Abstract
The modifiability of neuronal response plasticity is called “metaplasticity.” In suppressing synaptic inhibition and facilitating induction of long-term excitatory synaptic plasticity, endocannabinoids (eCBs) act as agents of metaplasticity. We now report the discovery of a calcium-dependent mechanism that regulates eCB mobilization by metabotropic glutamate receptor (mGluR) activation. The switch-like mechanism primes cells to release eCBs and requires a transient rise in intracellular Ca2+ concentration ([Ca2+]i) but not concurrent activation of mGluRs. Conversely, short-term, [Ca2+]i- dependent eCB release can be persistently enhanced by mGluR activation. Hence, eCBs are also objects of metaplasticity, subject to higher levels of physiological control.
Keywords: 2-arachidonyl glycerol, calcium, GABA, metabotropic glutamate, muscarinic
Endocannabinoids (eCBs) are important signaling molecules that modulate synaptic strength throughout the CNS (for reviews, see refs. 1–4). They control both short- and long-term forms of synaptic plasticity and are implicated in many animal behaviors. eCBs, which are fatty acid derivatives, are synthesized directly by enzymatic action on cellular plasma membrane phospholipids. Because they are not stored before use, eCBs are said to be available “on-demand.” Although the first identified eCB was anandamide, 2-arachidonyl glycerol (2-AG) is probably the principal ligand for cannabinoid receptors (CB1Rs) in much of the brain (5, 6). 2-AG synthesis is thought to proceed via phospholipase C (PLC) and the action of diacylglycerol lipase (DGL) on the PLC product, diacyglycerol (DAG). eCBs are retrograde messengers, and their release from postsynaptic cells may also be regulated. Physiological techniques cannot distinguish among synthesis, release, and transport processes, and we use the term “mobilization” to encompass all steps between stimulation of the eCB system and activation of CB1R. eCB mobilization occurs after either a rise in the intracellular Ca2+ concentration ([Ca2+]i) (7–9) or activation of G protein-coupled receptors (GPCRs), including dopaminergic (10, 11), metabotropic glutamatergic (12, 13), and muscarinic cholinergic (14) receptors.
In the hippocampus, CB1R is present in high concentrations on the synaptic terminals of certain GABAergic interneurons (15). Ca2+-dependent eCB mobilization transiently suppresses CA1 pyramidal cell evoked inhibitory postsynaptic currents (eIPSCs) (7, 8), a retrograde signal process called DSI (1). Group I metabotropic glutamate receptor (mGluR) activation potently stimulates eCB mobilization, thereby depressing eIPSCs (12, 13) or excitatory postsynaptic currents (EPSCs) for short (seconds to minutes) or long (minutes to hours) (16, 17) periods of time, depending on the stimulation conditions.
Various stimuli use different biochemical pathways for eCB mobilization, which can be designated eCBmGluR, eCBmAChR, and eCBCa. Pharmacological inhibitors of PLC abolish hippocampal long-term IPSC suppression (eCB-iLTD) initiated by group I mGluRs without affecting DSI (17, 18). PLCβ1 knockout mice lack both eCBmGluR and eCBmAChR, whereas DSI remains normal (19). Hashimotodani et al. (19) showed that GPCR-triggered eCB mobilization had a dose-dependent reliance on [Ca2+]i, and they proposed that PLCβ1 is a “coincidence detector” through which a simultaneous rise in [Ca2+]i and GPCR activation can initiate eCB mobilization. This model implies that the demand for eCBs is set by the degree of mGluR and [Ca2+]i coactivation. Nevertheless, fundamental questions about the regulation of eCBs remain unanswered, and it is not clear whether a coincidence detector is the only control mechanism.
We now report the discovery of a mode of regulation of eCBmGluR mobilization. We find that the mGluR–eCB pathway is subject to “priming” by a brief bolus rise in [Ca2+]i, that is, an initially ineffective treatment with an mGluR agonist becomes effective in mobilizing eCBs after an influx of Ca2+. The relationship between Ca2+ and mGluRs is reciprocal; i.e., prior activation of mGluRs enhances Ca2+-dependent eCB mobilization (eCBCa; i.e., DSI). Tests of the coincidence detection model revealed that it cannot account for priming. We conclude that there must be a higher level of control over eCBmGluR that will strongly influence eCB-dependent synaptic plasticity.
Results
Priming of mGluR-Mediated eCB Mobilization.
A simple on-demand model of eCB mobilization predicts that presentation of an appropriate stimulus will mobilize eCBs. However, if CA1 pyramidal cells had not first been tested for DSI, then the mGluR agonist dihydroxyphenylglycol (DHPG) did not elicit substantial eCBmGluR (i.e., eIPSC suppression ≥10%) in approximately half of the tested cells (39 of 77; e.g., Fig. 1a). The DHPG concentration was either 10 μM (n = 16 cells) or 50 μM (n = 61 cells). We considered three explanations for this surprising result: the cells could not mobilize eCBs, the mGluR-coupled eCB pathways were inoperative, or the interneuron terminals lacked CB1Rs. To distinguish among them, we gave a DSI trial to pyramidal cells that had not generated eCBmGluR and found that DSI was readily induced (Fig. 1a). This finding rules out two possibilities by showing that the pyramidal cells could mobilize eCBs and that CB1Rs were present. The deficiency appeared to be in the mGluR pathway.
