Abstract
Geriatricians have embraced the term “geriatric syndrome”, using it extensively to highlight the unique features of common health conditions in the elderly. Geriatric syndromes, such as delirium, falls, incontinence and frailty, are highly prevalent, multifactorial, and associated with substantial morbidity and poor outcomes. Nevertheless, this central geriatric concept has remained poorly defined. This article reviews criteria for defining geriatric syndromes, and proposes a balanced approach of developing preliminary criteria based on peer-reviewed evidence. Based on a review of the literature, four shared risk factors—older age, baseline cognitive impairment, baseline functional impairment, and impaired mobility—were identified across five common geriatric syndromes (pressure ulcers, incontinence, falls, functional decline, and delirium). Understanding basic mechanisms involved in geriatric syndromes will be critical to advancing research and developing targeted therapeutic options. However, given the complexity of these multifactorial conditions, attempts to define relevant mechanisms will need to incorporate more complex models, including a focus on synergistic interactions between different risk factors. Finally, major barriers have been identified in translating research advances, such as preventive strategies of proven effectiveness for delirium and falls, into clinical practice and policy initiatives. National strategic initiatives are required to overcome barriers and to achieve clinical, research, and policy advances that will improve quality of life for older persons.
Keywords: Geriatric syndromes, multifactorial, gerontology, policy
Introduction
The term “geriatric syndrome” is used to capture those clinical conditions in older persons that do not fit into discrete disease categories. Many of the most common conditions cared for by geriatricians, including delirium, falls, frailty, dizziness, syncope and urinary incontinence, are classified as geriatric syndromes. Nevertheless, the concept of the geriatric syndrome remains poorly defined.
While heterogeneous, geriatric syndromes share many common features. They are highly prevalent in older adults, especially the frail elderly. Their impact on quality of life and disability is substantial. Multiple underlying factors, involving multiple organ systems, tend to contribute to, and define, geriatric syndromes. As noted by Fried et al.(1), frequently the chief complaint does not represent the specific pathologic condition underlying the change in health status. In some cases, the two processes may involve distinct and distant organs with a disconnect between the site of the underlying physiologic insult and the resulting clinical symptom. For example, when an infection involving the urinary tract precipitates delirium, it is the altered neural function in the form of cognitive and behavioral changes which permits the diagnosis of delirium and determines many functional outcomes. The fact that these syndromes cross organ systems and discipline-based boundaries, along with their multifactorial nature, challenges traditional ways of viewing clinical care and research.
The concept of a geriatric syndrome has already facilitated the development of multicomponent intervention strategies and the establishment of ‘V’codes through the Centers for Medicare and Medicaid Services (CMS) for falls history. Nevertheless, the lack of a working definition has limited the usefulness of this term in clinical, research and policy arenas. Such a definition should seek to encompass the overarching clinical features which have led clinicians to apply this term to seemingly diverse conditions. Moreover, little progress has been made in developing a mechanistic understanding of common geriatric syndromes, with no agreement for how such research should be conducted.
The goals of this special article are to describe the advantages and limitations of establishing formal criteria for geriatric syndromes, to evaluate shared risk factors across five distinct geriatric syndromes, to propose potential mechanistic approaches for conducting basic to clinical translational research into geriatric syndromes, and to discuss local and national efforts to translate geriatric syndrome research to practice and policy. It is hoped that this article will help to catalyze further development in the field of geriatric syndromes—in clinical, research, and policy domains.
The Development of Formal Criteria for Geriatric Syndromes
The conceptualization of geriatric syndromes has been evolving over time(2). In general terms, a “syndrome” has been defined as “a group of signs and symptoms that occur together and characterize a particular abnormality”(3) or “the aggregate of symptoms and signs associated with any morbid process, and constituting together the picture of the disease”(2;4). Thus, in current medical usage, a syndrome refers to a pattern of symptoms and signs with a single underlying cause that may not yet be known(5) (Figure 1).
Figure 1.
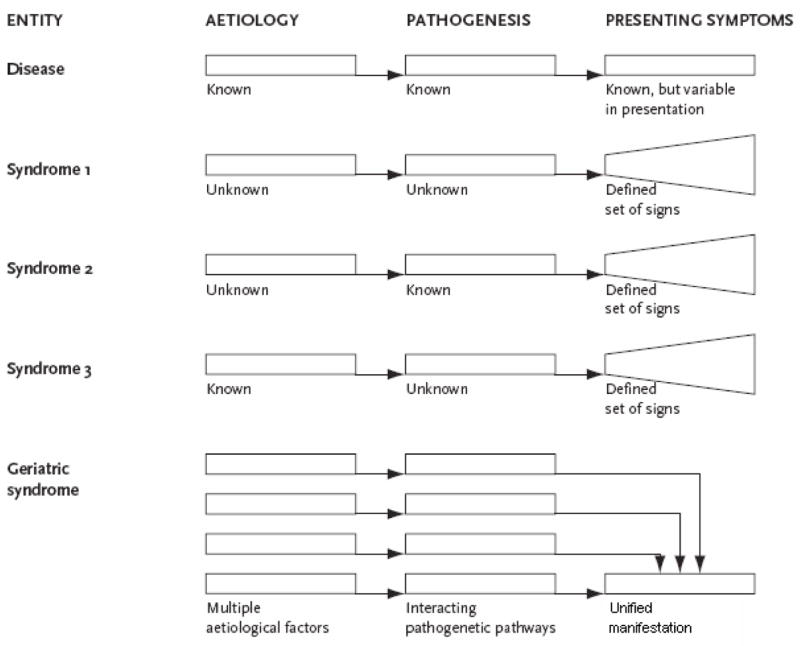
Schematic conceptual representation of clinical conditions defined by the terms “disease”, “syndrome” and “geriatric syndrome”, illustrating differences in numbers and complexity of relevant factors, including etiological risk factors, pathophysiologic mechanisms and presenting symptoms. Adapted with permission from Olde Rikkert et al. (5).
Geriatric syndromes, by contrast, refer to “multifactorial health conditions that occur when the accumulated effects of impairments in multiple systems render [an older] person vulnerable to situational challenges”(6). Thus, the geriatric usage of the term “syndrome” emphasizes multiple causation of a unified manifestation(2;5). With this usage, the conceptualization of geriatric syndromes aligns itself well with the concept of “phenotype”, defined as “the observable characteristics, at the physical, morphologic, or biochemical level, of an individual, as determined by the genotype and environment”(4). This concept emphasizes the multiple contributors to observable characteristics, such as the frailty phenotype(7).
Geriatric syndromes pose some special clinical considerations. First, for a given geriatric syndrome, multiple risk factors and multiple organ systems are often involved. Second, diagnostic strategies to identify the underlying causes can sometimes be ineffective, burdensome, dangerous, and costly. Finally, therapeutic management of the clinical manifestations can be helpful even in the absence of a firm diagnosis or clarification of the underlying causes.
Are there alternative options for terminology? Rather than “geriatric syndrome”, alternative terms might be “final common pathway” or “end product”. In this conceptualization, the geriatric syndrome represents the result of a series of processes or changes, suggesting multiple contributors. This conceptualization parallels other medical conditions like renal failure and hypertension, where multiple causes may contribute, where it may not always be appropriate to search for underlying cause(s), and where management does not always depend on the underlying cause(s).
Within medical research and practice, establishing formal criteria to define syndromes has a long tradition. Examples include criteria within rheumatology to define rheumatoid arthritis(8) and systemic lupus erythematosus(9); the National Institute of Neurological and Communicative Diseases and Stroke-Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) clinical criteria for Alzheimer’s Disease(10); and psychiatric diagnoses in the Diagnostic and Statistical Manuals(11). The advantages of such criteria include improved communication in both clinical and research settings, enhanced ability to directly compare syndromes between studies and to pool study findings, and the ability to create International Classification of Diseases (ICD-9) codes and billable diagnoses. The development of formalized criteria will also assist with creating unified concepts, to facilitate pathophysiologic studies and enhance the search for common mediators. For areas such as delirium and chronic fatigue syndrome, operational definitions have been developed that have facilitated research in these areas. Despite these advantages, premature establishment of formal criteria—without an adequate evidence base--can create rigid conceptualizations, stymie development and progress within the field, and lead to inappropriate application of concepts by clinicians and researchers with the potential for inaccurate diagnosis and therapeutic mismanagement. Examples of this phenomenon include the premature classification of diabetes as Type I and Type II, or the hyperlipidemias as Types I–V, which held back inquiry and progress for many years.
