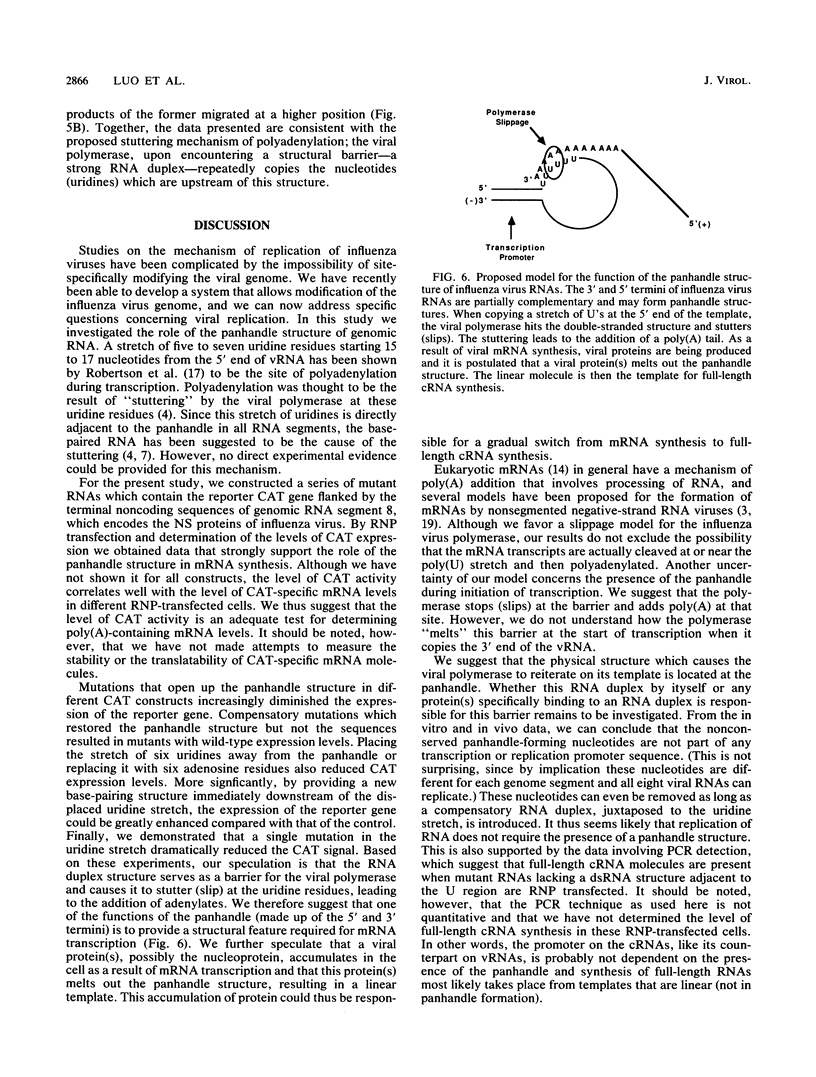
Abstract
Appropriate RNAs are transcribed and amplified and proteins are expressed after transfection into cells of in vitro-reconstituted RNA-protein complexes and infection with influenza virus as the helper. This system permits us to study the signals involved in transcription of influenza virus RNAs. For the analysis we used a plasmid-derived RNA containing the reporter gene for chloramphenicol acetyltransferase (CAT) flanked by the noncoding sequences of the NS RNA segment of influenza A/WSN/33 virus. Mutations were then introduced into both the 5' and 3' ends, and the resulting RNAs were studied to determine their transcription in vitro and their CAT expression activity in the RNA-protein transfection system. The results reveal that a stretch of uninterrupted uridines at the 5' end of the negative-strand RNA is essential for mRNA synthesis. Also, a double-stranded RNA "panhandle" structure generated by the 5'- and 3'-terminal nucleotides appears to be required for polyadenylation, since opening up of these base pairs diminished mRNA synthesis and eliminated expression of CAT activity by the mutant RNAs. Finally, it was shown that this double-stranded RNA structural requirement is not sequence specific, since a synthetic GC clamp can replace the virus-coded RNA duplex. The data suggest that the viral RNA polymerase adds poly(A) by a slippage (stuttering) mechanism which occurs when it hits the double-stranded RNA barrier next to the stretch of uridines.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Desselberger U., Racaniello V. R., Zazra J. J., Palese P. The 3' and 5'-terminal sequences of influenza A, B and C virus RNA segments are highly conserved and show partial inverted complementarity. Gene. 1980 Feb;8(3):315–328. doi: 10.1016/0378-1119(80)90007-4. [DOI] [PubMed] [Google Scholar]
- Enami M., Luytjes W., Krystal M., Palese P. Introduction of site-specific mutations into the genome of influenza virus. Proc Natl Acad Sci U S A. 1990 May;87(10):3802–3805. doi: 10.1073/pnas.87.10.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A. J., Abraham G., Skehel J. J., Smith J. C., Fellner P. Influenza virus messenger RNAs are incomplete transcripts of the genome RNAs. Nucleic Acids Res. 1977 Dec;4(12):4197–4209. doi: 10.1093/nar/4.12.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda A., Uéda K., Nagata K., Ishihama A. RNA polymerase of influenza virus: role of NP in RNA chain elongation. J Biochem. 1988 Dec;104(6):1021–1026. doi: 10.1093/oxfordjournals.jbchem.a122569. [DOI] [PubMed] [Google Scholar]