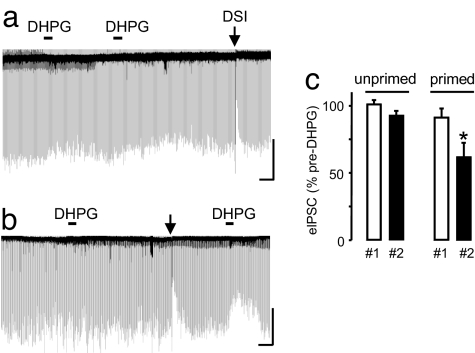
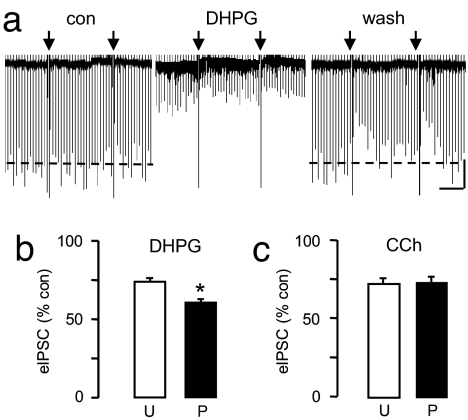
Fig. 1.
DSI primes mGluR-initiated eCB suppression of eIPSCs (eCBmGluR) in CA1 pyramidal cells. (a) DHPG (50 μM) was bath-applied to naïve cells (not previously tested for DSI) for 1 min, washed out, and reapplied for 1 min. eIPSCs (downward deflections, 10 μM NBQX and 50 μM AP-5 present), were only clearly suppressed by the DSI trial (1-s voltage step to 0 mV from −70 mV indicated by downward arrows in all figures), confirming the sensitivity of the cell to eCB responses. (Scale bars: 1.5 min/500 pA.) (b) DSI was induced between two DHPG applications; the second application markedly suppressed the eIPSCs. (Scale bars: 2 min/600 pA.) (c) Group data for a and b. eIPSCs were 100.8 ± 3.5% of control and 92.4 ± 3.17% of control for first and second DHPG applications in unprimed cells (n = 4). eIPSCs were 91 ± 7.5% of control and 61.8 ± 9.8% of control for first and second DHPG applications in primed cells (n = 4, P < 0.01).
The inability of high concentrations of mGluR agonists to trigger eCB mobilization has not been reported. In previous reports, cells were first tested for DSI before application of mGluR agonists, which was not done in the experiments of Fig. 1. To determine whether a preceding DSI trial affected subsequent eCBmGluR responses, we tested two groups of cells that were essentially unresponsive to the initial DHPG application. After washout of DHPG, only one group received a single DSI trial before a second DHPG application. The DSI trial (e.g., Fig. 1 b and c) invariably enabled the second DHPG application to trigger larger eCBmGluR. Initially unresponsive cells that did not receive an intervening DSI trial remained unresponsive to a second application (Fig. 1 a and c). Hence, neither repeated DHPG applications nor the passage of time led to eCBmGluR enhancement. The DSI trial seemed to be responsible. We refer to the enhancing effect of the trial on eCBmGluR as priming. In the presence of the CB1R antagonist SR141716A, eIPSCs were depressed to 93.8 ± 2.23% pre-“DSI trial” (SR141716A blocked DSI) and to 99.1 ± 4.46% afterward, n = 6, indicating that the DHPG-induced suppression is an eCB-mediated process. Priming of eCBmGluR did not depend on a high concentration of DHPG because it occurred when 10 μM DHPG was used [supporting information (SI) Fig. S1a]. Thus, 50 μM DHPG provides a stringent test for priming of eCBmGluR and was used unless otherwise noted.
There is a low level of CB1Rs on hippocampal glutamatergic terminals (20), and eCBs slightly affect excitatory transmission (21). After inhibiting GABAA (20 μM bicuculline) and GABABRs (50 μM CGP 35348) and removing the AMPA receptor antagonist 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline (NBQX), we tested whether priming would affect CB1R-mediated EPSC suppression. DHPG depressed the isolated EPSCs to 77.1 ± 4.95% of baseline before a 5-s depolarization-induced suppression (DSE)-inducing voltage step and to 76.9 ± 4.91% after it [not significant (NS), n = 12, data not shown]. Priming may be selective for inhibitory synapses.
Priming Is [Ca2+]i-Dependent but Does Not Require Concurrent mGluR Activation.