A balanced approach would be to develop preliminary criteria for select geriatric syndromes with an adequate evidence base by working committees assembled by professional organizations, such as the American Geriatrics Society (AGS). These preliminary criteria can be sent out for comment by other organizations and by the AGS membership. Once published, these criteria could be regularly updated and allowed to evolve over time. For research studies, these criteria would be helpful to compare research samples and results, to pool study findings, to modify, expand, or focus study samples, and to appropriately target interventions.
Shared Risk Factors for Distinct Geriatric Syndromes
A defining feature of geriatric syndromes is that multiple risk factors contribute to their etiology(3). Previous work has suggested that some geriatric syndromes might share underlying risk factors(6). We propose a unifying conceptual model for geriatric syndromes (Figure 2), demonstrating that shared risk factors may lead to these syndromes and to the overarching geriatric syndrome of frailty. While there is not yet a consensus definition, frailty is defined here as impairment in mobility, balance, muscle strength, cognition, nutrition, endurance, and physical activity(12). Frailty and the other geriatric syndromes may also feed-back to result in the development of more risk factors and more geriatric syndromes. These pathways in turn lead to the final outcomes of disability, dependence, and death. This conceptual model provides a unifying framework and holds important implications for elucidating both pathophysiologic mechanisms and management strategies.
Figure 2.
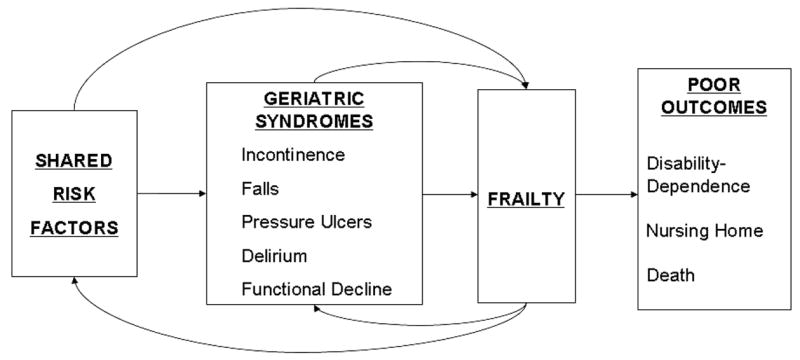
A unifying conceptual model demonstrates that shared risk factors may lead to geriatric syndromes, which may in turn lead to frailty, with feedback mechanisms enhancing the presence of shared risk factors and geriatric syndromes. Such self-sustaining pathways may result in poor outcomes involving disability-dependence, nursing home placement, and ultimately death, thus holding important implications for elucidating pathophysiologic mechanisms and designing effective intervention strategies.
While each geriatric syndrome is distinct, we hypothesized that they would have shared risk factors. Thus, we carried out a systematic review of the medical literature designed to examine previously identified risk factors for some common geriatric syndromes, and to identify common risk factors across all of these syndromes. We selected five geriatric syndromes for this investigation, based on the following criteria: they are common, associated with a high degree of morbidity, demonstrated to be preventable in some cases, and investigated with multiple previous risk factor studies. The five geriatric syndromes investigated were pressure ulcers, incontinence, falls, functional decline, and delirium.
Methods
A systematic review of the medical literature was conducted using PubMed from January, 1990 through December, 2005. The search was performed using key words and synonyms for each geriatric syndrome and the terms ‘risk factor’or ‘predictor’. Articles were selected based on review of their abstracts, which indicated that they were original articles that identified independent risk factors or a predictive model for the geriatric syndrome. Risk factors from each article were classified, and common risk factors across geriatric syndromes were identified.
Results
For pressure ulcers, 12 recent risk factors studies were identified(13–25), as summarized in Table 1. For incontinence, 9 recent risk factor studies were identified(26–34). Risk factors present in at least 2 studies included: older age (generally >65 years), high body mass index, functional impairment, impaired mobility, cognitive impairment or dementia, and use of physical restraints. For falls, 12 recent risk factors studies were identified(21;28;35–44).Risk factors present in at least 2 studies included older age, prior history of falls, functional impairment, use of a walking aid or assistive device, cognitive impairment or dementia, impaired mobility or low activity level, and balance abnormality. For functional decline, 12 recent risk factor studies were identified(21;45–55). Risk factors present in at least 2 studies included older age, previous falls, functional impairment, cognitive impairment or dementia, hospitalization, incident vascular event, depression, vision impairment, diabetes mellitus, and impaired mobility. For delirium, 36 risk factor studies were identified(56–89). Risk factors present in at least 2 studies included older age, cognitive impairment or dementia, psychoactive medication use, severe illness or multiple comorbidity, azotemia or dehydration, functional impairment, alcohol abuse, infection, metabolic derangement, and impaired mobility.
Table 1.
Risk Factors for Pressure Ulcers Based on a Systematic Literature Review*
| Reference | Age | LOS | Incontinence | Impaired mobility |
Low Weight |
Nutrition | Diabetes | Cognitive Impairment† |
Functional Impairment |
Other |
|---|---|---|---|---|---|---|---|---|---|---|
| 13 | X | X | Previous pressure ulcers | |||||||
| 14 | X | X | X | X | Cardiovascular disease or sepsis | |||||
| 15 | X | X | X | Anemia | ||||||
| 16 | X | X | X | X | ||||||
| 17 | X | X | X | X | Female | |||||
| 18 | X | X | Surgery | |||||||
| 19 | X | X | X | Male, moisture, friction | ||||||
| 20 | X | X | X | White, fecal incontinence, admitted from hospital | ||||||
| 21 | X | X | X | X | X | Male, poor physical condition | ||||
| 22 | X | X | X | X | Medical conditions | |||||
| 23 | X | X | X | Emergent admission | ||||||
| 24 | X | X | Nonblanchable erythema, dry skin, lymphopenia | |||||||
| 25 | X | X | X | X | Hypotension, fever |
Literature review from January 1990–December 2005.
Cognitive impairment includes decreased sensory perception.
Shaded area=shared risk factors across geriatric syndromes.
Shared risk factors identified consistently across all geriatric syndromes in this study included older age, functional impairment, cognitive impairment, and impaired mobility. While some risk factors (e.g., falls, diabetes) occurred across multiple geriatric syndromes, only the 4 identified risk factors occurred across all of the geriatric syndromes we examined.
Implications
This study has once again confirmed the multifactorial etiology of the common geriatric syndromes of pressure ulcers, incontinence, falls, functional decline, and delirium. Four shared risk factors have been identified across all of these geriatric syndromes, including older age, cognitive impairment, functional impairment, and impaired mobility. These findings raise the possibility of shared pathophysiologic mechanisms across these syndromes, such as multi-system dysregulation, inflammation, sarcopenia, and atherosclerosis. Importantly, at least 3 of these 4 risk factors are amenable to intervention, such as through preventive strategies to provide reorientation for cognitive impairment, or exercise, balance training, and mobilization to reduce functional impairment and impaired mobility. Testing of unified intervention strategies targeted towards these shared risk factors may prevent these common geriatric syndromes and frailty, along with their associated poor long-term outcomes.
Pathophysiology of Multifactorial Geriatric Syndromes
The research community can point to many accomplishments achieved by a bench-to-bedside translational approach(90;91), which has been most impressive when addressing inborn errors of metabolism(92). At the same time, there has been a growing awareness that optimum clinical care cannot be based entirely on a biological framework(1;93-95). This observation is particularly pertinent to the management of geriatric syndromes where it is imperative to also consider relevant social, spiritual and economic domains. While it is extremely difficult to study the pathophysiology of complex multifactorial geriatric syndromes, such studies must be undertaken if we are to have any chance at altering the natural history of these core contributors to late-life disability.
