Summary
Although caffeine is the most consumed psychoactive substance in the world, the extents of many of its effects are unknown. High doses of caffeine have been shown to activate the HPA axis while the effects of low to moderate doses have usually not been described in detail. Moreover, although several lines of evidence suggest that low doses of caffeine may restrain some negative affective states, the possible modulatory role of caffeine on HPA axis activation induced by a stressful stimulus has not been described. Thus, the present studies investigated the possible modulatory effects of low to moderate doses of caffeine on moderate to high HPA axis activation induced by different intensities of loud noise. First, in order to test this modulation, time courses for adrenocorticotropic hormone (ACTH) and corticosterone responses to loud noise stress and to caffeine were defined, in rats. Plasma ACTH and corticosterone levels peaked 30 min from the onset of noise presentation, and rapidly declined after noise termination. A low caffeine dose of 2 mg/kg significantly increased plasma corticosterone and ACTH levels 30 min following injections, but levels returned to baseline 60 min following injections. Caffeine doses of 30 mg/kg and higher elevated plasma hormone levels for at least 2 h. Doses of 2 or 10 mg/kg, however, did not modulate endocrine responses to loud noise presentation. It is concluded that although caffeine activates the HPA axis, low to moderate doses do not modulate HPA axis responses to stressful stimuli.
Keywords: ACTH, Corticosterone, HPA, Stress, Noise, Adenosine
1. Introduction
Caffeine is the most commonly consumed psychologically and physiologically active drug in the world. It is not ordinarily considered a drug of abuse, and government agencies have not imposed restrictions on caffeine consumption. However, while caffeine induces several well-documented positive effects such as increased alertness and cognition, caffeine can induce several potentially negative consequences. For example, it has been shown to induce anxiety in both animals and humans. In rats, high doses of caffeine induce anxiogenic behavior in the elevated plus maze (Lister, 1987; Baldwin et al., 1989; Jain et al., 1995; Bhattacharya et al., 1997). In addition, acute caffeine doses potentiate panic attacks in human volunteers with high anxiety (Bourin et al., 1998). Importantly, caffeine also elevates glucocorticoid levels in animals and humans. Acute caffeine increases plasma cortisol levels when injected into sleeping subjects (Lin et al., 1997) and elevates plasma corticosterone levels in rats (Nicholson, 1989).
Although the specific mechanism of caffeine-induced glucocorticoid release has yet to be elucidated, this effect is likely primarily mediated by central antagonistic actions at adenosine receptors that ultimately regulate the paraventricular nucleus of the hypothalamus, which in turn controls the pituitary-adrenocortical (HPA) system. In support of such a mechanism, only very high caffeine concentrations induced adrenocorticotropin hormone (ACTH) and corticosterone release from in vitro rat pituitary and adrenal gland tissues, respectively, as compared to effective systemic doses in vivo (Nicholson, 1989). In addition, blockade of corticotropin-releasing factor (CRF) release completely attenuated the caffeine-induced endocrine response in vivo, and only very high concentrations of caffeine induced CRF release from hypothalamic explants in vitro (Nicholson, 1989). Thus, caffeine likely activates the HPA axis via an interaction with centrally located adenosine receptors in hypothalamic afferent regions, eventually modulating CRF and HPA axis activity.
Importantly, several of caffeine's effects are biphasic. For example, in rats, caffeine has biphasic effects on locomotor activity (Svenningsson et al., 1995) and rotation behavior (Garrett and Holtzman, 1995), with low doses displaying an activating effect and high doses producing inhibitory effects. Additional evidence also suggests that caffeine may produce biphasic effects related to stress and anxiety. While high doses of caffeine consistently induce anxiety in rodents (Lister, 1987; Baldwin et al., 1989; Jain et al., 1995; Bhattacharya et al., 1997), a low dose of caffeine was ascribed a relaxing effect as indexed by the reduction of some neurotransmitter system activity in response to restraint stress (Carter et al., 1995; Yamato et al., 2002). Such putative anxiolytic or stress reducing effects have not, however, been tested directly using effector systems such as behavioral or neuroendocrine indices.
As mentioned above, high caffeine doses activate the HPA axis (Nicholson, 1989), but the effects of low to moderate doses of caffeine on HPA axis activation have not been studied systematically. In addition, although stressful stimuli were shown to attenuate some of the motor effects of caffeine (Meyer and Caston, 2005), the specific interactions of different doses of caffeine with neuroendocrine responses induced by stressful stimuli have not yet been reported. Thus, the present studies were designed to test the hypothesis that relatively low doses of caffeine might reduce HPA axis activation induced by moderately or highly stressful stimuli, as measured with plasma corticosterone and ACTH release. In order to test this potential central modulation of neuroendocrine response to stress by caffeine, we first characterized the time course of the endocrine response to loud noise and to different doses of acute caffeine, individually. We then tested the modulation of the HPA axis response to different levels of loud noise by different doses of caffeine.