DSI is triggered by a rise in [Ca2+]i, and if mGluRs are simultaneously activated, DSI is enhanced (13), and eCBmGluR increases (19). The binding of both Ca2+ and the G protein products of mGluR to PLCβ increases its activity, thereby enabling it to detect the coincidence of the two stimuli. A key feature of the coincidence detector is the narrow time window over which it operates: in CA1 pyramidal cells PLCβ1 only integrates the signals for ≈2 min after a 5-s depolarizing voltage step (19). To test the hypothesis that the coincidence detection mechanism accounts for eCBmGluR priming, we measured the temporal window of the priming process by giving a DSI step after an ineffectual DHPG application and reapplying DHPG 3–23 min later (mean 11.5 ± 2.74 min, n = 7). The response to the second DHPG application was increased in every case (Fig. 2 a–c). The finding that the t½ for [Ca2+]i decay after a DSI step is <40 s (14) implies that [Ca2+]i elevation and mGluR activation need not overlap.
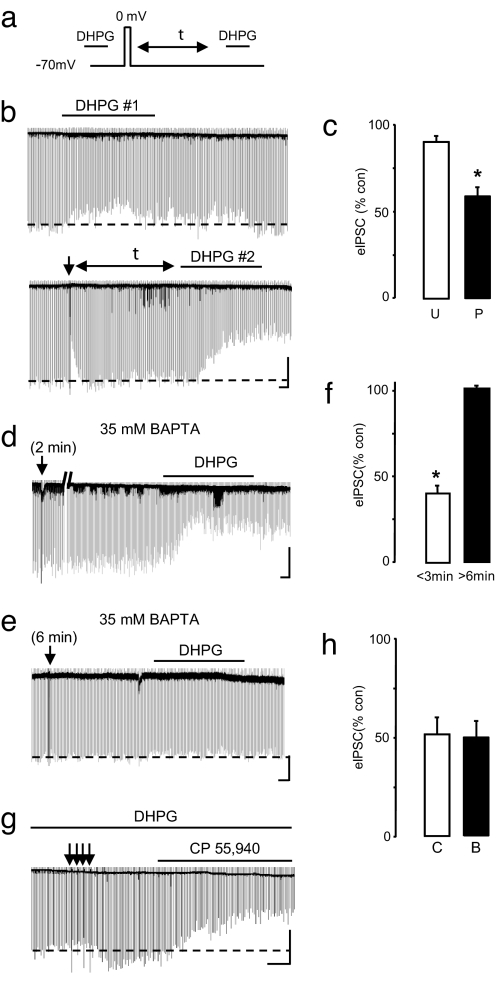
Fig. 2.
Priming of eCBmGluR is Ca2+-dependent but does not require concurrent rise in [Ca2+]i and mGluR activation. (a) Experimental protocol to test the coincidence detection hypothesis. DHPG was added to the bath for 10 min and washed out for 20 min. DSI was then induced, and DHPG was reapplied at different intervals (Δt) after DSI. (b) A continuous trace from a single cell showing eIPSCs before (Upper) and after (Lower) priming of eCBmGluR. (Scale bars: 45 s/400 pA.) (c) Group data for b. eIPSCs from unprimed (U) cells during DHPG application are 90.4 ± 2.79% of control (n = 7). eIPSCs in primed (P) cells during DHPG application (mean Δt of 11.5 ± 2.74 min), measured 59.3 ± 5.97% of control (P < 0.001). (d and e) The pipettes contained 35 mM BAPTA. (d) A DSI trial <3 min after whole-cell break-in resulted in priming; eIPSCs were reduced by the second DHPG application to 40 ± 5% of control (n = 4; P < 0.01). (e) DSI trial at >6 min after whole-cell break-in did not prime the cell; eIPSCs were 101 ± 1% of control (n = 7). (Scale bars: 50 s/350 pA.) (f) Group data for d and e. (g and h) Elimination eCBmGluR priming by BAPTA (35 mM) loading of cells did not prevent activation of CB1R by the CB1R agonist CP 55,940 (1 μM). In control cells (C), eIPSCs were reduced to 51.9 ± 9.19% of baseline (n = 5). In BAPTA-loaded cells (B), eIPSCs were reduced to 50.4 ± 7.76% of baseline (n = 4).
Although these results appeared incompatible with coincidence detection, the relevant Ca2+ for DSI induction might not have been detected by bulk [Ca2+]i measurements. Therefore, as another test, we included 35 mM 1,2-bis(2-aminophenoxy) ethane-N,N,N′,N′-tetraacetate (BAPTA) in the whole-cell pipette and delivered a DSI step at various times after break-in. If given <3 min after break-in (mean 1.95 min; n = 4), i.e., before internal equilibration of BAPTA, the DSI step primed eCBmGluR, even when DHPG was not bath-applied until 20 min later (Fig. 2 d and f). During this long interval, BAPTA will have equilibrated in the cell and chelated excess Ca2+; therefore, this result confirms that coincidence between a [Ca2+]i rise and mGluR activation is not required for priming eCBmGluR. However, if the DSI step was given >6 min after break-in (mean 8.06 min, n = 12), when there was higher and more uniform [BAPTA] in the cell, then neither DSI nor eCBmGluR could be elicited (Fig. 2 e and f). Hence, priming is Ca2+-dependent, but the primed state endures long after [Ca2+]i has returned to baseline.
