Abstract
The anxiogenic neuropeptide, corticotropin-releasing factor (CRF), has a complex effect on intermale aggression. CRF receptor 1 (CRFR1) is the primary receptor for CRF and in this study, we examined in detail isolation-induced intermale aggression in CRFR1 deficient mice. All mice contained a mixed 50:50 inbred/outbred background to improve aggressive performance. Mice were isolated for 4 weeks prior to two consecutive days of aggression testing using the resident-intruder paradigm. Mice were also tested for anxiety on the elevated plus maze. Relative to littermate wild-type (WT) controls, CRFR1-mutant mice exhibited normal levels of intermale aggression over the two test days in terms of percentage showing aggression, number of attacks, time aggressive, and latency to first attack. In terms of sites of attacks on intruders, CRFR1-deficient mice attacked the ventral portion of the mid-section (including belly) significantly less frequently than WT males on test day 1, but these differences did not reach significance on test day 2. No other differences in sites of attacks were observed. Tail rattling also did not differ between groups. Importantly, KO males showed decreased anxiety relative to WT mice (consistent with previous reports) as evidenced by spending significantly more time on the open arms and significantly less time on the closed arms of the elevated plus maze. Plus maze performance did not correlate with any measure of levels of aggression, suggesting a dissociation between altered levels of anxiety and aggressive performance. Taken together, the results suggest that the activation CRFR1 is not necessary for the normal production of isolation-induced intermale aggression.
Keywords: maternal aggression, CRH, mice, depression, fear, anxiety, CRF receptors
1. Introduction
The anxiogenic neuropeptide, corticotropin-releasing factor (CRF), is thought to be a major contributor to the behavioral responses to stress (fear and anxiety) by acting within the CNS (for reviews, see [23,34]). How CRF modulates intermale aggression, which is adaptive in rodents because it plays a role in establishing territories and increasing access to food and mates [6,20,29,40], is complex. For example, intracerebroventricular (icv) injections of CRF inhibits intermale aggression and increases defensive behavior in singly housed male mice that were tested as residents in a resident-intruder paradigm [26]. However, icv injections of CRF have no effect, but CRF injected into the amygdala elevates intermale aggression in singly housed rats tested in a neutral arena [10]. Consistent with this latter finding, oral administration of a CRFR1 antagonist impairs some aspects of intermale aggression and also decreases lateral attack frequency in hamsters using a resident-intruder paradigm [11]. Also, conditioned defeat behavior, which involves decreased expression of aggression with repeated defeats [19] may be controlled by central release of CRF. For example icv administration of a CRF antagonist prevents this response in hamsters when applied 30 min prior to testing [21] and in mice when the antagonist is injected into the amygdala immediately following a social defeat and ~24 hours prior to testing [32]. In some tests, acute stressors elevate aggression (termed stress or shock-induced aggression) and icv CRF elevates this form of aggression [36]. The effect of CRF on intermale aggression, then, can depend on context and site of CRF action.
In addition to its function within the CNS, CRF triggers peripheral increases in stress hormones (glucocorticoids) [38]. Within the CNS and elsewhere, CRF acts primarily on CRFR1 [4,35,41], but can activate with less efficacy CRF receptor 2 (CRFR2) [22,39]. CRF related peptides, urocortin (Ucn) 1 and Ucn 3, can also elicit different aspects of fear and anxiety (behavioral manifestations of stress) by acting within specific brain regions [3,18,23]. Ucn 1 and Ucn 3 may be the primary ligands for CRFR2, but Ucn 1 can also activate with lesser efficacy CRFR1 [24,25,31,39]. We recently showed that mice missing CRFR2 exhibit normal intermale aggression in a resident-intruder test in all aspects measured [13]. Thus, the presence of CRFR2 is not necessary for the normal production of intermale aggression using the resident-intruder paradigm. However, because some forms of aggression (e.g., stress-induced aggression) can be positively modulated by CRF, it is unclear how CRFR2 mice would perform in other paradigms of aggression testing.
A valuable approach for understanding the role of a gene in behavior is to examine behavioral changes when that gene is removed. CRFR1−/− (hereafter referred to as knockout (KO)) mice display decreased indices of fear and anxiety and elevated levels of CRF in the paraventricular nucleus, but not in amygdala or other regions [35]. CRFR2 expression is not altered in these mice. The KO mice also show normal growth and reproduction, but have lower than normal levels of glucocorticoids and glucocorticoid increases in response to stress are greatly impaired [35].
The aim of this study was to examine whether or how loss of CRFR1 affected intermale aggression using a standard resident-intruder paradigm. Because certain inbred strains, such as DBA and C57 (a common background for KO mice) show decreased intermale aggression relative to outbred mice [30], we examined the loss of the CRFR1 gene in a mixed inbred/outbred background that would be expected to heighten levels of intermale aggression. Given the complex effects CRF can have on intermale aggression described above and that loss of CRFR2 does not affect intermale aggression, our conservative prediction was that loss of the gene would have no effect on behavior. If some level of CRF acting on CRFR1 is necessary for intermale aggression, then it would be expected that intermale aggression would be decreased by the loss of the CRFR1 gene. If, though, CRF is entirely inhibitory of intermale aggression, then a possible elevation of aggression might be expected with loss of the primary receptor. Because KO mice have previously been shown to exhibit decreased indices of fear and anxiety as indicated on elevated plus maze performance, we also examined plus maze performance in WT and KO mice and compared this to aggressive responding.