2. Methods
2.1. Animals
Male Sprague-Dawly rats (225-250 g at arrival) from Harlan were used in these studies. They were kept on a 12:12 light/dark cycle (lights on at 7:00 am) in humidity and temperature controlled environment. Rats were kept in groups of four until surgery after which they were singly housed in polycarbonate cages with food and water available ad lib, and were allowed at least 1 week to acclimatize to the colony room before surgical or experimental manipulations. Rats were transferred to the experimental chambers, singly in polycarbonate cages (43×22×21 cm), the afternoon before the experiment for all experiments described. Animals were sacrificed by decapitation at the times indicated in each experiment. All rats were sacrificed between 9:30 and 10:30am to control for circadian variation of HPA axis hormones. All procedures performed were approved by the animal care and use committee of the University of Colorado.
2.2. Surgery: intraperitoneal (i.p.) catheterization
In order to avoid the stress of handling and direct intraperitoneal (i.p.) injections, rats employed in experiments 3-5 were fitted with i.p. catheters. Catheters were made from Silastic® tubing, 0.02″ i.d. and 0.037″ o.d. (Dow Corning, Midland, MI). They were 20 cm long with a 3 cm cuff at one end, made from a double ring of silicon medical adhesive (Dow Corning, Midland, MI). Rats were anaesthetized with sodium pentobarbital and a small incision was made in the peritoneal cavity, and the end of the catheter placed inside. The catheters were secured by tying a suture around the cuff and secured to the muscle layer of the abdominal wall. The same suture was used to close the incision in the abdominal wall. The catheter was exteriorized subcutaneously with the aid of a 10 G trocar at the dorsal nape of the neck. The catheter was secured via a pedestal (Plastics One, Inc., Roanoke, VA) anchored to the skull with dental cement. Wound clips were used to close the skin incisions. The length of the catheter between the cuff and pedestal provided slack within the body to allow for the animal's normal movements. Catheters were flushed with 0.8 ml of a 0.9% saline solution (i.p.), to ensure that they were patent. Animals were allowed to recover for at least 7 days before the experiment.
2.3. Corticosterone radioimmunoassay
The method for the assay has been described previously by Day and Akil (1999), and is routinely performed in the laboratory. Briefly, plasma was heat-treated to remove corticosterone from its binding protein. Plasma samples, controls, and standards were incubated overnight in phosphate buffer at 4 °C together with 3H-corticosterone (Amersham Pharmacia Biotech, Piscataway, NJ) and an antibody generated against corticosterone (courtesy of Dr S.J. Watson, University of Michigan). The samples were then extracted with phosphate buffer containing charcoal and dextran, centrifuged, and the supernatant counted for 3H using a liquid scintillation counter. Levels of corticosterone were calculated against a standard curve generated concurrently. All plasma from a given experiment was assessed simultaneously. The limits of corticosterone detection ranged from 0 to 80 ug/dl.
2.4. Adrenocorticotropic hormone (ACTH) radioimmunoassay
Blood plasma ACTH levels were quantified as directed in a radioisotopic kit from Nichols Institute Diagnostics. All plasma from a given experiment was assessed simultaneously. The limits of ACTH detection ranged from 1 to 1400 pg/ml.
2.5. Drug delivery
The afternoon before the experiment, animals were transported to the behavioral testing rooms, placed in sound attenuating chambers, and the catheters were connected to a syringe filled with saline, via PE tubing connecting a syringe outside of the chamber to a cage-top mounted fluid swivel (Instech, Plymouth Meeting, PA). Each cage contained a small 8 W light bulb that was kept on the same on-off schedule as the colony room. The next morning, animals were injected i.p. with caffeine or saline. All injections took place between 8:00 and 9:00 am to control for variations of HPA hormones. Caffeine was dissolved in 0.9% saline and injections were given as 1 ml/kg (body weight). This was immediately followed by a 0.5 ml saline injection to ensure 100% drug delivery.