Priming Affects eCB Mobilization, Not Activation of CB1R.
Priming could either enhance pyramidal cell generation of eCBmGluR or the responsiveness of the presynaptic CB1R. In the latter case, it would be impossible to activate CB1R in completely unprimed cells. To test this prediction, we again loaded cells with 35 mM BAPTA and did not stimulate them until >10 min after break-in to ensure that they remained unprimed. Neither DSI nor eCBmGluR could be elicited in these cells, confirming that they were loaded with BAPTA. However, the CB1R agonist CP 55,940 (1 μM) suppressed eIPSCs in unprimed cells to the same extent as in primed cells (Fig. 2 g and h), indicating that priming does not up-regulate CB1R. We conclude that some step or sequence of steps in the eCB mobilization process is the target of the priming process.
Accumulating evidence suggests that 2-AG, and not anandamide (AEA), is the active eCB in hippocampus; nevertheless, AEA could conceivably have a role in priming. The degradative enzyme for AEA, fatty-acid amide hydrolase (FAAH), can be inhibited by the FAAH inhibitor URB-597 (1 μM) (22) to test for the influence of AEA. In cells treated with URB-597, DHPG initially suppressed eIPSC to 85.8 ± 7.01% of baseline before a DSI trial, and to 50.1 ± 10.7% afterward (P < 0.05, n = 4, Fig. S1b). Priming evidently does not involve AEA.
eCB-iLTD Can Be Primed.
CB1R-dependent iLTD induction (17) requires mGluR activation lasting ≈10 min, or high-frequency synaptic stimulation (HFS), suggesting that different mechanisms are involved in iLTD than in transient eCBmGluR mobilization. In naïve cells that were unresponsive to a 10-min DHPG application, a DSI priming step enabled a second DHPG exposure to elicit prominent eCBmGluR and eCB-iLTD (Fig. 3 a and b). Without a prior DSI trial, a second DHPG application remained ineffective (n = 7). We also found that synaptic HFS had only slight and transient suppressive effects on eIPSCs in naïve cells, whereas after priming, HFS produced robust iLTD (Fig. 3 c and d).
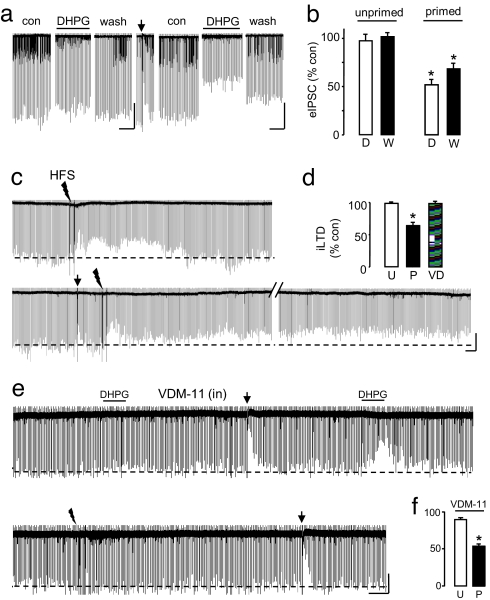
Fig. 3.
eCB-iLTD can be primed. (a) A 10-min DHPG (50 μM) treatment of a naïve cell caused minimal change in eIPSC amplitude and no iLTD. (Scale bars: 1 min/200 pA.) After a DSI trial, the cell displayed robust eIPSC suppression, and iLTD was present after a 20-min washout of DHPG. (Scale bars: 1 min/300 pA.) (b) Group data for a. eIPSCs were 97.5 ± 6.72% of baseline, and at 20 min after washout, 101.8 ± 3.68% of control in unprimed cells (n = M). Five of these cells were then given a DSI pulse, and the experiment was repeated. During the primed application period, DHPG reduced eIPSCs to 51.9 ±4.73% of control and iLTD was present after 20 min, with eIPSCs at 68.6 ± 5.30% of control (n = 5, P < 0.01). (c) Example showing eIPSC return to baseline 20 min after HFS (jagged arrow; 2 × 100-Hz trains of 0.1-ms stimuli separated by a 20-s interval, delivered to stratum radiatum) in a naïve cell, but after DSI, HFS induced iLTD. (Scale bars: 50 s/250 pA.) (d) Group data for c. eIPSCs after unprimed HFS measured 98.6 ± 0.71% of control (n = 4) and eIPSCs after primed HFS were 64.0 ± 5.72% of control (n = 4; P < 0.01). (e) Example of priming in a cell loaded with 10 μM VDM-11 via the recording pipette. DSI was not affected by VDM-11, and the second DHPG application produced a much larger response than the first. HFS did not induce iLTD in VDM-11-loaded cells (99.5 ± 2.9% of baseline, n = 6); bar labeled VD in d above. (Scale bars: 1min/300 pA.) (f) Group data for priming of DHPG effects on eIPSCs in VDM-11-loaded cells. eIPSCs were suppressed to 89.2 ± 1.97% of baseline by DHPG before DSI, and to 53.8 ± 2.65% afterward (P < 0.05, n = 4).
