Abstract
Nonmutated tissue differentiation antigens expressed by tumors are attractive targets for cancer immunotherapy, but the consequences of a highly effective antitumor immune response on self-tissue have not been fully characterized. We found that the infusion of ex vivo expanded adoptively transferred melanoma/melanocyte-specific CD8+ T cells that mediated robust tumor killing also induced autoimmune destruction of melanocytes in the eye. This severe autoimmunity was associated with the up-regulation of MHC class I molecules in the eye and high levels of IFN-γ derived from both adoptively transferred CD8+ T cells and host cells. Furthermore, ocular autoimmunity required the presence of the IFN-γ receptor on target tissues. Data compiled from >200 eyes and tumors in 10 independently performed experiments revealed a highly significant correlation (P < 0.0001) between the efficacy of tumor immunotherapy and the severity of ocular autoimmunity. Administration of high doses of steroids locally mitigated ocular autoimmunity without impairing the antitumor effect. These findings have particular importance for immunotherapies directed against self-antigens and highlight the need for targeting unique tumor antigens not expressed in critical tissues.
Keywords: cancer, immunotherapy, vaccine, adoptive cell transfer, IFN-γ
The identification of tumor-associated antigens (TAA) that can be specifically recognized by these T cells has led to the development of clinical trails aimed at eliciting antitumor immune responses. These TAA are generally categorized into unique/mutated, cancer testis, and nonmutated differentiation antigens. Although unique/mutated antigens may be the most specific targets for immunotherapy, their widespread usage has been limited, because they are generally not shared among patients, and previous detection methods have proven cumbersome (1). In contrast, differentiation antigens, in particular those specific to melanoma, are commonly shared between patients and are readily detectable (2). In addition, these melanocyte differentiation antigens (MDA) have been found to be targets of in vivo anticancer responses, and specific T cells can be easily isolated from both healthy donors and patients with cancer (3, 4). However, despite the ability to raise high levels of specific T cells (5, 6), the targeting of these differentiation antigens using vaccines alone has had only limited therapeutic success (7). The reasons for this lack of immunity are not well understood but may include limited access to cytokines, attenuated inflammatory stimuli, or the presence of regulatory cells, and has been reviewed extensively (8, 9).
The adoptive transfer of ex vivo expanded tumor infiltrating lymphocytes (TIL) after lymphodepletion has resulted in objective responses of ≈50% in patients with metastatic melanoma (10, 11). Nevertheless, the requirement for preexisting tumor-reactive T cells and the ability to expand them have limited the broad application of this therapy. Redirecting T cells against tumors using T cell antigen receptor (TCR)-based gene therapy may circumvent these limitations (12). Although TCR-based gene therapy might prove promising in targeting melanoma and may provide a gateway into nonmelanoma cancers, the relative importance of tumor target selection remains unclear.
Cancer immunotherapies targeting MDA, such as MART-1/Melan-A, gp100, and tyrosinase related protein-1, have been associated with the development of autoimmune vitiligo (13, 14). Here, we show that the administration of more potent antitumor regimens that include lymphodepletion before adoptive transfer of gp100-specific T cells along with a vaccine and high doses of IL-2 causes not only vitiligo but also autoimmunity destruction of melanocytes in the eye. These findings may have critical importance in the design of immunotherapies targeting nonmutated shared-tumor antigens.
Results
MDA-Specific CD8+ T Cells Trigger Ocular Autoimmunity.
Previously, we found that recombinant vaccinia virus (RVV) encoding the MDA TRP-1 could protect mice from tumor challenge and induce skin melanocyte disruption, vitiligo (13). However, these vaccines were unable to effectively treat established tumors in either mouse or human (7). Interestingly, although the eye contains melanocytes, we never observed any ocular autoimmune manifestations (13). We attributed these findings to nominal MHC expression and the immune privilege status of the eye (15).
Although vaccines were unable to treat established tumors, immunization with rVV encoding the MDA gp100 was capable of raising CD8+ gp100-specific T cells (16), which we used to clone a T cell receptor and develop the transgenic mouse termed pmel-1 (17). Subsequently, we found that recombinant poxviral immunization in conjunction with adoptive transfer of gp100-specific pmel-1 T cells and IL-2 could result in the regression of large, nonimmunogenic, established tumors and autoimmune depigmentation of the skin (vitiligo) (18). During this response, pmel-1 T cells were found in multiple tissues, including gp100+ (B16) and gp100− (MCA205) tumors (18). Despite this ubiquitous trafficking, we observed only pmel-1 IFN-γ release and tumor regression in B16 and not the MCA205 tumor. Interestingly, we also observed pmel-1 T cell infiltration in the immune privileged eye.