2.6. Experiment 1–time of peak endocrine response to loud noise
The afternoon before the experiment, rats were transported to the behavioral testing room and placed in ventilated double wooden (1.00″ plywood board) acoustic chambers, with outer chamber lined with 1.00″ insulation (Celotex TM). The internal dimensions of the inner box were 23.5″ (w)×15″ (d)×15″ (h), allowing placement of a polycarbonate cage inside. In order to determine the specific timing of endocrine responses to audiogenic stress, animals (n=5/group) were given either no noise, or 2, 4, 8, 15, 30, or 60 min noise (105 dBA, SPL). Noise was generated by a general radio (#1381) solid-state random noise generator, amplified by a Pyramid Studio Pro amplifier (#PA-600X) and delivered via a 6″×9″ Optimus speaker fixed in the middle of the ceiling of the chamber. Rats were sacrificed and trunk blood was collected for ACTH and corticosterone analysis immediately after noise termination.
2.7. Experiment 2–duration of endocrine response to loud noise
Animals (n=6/group) received 30 min of loud noise (105 dBA, SPL) which was produced as described above. Animals were sacrificed and trunk blood collected either immediately (0), or 15, 30, 60 or 120 min following the termination of the noise. An additional group served as a no noise control (C).
2.8. Experiment 3—time course of endocrine responses to different doses of caffeine
The time course for plasma corticosterone responses to caffeine was investigated between 30 min and 2 h following caffeine injections and ACTH was measured between 30 and 90 min following injections. Animals were sacrificed at various time points (30, 60, 90 and 120 min), and doses of 2, 10, and 30 mg/kg of caffeine or vehicle were employed (n=5-7 per group), which are roughly equivalent to approximately 1, 3 and 9 cups of coffee, respectively (Fredholm et al., 1999). These doses were employed because traditionally doses of 30 mg/kg and higher have been considered high doses not relevant to human consumption, but doses lower than 20 mg/kg did not activate the HPA axis 2 h following injections but were not studied at earlier time points (Nicholson, 1989). A dose similar to the lowest dose employed (2 mg/kg) was ascribed a relaxant effect by Yamato et al. (2002), and to our knowledge, no other studies have reported effects at doses lower than 2 mg/kg. In a pilot study an additional group was injected with 60 mg/kg caffeine and sacrificed 2 h following injection, but the corticosterone response for this group was not different from the group injected with 30 mg/kg caffeine.
2.9. Experiment 4—stress response experiment
To determine the effects of low and moderate doses of caffeine on stress responsiveness, animals underwent audiogenic stress in the presence or absence of acute caffeine. Animals were injected with caffeine (2 or 10 mg/kg) or saline and 30 min later exposed to 30 min of white noise (80 or 105 dB) (n=5/group for all groups except n=10/group for the group that received 105 dB noise and vehicle injections). These specific noise intensities were employed to induce moderate and high HPA axis activation as previously reported by Campeau & Watson (1997). Trunk blood was obtained 1 h following injections (immediately after noise termination) to determine plasma levels of corticosterone and ACTH, based on the time course results of experiments 1, 2, and 3.
2.10. Statistical analysis
All results were expressed as means ±SEMs. They were analyzed with one- or two-way ANOVA followed by post-hoc analysis for individual group differences using Tukey two tailed t-tests. All p-values <.05 were considered significant.
3. Results
3.1. Experiment 1—time of peak endocrine response to loud noise
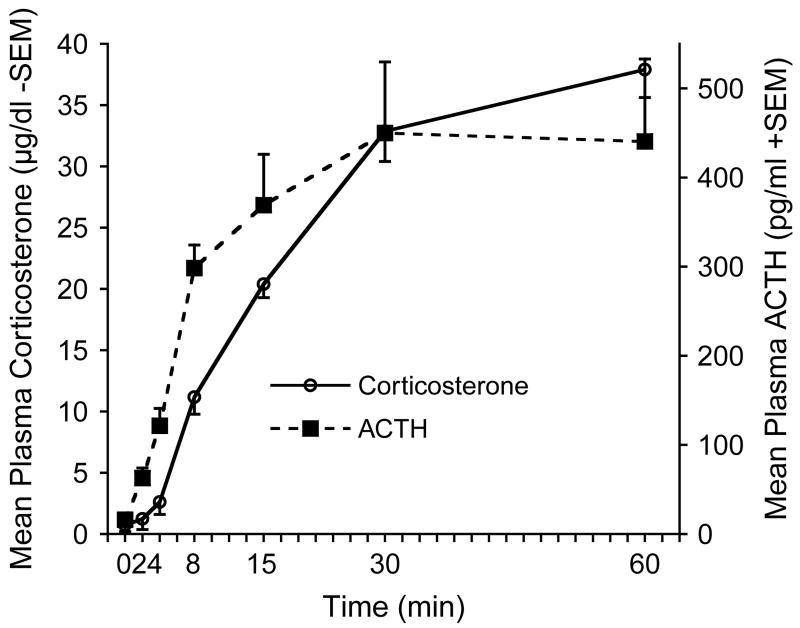
Plasma ACTH and corticosterone levels were significantly elevated in response to loud noise (105 dBA SPL) exposure (F6,28=102.52, and 11.98, p<.0001, for corticosterone and ACTH, respectively), as depicted in Fig. 1. Corticosterone levels were reliably elevated beginning at 8 min into the noise session (p<.05). The 30 and 60 min groups showed higher coritcosterone than all other time points (all p<.05) but not different from each other. ACTH levels were also reliably elevated at 8 min (p<.05). The 15, 30, and 60 min groups were not different from each other, but were all higher than the shorter time points (0, 2, and 4 min) (all p<.05). These results suggest that 30 min from the noise onset is an ideal time to detect maximum ACTH and corticosterone responses to the loud noise.