Priming Is Not Blocked by Inhibition of eCB Transport, PLC, or DGL.
The eCB transporter inhibitor VDM-11 prevents exogenous eCBs loaded into cells from exiting them, and it prevents induction of mGluR-dependent iLTD (23). To determine whether the transport process might be the target of priming, we loaded pyramidal cells with 10 μM VDM-11, but we found that priming of DHPG responses could still be elicited (Fig. 3 e and f). Intracellular VDM-11 prevented the induction of hippocampal HFS-iLTD (e.g., Fig. 3e; group data in Fig. 3d), confirming the efficacy of VDM-11.
The experiments of Fig. 2 suggest that a [Ca2+]i-dependent step or steps in the eCB mobilization process could be involved in priming. The most likely candidates would be PLCβ and DGL, which are [Ca2+]i-dependent enzymes activated by group I mGluRs and involved in 2-AG synthesis (6). In tissue-cultured hippocampal cells, extracellular application of the PLC antagonist U73122 abolishes the ability of mGluR or mAChR agonists to activate PLCβ1 (19); and in acute slices, U73122 abolishes eCBmGluR iLTD (17, 18). However, we found that neither intra- nor extracellular application of U73122 inhibited eCBmGluR and that the DGL inhibitor RHC 80267 (60 μM) did not block eCBmGluR (18). To try to ensure that the cells had not been primed before these tests, we incubated slices for >1 h before and after transferring them to the recording chamber with 6 μM U73122 or 60 μM RHC 80267, and we included the drugs in the patch pipette as well. Nevertheless, these treatments did not prevent priming (Fig. S2).
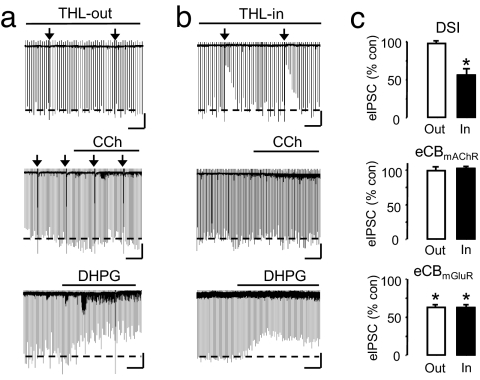
RHC 80267 is a comparatively weak DGL inhibitor in biochemical assays whereas tetrahydrolipstatin, tetrahydrolipstatin (THL; orlistat), is more potent (24), and it inhibits some eCB responses (18, 25–27). We found that extracellular application of 10 μM THL reduced DSI and virtually abolished the carbachol (CCh)-induced eCBmAChR response without preventing eCBmGluR priming (Fig. 4 a and c). Interestingly, internal THL, also 10 μM, blocked eCBmAChR but not DSI or eCBmGluR; Fig. 4 b and c and previous evidence (18) suggest that the three eCB pathways are not identical. Because THL antagonizes lipase activities downstream of Ca2+ (24), these experiments are compatible with the concept that a rise in [Ca2+]i primes cells independently of DGL. Moreover, the priming mechanism appears to be distinct from the Ca2+-dependent and eCBmAChR pathways.
Fig. 4.
Priming does not depend on DGL activation. (a) Bath application (10 μM THL-out) of THL (DGL inhibitor; Sigma) blocks DSI (97.5 ± 1.26% of baseline, NS, n = 8) and eCBmAChR (1 μM CCh) (eIPSCs were 99.1 ± 5.23% of baseline with THL-out, NS, n = 6). (Scale bars: 1 min/250 pA.) THL-out does not prevent eCBmGluR. DHPG (50 μM) given after DSI trials (data not shown) reduced eIPSCs to 63.0 ± 3.71% of baseline with THL-out (n = 4, P < 0.01). (Scale bars: 1 min/200 pA.) (b) Intracellular infusion of 10 μM THL (THL-in) did not prevent DSI (Top Center) (eIPSCs reduced to 55.9 ± 6.97% of baseline, n = 3, P < 0.05) or eCBmGluR (Bottom Center) (eIPSCs reduced to 62.6 ± 3.77% of baseline, NS from eCBmGluR with THL-out, n = 3, P > 0.05), but it did prevent eCBmAChR (Middle Center) (eIPSCs were 102.4 ± 1.02% of baseline, n = 3, NS, P > 0.1). (Scale bars: 30 s/250 pA.) (c) Mean group data for the experiments in each row.
eCBmAChR Does Not Require Priming.