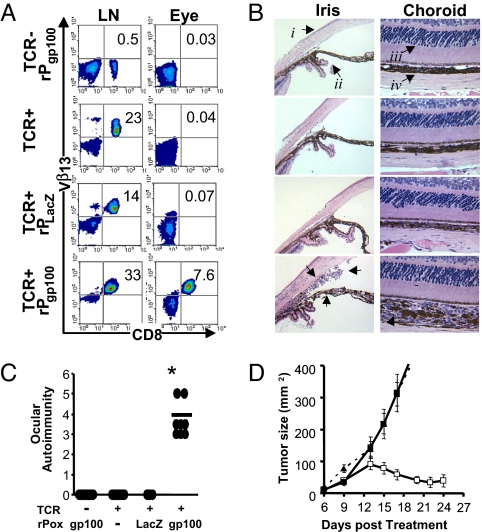
To explore the role of MDA-specific CD8+ T cells in ocular trafficking, autoimmunity, and tumor regression, TCR Tg mice were immunized with recombinant poxvirus encoding the relevant gp100 or irrelevant β-galactosidase antigen with concomitant IL-2. These mice were then evaluated for infiltration of pmel-1 T cells (Vβ13+ CD8+) and induction of immunity in the eye. We found that 7.6% of all cells in the eye were pmel-1 T cells after relevant antigen immunization, which was not observed in any other group despite the predominance of MDA-specific CD8+ T cells in the lymph node (>95% of all CD8+ T cells) (Fig. 1A). Histological examination of the eyes from these mice revealed a profound cellular infiltrate and considerable damage to the iris (iridiocyclitis), ciliary body, and choroid (choroiditis), with vitreous permeation (vitritis; data not shown) (Fig. 1B). Induction of ocular autoimmunity is illustrated using a masked scoring scheme described in the methods (Fig. 1C). Similar to eye autoimmunity, tumor regression was observed only in pmel-1 transgenic mice that were immunized with recombinant poxvirus encoding gp100 (Fig. 1D).
Fig. 1.
Induction of ocular autoimmunity during effective tumor destruction. (A) Flow cytometric analysis of Vβ13+CD8+ MDA-reactive pmel-1 T cells in the inguinal lymph node or eye 5 days after exogenous IL-2 and recombinant poxviral immunization of pmel-1 (TCR+) or WT (TCR−) mice. (B) Eyes from A were H&E-stained and examined for changes in morphology of the cornea (i), iris (ii), photoreceptors (iii), or choroid (iv). Arrows highlight cellular infiltrates in pmel-1 mice receiving recombinant gp100 poxvirus and IL-2. Data are representative of two independently identically performed experiments. (C) Ocular autoimmunity at day 5 after IL-2 and vaccination were assessed by using a masked ocular autoimmunity score as described in Methods from two independently performed experiments, *, P > 0.0001 vs. pmel-1 without vaccination. (D) Treatment of established tumors in pmel-1 transgenics vaccinated with exogenous IL-2 and recombinant gp100 poxvirus (□), without vaccination (■), or vaccinated WT littermates (▴), representative of two independent experiments.
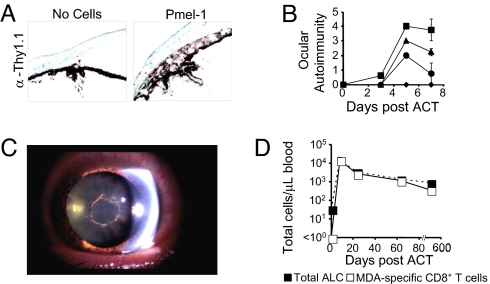
To further explore the role of MDA-specific CD8+ T cells in ocular and tumor immunity, we adoptively transferred congenic pmel-1 CD8+ Thy1.1+ T cells into tumor-bearing WT (Thy1.2+) recipients with relevant poxvirus. Flow cytometry and immunohistochemistry revealed the infiltration of actively dividing pmel-1 T cells [supporting information (SI) Fig. S1] localized in the eye (Fig. 2A). The transfer of increasing numbers of pmel-1 T cells augmented the severity of autoimmunity (P < 0.0001, 9 × 106 vs. 1 × 105). The first evidence of ocular autoimmunity was measured on day 3 and was fully evident on day 5 (Fig. 2B), lasting for at least 8 months after ACT (data not shown).