Figure 1.
Mean levels of plasma corticosterone (μg/dl−SEM) and ACTH (pg/ml+SEM) evoked in rats given up to 60 min of loud noise (105 dBA, SPL) at different time points (2, 4, 8, 15, 30, and 60 min). The 0 min group was not given any noise presentation. Levels of plasma ACTH rose quickly following the beginning of noise presentation and rose steadily until 30 min. Corticosterone plasma levels followed a dynamic of release similar but slightly delayed compared to ACTH release.
3.2. Experiment 2–duration of endocrine response to loud noise
Upon termination of 30 min of loud noise, the endocrine indices were reduced to control values within 60 min of noise cessation (F5,30=80.75, and 24.99, p<.0001, for corticosterone and ACTH, respectively), as depicted in Fig. 2. Although corticosterone levels trailed ACTH levels by approximately 15 min, the 60 and 120 min time point groups for both hormones were not significantly different from the control values (p>.05). The 30 min ACTH recovery time point group was also similar to the control group mean (p>.05), indicating a quick return to basal levels following noise termination. Furthermore, 15 min after noise termination ACTH was reliably lower than immediately upon noise termination (time=0 min) p<.05. Thus, in order to detect corticosterone and ACTH responses to noise stress, the blood plasma must be collected immediately after noise termination because of the especially rapid return to baseline for plasma ACTH levels.
Figure 2.
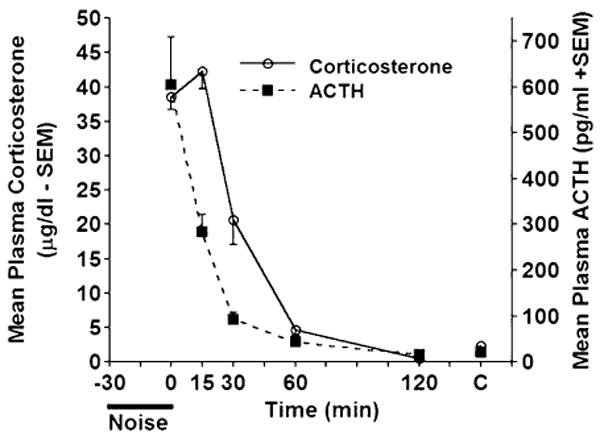
Mean levels of plasma corticosterone (μg/dl−SEM) and ACTH (pg/ml+SEM) following the termination of 30 min of loud noise (105 dBA, SPL) at different time points (0, 15, 30, 60 and 120 min). The 0 min group was given 30 min loud noise presentation. Rats not given noise, but sacrificed at the same time as the experimental rats served as controls (C at the far right of the graph). Levels of plasma ACTH were already significantly reduced 15 min following noise termination. The reduction in plasma corticosterone levels lagged that of ACTH, and was evident beginning at the 30 min time point.
3.3. Experiment 3–time course of endocrine responses to different doses of caffeine
The time course for corticosterone responses was characterized between 30 and 120 min after injections of caffeine (2, 10 and 30 mg/kg) (n=5-7 per group) and between 30 and 90 min following injections ACTH levels were measured (n=3-6 per group). The plasma corticosterone levels are shown in Fig. 3a. Two-way ANOVA revealed a significant effect of dose (F(3,74)=21.472, p<.0001) that was not significantly dependent on time (F(9,74)=1.068, p<.474). Animals that received doses of 30 and 10 mg/kg displayed significantly higher corticosterone levels than saline injected controls between 30 and 90 min (all p<.001), but remained elevated 2 h following injection only for animals that received 30 mg/kg caffeine (p<.001). Furthermore, the 30 mg/kg caffeine dose produced higher corticosterone levels than the 10 mg/kg dose at 60 and 90 min (p<.05). Rats injected with 2 mg/kg caffeine displayed corticosterone levels significantly higher than controls but only at the 30 min time point (p<.001). The corticosterone elevation displayed by the 2 mg/kg group at 30 min was still lower than that displayed by the 30 mg/kg group (p<.05). Plasma ACTH levels are shown in Fig. 3b. Caffeine doses significantly increased plasma ACTH levels (F(3,48)=11.28, p<.0001), but the main effect of time between sacrifice and injection failed to reach a statistically significant level over the observed time period (F(6,48=1.293, p=.28)). Differences between animals which received 10 and 30 mg/kg failed to reach statistical significance at all time points measured. Both doses had significantly higher values than saline injected controls throughout the time course (all p<.001). Plasma ACTH was elevated by a dose of 2 mg/kg at 30 min (p<.001), but at 60 and 90 min levels had dropped to control values. Thus, a more complete time course at low to moderate doses of caffeine does reveal HPA axis activation with shorter time to peak, even at a dose of 2 mg/kg.