Priming could still involve steps upstream of PLC and DGL that are common to both eCBmGluR and eCBmAChR. In this case, the eCBmAChR responses would also require priming. We tested the eCBmAChR responses with the same protocol that was used for priming of the eCBmGluR pathway: a 1-min application of 0.2 μM CCh followed by a single DSI trial and a second application of CCh. The eIPSCs were suppressed to 83.5 ± 1.85% of control before DSI and to 74.6 ± 7.05% of control afterward (NS, P > 0.2, paired t test, n = 6, data not shown). Hence, eCBmAChR was not subject to priming.
Priming of DSI by mGluR.
If priming represents a specific interaction between Ca2+ and the eCBmGluR pathways, then they might interact reciprocally in the priming process. This model predicts that prior mGluR activation would persistently potentiate DSI. To test this idea, we administered three DSI trials in cells (n = 10) and then bath-applied 50 μM DHPG for <5 min to avoid inducing iLTD. We adjusted the voltage step to produce moderate DSI (≤40 reduction in eIPSC) so that an enhancement could be detected. DHPG was then washed out of the bath, while the mGluR antagonists, LY367385 (100 μM) and 2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP) (10 μM) were applied to halt the action of DHPG. DSI was evoked every 1.5 min throughout. Within 10 min of the start of DHPG washout, the eIPSCs had recovered to control levels; however, we continued washing for 10 more min. At this point, DSI was still significantly increased (Fig. 5 a and b). Control DSI reduced eIPSCs to 74.0 ± 2.21% of baseline, whereas post-DHPG DSI reduced them to 60.5 ± 1.98% of baseline (P < 0.001; paired t test, n = 9). Hence, there could be a reciprocal relationship between the eCBmGluR and eCBCa pathways via the priming pathway. Another prediction is that eCBmAChR, which does not require priming, would not cause lasting enhancement of DSI. Indeed, the effects of a 4-min application of 1 μM CCh, which strongly suppressed eIPSCs and enhanced DSI, were completely reversed when CCh was washed from the chamber (and the mAChR antagonist, atropine, 1 μM, was added; Fig. 5c). Control DSI reduced the mean eIPSCs to 72.0 ± 3.56% of baseline; post-CCh DSI reduced eIPSCs to 73.9 ± 3.86% of baseline (NS, P > 0.5, paired t test, n = 7).
Fig. 5.
Priming of DSI by prior activation of mGluR. (a) Example in which minimal DSI was evoked at 90-s intervals; 50 μM DHPG was then applied for 4 min and washed out while 100 μM LY367385 and 10 μM MPEP were washed in. The right portion of the trace was taken 20 min later. (Scale bars: 1 min/200 pA.) (b) Group data, n = 9; P < 0.001. (c) Group data showing that 1 μM CCh, applied for 4 min, did not produce comparable lasting enhancement of DSI after being washed out for 20 min while1 μM atropine, was washed in (NS, n = 7).
Discussion
This article reports a powerful mechanism for regulating eCB mobilization. We show that eCBmGluR was controlled by a Ca2+-dependent priming process that was turned on quickly and remained active for long periods. Sensitivity to pharmacological antagonists and intracellular Ca2+ chelators, as well as timing requirements, distinguished the priming mechanism from the usual model of 2-AG synthesis and release (6) and from the PLC coincidence detection mechanism (19). Priming was not caused by up-regulation of CB1R; rather, it affected the coupling between mGluR activation and eCB mobilization. Both short-term and long-term eCBmGluR responses could be primed. Primed cells were more susceptible to iLTD induction by physiological stimuli and are therefore more likely to undergo long-term potentiation (LTP) (17).
We also demonstrated a LTP of DSI caused by prior mGluR, but not mAChR, activation. By suppressing IPSP/Cs, DSI can facilitate LTP expression (28). Thus, DSI potentiation is another means by which endocannabinoids can take part in metaplasticity. A recent report (29) shows potentiation of DSI by low-frequency field potential stimulation, perhaps representing synaptic activation of the same mGluR-dependent priming mechanism. Unlike priming, febrile seizures (30) or tetanic stimulation (31) potentiate DSI by up-regulation of CB1Rs, thus underscoring the varieties of endocannabinoid plasticity.
Our observations do not contradict previous studies of eCBmGluR. It has been conventional to test for DSI (or DSE) before any other manipulations (e.g., 7, 13, 17, 19) to reduce time lost on unresponsive cells. We suggest that these initial tests may have primed the cells. Cells that respond with eCBmGluR without explicit experimental priming might have been preprimed naturally, or they may have been inadvertently primed (e.g., from Ca2+ influx during break-in). Indeed, it is critical that establishment of whole-cell recording be done as gently as possible; cells that undergo instabilities during break-in or those requiring high holding currents are usually found to be primed.