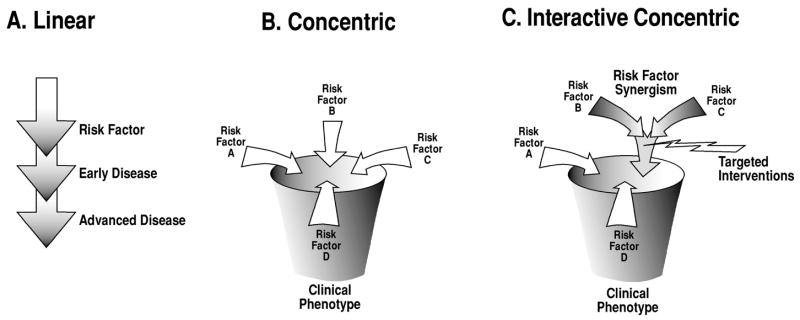
The pathophysiology of many non-geriatric conditions can be viewed along a traditional linear model (A in Figure 3). For example, a genetic alteration can lead to a disease process involving one organ system. In other cases, a clinical cluster of diseases involving multiple organ systems may develop(96). Although the term “syndrome” has been applied to genetic conditions with a multi-organ phenotype, the linear model is still applicable, since a direct relationship exists between altered genetics and the clinical phenotype(96). However, this linear model does not lend itself well to the study of common diseases such as diabetes, hypertension, atherosclerosis or cancer, which can only rarely be attributed to a single gene alteration. Moreover, this model also fails to incorporate the types of non-biological considerations discussed above. The concentric model (B in Figure 3) has been proposed as a means of highlighting the complexity of oncogenesis, together with the belief that the targeting of multiple pathways contributing to tumor survival and growth will improve treatment outcomes(97). We propose that this model can be adapted to study the pathophysiology of geriatric syndromes since it permits one to incorporate the multifactorial complexity inherent in these conditions.
Figure 3.
Mechanistic research addressing the pathophysiology of complex multifactorial geriatric syndromes will require the development of new conceptual models. The traditional linear model (A) has proven highly effective for the discovery of pathophysiologically relevant mechanisms in conditions such as inborn errors of metabolism, yet it does not adequately capture the multifactorial nature of geriatric syndromes. The concentric model (B) was developed by cancer researchers as a means of designing more effective cancer treatments by targeting multiple distinct oncogenic pathways(97). This approach may also not be suitable for geriatric syndromes since interventions targeting only one risk factor would address only a small portion of the overall risk for such conditions, while multi-component pharmaceutical interventions risk being unfocussed and could lead to adverse effects typically associated with geriatric polypharmacy. We propose an interactive concentric model (C) as a means of reconciling the need for mechanistic research with the conditions’multifactorial complexity, by focusing on pathways associated with risk factor synergisms, thus offering a locus for the design of targeted interventions. Modified from Decker and Sausville(97)
The above model is also attractive in that it permits us to address the pathophysiology of geriatric syndromes in a manner which reflects the complex interactions between an individual’s vulnerabilities and exposure to specific challenges. Even young and robust older individuals will fall, will develop cognitive deficits, or will become incontinent if challenged with a sufficiently great force, anticholinergic dose or physical restraint. The nature of such enhanced vulnerability is starting to be captured in frailty, falls, delirium and incontinence research. For example, multiple risk factors including sedative use, cognitive impairment, lower extremity disability, palmomental reflex, abnormalities of balance and gait, as well as foot problems all enhance the risk of falls(98). The risk increases linearly with the number of risk factors in the model, ranging from 8% for none to 78% in the presence of 4 or more risk factors(98). While this has led to innovative efforts incorporating multicomponent elements into strategies for the prevention of key geriatric syndromes (99;100), it has been difficult to conceptualize pathophysiologic studies to investigate such complex multifactorial conditions or to envision biologically-based treatments which could alter their natural history.
Traditional translational research is poorly-suited to address the pathophysiology of geriatric syndromes. First of all, it is possible to undertake careful research without establishing cause and effect since simple correlations between molecular changes and clinical outcomes may not establish causality even when demonstrated prospectively(101). In many ways, the use of genetically-modified animals (largely mice) has revolutionized the conduct of research designed to address the pathophysiology of complex conditions, such as osteoporosis(102;103) and Alzheimer’s disease(104) by linking the presence or absence of a gene to a specific phenotype. These technological advances have led to a great increase in the use of mice in such research. For example, PubMed citations using mice to study osteoporosis increased 25-fold from 1975-85 to 1995-2005, while mouse studies relevant to Alzheimer’s disease increased 50-fold. Such approaches will continue to grow, since approximately 10,000 of the nearly 25,000 genes in the mouse genome have already been deleted with knockout mouse mutations, and many other mutations are expected to become available in the near future(105).
Nonetheless, attempts to define the pathophysiology of complex multifactorial geriatric syndromes using current approaches can be problematic. For example, a decision to focus all efforts on a single risk factor may lack geriatric relevance since it addresses only a small portion of the overall risk and fails to consider other risk factors. By contrast, any research attempt to address all relevant risk factors runs the risk of being unfocussed. Moreover, unlike the use of multicomponent behavioral strategies for prevention, multicomponent strategies involving many biological interventions targeting different pathways could lead to unacceptable adverse effects in the frail elderly, given the well-established risk of polypharmacy in this population(106). If we are to develop strategies for altering the natural history of common geriatric syndromes, it will be essential to reconcile the need for defining relevant mechanisms with the underlying multifactorial complexity.
In spite of the enormity of the task, several promising directions need to be explored. One strategy involves capitalizing on the fact that some interventions exert highly specific effects on restricted populations of cells, while the effects of other strategies are more “pleiotropic” involving sometimes varying effects across many different cells and tissues. Examples of such potentially beneficial pleiotropic interventions include hormones, statins, antioxidants, as well as behavioral modifications such as exercise, improved nutrition and weight loss. Not only is it essential to test such interventions, it is also imperative to explore the basic mechanisms by which each exerts effects which are both pleiotropic and beneficial in the context of specific geriatric syndromes. While few investigators have pursued the development of animal models of individual geriatric syndromes, such a possibility should not be summarily dismissed. For example, the vulnerability of commonly used inbred mouse strains such as C57BL6J to develop a specific phenotype has been used in osteoporosis, diabetes, and atherosclerosis research(107;108). More recently, it has become apparent that the pattern of aging may vary greatly between different strains and that individual strains may exhibit a vulnerability to develop phenotypic features typical of geriatric syndromes, such as sarcopenic obesity(109).
Another approach involves an evaluation of the interactions between different risk factors in what could be termed the interactive concentric model of geriatric syndrome pathophysiology (C in Figure 3). Investigators are beginning to identify interactive synergisms between different risk factors for individual geriatric syndromes. For example, Cappola et al. have shown that the combination of low IGF-1 and high IL-6 levels in the same individual confers a higher risk for progressive disability and mortality in older women in a manner which suggests the presence of interactive synergisms between these two risk factors(110). It remains to be seen whether IGF-1 and IL-6 are actual mediators of relevant biological effects or markers of some other process. Nevertheless, these findings may have important clinical implications. The presence of such synergy implies that the pathways by which each of these risk factors contributes to progressive disability may biologically interact. The presence of such biological overlap between distinct risk factors (large arrow in Figure 3), may offer unique opportunities for making sense of this complexity and for identifying priority targets for developing clinically useful interventions.
Detrusor underactivity, a multifactorial geriatric condition which contributes to urinary retention in the frail elderly(111), is defined by detrusor muscle loss, fibrosis and axonal degeneration in human bladder biopsies(111;112). In animal studies using genetically modified mice, MIF (macrophage migration inhibitory factor), an atypical and abundant uroepithelial cytokine, has been implicated in the pathways by which two different risk factors--urinary retention/outlet obstruction(113) and lack of estrogen(114)--mediate bladder muscle loss and fibrosis. Moreover, aging, as well as comorbidities such as urinary tract infections(115), may also mediate their effects on detrusor underactivity via this pathway. Thus, it may be possible to use preclinical animal, as well as theoretical, models in an effort to define the efficacy of interventions designed to target such shared pathways in geriatric syndromes, analogous to the methods used by oncologists to anticipate the effects of drug combinations(116).
Linking Geriatric Syndrome Research to Practice and Policy
While research on geriatric syndromes has helped to clarify risk factors and to establish effective intervention strategies, by and large the results based on this evidence have failed to translate into clinical practice. The translation of geriatric syndrome research into practice faces unique challenges, which may heighten the barriers to evidence-based implementation(117). Beyond the complex nature of geriatric syndromes, numerous factors pose barriers to dissemination, including: 1) the lack of commonly accepted definitions for the recognition, diagnosis and coding of geriatric syndromes; 2) the lack of simple, measurable interventions; 3) the need for substantial provider time and longitudinal follow-up to intervene and assess effectiveness—i.e. intervention for geriatric syndromes requires human capital, rather than simply a new drug or technology; 4) the interventions often require new behaviors or attitude shifts on the part of the patient and/or the provider(s) (such as working with interdisciplinary teams, or focusing on identifying contributors like urinary tract infection or drugs rather than ordering a brain scan for a delirious patient), and often require system-wide changes across extended systems of care with coordination across multiple disciplines; 5) there are not enough champions for these interventions, particularly in the face of many other competing clinical demands and mandates; and 6) the multifactorial nature of the geriatric syndromes—requiring a coordinated, multifaceted approach—does not adhere with the traditional disease model which drives most medical practice (94).