Figure 3.
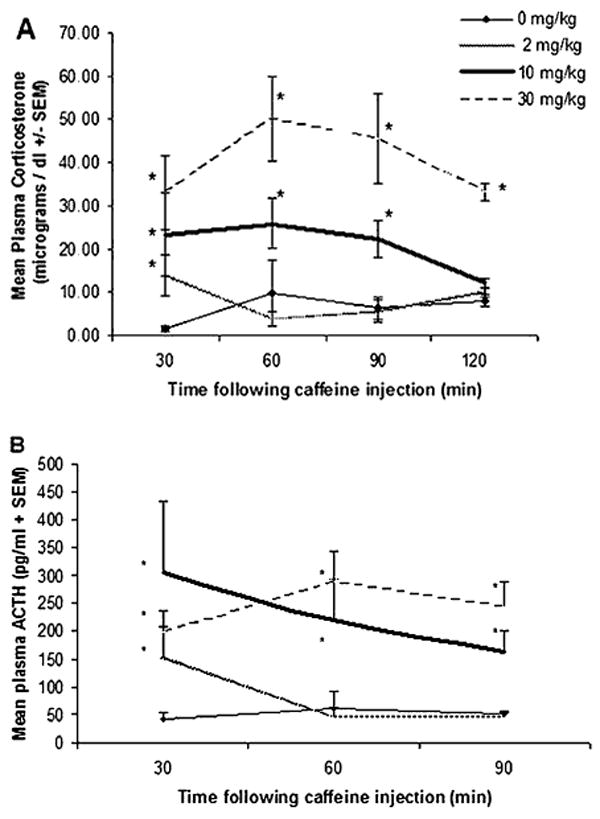
Different groups of rats received different doses of caffeine and were sacrificed at different times following injection. A, Mean plasma corticosterone levels (μg/dl±SEM). B, Mean plasma adrenocorticotropic hormone (ACTH) levels (pg/ml+SEM). Asterisks indicate significant differences between saline vs caffeine-injected groups measured at the same time.
3.4. Experiment 4–modulation of stress-induced response by caffeine
Experiment 4 was conducted in order to test the putative modulation by acute doses of caffeine on endocrine responses to a psychologically stressful event. Animals were either kept at ambient noise levels (approximately 60 dB), or exposed to white noise (80, or 105 dB) for 30 min which began 30 min after an injection of 2 or 10 mg/kg of caffeine or vehicle (saline), and plasma corticosterone and ACTH were measured 1 h after injection, immediately upon noise termination. This arrangement allowed measurement of HPA axis activation at a time when noise induces a maximal ACTH and corticosterone response, but the effect of 2 mg/kg caffeine was found to be minimal at this post-injection time (Experiment 3). The results are depicted in Fig. 4. Animals exposed to 80 or 105 dB white noise displayed significantly higher corticosterone and ACTH levels than no noise controls (all p<.0001). Saline injected animals that were exposed to 80 dB noise had significantly lower plasma ACTH levels than saline injected animals exposed to 105 dB noise (p<.05). However, corticosterone levels did not significantly vary between these groups. Caffeine injections did not significantly affect the corticosterone or ACTH response to noise when compared to the saline injected controls exposed to the same intensity of noise. The difference in the increase in ACTH levels induced by 80 dB noise did not reach statistical significance between saline injected controls and animals that received 2 and 10 mg/kg caffeine (p=.2 and .1, respectively). Thus, a moderate dose of caffeine (10 mg/kg) induced a mild increase in the ACTH elevation induced by 80 dB loud noise, but this effect is likely an additive one between the two conditions because 10 mg/kg caffeine alone induce a significant rise in ACTH levels in the ambient noise condition. Importantly an expected attenuation of the endocrine response to loud noise by a low dose of caffeine (2 mg/kg) was not observed at any noise intensity measured.
Figure 4.