Fig. 2.
Ocular autoimmunity is associated with the presence of MDA-reactive CD8+ T cells in mice and humans. (A) Eyes from mice that received vaccination with or without the adoptive transfer of congenic pmel-1 Thy1.1+ T cells (P) were sectioned and stained with isotype (α IgG) or α Thy1.1 antibodies using immunohistochemistry. (B) Increase in ocular autoimmunity with escalating numbers of adoptively transferred pmel-1 at 0 (♦), 1 × 105 (●), 1 × 106 (▴), or 9 × 106 (■) T cells per mouse (n = 3 mice per group) with vaccination. (C) Anterior ocular inflammation in a metastatic melanoma patient after lymphodepletion then adoptive transfer of TIL and IL-2. (D) Flow cytometric analysis and enumeration of Mart-1/Melan-A tetramer positive CD8+ T cells from this patient's PBL after ACT.
Concurrently, several metastatic melanoma patients receiving adoptive immunotherapy of TIL and IL-2 after lymphodepleting conditioning developed ocular autoimmune manifestations (10). In one patient, we observed inflammation in the anterior chamber in both the left and right eyes that required steroid intervention for >30 months (Fig. 2C). In addition, this patient developed hair depigmentation (poliosis). Interestingly, MDA-specific Mart-1/Melan-A CD8 T cells were found in the peripheral blood lymphocytes (PBL) of this patient and accounted for >95% of all lymphocytes during the onset of the ocular autoimmune manifestations; this patient experienced a complete objective clinical response (Fig. 2D) (10). We found that administration of periocular steroids attenuated ocular autoimmunity in mice but did not impair the efficacy of tumor immunotherapy (Fig. S2).
Severe Autoimmunity Requires the Presence of IFN-γ Receptor on Target Tissues.
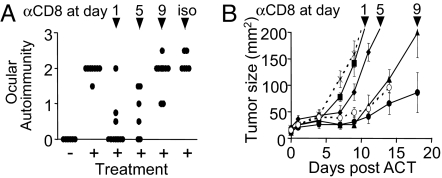
Although CD4+ T cells have been implicated in the induction of autoimmunity against naturally processed antigens in immune privileged sites such as the eye, the role of CD8+ T cells has not been well characterized. To evaluate the role of CD8+ T cells in self/tumor-specific immunity, CD8-depleting antibody was administered after the ACT pmel-1 T cells in Rag-1−/− mice. At early time points, depletion of CD8+ T cells abrogated both ocular and tumor immunity (Fig. 3 A and B). Removal of CD8+ T cells at later time points had less impact on immunity. Although these data demonstrate the role of MDA-reactive CD8+ T cells in the induction of immunity in the eye, it remained unclear how immune privilege was being abrogated in tissue that expresses low basal levels of MHC class I (15).
Fig. 3.
Ocular and tumor immunity is CD8+ T cell-dependent. (A) Evaluation of ocular autoimmunity 15 days after ACT of CD8+ enriched pmel-1 T cells into Rag1−/− T and B cell-deficient mice and CD8 depletion (100 μg per mouse) at times indicated. (B) Evaluation of tumor growth after CD8 depletion at days: 1 (■), 5 (♦), 9 (▴), isotype (○), no antibody (●), or no pmel-1 (X) (n = 5 mice per group). Data are representative of two independently performed experiments.
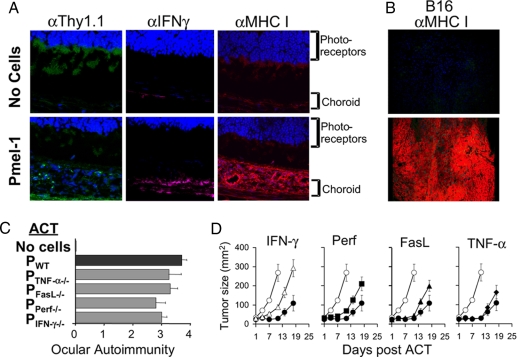
To evaluate the role of MHC class I in this CD8+ T cell-mediated immunity, we stained eyes from mice that received ACT of pmel-1 with antibodies against Thy1.1+ with the Thy1.1+, MHC class I, and an inducer of MHC, IFN-γ. We observed positive staining for Thy1.1, the presence of IFN-γ, and pronounced staining of MHC class I in the eyes of treated mice (Fig. 4A). In B16 melanoma, after ACT, there was marked expression of MHC class I, which was not observed without treatment (Fig. 4B). Not surprisingly, the removal of MHC class I or TAP in the eye (B6 bone marrow chimera) abrogated ocular immunity during a productive immunotherapy (Fig. S3).