2. Materials and methods
2.1 Animals
CRFR1-deficient male mice in an inbred C57BL/6 background [35] were produced by crossings of heterozygote CRFR1 (+/−) mice (The Jackson Laboratory, Bar Harbor, ME). Mutant males were then crossed with females (outbred hsd:ICR strain) selectively bred to exhibit high levels of maternal aggression. Heterozygote CRFR1 (+/−) mice (with mixed inbred and outbred backgrounds) were then bred to produce WT and knockout KO male mice used in this study. Offspring were weaned at 21 days and male siblings were group housed until pairing as adults. All genotyping occurred after 21 days. Thus, WT and KO males were siblings and were exposed to the same maternal and post maternal environment. All WT and KO mice were given ad lib access to regular rodent chow and tap water. For studying intermale aggression, WT and KO males (beginning at ~50 days old) were housed individually for four weeks prior to testing (described below). Intruder male mice (hsd:ICR strain; Harlan, Madison, WI) were sexually naïve and group-housed (4 animals/cage). Intruder males (~ 2 months old) were given ad lib access to regular chow. Intruder males were never used more than once per day and used for ~ 3 tests each. All animals were housed on a 14:10 light/dark cycle with lights on at 0600 CST. The longer photoperiod (relative to 12:12 light/dark) allowed for greater separation of diurnal testing from either lights on or lights off (times of physiological and behavioral changes in mice). Daytime testing was performed because robust aggression can be detected during this period in male mice [12,26,28].
2.2 Genotyping
Mice were genotyped by PCR using sense WT (5′-TCT CAG GAT TGC TAA GTT CAG-3′), sense KO (5′-AAC TTC CTG ACT AGG GG -3′), and a common antisense primer (5′-ACT GCT AGT GTG ATG TCC TGC -3′). Reactions were run with purified DNA from ear snips and analyzed according to vendor protocol (The Jackson Laboratory).
2.3 Intermale aggression testing
Following four weeks of isolation, each male (n = 19 for WT; n = 21 for KO) was tested for intermale aggression for two consecutive days for 5 min each between 0900 and 1300 h. An intruder (hsd:ICR strain) male mouse was placed in the resident’s (WT or KO’s) home cage and each test session was recorded on videotape. WT and KO mice were always alternately tested on the same day such that the intruder mice from the same cage were used equally to test both genotypes. Thus, any previous fighting experience of intruders that may have affected outcome was evenly divided among the two groups. Further, in this study the intruder mice were not aggressive. Out of 80 total tests, no intruder mice initiated aggression and hence had to be removed from testing (removing aggressive intruders immediately from testing is standard procedure and is always noted if it occurs). Quantification was performed off-line from videotapes using pen and paper. Aggression scoring was conducted by individuals blind to experimental conditions and treatments. For quantification of intermale aggression the following features were measured: latency to first attack, number of attacks, and total duration of attacks [14,15]. The amount of time attacking different regions of the male (including head/neck, flank/back, or the ventral portion of the midsection, including belly) and the amount of time lunging or clawing (without physical contact) were recorded. Additionally, the number of tail rattling events and the total duration of tail rattling was recorded.
2.4 Elevated plus maze
The plus-maze apparatus was made of black Plexiglas and had two open arms (35 × 5 cm) and two enclosed arms of the same size with walls 15 cm high. The apparatus was elevated 70 cm above the ground. The arms were connected by a central square (5 × 5 cm). Indirect lighting in an otherwise dark room was adjusted to provide a standard of 6.0 lux for each test. All testing was conducted on the day following the second aggression test between 0900 and 1300h. Mice were tested individually in 5 min sessions. Each mouse was removed from the home cage and was placed on the center platform facing an open arm to initiate the test session. Behaviors scored were the number of entries to open or closed arms and the time spent in the middle square or open or closed arms. Arm entries were defined as entry of all four paws into the arm. All test sessions were videotaped and scored by individuals blind to experimental condition.
2.5 Data analysis
Intermale aggression variables were analyzed using a one-way ANOVA. In the cases where the distribution of the differences between WT and KO were not normally distributed, then a nonparametric ANOVA on ranks (Dunn’s Method or Kruskal-Wallis) was used. For statistical analysis Sigma Stat software was used (SPSS Inc., Chicago IL). Lunging and clawing is a mild form of aggression that does not include contact and was not included in analysis of overall levels of aggression. However, this measure was included in a separate analysis of total breakdown of different forms of agonistic behaviors within WT and KO mice. In the case of time to first attack, if an animal was not aggressive (no aggression shown during the test period), a time of 300 sec was assigned (the maximum possible for the test). Pearson correlations were run between all dependent aggressive and all plus maze measures to identify possible links between these two traits.