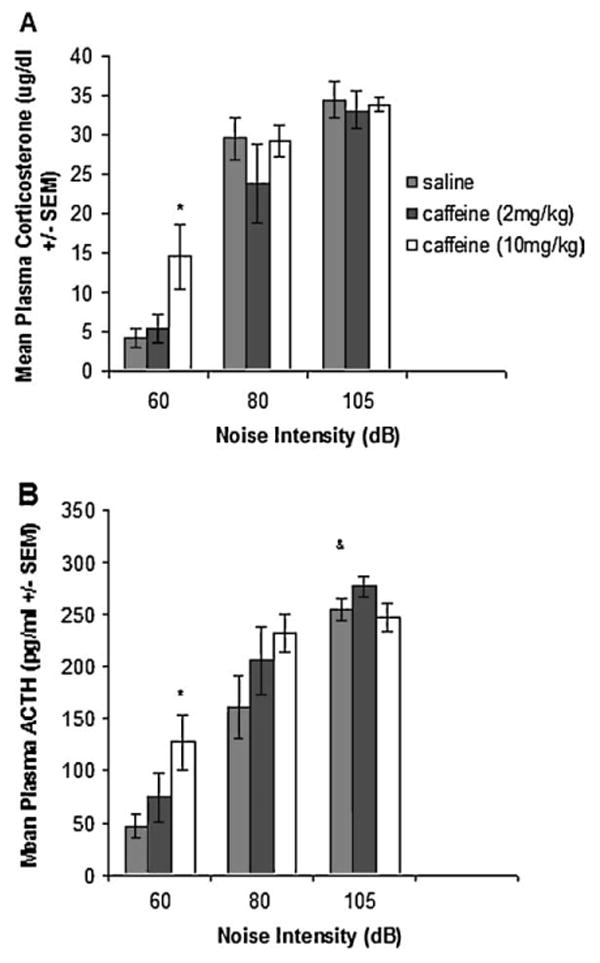
Different groups of rats either received no noise (60 dB background condition) or were exposed to 30 min of different intensities (80 and 105 dB) of loud white noise 30 min after receiving a 2 or 10 mg/kg caffeine injection. A, Mean plasma corticosterone levels (μg/dl±SEM). B, Mean plasma ACTH levels (pg/ml±SEM). Asterisks indicate significant differences between saline vs caffeine-injected groups exposed to the same noise intensity. (&) Indicates significant differences between saline injected rats at 80 vs 105 dB (p<.05).
4. Discussion
Generally, the results of the present studies show that acute high and moderate doses of caffeine consistently activate the HPA axis and a dose as low as 2 mg/kg activates the HPA axis 30 min post-injection. Additionally, loud white noise induces temporally predictable HPA axis activation. Caffeine, however, did not significantly modulate the HPA axis responsiveness to white noise stress.
The results of Experiment 1 indicate that plasma corticosterone and ACTH levels quickly begin to rise upon initiation of loud noise, and maintained high, non-decremental levels for at least 1 h if the noise is kept on. The levels of ACTH and corticosterone also declined rapidly, such that by 15 min after noise termination, ACTH levels were already significantly reduced compared to their peak values, with corticosterone declining with a 15-min lag period compared to ACTH, as shown in Experiment 2. Hormone levels were not significantly different from non-stressed values by 60 min after stress cessation. This pattern of endocrine release is similar to that previously reported in response to noise (Britton et al., 1992), but slower than in response to immobilization, restraint, ether, or electric shocks (Hauger et al., 1988; Keller-Wood and Dallman, 1984; Rivier and Vale, 1987; Kovacs and Sawchenko, 1996). These results emphasize the fact that dynamic HPA axis regulation can be quite different given the precise nature of the stress situation. Together, Experiments 1 and 2 suggests that maximum endocrine responses to noise can be measured 30 min after the initiation of loud noise presentation and immediately following its termination.
The present corticosterone responses to caffeine measured at 2 h are consistent with those previously observed (Nicholson, 1989). Doses of 30 mg/kg and higher, but not 10 mg/kg and lower, induced significant elevations in basal plasma corticoster-one levels 2 h following caffeine injections. It was possible, however, that lower doses of caffeine would induce hormonal responses at earlier time points. Indeed, the present time course shows that plasma corticosterone and ACTH levels remain elevated from 30 to 90 min but return to baseline 2 h following a 10 mg/kg caffeine injection. Importantly, a low dose of caffeine (2 mg/kg) elevated corticosterone and ACTH levels 30 min but not 60 min following the injection. These results thus extend the findings of Nicholson (1989) in that caffeine induces HPA axis activation, but at much lower doses than previously reported. Because the hormone levels return to baseline 60 min following a 2 mg/kg injection, any caffeine induced modulation of endocrine responses to other events could perhaps be detected at this time.
