Abstract
Eyes with age-related macular degeneration (AMD) demonstrate accumulation of specific deposits and extracellular matrix (ECM) molecules under the retinal pigment epithelium (RPE). AMD is about two times more prevalent in aging postmenopausal women. Therefore we studied whether 17β-estradiol (E2) modulates the expression and activity of the trimolecular complex (MMP-2, TIMP-2 and MMP-14), molecules which are of major importance for ECM turnover in RPE. We used cell lines isolated from estrogen receptor knockout mice (ERKO) to determine which ER (estrogen receptor) subtype was important for ECM regulation in RPE cells.
We found that mouse RPE sheets had higher baseline MMP-2 activity in the presence of ERβ. This correlated with higher MMP-2 activity in RPE cell lines isolated from ERKOα mice. Exposure to E2 increased MMP-2 activity in mouse RPE cell lines. In addition E2 increased transcriptional activation of the MMP-2 promoter through a functional Sp1 site which required the presence of ERβ, but not ERα. E2 also maintained levels of pro MMP-2, and MMP-14 and TIMP-2 activity after oxidant injury. Since the direct effects of E2 on MMP-2 transcriptional activation and the regulation of the trimolecular complex after oxidant induced injury requires ERβ, this receptor subtype may have a role as a potential therapeutic target to prevent changes in activation of MMP-2.
Keywords: Estrogen receptor, Age related macular degeneration, extracellular matrix, estrogen, matrix metalloproteinases, tissue inhibitors of metalloproteinases
Introduction
Age-related macular degeneration (AMD) is the most important cause of central vision loss in the elderly. It has been estimated to affect over 10 million individuals in the United States alone and there are estimates this will increase to 30 million by the year 2020 (Fine et al., 2000;Friedman et al., 2004). Early dry AMD is characterized by progressive thickening and accumulation of extracellular matrix (ECM) deposits under the retinal pigmented epithelial cells (RPE) (Berger et al., 1999;Green, 1999). While the mechanisms of deposit formation are complex, dsyregulation of ECM molecules most likely plays a key role. ECM in most tissues undergoes continuous turnover of collagen and other matrix components by a tightly regulated balance in production of matrix molecules like collagen IV, matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) (Corcoran et al., 1996). In general, MMPs degrade ECM components, activate other MMPs and participate in ECM homeostasis (Nagase et al., 2006). In particular proMMP-2, MMP14 and TIMP-2 form a trimolecular complex resulting in MMP-2 activation (Nagase et al., 2006). RPE cells synthesize fibronectin, collagens, and many other molecules important for the formation of its basement membrane and repair of Bruch’s membrane (Campochiaro et al., 1986). Active MMP-2 is the major RPE enzyme for the degradation of collagen types I and IV, and laminin, all essential components of Bruch’s membrane (Ahir et al., 2002;Eichler et al., 2002;Marin-Castano et al., 2003;Padgett et al., 1997). In addition, MMP-14 has a direct effect on collagen type I and III degradation (Itoh and Seiki, 2004).
We have previously shown that loss of RPE MMP-2 activity correlated with deposit severity in vivo, when mice were exposed to blue light, a surrogate for sunlight and acute oxidant injury. In vitro, exposure of human RPE cells to MPO/H2O2, relevant oxidant molecules, leads to non-lethal blebbing without cell death and decreased MMP-2 activity (Marin-Castano et al., 2005). Our most recent data suggests that a decrease in the trimolecular complex; pro MMP-2, MMP-14 and TIMP-2 results in the decreased MMP-2 activity (Elliot et al., 2006).
Estrogens, in particular, have potent regulatory influences on collagen and MMP expression, molecules important for ECM homeostasis (Lei et al., 1998;Potier et al., 2001) in the RPE as well as other organs. Estrogen action is mediated via the two estrogen receptor (ER) subtypes, ERα and ERβ. We have previously reported that both estrogen receptor subtypes α and β are expressed in vitro and in vivo in human RPE cells (Marin-Castano et al., 2003). The ER subtypes have unique roles in estrogen-dependent gene regulation and the transcriptional activities of ERα and ERβ differ depending on the cell and tissue context (Hall and McDonnell, 1999). The majority of tissues express predominantly one ER subtype over another, however expression of both ERα and ERβ in the same cell type has been reported for instance in neurons and thymocytes (Greco et al., 2001;Mor et al., 2001). Therefore the presence of both subtypes would predict a more complex regulation of estrogen-mediated gene expression.