3. Results
3.1 Intermale aggression
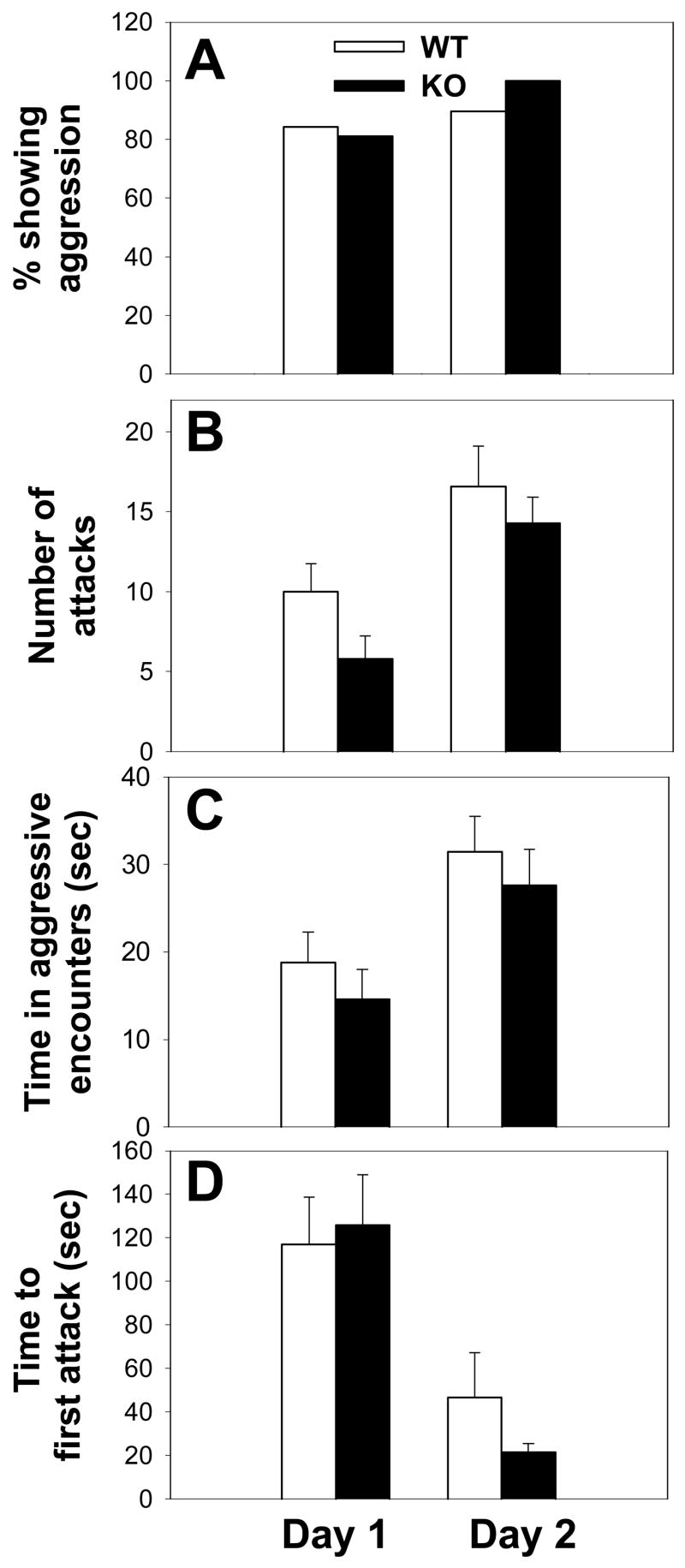
Intermale aggression did not differ between KO and WT mice on test day 1 in terms of percentage showing aggression (H(1,39) = 0.00; p = 0.789; ANOVA on Ranks) (Fig. 1A), number of attacks (H(1,39) = 3.03; p = 0.082; ANOVA on Ranks) (Fig. 1B), time in aggressive encounters (H(1,39) = 1.96; p = 0.161; ANOVA on Ranks) (Fig. 1C), or time to first attack (H(1,39) = 0.00; p = 0.978; ANOVA on Ranks) (Fig. 1D). Consistent with results of day 1, no differences in aggression were found between WT and KO mice on day 2 in terms of percentage showing aggression (H(1,39) = 2.26; p = 0.132; ANOVA on Ranks) (Fig. 1A), number of attacks (H(1,39) = 0.32; p = 0.569; ANOVA on Ranks) (Fig. 1B), time in aggressive encounters (F(1,39) = 0.43; p = 0.513; one-way ANOVA) (Fig. 1C), or time to first attack (H(1,39) = 0.00; p = 0.978; ANOVA on Ranks) (Fig. 1D).
Fig. 1.
Analysis of intermale aggression in WT and KO mice. Using a resident-intruder paradigm, WT and KO mice show similar profiles in terms of the percentage of males showing any aggression (A), the average number of attacks (B), the average amount of time in aggressive encounters (C), and the average latency to first attack (D) when examined on either test day 1 or 2. Bars represent means ± SE. White bars indicate WT mice and black bars indicate KO mice. Data that were non-normally distributed were examined via non-parametric tests (see Methods and Results for more details).
In terms of the breakdown of total agonistic behavior on test day 1, WT and KO mice did not differ in terms of percentage of attacks to the back or flank region (H(1,33) = 2.07; p = 0.149; ANOVA on Ranks) (Fig. 2A), or to the head or neck region (H(1,33) = 1.56; p = 0.210; ANOVA on Ranks) (Fig. 2B) although there was a trend towards increased attacks to the back or flank region in KO mice. In contrast, on day 1 WT mice exhibited significantly greater percentage attacks to the ventral portion of the mid-section (including belly) relative to KO mice (H(1,33) = 4.54; p = 0.033; ANOVA on Ranks) (Fig. 1C). On test day 2 no differences existed between groups in terms of percentage of attacks to the back or flank region (F(1,37) = 0.06; p = 0.803; one-way ANOVA) (Fig. 2A), to the head or neck region (H(1,37) = 0.08; p = 0.777; ANOVA on Ranks (Fig. 2B), or the ventral portion of the mid-section (including belly) (H(1,37) = 0.06; p = 0.799; ANOVA on Ranks) (Fig. 2C). Lunging or clawing was absent in most mice or occurred at an extremely low level (< 2%) and was not included in analysis.