In this section, the evidence-practice gap will be examined for two common geriatric syndromes, delirium and falls. Two examples of local efforts to translate research into practice for these conditions will be highlighted, along with the barriers to dissemination. Finally, a description of national efforts to link research to practice through policy initiatives will be provided.
Delirium
Overview
Delirium, defined as an acute decline in attention and global cognitive functioning, is a common and life-threatening problem for hospitalized older patients. Occurring in 14–56% of patients, delirium is associated with hospital mortality rates of 22–76%(118). Despite its clinical importance, delirium is unrecognized in 66–70% of patients(119) and is documented in the medical record of only 3% of patients when present(120). This lack of recognition has precluded effective intervention for delirium. Several recent intervention trials(99;121–124) have documented that 30–40% of delirium may be preventable, and intervention may also reduce delirium duration.
Intervention Strategy and Dissemination Process
The Hospital Elder Life Program (HELP) has been demonstrated to be effective for prevention of delirium and functional decline(99;125), and to be cost-effective for both acute hospital costs(126) and long-term nursing home placement(127). Moreover, a HELP dissemination site demonstrated reduction in delirium rates and hospital costs in a quality improvement study(128). Since 1999, the HELP Dissemination Program has been established to facilitate the translation of research into practice by providing assistance with the implementation of HELP at other hospitals. Interested sites purchase the HELP dissemination package, which includes program manuals, business tools, training videotapes or compact discs, and tracking software. Sites receive ongoing support from the HELP Dissemination Team, the HELP website (http://www.hospitalelderlifeprogram.org) with its program resources and on-line discussion forum, semiannual special interest group meetings, and annual HELP conference.
Barriers to Dissemination
In two qualitative studies(129;130), Bradley et al identified common challenges to translating the HELP model into practice. The initial study(129) examined challenges in initial implementation of the intervention, and identified six common challenges: gaining internal support despite differing goals of administration and clinical staff; ensuring effective clinician leadership; integrating with existing geriatric programs (coordination vs. competition); balancing program fidelity with local resources (reduce duplication; adaptation); documenting positive outcomes despite limited resources for research, and maintaining momentum despite unrealistic timeframes, limited resources, and staff turnover. In a subsequent study, Bradley et al.(130) identified 3 common challenges to sustaining the intervention: presence of clinical leadership, adaptation to local circumstances, and obtaining long-term funding. Obtaining long-term funding represented the ultimate challenge across all sites, and most successful sites demonstrated local benefits and elicited funding through the hospital’s operating budget.
Falls
Overview
Falls pose a serious health problem for older persons, occurring in 30% of adults over age 65 and 40% over age 80(98;131). Falls are the leading cause of unintentional injury, which ranks as the sixth leading cause of death among the elderly(131). In addition, falls lead to functional decline, hospitalization, institutionalization, and increased healthcare costs(98;131). To date, over 60 intervention trials have been conducted including multifactorial targeted risk factor intervention studies(132), which have resulted in an approximately 30% relative risk reduction in fall rate. Moreover, fall prevention has been demonstrated to be cost-effective, and perhaps cost-saving(132). Despite this evidence, fall prevention has been largely neglected in clinical practice. A recent survey of primary care providers documented that only 37% ask patients about falls(133).
Dissemination Process
In a local effort to catalyze translation of research into practice, the Connecticut Collaboration for Fall Prevention (CCFP) was implemented to disseminate current evidence throughout the health care system in the greater Hartford, Connecticut area and to embed fall risk evaluation and management into practice by changing knowledge, attitudes, and behavior of health care providers (134). Health care providers targeted for these efforts included emergency departments in 7 local hospitals, 212 primary care offices (>500 physicians), 26 home care agencies (>200 staff), and 130 rehabilitation centers (>300 physical therapists). Practice materials on fall risk evaluation and management were developed and circulated, including checklists, manuals, passbooks, and website (http://www.fallprevention.org/). A variety of professional behavior change strategies(134;135) were implemented, including general methods to heighten fall awareness and targeted methods to increase fall-related practices. The methods to heighten fall awareness included media presentations (e.g., television, radio, newspaper), monthly newsletters, posters and brochures at clinical and community sites, lectures and in-services, and efforts to publicize the website. The methods to increase fall-related practices included working groups to facilitate buy-in from local leaders, repeated contacts with providers (i.e., academic detailing(136)), and patient-mediated strategies (i.e., encouraging direct patient requests for fall management).
Barriers to Dissemination
Through interviews with CCFP working groups(134), barriers to the translation of evidence-based strategies were identified, including knowledge, attitudinal, and organizational barriers. Knowledge barriers included that both providers and seniors were not aware of the preventable nature of falls; providers were not familiar with geriatrics or lacked expertise in fall prevention; providers were not aware of the expertise of other providers who would represent appropriate referral resources; and the false perception that fall risk evaluation and management were not allowed by Medicare. Attitudinal barriers included the lack of importance assigned to falls by providers; providers believing they were already addressing the problem; and patients not requesting attention to their falls. Finally, organizational barriers included the fragmented, uncoordinated nature of our healthcare system; rapid turnover of providers; arcane Medicare reimbursement system for fall-related services; healthcare focusing on diseases rather than multifactorial geriatric syndromes; competing demands on providers who are bombarded with guidelines; and the lack of a mandate to address falls in clinical practice. Clearly, local efforts can go only so far without a national push.
National Efforts to Translate Research into Practice and Policy
“Knowing is not enough; we must apply. Willing is not enough; we must do.” – Goethe
Table 2 provides examples of national efforts to translate research into practice and policy for geriatric syndromes (117). Examples provided span the areas of educational and clinical efforts, quality improvement approaches, accreditation standards, reimbursement and payment policies, and legislative policies. While many other initiatives exist, Table 2 provides a few representative examples of areas that are initiated or in process. These examples provide a valuable framework to further address the full scope of common geriatric syndromes.
Table 2.
Examples of National Efforts to Translate Research into Practice and Policy*
| Area | Description | Examples |
|---|---|---|
| Educational and Clinical Efforts |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Quality Improvement Approaches |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Accreditation Standards |
|
|
| Reimbursement and Payment Policies |
|
|
|
|
|
| Legislative Policies |
|
|
AGS=American Geriatrics Society; NCOA=National Council on Aging; ABIM=American Board of Internal Medicine; AARP=American Association for Retired Persons; ACOVE=Acute Care for Vulnerable Elders; AHRQ=Agency for Healthcare Research and Quality; NCQA=National Committee for Quality Assurance; MedPAC=Medicare Payment Advisory Commission; JCAHO=Joint Commission on Accreditation of Healthcare Organizations; ICD=International Classification of Diseases; CMS=Centers for Medicare and Medicaid Services; AMA=American Medical Association
Medicare reimbursement for common geriatric syndromes remains a critical issue which will need to be addressed to provide appropriate healthcare for these conditions. Considerable work will be needed to accomplish this goal. Payers are concerned about the potential cost of services, the potential for fraud and abuse, the fact that coordination of services are typically not covered by Medicare, and the statutory limitations on coverage (i.e., Medicare usually covers only acute episodes of care). Providers report challenges in providing care to older patients because Medicare is not accustomed to handling the multi-provider, multi-setting model needed to address geriatric syndromes; component services may be processed by different types of Medicare contractors; and variable interpretation of Medicare policies occurs among carriers and intermediaries. Clearly, these are areas that must be better addressed to provide optimal care for patients with geriatric syndromes.
Summary
Geriatric syndromes represent common, serious conditions for older persons, holding substantial implications for functioning and quality of life. In large part, these conditions are most prevalent in the older population, and thus, pose distinctive challenges for clinicians caring for this population. The lack of formal criteria to define geriatric syndromes has limited progress in the field. We propose that a more formal recognition of the concepts underlying geriatric syndromes, supported by an improved dialogue between different disciplines, is needed to ensure future progress.
Geriatric syndromes are multifactorial, and shared risk factors—including older age, cognitive impairment, functional impairment, and impaired mobility—were demonstrated across the common geriatric syndromes of pressure ulcers, incontinence, falls, functional decline, and delirium. These findings support the likelihood of shared pathophysiologic mechanisms, and raise the possibility of a unified approach to prevention of these syndromes.