- Hsu M. T., Parvin J. D., Gupta S., Krystal M., Palese P. Genomic RNAs of influenza viruses are held in a circular conformation in virions and in infected cells by a terminal panhandle. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8140–8144. doi: 10.1073/pnas.84.22.8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T. S., Palese P., Krystal M. Determination of influenza virus proteins required for genome replication. J Virol. 1990 Nov;64(11):5669–5673. doi: 10.1128/jvi.64.11.5669-5673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G. X., Chao M., Hsieh S. Y., Sureau C., Nishikura K., Taylor J. A specific base transition occurs on replicating hepatitis delta virus RNA. J Virol. 1990 Mar;64(3):1021–1027. doi: 10.1128/jvi.64.3.1021-1027.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luytjes W., Krystal M., Enami M., Parvin J. D., Palese P. Amplification, expression, and packaging of foreign gene by influenza virus. Cell. 1989 Dec 22;59(6):1107–1113. doi: 10.1016/0092-8674(89)90766-6. [DOI] [PubMed] [Google Scholar]
- Palese P. The genes of influenza virus. Cell. 1977 Jan;10(1):1–10. doi: 10.1016/0092-8674(77)90133-7. [DOI] [PubMed] [Google Scholar]
- Parvin J. D., Palese P., Honda A., Ishihama A., Krystal M. Promoter analysis of influenza virus RNA polymerase. J Virol. 1989 Dec;63(12):5142–5152. doi: 10.1128/jvi.63.12.5142-5152.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Kilbourne E. D. RNAs of influenza A, B, and C viruses. J Virol. 1976 May;18(2):738–744. doi: 10.1128/jvi.18.2.738-744.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. S. 5' and 3' terminal nucleotide sequences of the RNA genome segments of influenza virus. Nucleic Acids Res. 1979 Aug 24;6(12):3745–3757. doi: 10.1093/nar/6.12.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. S., Schubert M., Lazzarini R. A. Polyadenylation sites for influenza virus mRNA. J Virol. 1981 Apr;38(1):157–163. doi: 10.1128/jvi.38.1.157-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Herman R. C., Lazzarini R. A. Site on the vesicular stomatitis virus genome specifying polyadenylation and the end of the L gene mRNA. J Virol. 1980 May;34(2):550–559. doi: 10.1128/jvi.34.2.550-559.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharmeen L., Taylor J. Enzymatic synthesis of RNA oligonucleotides. Nucleic Acids Res. 1987 Aug 25;15(16):6705–6711. doi: 10.1093/nar/15.16.6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Hay A. J. Nucleotide sequences at the 5' termini of influenza virus RNAs and their transcripts. Nucleic Acids Res. 1978 Apr;5(4):1207–1219. doi: 10.1093/nar/5.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura A., Tobita K., Kilbourne E. D. Isolation and preliminary characterization of temperature-sensitive mutants of influenza virus. J Virol. 1972 Oct;10(4):639–647. doi: 10.1128/jvi.10.4.639-647.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]