Fig. 2.
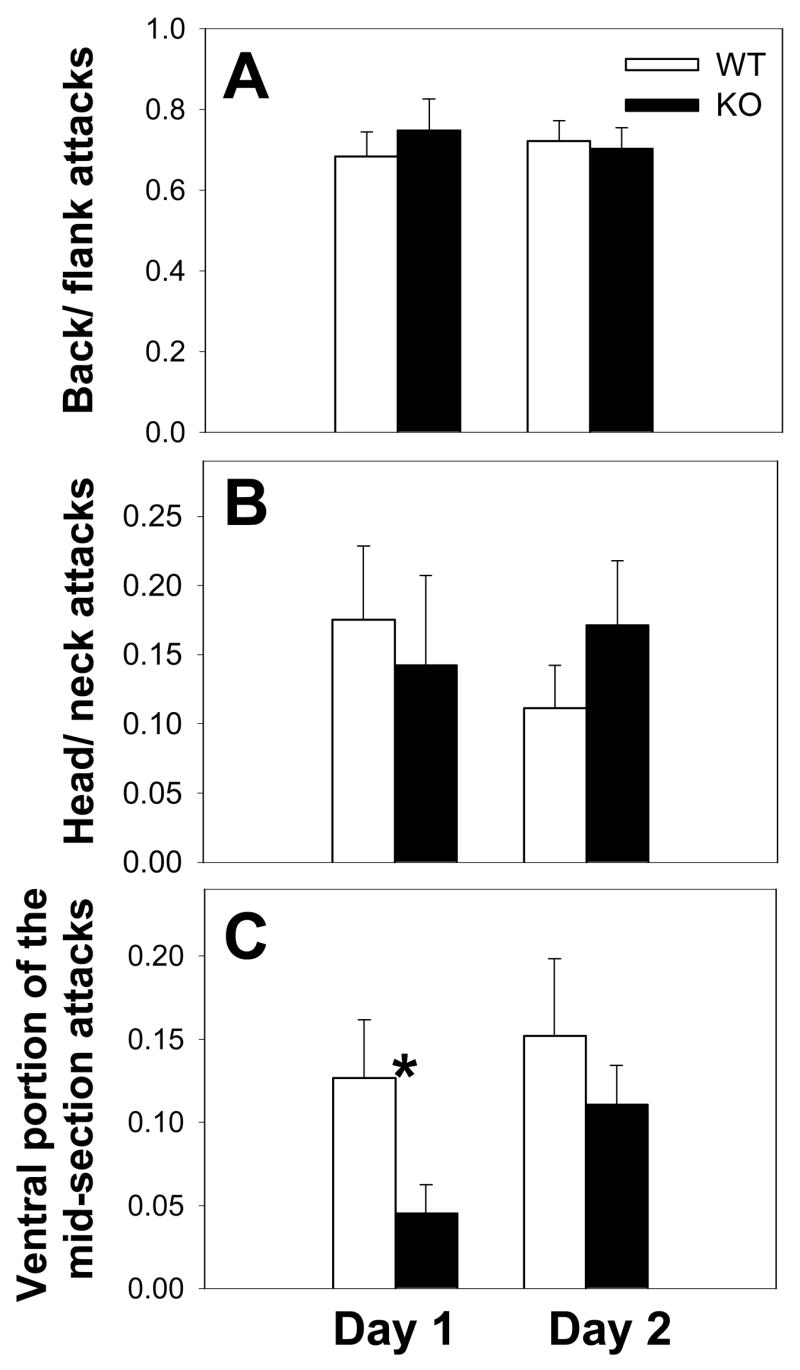
Analysis of intermale aggression in WT and KO mice in terms of proportion of attacks on intruders. WT and KO mice show similar profiles in terms of proportion of attacks to the back or flank (A) or the head or the neck (B) on either test day. In contrast, KO mice exhibit a significantly lower proportion of attacks to the ventral portion of the mid-section (including belly) on test day 1, but not on test day 2 (C). Bars represent means ± SE. White bars indicate WT mice and black bars indicate KO mice. Data that were non-normally distributed were examined via non-parametric tests (see Methods and Results for more details). * = p<0.05; one-way ANOVA on ranks for (C).
On day 1, both genotypes produced a similar number of tail rattling events (WT mean = 1.3 ± 0.5; KO mean = 1.4 ± 0.5;) (H(1,33) = 0.5; p = 0.444; ANOVA on ranks) and spent a similar amount of time tail rattling (WT mean = 1.7 ± 0.7 sec; KO mean = 3.1 ± 1.4 sec;) (H(1,33) = 0.7; p = 0.379; ANOVA on ranks). On day 2, no differences existed between genotype in terms of either number of tail rattling events (WT mean = 3.1 ± 0.8; KO mean = 2.8 ± 0.8) (H(1,33) = 0.0; p = 0.887; ANOVA on ranks) or time tail rattling (WT mean = 6.8 ± 2.7 sec; KO mean = 5.0 ± 1.7 sec) (H(1,33) = 0.1; p = 0.697; ANOVA on ranks).