Studies designed to elucidate the pathophysiology of geriatric syndromes are essential, but must embrace the complex and multifactorial nature of these conditions. Identifying shared common ground or mechanisms will represent a major advance. Simple linear models linking one cause to one effect are not likely to suitably address these conditions. More complex models, such as concentric models proposed in oncology, should incorporate both multiple potential pathways to the outcome, as well as the potential for interaction or synergisms between pathways or causes.
Even with substantial progress in clarifying risk factors and intervention strategies for some common geriatric syndromes, such as delirium and falls, these advances have failed to translate widely into clinical practice or policy initiatives. Dissemination programs have been established for delirium and fall prevention, and success and barriers to dissemination have been systematically evaluated. Barriers still exist at patient, provider, and organizational levels.
Table 3 presents a call to action to enhance progress in geriatric syndromes. The challenge of caring for our older population, as exemplified by these common geriatric syndromes, will require paradigm shifts and new approaches to optimize care. These challenges will stretch all of us, as consumers, providers, payers, and policy-makers, to improve our health care system to better address the needs of our rapidly aging population.
Table 3.
A Call to Action to Enhance Progress in Geriatric Syndromes
Clinical:
Research:
Policy:
|
Acknowledgments
This work was presented in part as a Symposium at the American Geriatrics Society 2006 Annual Scientific Meeting. The authors would like to thank the AGS Research Committee for sponsorship of this symposium. Dr. Inouye is supported in part by Grants K24AG00949 and R21AG025193 from the National Institute on Aging, and by the Milton and Shirley F. Levy Family Chair. Dr. Studenski is supported in part by Grant K07AG023641 and by the Pittsburgh OAIC (P30AG024827). Dr. Tinetti is supported in part by a grant from the Patrick and Catherine Weldon Donaghue Medical Research Foundation, by the Yale OAIC (P30AG021342) and by the Gladys Phillips Crofoot Chair. Dr. Kuchel is supported in part by a grant from the Patrick & Catherine Weldon Donaghue Medical Research Foundation and by the Travelers in Geriatrics and Gerontology.
Footnotes
Sponsors’ Role: The sponsors played no role in design, methods, recruitment, data collection, analysis, or preparation of the paper.
Financial Disclosures:
Dr. Sharon Inouye: None
Dr. Stephanie Studenski: Dr. Studenski has served as a consultant for Merck and Amgen, and has had an unrelated research project funded by Eli Lilly and Co.
Dr. Mary Tinetti: None
Dr. George Kuchel: Dr. Kuchel has served as a consultant for Johnson and Johnson; Pfizer; and Odyssey Pharmaceuticals.
References
- 1.Fried LP, Storer DJ, King DE, et al. Diagnosis of illness presentation in the elderly. J Am Geriatr Soc. 1991;39:117–123. doi: 10.1111/j.1532-5415.1991.tb01612.x. [DOI] [PubMed] [Google Scholar]
- 2.Flacker JM. What is a geriatric syndrome anyway? J Am Geriatr Soc. 2003;51:574–576. doi: 10.1046/j.1532-5415.2003.51174.x. [DOI] [PubMed] [Google Scholar]
- 3.Merriam-Webster Online Dictionary. 2006.
- 4.Stedman's Medical Dictionary. Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 5.Olde Rikkert MG, Rigaud AS, van Hoeyweghen RJ, et al. Geriatric syndromes: medical misnomer or progress in geriatrics? Neth J Med. 2003;61:83–87. [PubMed] [Google Scholar]
- 6.Tinetti ME, Inouye SK, Gill TM, et al. Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes. JAMA. 1995;273(3):1348–1353. [PubMed] [Google Scholar]
- 7.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 8.ROPES MW, BENNETT GA, Cobb S, et al. Proposed diagnostic criteria for rheumatoid arthritis. Ann Rheum Dis. 1957;16:118–125. doi: 10.1136/ard.16.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trimble RB, Townes AS, Robinson H, et al. Preliminary criteria for the classification of systemic lupus erythematosus (SLE). Evaluation in early diagnosed SLE and rheumatoid arthritis. Arthritis Rheum. 1974;17:184–188. doi: 10.1002/art.1780170212. [DOI] [PubMed] [Google Scholar]
- 10.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 11.Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. 4. American Psychiatric Publishing; 2000. [Google Scholar]
- 12.Ferrucci L, Guralnik JM, Studenski S, et al. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. 2004;52:625–634. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 13.Defloor T, Clark M, Witherow A, et al. EPUAP statement on prevalence and incidence monitoring of pressure ulcer occurrence. J Tissue Viability. 2005;15:20–27. doi: 10.1016/s0965-206x(05)53004-3. [DOI] [PubMed] [Google Scholar]
- 14.Chan EY, Tan SL, Lee CK, et al. Prevalence, incidence and predictors of pressure ulcers in a tertiary hospital in Singapore. J Wound Care. 2005;14:383–388. doi: 10.12968/jowc.2005.14.8.26820. [DOI] [PubMed] [Google Scholar]
- 15.Chauhan VS, Goel S, Kumar P, et al. The prevalence of pressure ulcers in hospitalised patients in a university hospital in India. J Wound Care. 2005;14:36–37. doi: 10.12968/jowc.2005.14.1.26724. [DOI] [PubMed] [Google Scholar]
- 16.Gunningberg L. Risk, prevalence and prevention of pressure ulcers in three Swedish healthcare settings. J Wound Care. 2004;13:286–290. doi: 10.12968/jowc.2004.13.7.26638. [DOI] [PubMed] [Google Scholar]
- 17.Lindgren M, Unosson M, Krantz AM, et al. Pressure ulcer risk factors in patients undergoing surgery. J Adv Nurs. 2005;50:605–612. doi: 10.1111/j.1365-2648.2005.03441.x. [DOI] [PubMed] [Google Scholar]
- 18.Stausberg J, Kroger K, Maier I, et al. Pressure ulcers in secondary care: incidence, prevalence, and relevance. Adv Skin Wound Care. 2005;18:140–145. doi: 10.1097/00129334-200504000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Fisher AR, Wells G, Harrison MB. Factors associated with pressure ulcers in adults in acute care hospitals. Holist Nurs Pract. 2004;18:242–253. doi: 10.1097/00004650-200409000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Baumgarten M, Margolis D, Gruber-Baldini AL, et al. Pressure ulcers and the transition to long-term care. Adv Skin Wound Care. 2003;16:299–304. doi: 10.1097/00129334-200311000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Dunlop DD, Manheim LM, Sohn MW, et al. Incidence of functional limitation in older adults: the impact of gender, race, and chronic conditions. Arch Phys Med Rehabil. 2002;83:964–971. doi: 10.1053/apmr.2002.32817. [DOI] [PubMed] [Google Scholar]
- 22.Margolis DJ, Knauss J, Bilker W, et al. Medical conditions as risk factors for pressure ulcers in an outpatient setting. Age Ageing. 2003;32:259–264. doi: 10.1093/ageing/32.3.259. [DOI] [PubMed] [Google Scholar]
- 23.Eachempati SR, Hydo LJ, Barie PS. Factors influencing the development of decubitus ulcers in critically ill surgical patients. Crit Care Med. 2001;29:1678–1682. doi: 10.1097/00003246-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Allman RM, Goode PS, Patrick MM, et al. Pressure ulcer risk factors among hospitalized patients with activity limitation. JAMA. 1995;273:865–870. [PubMed] [Google Scholar]
- 25.Bergstrom N, Braden B. A prospective study of pressure sore risk among institutionalized elderly. J Am Geriatr Soc. 1992;40:747–758. doi: 10.1111/j.1532-5415.1992.tb01845.x. [DOI] [PubMed] [Google Scholar]
- 26.Jackson SL, Scholes D, Boyko EJ, et al. Urinary incontinence and diabetes in postmenopausal women. Diabetes Care. 2005;28:1730–1738. doi: 10.2337/diacare.28.7.1730. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins KR, Fultz NH. Functional impairment as a risk factor for urinary incontinence among older Americans. Neurourol Urodyn. 2005;24:51–55. doi: 10.1002/nau.20089. [DOI] [PubMed] [Google Scholar]