The results are not incompatible with the coincidence detection model, although they raise questions about its role. The model emerged from work on tissue-cultured cells from wild-type and PLCβ1 knockout animals; thus, developmental changes may reduce direct dependence of the eCB system on PLCβ in slices. Alternatively, PLCβ could be upstream of the eCB signaling process, such that its constitutive elimination removes essential substrates of eCB signaling. Hashimotodani et al. (19) found that U73122 thoroughly blocked PLCβ1 as measured by a TRPC6 assay, but side effects confounded their tests of U73122 on eCBmGluR. U73122 does not have adverse effects in slices, and we find that the PLCβ antagonist does not inhibit eCB priming or mobilization (see also refs. 17 and 18).
Resolution of these complex issues will require a full understanding of the biochemical pathways of eCB synthesis, and differences may exist among preparations and brain regions. U73122 blocks 2-AG synthesis in hippocampal and corticostriatal cells (32). In the cerebellum, PLCβ4 is essential for eCBmGluR signaling (26), and U73122 may reduce DSI (33), although this latter possibility is disputed (34). In the neocortex, extracellular U73122 reduces stimulation-induced eCB-LTD (35), but U73122 is ineffective in ventral tegmental area (VTA) (25). Ca2+ and mGluRs may interact through other mechanisms besides PLCβ to facilitate eCB mobilization, although complex effects of U73122 (36) preclude firm conclusions based on its use. Intracellular DGL inhibition blocks stimulus train-induced DSE in VTA and cerebellum (27), and a DGL inhibitor reduces DSE in autaptic hippocampal cultures (37). Interestingly, extracellular application of neither RHC 80627 nor THL reduced cerebellar DSE (27). DGLα is found in synaptic spines apposed to glutamate terminals but is not near GABA terminals (38). The insensitivity of eCB-dependent EPSC suppression to priming may reflect these or other distinctions between the synapses.
Finally, our results suggest that the interpretation of on-demand when applied to eCB mobilization can be modified. Mobilization of eCBs is sensitive to prior neuronal activity. The requirement for a cell to have experienced a significant transient increase in [Ca2+]i before it can mount a robust eCBmGluR response constrains the eCB system. In effect, priming represents a hebbian condition requiring both postsynaptic (rise in [Ca2+]i) and presynaptic (release of glutamate) factors for the mobilization of eCBmGluR. Behaviorally, priming would seem to be ideally suited for an attentional function that sets the stage for subsequent eCB-assisted, associational learning processes. The growing number of eCB-mediated functions in physiological and pathophysiological phenomena makes understanding the mechanisms of eCB metaplasticity (39) an important challenge.
Materials and Methods
Animal handling procedures were approved by the University of Maryland School of Medicine IACUC. Conventional hippocampal slices were used. Male rats, 5–7 weeks old, were heavily sedated with isoflurane and decapitated. Slices, 400 μm thick, were cut on a Vibratome (Tech Products) in an ice-cold bath solution and then stored at room temperature for >1 h before transfer to the recording chamber (40). The extracellular recording solution contained 120 mM NaCl, 3 mM KCl, 2.5 mM CaCl2, 2 mM MgSO4, 1 mM NaH2PO4, 25 mM NaHCO3, and 20 mM glucose, and it was bubbled with 95% O2, 5% CO2 (pH 7.4) at 30°C. Ionotropic glutamate responses were blocked with either 2 mM kynurenic acid or 50 μM AP-5 plus 10 μM NBQX. Whole-cell pipettes contained 90 mM CsCH3SO3, 10 mM Hepes, 0.2 mM BAPTA, 0.3 mM Tris-GTP, 4 mM Mg-ATP, 50 mM CsCl, 1 mM MgCl2, and 5 mM QX-314 (pH 7.25). An Axopatch 1C and pClamp 8.0 (Axon Instruments) were used. Extracellular stimulation (100-μs pulses at 0.25 Hz) were delivered in stratum radiatum because doing so induces large, eCB-sensitive eIPSCs, (e.g., 1), and most of the CCK- and CB1R-expressing interneurons are in this region (3). Cells were voltage-clamped at −70 mV, and DSI was induced by a 1- or 2-s voltage step to 0 mV. Electrode resistances in the bath were 3–6 MΩ. Statistical tests among groups were done with ANOVA followed by Holm–Sidak post hoc tests (SigmaStat). Paired t tests were used for single comparisons, except as noted. The significance level for all tests was P < 0.05 (*). Group means ± SEMs are shown for display purposes.
Supplementary Material
Acknowledgments.
We thank Scott Thompson, Thomas Blanpied, Miranda Karson, and Carlos Lafourcade for comments on a draft of this article. This work is part of the Ph.D. thesis of D.A.E. and was supported by National Institutes of Health Grants R01 NS30219 and DE140625 (to B.E.A.) and a Canadian Institutes of Health Research Doctoral Research Award fellowship (to D.A.E.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803558105/DCSupplemental.