3.2 Elevated plus maze test
In terms of elevated plus maze performance, relative to WT, KO mice showed a trend towards more open arm entries (F(1,39) = 3.88; p = 0.056; one-way ANOVA) (Fig. 3A) and spent significantly more time in open arms (F(1,39) = 4.14; p = 0.049; one-way ANOVA) (Fig. 3B). Further, KO mice showed a trend towards a shorter latency to first enter an open arm (in sec) (WT median = 52.0; KO median = 30.0), but these differences did not reach significance (H(1,39) = 2.97; p = 0.084; ANOVA on ranks). KO mice and WT mice had a similar number of visits to closed arms (F(1,39) = 0.00; p = 0.939; one-way ANOVA) (Fig. 3C), but KO spent significantly less time on closed arms (F(1,39) = 8.36; p = 0.006; one-way ANOVA) (Fig. 4D). Additionally, KO mice spent significantly more time in the middle square relative to WT mice (WT mean = 71.8 ± 6.6 sec; KO mean = 93.6 ± 7.3 sec) (F(1,39) = 4.78; p = 0.035; one-way ANOVA). Both mice made a similar number of entries to middle squares (WT mean = 14.5 ± 0.9 sec; KO mean = 16.2 ± 1.0 sec) (F(1,39) = 1.5; p = 0.227; one-way ANOVA).
Fig. 3.
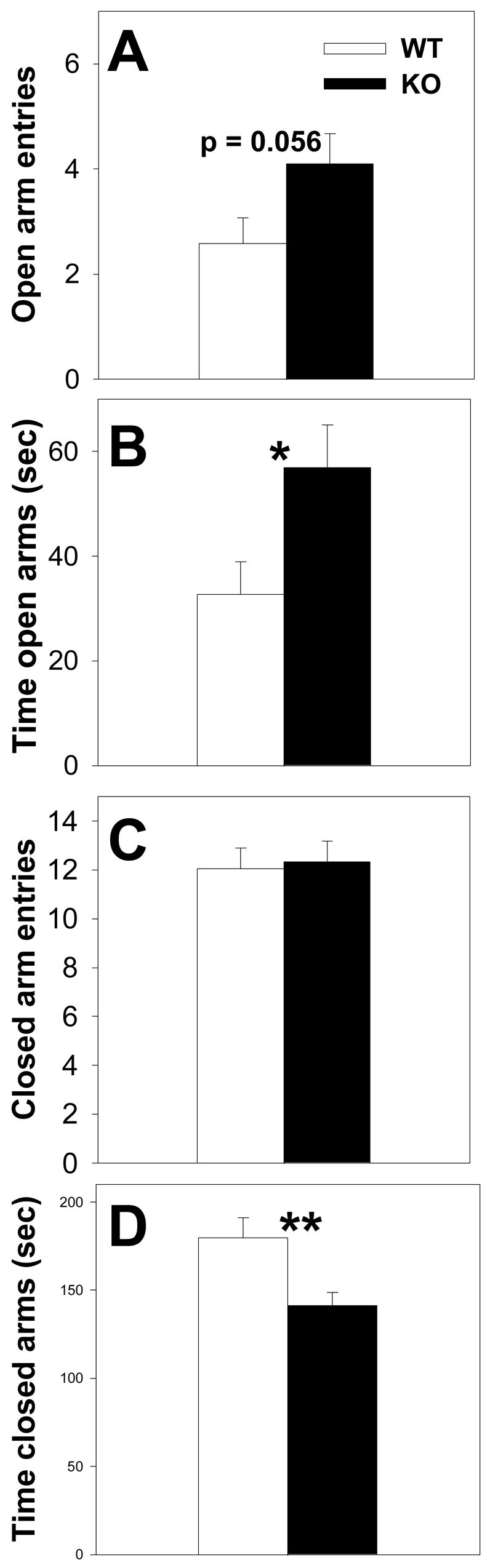
Elevated plus maze performance in WT and KO mice. KO showed a trend towards more entries to the open arm relative to WT mice, but the differences between groups did not reach significance (A). KO mice spent significantly more time on open arms relative to WT mice (B), made an equal number of visits to closed arms (C), but spent significantly less time on the closed arms (D). Bars represent means ± SE. White bars indicate WT mice and black bars indicate KO mice. * = p<0.05; * = p<0.01; one-way ANOVA for (A), (B), and (D).
Pearson correlations examinations of plus maze and aggressive performance using either all mice combined or separate WT and KO groups revealed no significant correlations between any of the two measures (data not shown).
4. Discussion
In the present study, we show that KO mice missing CRFR1 exhibit normal levels of isolation induced intermale aggression. A significantly lower percentage of attacks to the ventral portion of the mid-section (including belly) of intruders by KO mice on test day 1 (but not test day 2) was the only difference noted between genotypes. The finding that KO mice show increased time on open arms and decreased time on closed arms of the elevated plus maze (consistent with previous published reports) suggest that whatever fear or anxiety pathways altered in KO mice do not influence resident-intruder intermale aggression. Thus, a dissociation of plus maze performance and this form of aggression is found. However, plus maze testing occurred following aggression tests and differences in stress reactivity to aggression testing between genotype could have affected anxiety performance. Use of other anxiety tests, especially those that themselves produce less stress on the subjects (e.g., open field test), could be performed prior to aggression testing as alternative approach to examining links between anxiety and aggression.