- 28.Mecocci P, von SE, Cherubini A, et al. Cognitive impairment is the major risk factor for development of geriatric syndromes during hospitalization: results from the GIFA study. Dement Geriatr Cogn Disord. 2005;20:262–269. doi: 10.1159/000087440. [DOI] [PubMed] [Google Scholar]
- 29.Nelson RL, Furner SE. Risk factors for the development of fecal and urinary incontinence in Wisconsin nursing home residents. Maturitas. 2005;52:26–31. doi: 10.1016/j.maturitas.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Rohr G, Stovring H, Christensen K, et al. Characteristics of middle-aged and elderly women with urinary incontinence. Scand J Prim Health Care. 2005;23:203–208. doi: 10.1080/02813430500362803. [DOI] [PubMed] [Google Scholar]
- 31.Saxer S, Halfens RJ, Muller M, et al. Risk factors for urinary incontinence in nursing home residents. Swiss Med Wkly. 2005;135:495–502. doi: 10.4414/smw.2005.10973. [DOI] [PubMed] [Google Scholar]
- 32.Wu J, Baguley IJ. Urinary retention in a general rehabilitation unit: prevalence, clinical outcome, and the role of screening. Arch Phys Med Rehabil. 2005;86:1772–1777. doi: 10.1016/j.apmr.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Landi F, Cesari M, Russo A, et al. Potentially reversible risk factors and urinary incontinence in frail older people living in community. Age Ageing. 2003;32:194–199. doi: 10.1093/ageing/32.2.194. [DOI] [PubMed] [Google Scholar]
- 34.Palmer MH, Baumgarten M, Langenberg P, et al. Risk factors for hospital-acquired incontinence in elderly female hip fracture patients. J Gerontol A Biol Sci Med Sci. 2002;57:M672–M677. doi: 10.1093/gerona/57.10.m672. [DOI] [PubMed] [Google Scholar]
- 35.Bergland A, Jarnlo GB, Wyller TB. Self-reported walking, balance testing and risk of fall among the elderly. Tidsskr Nor Laegeforen. 2006;126:176–178. [PubMed] [Google Scholar]
- 36.Arnold CM, Busch AJ, Schachter CL, et al. The relationship of intrinsic fall risk factors to a recent history of falling in older women with osteoporosis. J Orthop Sports Phys Ther. 2005;35:452–460. doi: 10.2519/jospt.2005.35.7.452. [DOI] [PubMed] [Google Scholar]
- 37.Fischer ID, Krauss MJ, Dunagan WC, et al. Patterns and predictors of inpatient falls and fall-related injuries in a large academic hospital. Infect Control Hosp Epidemiol. 2005;26:822–827. doi: 10.1086/502500. [DOI] [PubMed] [Google Scholar]
- 38.Gill T, Taylor AW, Pengelly A. A population-based survey of factors relating to the prevalence of falls in older people. Gerontology. 2005;51:340–345. doi: 10.1159/000086372. [DOI] [PubMed] [Google Scholar]
- 39.Horikawa E, Matsui T, Arai H, et al. Risk of falls in Alzheimer's disease: a prospective study. Intern Med. 2005;44:717–721. doi: 10.2169/internalmedicine.44.717. [DOI] [PubMed] [Google Scholar]
- 40.Kose N, Cuvalci S, Ekici G, et al. The risk factors of fall and their correlation with balance, depression, cognitive impairment and mobility skills in elderly nursing home residents. Saudi Med J. 2005;26:978–981. [PubMed] [Google Scholar]
- 41.Kallin K, Gustafson Y, Sandman PO, et al. Factors associated with falls among older, cognitively impaired people in geriatric care settings: a population-based study. Am J Geriatr Psychiatry. 2005;13:501–509. doi: 10.1176/appi.ajgp.13.6.501. [DOI] [PubMed] [Google Scholar]
- 42.Murray KJ, Hill K, Phillips B, et al. A pilot study of falls risk and vestibular dysfunction in older fallers presenting to hospital emergency departments. Disabil Rehabil. 2005;6;27:499–506. doi: 10.1080/09638280400018486. [DOI] [PubMed] [Google Scholar]
- 43.Shumway-Cook A, Ciol MA, Gruber W, et al. Incidence of and risk factors for falls following hip fracture in community-dwelling older adults. Phys Ther. 2005;85:648–655. [PubMed] [Google Scholar]
- 44.van DC, Gruber-Baldini AL, Zimmerman S, et al. Dementia as a risk factor for falls and fall injuries among nursing home residents. J Am Geriatr Soc. 2003;51:1213–1218. doi: 10.1046/j.1532-5415.2003.51404.x. [DOI] [PubMed] [Google Scholar]
- 45.Ishizaki T, Yoshida H, Suzuki T, et al. Effects of cognitive function on functional decline among community-dwelling non-disabled older Japanese. Arch Gerontol Geriatr. 2006 Jan;42:47–58. doi: 10.1016/j.archger.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Wu HY, Sahadevan S, Ding YY. Factors associated with functional decline of hospitalised older persons following discharge from an acute geriatric unit. Ann Acad Med Singapore. 2006;35:17. [PubMed] [Google Scholar]
- 47.Cornette P, Swine C, Malhomme B, et al. Early evaluation of the risk of functional decline following hospitalization of older patients: development of a predictive tool. Eur J Public Health. 2006;16:203–208. doi: 10.1093/eurpub/cki054. [DOI] [PubMed] [Google Scholar]
- 48.Dunlop DD, Semanik P, Song J, et al. Risk factors for functional decline in older adults with arthritis. Arthritis Rheum. 2005;52:1274–1282. doi: 10.1002/art.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamper AM, Stott DJ, Hyland M, et al. Predictors of functional decline in elderly people with vascular risk factors or disease. Age Ageing. 2005;34:450–455. doi: 10.1093/ageing/afi137. [DOI] [PubMed] [Google Scholar]
- 50.Spiers NA, Matthews RJ, Jagger C, et al. Diseases and impairments as risk factors for onset of disability in the older population in England and Wales: findings from the Medical Research Council Cognitive Function and Ageing Study. J Gerontol A Biol Sci Med Sci. 2005;60:248–254. doi: 10.1093/gerona/60.2.248. [DOI] [PubMed] [Google Scholar]
- 51.Stel VS, Smit JH, Pluijm SM, et al. Consequences of falling in older men and women and risk factors for health service use and functional decline. Age Ageing. 2004;33:58–65. doi: 10.1093/ageing/afh028. [DOI] [PubMed] [Google Scholar]
- 52.Gill TM, Allore H, Guo Z. Restricted activity and functional decline among community-living older persons. Arch Intern Med. 2003;163:1317–1322. doi: 10.1001/archinte.163.11.1317. [DOI] [PubMed] [Google Scholar]
- 53.Wang L, van BG, Kukull WB, et al. Predictors of functional change: a longitudinal study of nondemented people aged 65 and older. J Am Geriatr Soc. 2002;50:1525–1534. doi: 10.1046/j.1532-5415.2002.50408.x. [DOI] [PubMed] [Google Scholar]
- 54.Beland F, Zunzunegui MV. Predictors of functional status in older people living at home. Age Ageing. 1999;28:153–159. doi: 10.1093/ageing/28.2.153. [DOI] [PubMed] [Google Scholar]
- 55.Mor V, Wilcox V, Rakowski W, et al. Functional transitions among the elderly: patterns, predictors, and related hospital use. Am J Public Health. 1994;84:1274–1280. doi: 10.2105/ajph.84.8.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson K, Broadhurst C, Diver M, et al. Plasma insulin growth factor-1 and incident delirium in older people. Int J Geriatr Psychiatry. 2005;20:154–159. doi: 10.1002/gps.1265. [DOI] [PubMed] [Google Scholar]
- 57.Yamagata K, Onizawa K, Yusa H, et al. Risk factors for postoperative delirium in patients undergoing head and neck cancer surgery. Int J Oral Maxillofac Surg. 2005;34:33–36. doi: 10.1016/j.ijom.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 58.Blondell RD, Powell GE, Dodds HN, et al. Admission characteristics of trauma patients in whom delirium develops. Am J Surg. 2004;187:332–337. doi: 10.1016/j.amjsurg.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 59.Kagansky N, Rimon E, Naor S, et al. Low incidence of delirium in very old patients after surgery for hip fractures. Am J Geriatr Psychiatry. 2004;12:306–314. [PubMed] [Google Scholar]
- 60.Santos FS, Velasco IT, Fraguas R., Jr Risk factors for delirium in the elderly after coronary artery bypass graft surgery. Int Psychogeriatr. 2004;16:175–193. [PubMed] [Google Scholar]