The prevalence of dry AMD in aged women (estrogen deficient) is at least twice that of dry disease in men, and the frequency of progression into severe AMD is also greater. In mice, estrogen deficiency increases susceptibility to sub-retinal deposit formation in vivo (Espinosa-Heidmann et al., 2005). This led us to postulate that loss of estrogen regulation of MMP-2 activity either directly or via regulation of MMP-14 and TIMP-2 may be responsible for the development of sub-retinal deposits. Therefore, we isolated RPE cell lines from estrogen receptor knockout (ERKO) mice and their wildtype littermates in order to study the impact of the ER subtypes individually or together on matrix regulation. These cells retained their in vivo phenotype in culture as shown by the stable presence of RPE cell markers (Catanuto et al., 2007). Transient oxidant injury decreased MMP-2, MMP-14 and TIMP-2 activity however this decrease was prevented by physiologic concentrations of E2 only in the presence of ERβ. These results were ER-dependent since the complete ER antagonist, ICI 182780, prevented this protection. Transient transfection studies with an MMP-2 promoter construct, showed a direct effect of E2 on MMP-2 transcriptional activation if both subtypes or ERβ alone were present. These data suggest that ERβ protects against oxidant mediated decrease of MMP-2, TIMP-2 and MMP-14 and that ERα may have opposing effects. ER-dependent protection against oxidant injury-induced MMP-2 decrease occurs not only through regulation of the trimolecular complex but also through transcriptional regulation of the MMP-2 promoter.
Animals and Experimental Protocol
Female estrogen receptor knockout (ERKO) mice and wildtype littermates on a C57BL/6 background were obtained from the National Institute of Environmental Health. The guidelines of the ARVO Statement for the Use and of Animals in Ophthalmic and Vision Research were followed, and the Division of Veterinary Resources (University of Miami, Miller School of Medicine) approved all experiments. As described previously, the mice were fed a high-fat chow (Diet 5015; PMI Nutrition International Test Diet) for a total of eight months. Mice were sacrificed at 14 months of age and the eyes immediately removed for recovery of RPE sheets for cell culture.
RPE Isolation
RPE sheets were isolated as previously described (Catanuto et al., 2007). Briefly, the eyes were enucleated placed in a dish containing PBS (1x) and opened by a circumferential incision at the ora serrata. The anterior segment was removed, and the vitreous-retina was separated from the RPE and choroid eyecup. The remaining eyecup was incubated with dispase at 37°C for 10 minutes. The RPE sheets were transferred into 24 well plates to develop cell lines. An aliquot from the isolated RPE cells were stained with mouse anti-cytokeratin-18 antibody (Sigma, St. Louis, MO) and with mouse anti-endothelial cell (CD146) monoclonal antibody (Chemicon International, Inc., Temecula, CA) to ensure that there was no contamination with endothelial cells. For MMP-2 and TIMP activity assays, protein was extracted from RPE sheets and a protein assay preformed as previously described (Elliot et al., 2006)
Cell characterization and immortalization
RPE sheets were allowed to grow to confluence in DMEM:F12, 10% FBS. At confluence cells were trypsinized and divided into two groups. One group was allowed to grow and was subsequently frozen while the other group was immortalized with replication-deficient retrovirus containing human papilloma virus 16 E6/E7 (Fontijn et al., 1995). All cell lines were stained with RPE65 a marker of RPE cells. In addition epithelial cell markers ZO1 and ezrin staining were assessed and cells were subjected to flow cytometry (Catanuto et al., 2007). Western analysis was performed to detect RPE65 protein. Immortalized cells were used between passages 5–15.
Oxidant injury
RPE cell lines were treated with 10 microunits of myleoperoxidase (MPO, ) followed by 100µM H2O2 as previously described (Marin-Castano et al., 2003). 6 hours later, cells were washed and exposed to 1.0 % charcoal stripped serum. 24 hours after treatment RPE cells were collected and processed. As described for ARPE-19 cells this treatment provides a non-lethal injury as assessed by cell number and a viability assay (Marin-Castano et al., 2005)
MMP-2 and TIMP-2 Activity
RPE cell supernatants were collected and the protein concentration of the corresponding cell layer determined. MMP-2 activity was assessed with 10% zymogram gels (Invitrogen Corp., Carlsbad, CA), and TIMP-2 activity with reverse zymography gels as described previously (Cousins et al., 2003). In addition, MMP-2 and TIMP-2 were measured using the human Biotrak activity assay which recognizes mouse MMP-2 or TIMP-2 respectively and normalized to cell number (Amersham Biosciences, Piscataway, NJ).