The results from this study are consistent with previous work in which we found that isolation-induced intermale aggression is not altered in CRFR2-deficient mice [13]. The simplest explanation for the normal knockout phenotype in this or the previous study, is that isolation-induced intermale aggression is not dependent upon CRFR1 or CRFR2. Earlier studies have indicated CRF can inhibit this form of aggression with icv injections [26] or elevate it when injected into the amygdala [10]. If CRFR1 activation is relatively low in these males, the lack of behavioral change with loss of a gene may simply reflect a floor effect. Under this scenario, other neuromodulators (besides those that activate CRFR1) would be involved in positively regulating aggression. At higher levels of activation, CRFR1 activity could still be involved in both positively and negatively regulating the behavior along an inverted U-shaped curve. This would explain why CRF can both inhibit and activate aggression levels depending on levels and sites of injection. Because CRF and its related peptides, Ucn 1 and 3, can activate CRFR2 [24,25,31,39], it is possible that increased activation of CRFR2 compensates for the loss of CRFR1. In the original examination of the KO mice, no differences in CRFR2 were observed [35]. However, a dynamic alteration in CRFR1 transcription and translation can occur following simple manipulations [1], so dynamic changes in expression of CRFR2 in lactating KO mice could also be expected. An examination of intermale aggression in double knockouts of both receptors would help examine how the receptors might work together to regulate aggression.
Besides isolation induced aggression (tested here), CRF is thought to regulate both conditioned defeat [21] and anxiety-induced aggression [36]. In the former case, a CRF receptor antagonist ameliorates conditioned defeat, so it would be interesting to see whether or how this form of aggression is altered in KO mice. For anxiety-induced aggression, it would also be interesting to see if this form of aggression was decreased in KO mice.
Previous work in squirrel monkeys found a CRF receptor antagonist decreased aggressive behaviors exhibited at a mirror stimulus [42]. Recent work in male hamsters found a CRFR1 antagonist, SSR125543A, administered orally lengthened latency to attack and also decreased lateral type attacks that can include a sideways approach to the intruder [11]. The only difference between genotype in terms of aggression involved the sites of attack, with KO mice attacking the ventral portion of the mid-section (including belly) less frequently on the first of two test days. Attacks to this region and also to the back/flank regions, especially in rats, have been termed offensive aggression, whereas attacks to the face/neck region have been termed defensive attacks [5]. Our finding that intermale aggression levels are not altered by genotype, but only shift in this one measure on one of two days, suggests that the loss of CRFR1 may have a subtle, but interesting effect on the final output of aggression. When the sisters of the males in this study were examined for maternal aggression, attacks to the ventral portion of the mid-section (including belly) were also reduced in KO mice, but overall levels of aggression did not differ (S.C. Gammie and S.A. Stevenson, unpublished observations). Hence, two studies now suggest that decreased attacks to this region occur with loss of CRFR1. Why attacks to the ventral portion of the mid-section (including belly) of the intruder would be specifically altered in KO mice is not known, but differences in stress reactivity or glucocorticoid levels in KO mice is one possibility (see below). Additional studies that employ site-directed injections of CRFR1 antagonists and a careful ethological examination of aggressive sites of attack would be one approach in determining whether or how CRFR1 may regulate final aggressive output.
CRFR1-deficient mice have a normal pattern of growth, but exhibit lower than normal levels of glucocorticoids relative to WT mice, although a significant increase in corticosterone in response to a stressor still occurs in KO mice [35]. Given differences in corticosterone between genotype, we cannot rule out a role for glucocorticoids in our findings. Interestingly, in hamsters higher attacks to the belly region occur with transitions to adulthood from puberty and exposure to stressors (or glucocorticoids) accelerates the production of attacks to the region [9,43,44]. Thus, lower attacks to the ventral portion of the mid-section (including belly) of the intruder by KO mice would be consistent with lower glucorticoid activity in these mice. However, decreased [16] or increased [2,17] levels of glucocorticoids can heightened intermale aggression in rats and mice, so it is not clear how altering glucocorticoids in the KO mice would affect aggressive output. Glucocorticoid replacement was unable to rescue anxiety measure differences between genotypes [35], so altering steroids in these mice may have little or no impact on aggression as well. Given the interesting effects of glucocorticoids on aggression, future studies that regulate corticosterone levels in these mice and then examine aggressive output could help clarify some of these issues.
A drawback of knock-out studies is that the deletion of a gene may have developmental or compensatory effects that are separable from the functional use of the protein product as adults [27]. Further, inbred mice are used as a background for most knockouts in mice and although these provide genetic consistency, some of these strains (e.g., C57) impart decreased performance in certain behaviors, including in intermale aggression, relative to outbred mice [30]. By crossing the deletion into an outbred stock, we were able to produce mice with hybrid vigor (50 % inbred: 50% outbred background). Because the genome of inbred mice has been reduced to single alleles, genetic interactions are decreased and it has been suggested that examinations of missing genes on a more variable, outbred background may be more relevant to understanding the role of genes in humans [37]. The outbred mice (hsd:ICR strain) used here had been selected for high maternal aggression for over 7 generations, but to date no evidence suggests alterations in isolation-induced intermale aggression due to selection (S.C. Gammie and S.A. Stevenson, unpublished observations). Because brothers (WT and KO) from the same heterozygote dams were tested, environmental variability was minimized. Thus, we were able to produce mice that exhibited high levels of intermale aggression which allowed for better examination of how loss of the gene affected behavior. The levels of aggression were robust in WT and KO mice and the finding of a jump in aggression from test day 1 to day 2 (Fig. 1) is consistent with other studies in intermale aggression showing the same pattern [7,8,33]. Given that genetic background can affect behavioral phenotype in knockout mice [37], it will be valuable in future studies to examine aggressive responding in CRFR1 mutant mice with different genetic backgrounds (including inbred and outbred strains).