Fig. 4.
Ocular and tumor immunity is associated with IFN-γ and the up-regulation of MHC class I. (A) In situ detection of pmel-1, IFN-γ, and MHC class I in the eye after ACT using confocal microscopy. (B) Detection of enhanced MHC class I expression in tumor 5 days after ACT of pmel-1 using confocal microscopy. Images are representative of multiple fields. (C) The induction of ocular autoimmunity was blindly evaluated 5 days after the adoptive transfer of WT, FasL, TNF-α, perforin or IFN-γ-deficient pmel-1 T cells in WT tumor-bearing mice. (D) Tumor growth was assessed after the ACT of pmel-1 WT T cells (●) or pmel-1 T cells deficient in IFN-γ (▴), perforin (▵), FasL (♦), TNF-α (■), or no cells (○). Data are representative of two independently performed experiments.
To further evaluate the role of IFN-γ and other known T cell effector molecules, we infused pmel-1 T cells deficient in either IFN-γ, perforin, FasL, or TNF-α into tumor-bearing mice (19). We observed that ACT of T cells deficient in IFN-γ or perforin resulted in a slight decrease in ocular and tumor immunity (Fig. 4 C and D) (P < 0.05, P > 0.01 vs. pmel-1 WT), whereas removal of FasL or TNF-α had little or no impairment to self/tumor immunity. It was surprising that the removal of T cell-derived IFN-γ had minimal detriment, considering there was a profound increase and dependence on MHC class I for the induction of immunity.
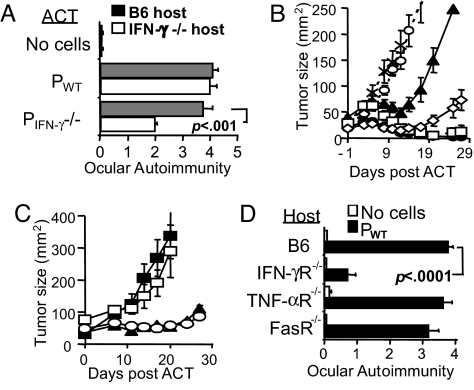
It is possible that endogenous sources of IFN-γ could be contributing to tumor and ocular immunity in the absence of effector T cell IFN-γ (20). To explore this possibility, we adoptively transferred IFN-γ-deficient pmel-1 T cells into IFN-γ−/− or WT B16-bearing hosts. ACT of pmel-1 IFN-γ−/− T cells into IFN-γ−/− hosts resulted in a significant decrease in ocular (Fig. 5A) and tumor immunity (Fig. 5B), whereas the transfer of pmel-1 IFN-γ−/− T cells into WT hosts or WT pmel-1 T cells into IFN-γ hosts resulted in only minimal detriment. Interestingly, the genetic absence of IFN-γ did not completely abrogate immunity; this might be due to enhanced effector cytokine production and proliferation observed in pmel-1 T cells deficient in IFN-γ (Fig. S4).
Fig. 5.
Ocular autoimmunity requires the presence of IFN-γ-receptor on host tissue. (A and B) Pmel-1 and host IFN-γ-dependent ocular and tumor immunity. (A) Assessment of ocular immunity after the ACT of pmel-1 WT or IFN-γ −/− T cells into WT or IFN-γ −/− recipients. (B) Tumor therapy after the ACT of pmel-1 WT into B6 (●) or IFN-γ− −/− (□) hosts, ACT of pmel-1 IFN-γ− −/− into B6 (◇) or IFN-γ −/− (▴) hosts, no cells B6 (X) or IFN-γ−/− (○) hosts. (C) Tumor therapy after the ACT of pmel-1 T cells and the administration IFN-γ neutralizing antibody (■), isotype (▴), PBS (○), or no cells (□). (D) Target tissue expression of IFN-γ receptor, but not TNF-α or Fas receptor, dictates immunity in the eye. Evaluation of ocular autoimmunity in B6 bone marrow reconstituted WT, IFN-γR, TNF-αR, or FasR-deficient chimeric recipients 5 days after ACT with pmel-1 (■) or no cells (□). Each group contains at least five mice per group, and data are representative of at least two independently performed experiments.