More to the point, caffeine did not significantly modulate the peak endocrine responses to loud white noise, irrespective of doses. The higher corticosterone and ACTH release induced by the 10 mg/kg caffeine dose, especially in the background (60 dB) noise condition is likely a reflection of the ability of caffeine at this dose to induce HPA axis activation basally, as shown in Experiment 3, but not by reducing the threshold to stressful events. A number of prior results suggested that a low dose of caffeine may produce an attenuation of the HPA axis response to stressful stimuli: first, because of the biphasic effects of caffeine reported in some responses (Svenningsson et al., 1995; Garrett and Holtzman, 1995); second, the finding that a similar low dose (2 mg/kg) attenuated the restraint-induced elevations in hippocampal serotonin and dopamine release (Yamato et al., 2002), despite the fact that caffeine alone elevated these neurotransmitters in the hippocampus (Carter et al., 1995); and finally, the existence of a putative mechanism whereby antagonism at adenosine A2a receptors might produce different effects than antagonism at the A1 receptors (Fredholm et al., 1999), as indicated by the findings that only high doses of caffeine (10 mg/kg and above) induce anxiety in rodents (Lister, 1987; Baldwin et al., 1989; Jain et al., 1995; Bhattacharya et al., 1997), while selective blockade of A1 receptors (Florio et al., 1998) but not A2a receptors (El Yacoubi et al., 2000) was reported to induce anxiety. These central actions of caffeine might have been expected to generalize to regulation of the hypothalamic paraventricular nucleus. We demonstrate here, however, that low doses of caffeine do not attenuate peak HPA axis responses to stress. Thus, the previously observed modulation of hippocampal neurotransmitter levels by low doses of caffeine appear independent from HPA axis regulation. This result is unlikely to be simply explained by a general inability to attenuate noise-induced HPA axis activation, as this has been demonstrated in response to other drug treatments (Gue et al., 1987). Overall, therefore, these results do not support the hypothesis that even low doses of caffeine may restrain a variety of negative affective states. It should be noted that area under the curve assessed at different time points would provide a more accurate determination of hormonal release with this drug treatment, as compared to determination from a single time point as performed in the present experiment (Day and Akil, 1999; Garcia et al., 2000). Thus, the lack of change in peak amplitude neuroendocrine responses observed in the present study does not rule out possible regulation of the onset and/or offset of these responses, which the present study did not evaluate.
It should also be noted that the hormonal responses to noise intensities were more sensitive than previously observed (Campeau and Watson, 1997). Thus, a ceiling effect on the hormonal responses could have been a limitation to observe a caffeine induced sensitization to loud noise stress. Thus, the lowest noise intensity was employed which produced a significantly lower rise in plasma ACTH than the higher intensity. Still the corticosterone and ACTH responses to noise were not significantly different between saline and caffeine injected rats. The fact that the ACTH response to 80 dB noise was slightly elevated in caffeine injected rats is likely an additive effect between the different stimuli because 10 mg/kg of caffeine alone significantly elevated plasma hormone levels.
In conclusion, a wide range of caffeine doses activate the HPA axis. However, although it may still be a matter of debate whether low doses of caffeine modulate affective states, low to moderate doses do not clearly modulate peak endocrine responses to other stressful stimuli.
Acknowledgments
This work was supported by grant MH065327 and MH068016 (SC) and a Howard Hughes Biological Sciences Undergraduate Initiative grant (MDP).