Acknowledgments
This work was supported by National Institutes of Health Grant R01MH066086 to S.C.G. The authors wish to thank Emily Bethea, Kelly Clinkenbeard, Michael Foley, and Allen Irgens for technical assistance and Kate Skogen and Jeff Alexander for animal care.
References
- 1.Aguilera G, Nikodemova M, Wynn PC, Catt KJ. Corticotropin releasing hormone receptors: two decades later. Peptides. 2004;25:319–29. doi: 10.1016/j.peptides.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Al-Maliki S. Influences of stress-related hormones on a variety of attack behaviour in laboratory mice. In: McConnell P, editor. Adaptive Capabilities of the Nervous System. Vol. 53. Elsevier; Amsterdam: 1980. pp. 421–426. [DOI] [PubMed] [Google Scholar]
- 3.Bakshi VP, Smith-Roe S, Newman SM, Grigoriadis DE, Kalin NH. Reduction of stress-induced behavior by antagonism of corticotropin-releasing hormone 2 (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. J Neurosci. 2002;22:2926–35. doi: 10.1523/JNEUROSCI.22-07-02926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bittencourt JC, Sawchenko PE. Do centrally administered neuropeptides access cognate receptors?: an analysis in the central corticotropin-releasing factor system. J Neurosci. 2000;20:1142–56. doi: 10.1523/JNEUROSCI.20-03-01142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanchard RJ, Blanchard DC. The organization and modeling of animal aggression. In: Brain PF, Benton D, Sijthoff, Noordhoff, Alphen aan den Rijn, editors. The biology of aggression. 1981. pp. 529–561. [Google Scholar]
- 6.Brain P. Differentiating types of attack and defense in rodents. In: Brain P, Benton D, editors. Multidisciplinary approaches to aggression research. Elsevier; Amsterdam: 1981. pp. 53–78. [Google Scholar]
- 7.Burright RG, Freeman MJ, Donovick PJ. Repeated tests of intermale aggression in mice (Mus musculus) are influenced by housing and test conditions. J Comp Psychol. 1988;102:303–5. doi: 10.1037/0735-7036.102.4.303. [DOI] [PubMed] [Google Scholar]
- 8.Cairns RB, MacCombie DJ, Hood KE. A developmental-genetic analysis of aggressive behavior in mice: I. Behavioral outcomes J Comp Psychol. 1983;97:69–89. [PubMed] [Google Scholar]
- 9.Delville Y, David JT, Taravosh-Lahn K, Wommack JC. Stress and the development of agonistic behavior in golden hamsters. Horm Behav. 2003;44:263–70. doi: 10.1016/s0018-506x(03)00130-2. [DOI] [PubMed] [Google Scholar]
- 10.Elkabir DR, Wyatt ME, Vellucci SV, Herbert J. The effects of separate or combined infusions of corticotrophin- releasing factor and vasopressin either intraventricularly or into the amygdala on aggressive and investigative behaviour in the rat. Regul Pept. 1990;28:199–214. doi: 10.1016/0167-0115(90)90018-r. [DOI] [PubMed] [Google Scholar]
- 11.Farrokhi C, Blanchard DC, Griebel G, Yang M, Gonzales C, Markham C, Blanchard RJ. Effects of the CRF1 antagonist SSR125543A on aggressive behaviors in hamsters. Pharmacology Biochemistry and Behavior. 2004;77:465–9. doi: 10.1016/j.pbb.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Gammie SC, Hasen NS, Rhodes JS, Girard I, Garland T., Jr Predatory aggression, but not maternal or intermale aggression, is associated with high voluntary wheel-running behavior in mice. Horm Behav. 2003;44:209–21. doi: 10.1016/s0018-506x(03)00140-5. [DOI] [PubMed] [Google Scholar]
- 13.Gammie SC, Hasen NS, Stevenson SA, Bale TL, D’Anna KD. Elevated stress sensitivity in corticotropin-releasing factor receptor 2 deficient mice decreases maternal, but not intermale aggression. Behav Brain Res. 2005;160:169–77. doi: 10.1016/j.bbr.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 14.Gammie SC, Huang PL, Nelson RJ. Maternal aggression in endothelial nitric oxide synthase-deficient mice. Horm Behav. 2000;38:13–20. doi: 10.1006/hbeh.2000.1595. [DOI] [PubMed] [Google Scholar]
- 15.Gammie SC, Nelson RJ. Maternal aggression is reduced in neuronal nitric oxide synthase-deficient mice. J Neurosci. 1999;19:8027–8035. doi: 10.1523/JNEUROSCI.19-18-08027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halasz J, Liposits Z, Kruk MR, Haller J. Neural background of glucocorticoid dysfunction-induced abnormal aggression in rats: involvement of fear- and stress-related structures. Eur J Neurosci. 2002;15:561–9. doi: 10.1046/j.0953-816x.2001.01883.x. [DOI] [PubMed] [Google Scholar]
- 17.Haller J, Halasz J, Mikics E, Kruk MR, Makara GB. Ultradian corticosterone rhythm and the propensity to behave aggressively in male rats. J Neuroendocrinol. 2000;12:937–40. doi: 10.1046/j.1365-2826.2000.00568.x. [DOI] [PubMed] [Google Scholar]
- 18.Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF. The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. J Neurosci. 2002;22:1020–6. doi: 10.1523/JNEUROSCI.22-03-01020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM. Conditioned defeat in male and female Syrian hamsters. Horm Behav. 2003;44:293–9. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Hurst JL. Mating in free-living wild house mice (Mus-domesticus) J Zool. 1986;210:623–628. [Google Scholar]