To obviate any potential compensation by pmel-1 IFN-γ−/− T cells, we administered IFN-γ neutralizing antibody after ACT. We observed complete abrogation of tumor immunity (Fig. 5C) and a significant reduction of ocular autoimmunity, P = 0.0002 (Fig. S5). The decrease of ocular autoimmunity after the administration IFN-γ neutralizing antibody was primarily due to abrogation of anterior, but not posterior, immunity, perhaps because of the inability of the antibody to penetrate the tight junctions of the choroid (15). In an effort to circumvent difficulties with antibody translocation and to better explore the role of effector molecules on target tissue, we generated lethally irradiated IFN-γ receptor (R), FasR-, or TNF-αR-deficient B6 reconstituted chimeras. Here, eye tissues of nonhematopoietic origin, e.g., melanocytes of the iris and choriod, lack the ability to receive signaling of these effector molecules, whereas signaling in cells of hematopoietic origin and transplanted tumor is preserved. Tumor regression was observed in all treated chimeras (data not shown). Interestingly, we found that ocular autoimmunity was significantly abrogated in treated IFN-γR-deficient chimeras, P < 0.0001 (Fig. 5D), but not in treated FasR or TNF-αR-deficient chimeras. Taken together, these data indicate that the induction of immunity in the eye depended on both adoptively transferred CD8+ T cell and IFN-γ from endogenous sources such as CD4+ T cells or natural killer cells. Moreover, ocular immunity absolutely required response through the IFN-γ receptor on eye target tissue of nonhematopoietic origin.
Increased Efficacy of Cancer Immunotherapy Aggravates Ocular Autoimmunity.
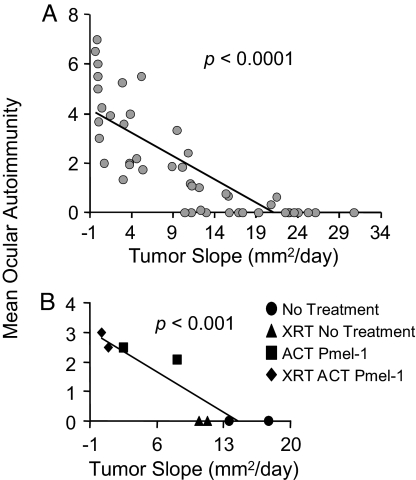
To further characterize the relationship between ocular and tumor immunity, we plotted the mean ocular autoimmunity score (day 5) vs. the tumor slope (mm2/day) of 46 different treatment groups from 10 independently performed experiments. This totaled >200 eyes and 200 tumor-bearing mice, with all experiments performed included at the time of analysis. Here, linear regression analysis demonstrated a significant correlation (P < 0.0001) between a successful tumor therapy and severity of ocular autoimmunity (Fig. 6A). Treatment groups that had rapidly growing tumors, ≈20 mm2/day, had no autoimmunity, whereas treatment groups with regressing tumors approximately −1 mm2 per day developed autoimmunity. Lymphodepletion before adoptive transfer of pmel-1 T cells augmented both tumor regression and the severity of ocular autoimmunity (P < 0.001) (Fig. 6B) (21). Thus, in the present model, the efficacies of the antitumor immune therapies were directly correlated with the induction of autoimmunity in the eye.
Fig. 6.
Improved tumor therapy correlates with the severity of ocular autoimmunity. (A) Mean ocular immunity score (day 5) vs. mean tumor slope for each group. Data are representative of 10 independent experiments, 46 treatment groups, 218 eyes, and >250 tumors. (B) Ablation augments ocular and tumor immunity. Pmel-1 ACT with (♦) or without (■) prior ablation or no pmel-1 with (▴) or without prior ablation (●). n = 56 mice, combination of two independent experiments.
Discussion
Melanocyte differentiation antigens have been used extensively as targets for immunotherapy, because they are generally shared among patients and MDA-specific T cells and have been found to be targets of immune responses. However, despite the ability to raise high levels of specific T cells (6), the targeting of these differentiation antigens has met only limited therapeutic success (7). Recently, adoptive transfer of ex vivo expanded tumor infiltrating lymphocyte has resulted in >50% objective clinical responses in patients with metastatic melanoma. In addition to vitiligo, we observed ocular autoimmunity in 14% of the patients (10). One patient who developed ocular autoimmunity had Mart-1/Melan-A tetramer-positive CD8+ T cells at >90% of all lymphocytes. In the murine setting, we unequivocally show that MDA-specific CD8+ T cells can mediate tumor regression and induce autoimmunity in the immune privileged eye. This finding is in contrast to previous work, where CD4+ T cells or macrophages have been reported to be principal mediators in the induction of ocular autoimmunity, whereas CD8+ T cells were described to have less of a role (15).