References
- Baldwin HA, Johnston AL, File SE. Antagonistic effects of caffeine and yohimbine in animal tests of anxiety. European Journal of Pharmacology. 1989;159:211–215. doi: 10.1016/0014-2999(89)90709-7. [DOI] [PubMed] [Google Scholar]
- Bhattacharya SK, Satyan KS, Chakrabarti A. Anxiogenic action of caffeine: an experimental study in rats. Journal of Psychopharmacology. 1997;11:219–224. doi: 10.1177/026988119701100304. [DOI] [PubMed] [Google Scholar]
- Bourin M, Baker GB, Bradwejn J. Neurobiology of panic disorder. Journal of Psychosomatic Research. 1998;44:163–180. doi: 10.1016/s0022-3999(97)00203-1. [DOI] [PubMed] [Google Scholar]
- Britton KT, Segal DS, Kuczenski R, Hauger R. Dissociation between in vivo hippocampal norepinephrine response and behavioral/neuroendocrine responses to noise stress in rats. Brain Research. 1992;574:125–130. doi: 10.1016/0006-8993(92)90808-m. [DOI] [PubMed] [Google Scholar]
- Campeau S, Watson SJ. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. Journal of Neuroendocrinology. 1997;9:577–588. doi: 10.1046/j.1365-2826.1997.00593.x. [DOI] [PubMed] [Google Scholar]
- Carter AJ, O'Connor WT, Carter MJ, Ungerstedt U. Caffeine enhances acetylcholine release in the hippocampus in vivo by a selective interaction with adenosine A1 receptors. Journal of Experimental Therapeutics. 1995;273:637–642. [PubMed] [Google Scholar]
- Day HE, Akil H. Evidence that cholecystokinin receptors are not involved in the hypothalamic-pituitaty-adrenal response to intraperitoneal administration of interleukin-1beta. Journal of Neuroendocrinology. 1999;7:561–568. doi: 10.1046/j.1365-2826.1999.00358.x. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM. The anxiogenic-like effect of caffeine in two experimental procedures measuring anxiety in the mouse is not shared by selective A(2A) adenosine receptor antagonist. Psychopharmacology. 2000;148:153–163. doi: 10.1007/s002130050037. [DOI] [PubMed] [Google Scholar]
- Florio C, Prezioso A, Papaioannou A, Vertua R. Adenosine A1 receptors modulate anxiety in CD1 mice. Psychopharmacology. 1998;136:311–319. doi: 10.1007/s002130050572. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacological Reviews. 1999;51:83–133. [PubMed] [Google Scholar]
- Garcia A, Marti O, Valles A, Dal-Zotto S, Armario A. Recovery of the hypothalamic-pituitary-adrenal response to stress, Effect of stress intensity, stress duration and previous stress exposure. Neuroendocrinology. 2000;72:114–125. doi: 10.1159/000054578. [DOI] [PubMed] [Google Scholar]
- Garret BE, Holtzman SG. Does adenosine receptor blockade mediate caffeine-induced rotational behavior? Journal of Pharmocology an Experimental Therapeutics. 1995;274:207–214. [PubMed] [Google Scholar]
- Gue M, Fioramonti J, Frexinos J, Alvinerie M, Bueno L. Influence of acoustic stress by noise on gastrointestinal motility in dogs. Digestive Disorders Sciences. 1987;32:1411–1417. doi: 10.1007/BF01296668. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Millan MA, Lorang M, Harwood JP, Aguilera G. Corticotropin-releasing factor receptors and pituitary adrenal responses during immobilization stress. Endocrinology. 1988;123:396–405. doi: 10.1210/endo-123-1-396. [DOI] [PubMed] [Google Scholar]
- Jain N, Kemp N, Adeyemo O, Buchanan P, Stone TW. Anxiolytic activity of adenosine receptor activation in mice. British Journal of Pharmacology. 1995;116:2127–2133. doi: 10.1111/j.1476-5381.1995.tb16421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocrinology Reviews. 1984;5:1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ, Sawchenko PE. Sequence of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons. Journal of Neuroscience. 1996;16:262–273. doi: 10.1523/JNEUROSCI.16-01-00262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AS, Uhde TW, Slate SO, McCann UD. Effects of intravenous caffeine administered to healthy males during sleep. Depression and Anxiety. 1997;5:21–28. [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology. 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Meyer L, Caston J. Repeated stress alters caffeine action on motor coordination in C57Bl6/J male mice. Brain Research. 2005;1039:171–176. doi: 10.1016/j.brainres.2005.01.053. [DOI] [PubMed] [Google Scholar]
- Nicholson SA. Stimulatory effect of caffeine on the hypothalamo-pituitary-adrenocortical axis in the rat. Journal of Endocrinology. 1989;122:535–543. doi: 10.1677/joe.0.1220535. [DOI] [PubMed] [Google Scholar]
- Rivier C, Vale W. Diminished responsiveness of the hypothalamic-pituitary adrenal axis of the rat during exposure to prolonged stress: a pituitary-mediated mechanism. Endocrinology. 1987;121:1320–1328. doi: 10.1210/endo-121-4-1320. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nomikos GG, Fredholm BB. Biphasic changes in locomotor behavior and in expression of mRNA for NGFI-A and NGFI-B in rat striatum following acute caffeine administration. Journal of Neuroscience. 1995;15:7612–7624. doi: 10.1523/JNEUROSCI.15-11-07612.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamato T, Yamasaki S, Misumi Y, Kino M, Obata T, Aomine M. Modulation of the stress response by coffee: an in vivo microdialysis study of hippocampal serotonin and dopamine levels in rat. Neuroscience Letters. 2002;332:87–90. doi: 10.1016/s0304-3940(02)00828-5. [DOI] [PubMed] [Google Scholar]