- 21.Jasnow AM, Banks MC, Owens EC, Huhman KL. Differential effects of two corticotropin-releasing factor antagonists on conditioned defeat in male Syrian hamsters (Mesocricetus auratus) Brain Res. 1999;846:122. doi: 10.1016/s0006-8993(99)02007-7. [DOI] [PubMed] [Google Scholar]
- 22.Kishimoto T, Pearse RV, 2nd, Lin CR, Rosenfeld MG. A sauvagine/corticotropin-releasing factor receptor expressed in heart and skeletal muscle. Proc Natl Acad Sci U S A. 1995;92:1108–12. doi: 10.1073/pnas.92.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–52. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- 24.Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;98:7570–5. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, Vaughan J, Sawchenko PE, Vale WW. Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotrophin-releasing factor receptor expression. J Neurosci. 2002;22:991–1001. doi: 10.1523/JNEUROSCI.22-03-00991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mele A, Cabib S, Oliverio A, Melchiorri P, Puglisi-Allegra S. Effects of corticotropin releasing factor and sauvagine on social behavior of isolated mice. Peptides. 1987;8:935–8. doi: 10.1016/0196-9781(87)90083-0. [DOI] [PubMed] [Google Scholar]
- 27.Nelson RJ. The use of genetic “knockout” mice in behavioral endocrinology research. Horm Behav. 1997;31:188–96. doi: 10.1006/hbeh.1997.1381. [DOI] [PubMed] [Google Scholar]
- 28.Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL, Dawson TM, Snyder SH. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature. 1995;378:383–6. doi: 10.1038/378383a0. [DOI] [PubMed] [Google Scholar]
- 29.Parmigiani S, Ferrari PF, Palanza P. An evolutionary approach to behavioral pharmacology: using drugs to understand proximate and ultimate mechanisms of different forms of aggression in mice. Neurosci Biobehav Rev. 1998;23:143–53. doi: 10.1016/s0149-7634(98)00016-5. [DOI] [PubMed] [Google Scholar]
- 30.Parmigiani S, Palanza P, Rogers J, Ferrari PF. Selection, evolution of behavior and animal models in behavioral neuroscience. Neurosci Biobehav Rev. 1999;23:957–69. doi: 10.1016/s0149-7634(99)00029-9. [DOI] [PubMed] [Google Scholar]
- 31.Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001;98:2843–8. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robison CL, Meyerhoff JL, Saviolakis GA, Chen WK, Rice KC, Lumley LA. A CRH1 antagonist into the amygdala of mice prevents defeat-induced defensive behavior. Ann N Y Acad Sci. 2004;1032:324–7. doi: 10.1196/annals.1314.052. [DOI] [PubMed] [Google Scholar]
- 33.Ryan V, Wehmer F. Effect of postnatal litter size on adult aggression in the laboratory mouse. Dev Psychobiol. 1975;8:363–70. doi: 10.1002/dev.420080410. [DOI] [PubMed] [Google Scholar]
- 34.Smagin GN, Heinrichs SC, Dunn AJ. The role of CRH in behavioral responses to stress. Peptides. 2001;22:713–24. doi: 10.1016/s0196-9781(01)00384-9. [DOI] [PubMed] [Google Scholar]
- 35.Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- 36.Tazi A, Dantzer R, Le Moal M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist blocks stress-induced fighting in rats. Regul Pept. 1987;18:37–42. doi: 10.1016/0167-0115(87)90048-6. [DOI] [PubMed] [Google Scholar]
- 37.Toth M. 5-HT1A receptor knockout mouse as a genetic model of anxiety. Eur J Pharmacol. 2003;463:177–8. doi: 10.1016/s0014-2999(03)01280-9. [DOI] [PubMed] [Google Scholar]
- 38.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–7. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 39.Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–92. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- 40.Vom Saal FS, Howard LS. The regulation of infanticide and parental behavior: implications for reproductive success in male mice. Science. 1982;215:1270–2. doi: 10.1126/science.7058349. [DOI] [PubMed] [Google Scholar]
- 41.Weninger SC, Dunn AJ, Muglia LJ, Dikkes P, Miczek KA, Swiergiel AH, Berridge CW, Majzoub JA. Stress-induced behaviors require the corticotropin-releasing hormone (CRH) receptor, but not CRH. Proc Natl Acad Sci U S A. 1999;96:8283–8. doi: 10.1073/pnas.96.14.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winslow JT, Newman JD, Insel TR. CRH and alpha-helical-CRH modulate behavioral measures of arousal in monkeys. Pharmacology Biochemistry and Behavior. 1989;32:919–26. doi: 10.1016/0091-3057(89)90059-2. [DOI] [PubMed] [Google Scholar]
- 43.Wommack JC, Salinas A, Delville Y. Glucocorticoids and the development of agonistic behaviour during puberty in male golden hamsters. J Neuroendocrinol. 2005;17:781–7. doi: 10.1111/j.1365-2826.2005.01369.x. [DOI] [PubMed] [Google Scholar]
- 44.Wommack JC, Taravosh-Lahn K, David JT, Delville Y. Repeated exposure to social stress alters the development of agonistic behavior in male golden hamsters. Horm Behav. 2003;43:229–36. doi: 10.1016/s0018-506x(02)00029-6. [DOI] [PubMed] [Google Scholar]