- 61.Yoshimura Y, Kubo S, Shirata K, et al. Risk factors for postoperative delirium after liver resection for hepatocellular carcinoma. World J Surg. 2004;28:982–986. doi: 10.1007/s00268-004-7344-1. [DOI] [PubMed] [Google Scholar]
- 62.Wang SG, Lee UJ, Goh EK, et al. Factors associated with postoperative delirium after major head and neck surgery. Ann Otol Rhinol Laryngol. 2004;113:48–51. doi: 10.1177/000348940411300111. [DOI] [PubMed] [Google Scholar]
- 63.Bohner H, Hummel TC, Habel U, et al. Predicting delirium after vascular surgery: a model based on pre- and intraoperative data. Ann Surg. 2003;238:149–156. doi: 10.1097/01.sla.0000077920.38307.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ljubisavljevic V, Kelly B. Risk factors for development of delirium among oncology patients. Gen Hosp Psychiatry. 2003;25:345–352. doi: 10.1016/s0163-8343(03)00070-7. [DOI] [PubMed] [Google Scholar]
- 65.Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:76–81. doi: 10.1093/gerona/58.1.m76. [DOI] [PubMed] [Google Scholar]
- 66.Fann JR, Roth-Roemer S, Burington BE, et al. Delirium in patients undergoing hematopoietic stem cell transplantation. Cancer. 2002;1;95:1971–1981. doi: 10.1002/cncr.10889. [DOI] [PubMed] [Google Scholar]
- 67.Schneider F, Bohner H, Habel U, et al. Risk factors for postoperative delirium in vascular surgery. Gen Hosp Psychiatry. 2002;24:28–34. doi: 10.1016/s0163-8343(01)00168-2. [DOI] [PubMed] [Google Scholar]
- 68.Aldemir M, Ozen S, Kara IH, et al. Predisposing factors for delirium in the surgical intensive care unit. Crit Care. 2001;5:265–270. doi: 10.1186/cc1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Galanakis P, Bickel H, Gradinger R, et al. Acute confusional state in the elderly following hip surgery: incidence, risk factors and complications. Int J Geriatr Psychiatry. 2001;16:349–355. doi: 10.1002/gps.327. [DOI] [PubMed] [Google Scholar]
- 70.Litaker D, Locala J, Franco K, et al. Preoperative risk factors for postoperative delirium. Gen Hosp Psychiatry. 2001;23:84–89. doi: 10.1016/s0163-8343(01)00117-7. [DOI] [PubMed] [Google Scholar]
- 71.McCusker J, Cole M, Abrahamowicz M, et al. Environmental risk factors for delirium in hospitalized older people. J Am Geriatr Soc. 2001;49:1327–1334. doi: 10.1046/j.1532-5415.2001.49260.x. [DOI] [PubMed] [Google Scholar]
- 72.Dubois MJ, Bergeron N, Dumont M, et al. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27:1297–1304. doi: 10.1007/s001340101017. [DOI] [PubMed] [Google Scholar]
- 73.Dai YT, Lou MF, Yip PK, et al. Risk factors and incidence of postoperative delirium in elderly Chinese patients. gerontology. 2000;46:28–35. doi: 10.1159/000022130. [DOI] [PubMed] [Google Scholar]
- 74.Martin NJ, Stones MJ, Young JE, et al. Development of delirium: a prospective cohort study in a community hospital. Int Psychogeriatr. 2000;12:117–127. doi: 10.1017/s1041610200006244. [DOI] [PubMed] [Google Scholar]
- 75.Marcantonio ER, Goldman L, Orav EJ, et al. The association of intraoperative factors with the development of postoperative delirium. Am J Med. 1998;105:380–384. doi: 10.1016/s0002-9343(98)00292-7. [DOI] [PubMed] [Google Scholar]
- 76.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;20;275:852–857. [PubMed] [Google Scholar]
- 77.Fisher BW, Flowerdew G. A simple model for predicting postoperative delirium in older patients undergoing elective orthopedic surgery. J Am Geriatr Soc. 1995;43:175–178. doi: 10.1111/j.1532-5415.1995.tb06385.x. [DOI] [PubMed] [Google Scholar]
- 78.Foy A, O'Connell D, Henry D, et al. Benzodiazepine use as a cause of cognitive impairment in elderly hospital inpatients. J Gerontol A Biol Sci Med Sci. 1995;50:M99–106. doi: 10.1093/gerona/50a.2.m99. [DOI] [PubMed] [Google Scholar]
- 79.Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271:134–139. [PubMed] [Google Scholar]
- 80.Pompei P, Foreman M, Rudberg MA, et al. Delirium in hospitalized older persons: outcomes and predictors. J Am Geriatr Soc. 1994;42:809–815. doi: 10.1111/j.1532-5415.1994.tb06551.x. [DOI] [PubMed] [Google Scholar]
- 81.Inouye SK, Viscoli CM, Horwitz RI, et al. A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann Intern Med. 1993;119:474–481. doi: 10.7326/0003-4819-119-6-199309150-00005. [DOI] [PubMed] [Google Scholar]
- 82.Jitapunkul S, Pillay I, Ebrahim S. Delirium in newly admitted elderly patients: A prospective study. Q J Med. 1992;83:307–314. [PubMed] [Google Scholar]
- 83.Williams-Russo P, Urquhart BL, Sharrock NE, et al. Post-operative delirium: Predictors and prognosis in elderly orthopedic patients. J Am Geriatr Soc. 1992;40:759–767. doi: 10.1111/j.1532-5415.1992.tb01846.x. [DOI] [PubMed] [Google Scholar]
- 84.Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA. 1990;263:1097–1101. [PubMed] [Google Scholar]
- 85.Rockwood K. Acute confusion in elderly medical patients. J Am Geriatr Soc. 1989;37:150–154. doi: 10.1111/j.1532-5415.1989.tb05874.x. [DOI] [PubMed] [Google Scholar]
- 86.Rogers MP, Liang MH, Daltroy LH, et al. Delirium after elective orthopedic surgery: risk factors and natural history. Int J Psychiatry Med. 1989;19:109–121. doi: 10.2190/2q3v-hyt4-nn49-bpr4. [DOI] [PubMed] [Google Scholar]
- 87.Gustafson Y, Berggren D, Brannstrom B, et al. Acute confusional states in elderly patients treated for femoral neck fracture. J Am Geriatr Soc. 1988;36:525–530. doi: 10.1111/j.1532-5415.1988.tb04023.x. [DOI] [PubMed] [Google Scholar]
- 88.Williams MA, Campbell EB, Raynor WJ, Jr, et al. Predictors of acute confusional states in hospitalized elderly patients. Res Nurs Health. 1985;8:31–40. doi: 10.1002/nur.4770080107. [DOI] [PubMed] [Google Scholar]
- 89.Seymour DG, Henschke PJ, Cape RD, et al. Acute confusional states and dementia in the elderly: the role of dehydration/volume depletion, physical illness and age. Age Ageing. 1980;9:137–146. doi: 10.1093/ageing/9.3.137. [DOI] [PubMed] [Google Scholar]
- 90.Rosenberg LE. The physician-scientist: an essential--and fragile--link in the medical research chain. J Clin Invest. 1999;103:1621–1626. doi: 10.1172/JCI7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goldstein JL, Brown MS. The clinical investigator: bewitched, bothered, and bewildered--but still beloved. J Clin Invest. 1997;99:2803–2812. doi: 10.1172/JCI119470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hamosh A, Scott AF, Amberger JS, et al. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005;33(Database issue):D514–D517. doi: 10.1093/nar/gki033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smith R. In search of “non-disease”. BMJ. 2002;324:883–885. doi: 10.1136/bmj.324.7342.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tinetti ME, Fried T. The end of the disease era. Am J Med. 2004;116:179–185. doi: 10.1016/j.amjmed.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 95.Scully JL. What is a disease? EMBO Rep. 2004;5:650–653. doi: 10.1038/sj.embor.7400195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scriver CR, Sly WS. The Metabolic and Molecular Bases of Inherited Disease. 8. New York: McGraw-Hill; 2000. [Google Scholar]
- 97.Decker S, Sausville EA. Preclinical modeling of combination treatments: fantasy or requirement? Ann N Y Acad Sci. 2005;1059:61–69. doi: 10.1196/annals.1339.024. [DOI] [PubMed] [Google Scholar]
- 98.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 99.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 100.Allore HG, Tinetti ME, Gill TM, et al. Experimental designs for multicomponent interventions among persons with multifactorial geriatric syndromes. Clin Trials. 2005;2:13–21. doi: 10.1191/1740774505cn067oa. [DOI] [PubMed] [Google Scholar]
- 101.Liebman MN. Opening Pandora's box: clinical data and the study of complex diseases. Sci STKE. 2002;2002:E20. doi: 10.1126/stke.2002.130.pe20. [DOI] [PubMed] [Google Scholar]