Interestingly, despite the predominance of MDA-specific T cells, unmanipulated pmel-1 transgenics do not develop spontaneous ocular autoimmunity or survive tumor challenge. These findings are recapitulated in humans, where patients experience tumor progression even in the presence of high levels of tumor-specific T cells (6). This lack of CD8+ T cell reactivity may be in part because of the relatively low MHC class I expression on melanin-containing tissues, poor antigen-MHC affinity (22, 23), antigen-presenting cell inactivation (15), or deficiencies in antigen processing (24). Other workers have found that this ignorant state can be reversed by the conditional up-regulation of MHC class I, resulting in autoimmune myositis (25). We observed a pronounced up-regulation of MHC class I in afflicted eyes and tumor after the ACT of pmel-1 T cells that was not observed in untreated mice.
Surprisingly, IFN-γ derived from adoptively transferred CD8+ T cells was by itself not required for the induction of either tumor or eye immunity, despite the dramatic up-regulation of MHC class I. Rather, immunity was found to be significantly impaired when both effector and host IFN-γ production were absent. The administration of IFN-neutralizing antibody abrogated both tumor and anterior ocular autoimmunity, negating any possible compensation from enhanced proliferation and cytokine release evidenced in IFN-γ-deficient pmel-1 T cells. Finally, ocular immunity was abolished in the absence of IFN-γ receptor on target tissue of nonhematopoietic origin. It was interesting that the removal of perforin, FasL, or TNF-α on the effector cells or removal of Fas or TNF-α receptor on the target tissue had no or only minimal detriment to immunity.
Our findings may have parallels with several ocular autoimmune diseases, such as Vogt–Koyanagi–Harada disease (26, 27). In this rare disease, patients develop immunity against melanocytes in the eye as well the skin and ear. MDA-specific CD8+ T cells can be found in the eyes of patients with this and other ocular autoimmune diseases against melanocytes (27). In our current study, we observed melanocyte destruction in the skin, although it was generally late onset (> 3 months) and erratic in distribution and thus was not compared with the induction of ocular or tumor immunity. Immunity in inner ear, which also contains melanocytes, was also not evaluated in our study.
Our data suggest that, as tumor immunotherapies improve, these autoimmune manifestations may become more prevalent. This is in concert with previous work where objective clinical responses and the induction of vitiligo were found to correlate in metastatic melanoma patients receiving high doses of IL-2 (28). In these studies, melanoma patients receiving high-dose IL-2 achieved objective responses of 15–20%, and autoimmunity against melanin-containing tissue was limited to vitiligo. Interestingly, melanoma patients in our current clinical trials receiving the ACT of TIL and lymphoconditioning achieve clinical responses of ≈50% and develop not only vitiligo but also autoimmunity in the immune-privileged melanin-containing eye (10).
New immunotherapies using TCR retroviral gene insertion allow us to redirect immune response against cancer (12, 20). As these therapies improve, either by increasing the avidity of the TCR (29) or by targeting prevalent antigens (30), we might observe an increase in these autoimmune manifestations. Indeed, recently, we have observed the induction of not only skin but also eye and ear immunity in patients receiving PBL transduced with highly avid TCRs against MDAs (unpublished observations). Although the autoimmune side effects of melanocyte/melanoma-targeted therapies have been manageable, the unintended autoimmunity of therapies targeting colorectal, brain, or lung cancer might prove more severe. Redirecting immune responses against cancer-testis or unique mutated tumor-specific antigens (3, 31–34), along with more advanced tumor screening methods, may prove critical as our immunotherapies advance (1, 35, 36).
Methods
Mice and Immunotherapy.
Pmel-1+CD45.1+, pmel-1+Thy1.1+, C57BL/6n, and Rag1−/− mice were bred and housed according to the guidelines of the Animal Care and Use Committee at National Institutes of Health. FasL−/−, TNF-α−/−, perforin−/−, and IFN-γ−/− mice were obtained from The Jackson Laboratory; Pmel-1 transgenics and crosses were confirmed by PCR analysis as described in ref. 18 and according to The Jackson Laboratory PCR protocols. For chimeras, β2m−/− (Taconic Farms), TAP1−/−, FasR−/−, TNF-αR1/2−/−, and IFN-γR−/− genotypes were confirmed by PCR (The Jackson Laboratory), irradiated with 10 Gy, injected i.v. with 5 × 106 C57BL/6 bone marrow, and used 12–16 weeks after transfer.