References
- 1.Alger BE. Retrograde signaling in the regulation of synaptic transmission: Focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- 2.Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- 3.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 4.Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- 5.Martin BR, Mechoulam R, Razdan RK. Discovery and characterization of endogenous cannabinoids. Life Sci. 1999;65:573–595. doi: 10.1016/s0024-3205(99)00281-7. [DOI] [PubMed] [Google Scholar]
- 6.Piomelli D. The molecular logic of endocannabinoid signaling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- 7.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 8.Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 9.Kreitzer AC, Regehr WG. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci. 2001;21(RC174):1–5. doi: 10.1523/JNEUROSCI.21-20-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giuffrida A, et al. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- 11.Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maejima T, Hasimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- 13.Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci. 2001;21(RC188):1–5. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katona I, et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- 17.Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: A novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- 18.Edwards DA, Kim J, Alger BE. Multiple mechanisms of endocannabinoid response initiation in hippocampus. J Neurophysiol. 2006;95:67–75. doi: 10.1152/jn.00813.2005. [DOI] [PubMed] [Google Scholar]
- 19.Hashimotodani Y, et al. Phospholipase Cβ serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–268. doi: 10.1016/j.neuron.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Kawamura Y, et al. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohno-Shosaku T, et al. Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J Neurosci. 2002;22:3864–3872. doi: 10.1523/JNEUROSCI.22-10-03864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci. 2004;7:697–698. doi: 10.1038/nn1262. [DOI] [PubMed] [Google Scholar]
- 23.Ronesi J, Gerdeman GL, Lovinger DM. Disruption of endocannabinoid release and striatal long-term depression by postsynaptic blockade of endocannabinoid membrane transport. J Neurosci. 2004;24:1673–1679. doi: 10.1523/JNEUROSCI.5214-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bisogno T, et al. Development of the first potent and specific inhibitors of endocannabinoid biosynthesis. Biochim Biophys Acta. 2006;1761:205–212. doi: 10.1016/j.bbalip.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Melis M, et al. Prefrontal cortex stimulation induces 2-arachidonoyl-glycerol-mediated suppression of excitation in dopamine neurons. J Neurosci. 2004;24:10707–10715. doi: 10.1523/JNEUROSCI.3502-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maejima T, et al. Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cβ4 signaling cascade in the cerebellum. J Neurosci. 2005;25:6826–6835. doi: 10.1523/JNEUROSCI.0945-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron. 2005;48:647–659. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Carlson G, Wang Y, Alger BE. Endocannabinoids facilitate the induction of LTP in the hippocampus. Nat Neurosci. 2002;5:723–724. doi: 10.1038/nn879. [DOI] [PubMed] [Google Scholar]
- 29.Zhu PJ, Lovinger DM. Persistent synaptic activity produces long-lasting enhancement of endocannabinoid modulation and alters long-term synaptic plasticity. J Neurophysiol. 2007;97:4386–4389. doi: 10.1152/jn.01228.2006. [DOI] [PubMed] [Google Scholar]
- 30.Chen K, et al. Long-term plasticity of endocannabinoid signaling induced by developmental febrile seizures. Neuron. 2003;39:599–611. doi: 10.1016/s0896-6273(03)00499-9. [DOI] [PubMed] [Google Scholar]
- 31.Chen K, et al. Prevention of plasticity of endocannabinoid signalling inhibits persistent limbic hyperexcitability caused by developmental seizures. J Neurosci. 2007;27:46–58. doi: 10.1523/JNEUROSCI.3966-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung K-M, et al. Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Mol Pharmacol. 2005;68:1196–1202. doi: 10.1124/mol.105.013961. [DOI] [PubMed] [Google Scholar]
- 33.Galante M, Diana MA. Group I metabotropic glutamate receptors inhibit GABA release at interneuron–Purkinje cell synapses through endocannabinoid production. J Neurosci. 2004;24:4865–4874. doi: 10.1523/JNEUROSCI.0403-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szabo B, et al. Depolarization-induced retrograde synaptic inhibition in the cerebellar cortex is mediated by 2-arachidonoylglycerol. J Physiol (Lond) 2006;577:263–280. doi: 10.1113/jphysiol.2006.119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nevian T, Sakmann B. Single spine Ca2+ signals evoked by coincident EPSPs and backpropagating action potentials in spiny stellate cells of layer 4 in the juvenile rat somatosensory barrel cortex. J Neurosci. 2004;24:1689–1699. doi: 10.1523/JNEUROSCI.3332-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horowitz LF, et al. Phospholipase C in living cells: Activation, inhibition, Ca2+ requirement, and regulation of M current. J Gen Physiol. 2005;126:243–262. doi: 10.1085/jgp.200509309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Straiker A, Mackie K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol (Lond) 2005;569:501–517. doi: 10.1113/jphysiol.2005.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katona I, et al. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abraham WC, Bear MF. Metaplasticity: The plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- 40.Nicoll RA, Alger BE. A simple chamber for recording from submerged brain slices. J Neurosci Methods. 1981;4:153–156. doi: 10.1016/0165-0270(81)90049-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.