- 102.Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lorenzo J. Interactions between immune and bone cells: new insights with many remaining questions. J Clin Invest. 2000;106:749–752. doi: 10.1172/JCI11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hock BJ, Jr, Lamb BT. Transgenic mouse models of Alzheimer's disease. Trends Genet. 2001;17:S7–12. doi: 10.1016/s0168-9525(01)02449-0. [DOI] [PubMed] [Google Scholar]
- 105.Waltz E. Price of mice to plummet under NIH's new scheme. Nat Med. 2005;11:1261. doi: 10.1038/nm1205-1261b. [DOI] [PubMed] [Google Scholar]
- 106.Frazier SC. Health outcomes and polypharmacy in elderly individuals: an integrated literature review. J Gerontol Nurs. 2005;31:4–11. doi: 10.3928/0098-9134-20050901-04. [DOI] [PubMed] [Google Scholar]
- 107.Drake TA, Schadt E, Hannani K, et al. Genetic loci determining bone density in mice with diet-induced atherosclerosis. Physiol Genomics. 2001;5:205–215. doi: 10.1152/physiolgenomics.2001.5.4.205. [DOI] [PubMed] [Google Scholar]
- 108.Toye AA, Lippiat JD, Proks P, et al. A genetic and physiological study of impaired glucose homeostasis control in C57BL/6J mice. Diabetologia. 2005;48:675–686. doi: 10.1007/s00125-005-1680-z. [DOI] [PubMed] [Google Scholar]
- 109.Kuchel GA, DuBose M, Gruman C, et al. Aging Patterns in Mouse Strains Used in Gene Deletion Studies: Implications for Sarcopenic Obesity. Journal of the American Geriatrics Society. 2006;54:S134–S135. [Google Scholar]
- 110.Cappola AR, Xue QL, Ferrucci L, et al. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab. 2003;88:2019–2025. doi: 10.1210/jc.2002-021694. [DOI] [PubMed] [Google Scholar]
- 111.Taylor JA, Kuchel GA. Detrusor Underactivity: Clinical features and pathogenesis of an underdiagnosed geriatric condition. Journal of the American Geriatrics Society. 2006 doi: 10.1111/j.1532-5415.2006.00917.x. In press. [DOI] [PubMed] [Google Scholar]
- 112.Elbadawi A, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction. II Aging detrusor: Normal versus impaired contractility. Journal of Urology. 1993;150:1657–1667. doi: 10.1016/s0022-5347(17)35867-6. [DOI] [PubMed] [Google Scholar]
- 113.Taylor JA, Zhu Q, Irwin B, et al. Null mutation in macrophage migration inhibitory factor (MIF) prevents muscle cell loss and fibrosis in partial bladder outlet obstruction. American Journal of Physiology: Renal, Fluid and Electrolyte Physiology. 2006 doi: 10.1152/ajprenal.00144.2006. In press. [DOI] [PubMed] [Google Scholar]
- 114.Kuchel GA, Zhu Q. Role of Immune and Endocrine Factors in Detrusor Underactivity and Urinary Retention. Journal of the American Geriatrics Society. 2006;54:S13. doi: 10.1111/j.1532-5415.2006.00917.x. [DOI] [PubMed] [Google Scholar]
- 115.Meyer-Siegler KL, Iczkowski KA, Vera PL. Macrophage Migration Inhibitory Factor is Increased in the Urine of Patients With Urinary Tract Infection: Macrophage Migration Inhibitory Factor-Protein Complexes in Human Urine. J Urol. 2006;174:1523–1528. doi: 10.1016/S0022-5347(05)00650-6. [DOI] [PubMed] [Google Scholar]
- 116.Gitler MS, Monks A, Sausville EA. Preclinical models for defining efficacy of drug combinations: mapping the road to the clinic. Mol Cancer Ther. 2003;2:929–932. [PubMed] [Google Scholar]
- 117.Tinetti ME, Gordon C, Lapin P, et al. Fall prevention: a case study of the challenges in adopting evidence-based geriatric care practices. The Gerontologist. 2006 doi: 10.1093/geront/46.6.717. In press. [DOI] [PubMed] [Google Scholar]
- 118.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354:1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 119.Inouye SK, Foreman MD, Mion LC, et al. Nurses’recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med. 2001;161:2467–2473. doi: 10.1001/archinte.161.20.2467. [DOI] [PubMed] [Google Scholar]
- 120.Inouye SK, Leo-Summers L, Zhang Y, et al. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53:312–318. doi: 10.1111/j.1532-5415.2005.53120.x. [DOI] [PubMed] [Google Scholar]
- 121.Naughton BJ, Saltzman S, Ramadan F, et al. A multifactorial intervention to reduce prevalence of delirium and shorten hospital length of stay. J Am Geriatr Soc. 2005;53:18–23. doi: 10.1111/j.1532-5415.2005.53005.x. [DOI] [PubMed] [Google Scholar]
- 122.Tabet N, Hudson S, Sweeney V, et al. An educational intervention can prevent delirium on acute medical wards. Age Ageing. 2005;34:152–156. doi: 10.1093/ageing/afi031. [DOI] [PubMed] [Google Scholar]
- 123.Milisen K, Foreman MD, Abraham IL, et al. A nurse-led interdisciplinary intervention program for delirium in elderly hip-fracture patients. J Am Geriatr Soc. 2001;49:523–532. doi: 10.1046/j.1532-5415.2001.49109.x. [DOI] [PubMed] [Google Scholar]
- 124.Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516–522. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 125.Inouye SK, Bogardus ST, Jr, Baker DI, et al. The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. Hospital Elder Life Program J Am Geriatr Soc. 2000;48:1697–1706. doi: 10.1111/j.1532-5415.2000.tb03885.x. [DOI] [PubMed] [Google Scholar]
- 126.Rizzo JA, Bogardus ST, Jr, Leo-Summers L, et al. Multicomponent targeted intervention to prevent delirium in hospitalized older patients: what is the economic value? Med Care. 2001;39:740–752. doi: 10.1097/00005650-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 127.Leslie DL, Zhang Y, Bogardus ST, et al. Consequences of preventing delirium in hospitalized older adults on nursing home costs. J Am Geriatr Soc. 2005;53:405–409. doi: 10.1111/j.1532-5415.2005.53156.x. [DOI] [PubMed] [Google Scholar]
- 128.Rubin FH, Williams JT, Lescisin DA, et al. Replicating the hospital elder life program in a community hospital and demonstrating effectiveness using quality improvement methodology. J Am Geriatr Soc. 2006;54:969–974. doi: 10.1111/j.1532-5415.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 129.Bradley EH, Schlesinger M, Webster TR, et al. Translating research into clinical practice: making change happen. J Am Geriatr Soc. 2004;52:1875–1882. doi: 10.1111/j.1532-5415.2004.52510.x. [DOI] [PubMed] [Google Scholar]
- 130.Bradley EH, Webster TR, Baker D, et al. After adoption: sustaining the innovation. A case study of disseminating the hospital elder life program. J Am Geriatr Soc. 2005;53:1455–1461. doi: 10.1111/j.1532-5415.2005.53451.x. [DOI] [PubMed] [Google Scholar]
- 131.Tinetti ME, Baker DI, McAvay G, et al. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med. 1994;331:821–827. doi: 10.1056/NEJM199409293311301. [DOI] [PubMed] [Google Scholar]
- 132.Guideline for the prevention of falls in older persons. American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. J Am Geriatr Soc. 2001;49:664–672. [PubMed] [Google Scholar]
- 133.Wenger WC, Tinetti ME, King MB. Perceptions of physicians on the barriers and facilitators to integrating fall risk evaluation and management into practice. Journal of General Internal Medicine. 2006;21:117–122. doi: 10.1111/j.1525-1497.2005.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Baker DI, King MB, Fortinsky RH, et al. Dissemination of an evidence-based multicomponent fall risk-assessment and -management strategy throughout a geographic area. J Am Geriatr Soc. 2005;53:675–680. doi: 10.1111/j.1532-5415.2005.53218.x. [DOI] [PubMed] [Google Scholar]
- 135.Chou WC, Tinetti ME, King MB, et al. Perceptions of physicians on the barriers and facilitators to integrating fall risk evaluation and management into practice. J Gen Intern Med. 2006;21:117–122. doi: 10.1111/j.1525-1497.2005.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Solomon DH, Van HL, Glynn RJ, et al. Academic detailing to improve use of broad-spectrum antibiotics at an academic medical center. Arch Intern Med. 2001;161:1897–1902. doi: 10.1001/archinte.161.15.1897. [DOI] [PubMed] [Google Scholar]