B16 (H-2b), a gp100+ spontaneous murine melanoma, was obtained from the National Cancer Institute tumor repository and maintained in media (18). Mice were implanted with B16 melanoma (18). At the time of ACT, mice (n ≥ 5 for all groups unless otherwise indicated) were injected i.v. with in vitro activated pmel-1 splenocytes (1–2 × 106 CD8+ Vβ13+ T cells), or CD8+ enriched where indicated (Miltenyi Biotec) and received 2 × 107 plaque-forming units of rVV or fowlpox encoding hgp100 or LacZ (Bernard Moss, National Institutes of Health). All mice were injected with rHIL-2 and lymphopenia-induced as described (18, 37). Where indicated, mice were injected i.p. with 100 μg of CD8-depleting antibody (53.6.7, BD PharMingen), 100 μg of anti-IFN-γ (XMG1.2), or 400 μg of periocular triamcinolone. Mice were randomized, and tumors were blindly measured by using digital calipers. The products of the perpendicular diameters are presented as mean ± SEM.
Immunofluoresence and Immunochemistry.
Mouse organs were harvested, homogenized, and labeled with mAbs (18). For in vivo proliferation, mice were injected i.p with 1–1.5 mg of 5-bromo-2-deoxyuridine (BrdU3; Sigma), killed, and organs homogenized, labeled, and analyzed (18). For ocular autoimmunity evaluation, eyes were enucleated 5 days after adoptive transfer unless otherwise indicated, fixed in 10% formalin, embedded in methylacrylate, sectioned via papillary-optic nerve axis, H&E-stained, and autoimmunity assessed in a masked fashion (by C.-C.C.). The ocular autoimmune score represents the sum of iridiocyclitis, choroiditis, and vitritus using the following scoring method: none = 0, mild = 1, moderate = 2, and severe = 3. Values are presented as mean ± SEM.
For immunohistochemistry and confocal microscopy, eyes were snap-frozen at −80°C and sectioned using a cryostat. Frozen sections were fixed in 4% formaldehyde in PBS, washed, and incubated with Biotin or FITC-conjugated anti-Thy1.1 10 μg/ml (BD PharMingen), PE-conjugated anti-IFN-γ 2 μg/ml (BD PharMingen), or biotin-conjugated H-2Db 10 μg/ml (eBiosciences). Biotin-stained samples were incubated with Cy5-conjugated streptavidin (Jackson ImmunoResearch). DAPI (Molecular Probes–Invitrogen) was used to stain cell nuclei. Images were obtained by using a Leica SP2 laser-scanning confocal microscope (Leica Microsystems) operated by the Biological Imaging Core, National Eye Institute, National Institutes of Health, Bethesda, MD.
Statistics.
Averages are presented as mean ± SEM. We performed factoral or repeated-measure ANOVA or linear regression analysis using the StatView software (SAS Institute), significance considered at P < 0.05.
Supplementary Material
Acknowledgments.
We thank Dr. Mercedes Campos, Katie Bergstrom, and Shirin Treadwell for expert technical assistance. This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. This study was performed in partial fulfillment of a Ph.D. in Biochemistry (to D.C.P.) at the George Washington University, Washington, DC.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710929105/DCSupplemental.
References
- 1.Kessler JH, Melief CJM. Identification of T-cell epitopes for cancer immunotherapy. Leukemia. 2007;21:1859–1874. doi: 10.1038/sj.leu.2404787. [DOI] [PubMed] [Google Scholar]
- 2.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 3.Ottaviani S, Zhang Y, Boon T, van der Bruggen P. A MAGE-1 antigenic peptide recognized by human cytolytic T lymphocytes on HLA-A2 tumor cells. Cancer Immunol Immunother. 2005;54:1214–1220. doi: 10.1007/s00262-005-0705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg SA, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lurquin C, et al. Contrasting frequencies of antitumor and anti-vaccine T cells in metastases of a melanoma patient vaccinated with a MAGE tumor antigen. J Exp Med. 2005;201:249–257. doi: 10.1084/jem.20041378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg SA, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–6176. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: Moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 9.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: Building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudley ME, et al. Adoptive cell transfer therapy after nonmyeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maine GN, Mule JJ. Making room for T cells. J Clin Invest. 2002;110:157–159. doi: 10.1172/JCI16166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan RA, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Overwijk WW, et al. Vaccination with a recombinant vaccinia virus encoding a “self” antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4(+) T lymphocytes. Proc Natl Acad Sci USA. 1999;96:2982–2987. doi: 10.1073/pnas.96.6.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yee C, et al. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of t cell-mediated vitiligo. J Exp Med. 2000;192:1637–1644. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- 16.Overwijk WW, et al. gp100/pmel 17 is a murine tumor rejection antigen: Induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Overwijk WW, et al. Tumor Regression and Autoimmunity after Reversal of a Functionally Tolerant State of Self-reactive CD8+ T Cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer DC, et al. Vaccine-stimulated, adoptively transferred CD8+ T cells traffic indiscriminately and ubiquitously while mediating specific tumor destruction. J Immunol. 2004;173:7209–7216. doi: 10.4049/jimmunol.173.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calzascia T, et al. TNF-alpha is critical for antitumor but not antiviral T cell immunity in mice. J Clin Invest. 2007;117:3833–3845. doi: 10.1172/JCI32567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smyth MJ, Cretney E, Kershaw MH, Hayakawa Y. Cytokines in cancer immunity and immunotherapy. Immunological Reviews. 2004;202:275–293. doi: 10.1111/j.0105-2896.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- 21.Paulos CM, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sette A, et al. The relationship between class I binding affinity and immunogenicity of potential cytotoxic T cell epitopes. J Immunol. 1994;153:5586–5592. [PubMed] [Google Scholar]
- 23.Peters B, Sette A. Integrating epitope data into the emerging web of biomedical knowledge resources. Nat Rev Immunol. 2007;7:485–490. doi: 10.1038/nri2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Restifo NP, et al. Identification of human cancers deficient in antigen processing. J Exp Med. 1993;177:265–272. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagaraju K, et al. Conditional up-regulation of MHC class I in skeletal muscle leads to self-sustaining autoimmune myositis and myositis-specific autoantibodies. Proc Natl Acad Sci USA. 2000;97:9209–9214. doi: 10.1073/pnas.97.16.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawakami Y, et al. T cell immune responses against melanoma and melanocytes in cancer and autoimmunity. Pigment Cell Research. 2000;13:163–169. doi: 10.1034/j.1600-0749.13.s8.29.x. [DOI] [PubMed] [Google Scholar]
- 27.Sugita S, Sagawa K, Mochizuki M, Shichijo S, Itoh K. Melanocyte lysis by cytotoxic T lymphocytes recognizing the MART-1 melanoma antigen in HLA-A2 patients with Vogt-Koyanagi-Harada disease. Int Immunol. 1996;8:799–803. doi: 10.1093/intimm/8.5.799. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg SA, White DE. Vitiligo in patients with melanoma: Normal tissue antigens can be targets for cancer immunotherapy. J Immunother Emphasis Tumor Immunol. 1996;19:81–84. [PubMed] [Google Scholar]
- 29.Gronski MA, et al. TCR affinity and negative regulation limit autoimmunity. Nat Med. 2004;10:1234–1239. doi: 10.1038/nm1114. [DOI] [PubMed] [Google Scholar]
- 30.Steinman RM. Dendritic cells: Versatile controllers of the immune system. Nat Med. 2007;13:1155–1159. doi: 10.1038/nm1643. [DOI] [PubMed] [Google Scholar]
- 31.Chen YT, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci USA. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jungbluth AA, et al. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer. 2001;92:856–860. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
- 33.Charalambous A, Oks M, Nchinda G, Yamazaki S, Steinman RM. Dendritic cell targeting of survivin protein in a xenogeneic form elicits strong CD4+ T cell immunity to mouse survivin. J Immunol. 2006;177:8410–8421. doi: 10.4049/jimmunol.177.12.8410. [DOI] [PubMed] [Google Scholar]
- 34.Loriot A, Boon T, De Smet C. Five new human cancer-germline genes identified among 12 genes expressed in spermatogonia. Int J Cancer. 2003;105:371–376. doi: 10.1002/ijc.11104. [DOI] [PubMed] [Google Scholar]
- 35.Coukos G, Conejo-Garcia JR, Roden RB, Wu TC. Immunotherapy for gynaecological malignancies. Expert Opin Biol Ther. 2005;5:1193–1210. doi: 10.1517/14712598.5.9.1193. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, et al. Integrative genomic analysis of phosphatidylinositol 3′-kinase family identifies PIK3R3 as a potential therapeutic target in epithelial ovarian cancer. Clin Cancer Res. 2007;13:5314–5321. doi: 10.1158/1078-0432.CCR-06-2660. [DOI] [PubMed] [Google Scholar]
- 37.Gattinoni L, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.