RPE cell lines were grown in medium containing 1.0 % charcoal stripped serum and treated with either vehichle (V), 17β-estradiol (1–10nM, E2), ICI 182,780 (10uM, ICI, a complete ER antagonist) or both E2 and ICI. The supernatant was diluted to normalize for protein quantity before the addition of 5x Laemmli buffer under nonreducing conditions so that the equivalent of supernatant from 20µg of cells was loaded. For RPE cell sheets 5µg of protein was electrophoresed. After electrophoresis, gels were washed and treated as previously described (Elliot et al., 2006). The gels were stained with Coomassie Blue and air dried. Densitometry, using NIH image version1.6 (National Institutes of Health, Bethesda, MD) was used to analyze relative MMP-2 or TIMP-2 activity. Each assay was repeated at least three times
MMP-14 activity
RPE cells were plated in 24 well plates and grown to confluence at which time cells were treated as above. After treatment the media was removed and replaced with 250µl of extraction buffer provided with the MMP-14 Biotrak Activity Assay System (Amersham Biosciences, Piscataway, NJ) and incubated at 4°C for 15 minutes. The supernatant was assayed for MMP-14 according to manufacturer’s directions for lower endogenous MMP-14 levels (assay range 0.125–4 ng/ml). Data were normalized to protein concentration for each sample.
Transfection studies
For transient transfection experiments, RPE cells were transfected with a luciferase-based reporter construct containing either the full length MMP-2 (a gift of Dr. Watkins, Oxford) or a common functional polymorphism (−1306C → T) leading to a deletion of an Sp1 site. Cells were transfected using Gene Porter as previously described (Karl et al., 2005). The corresponding empty vectors were used as controls. Transfection efficacy was adjusted by cotransfection with phosphorylated Rous sarcoma virus-β-galactosidase (0.3–0.5 µg/well). After transfection, cells were incubated for 24 h in the presence of vehicle, 1 nM E2, 10uM ICI, or ICI followed by E2. For luciferase and galactosidase assays, cells were lysed in 100 µl reporter lysis buffer at room temperature. Light emission was detected using a luminometer (AutoLumatPlus, PerkinElmer) after addition of luciferin to 40 µl cell lysate. Values were expressed as arbitrary light units normalized to the β-galactosidase activity of each sample
Statistical analysis
All in vitro experiments were performed in triplicate, and triplicate wells were collected. All values were expressed as mean ± SEM. One-way analysis of variance and Tukey’s multiple comparison post hoc tests were performed for the statistical analysis (GraphPad Prism, San Diego, CA). A value of P < 0.05 was considered significant.
Results
Isolation and characterization of mouse RPE cell lines
All of the mouse RPE cell lines retained their cobblestone phenotype, and expressed RPE65, a membrane associated marker of RPE cells in vivo, before and after immortalization (data not shown). The presence of RPE65 was confirmed by real-time PCR (data not shown) and western analysis (Catanuto et al., 2007). In addition ZO1 and actin immunofluorescent staining pattern was that of epithelial cells. Ezrin staining was shown to be apical. Since all cell lines retained their in vivo markers and cell cycle was not altered by immortalization (data not shown), all experiments were preformed on immortalized cells (Catanuto et al., 2007).
Baseline MMP-2 and TIMP-2 activity
At baseline pro (68kDa) and active (62kDa) MMP-2 were increased in RPE sheets freshly isolated from ERKOα mice compared to ERKOβ mice (Figure 1A). This was supported by ELISA data that showed baseline MMP-2 higher in RPE cell lines isolated from ERKOα mice (0.56 ± 0.008 OD units) compared to RPE cell lines isolated from ERKOβ mice (0.87±0.04 OD units, p<0.05). Reverse zymography revealed little difference in baseline TIMP-2 (22kDa, lower band) activity (Figure 1B) which was confirmed by ELISA (ERKOβ; 0.149 ± 0.018 OD units vs. ERKOα; 0.156 ± 0.025 OD units).
Figure 1. MMP-2 and TIMP-2 activity or freshly isolated RPE sheets isolated from ERKO mice and their wildtype littermates.
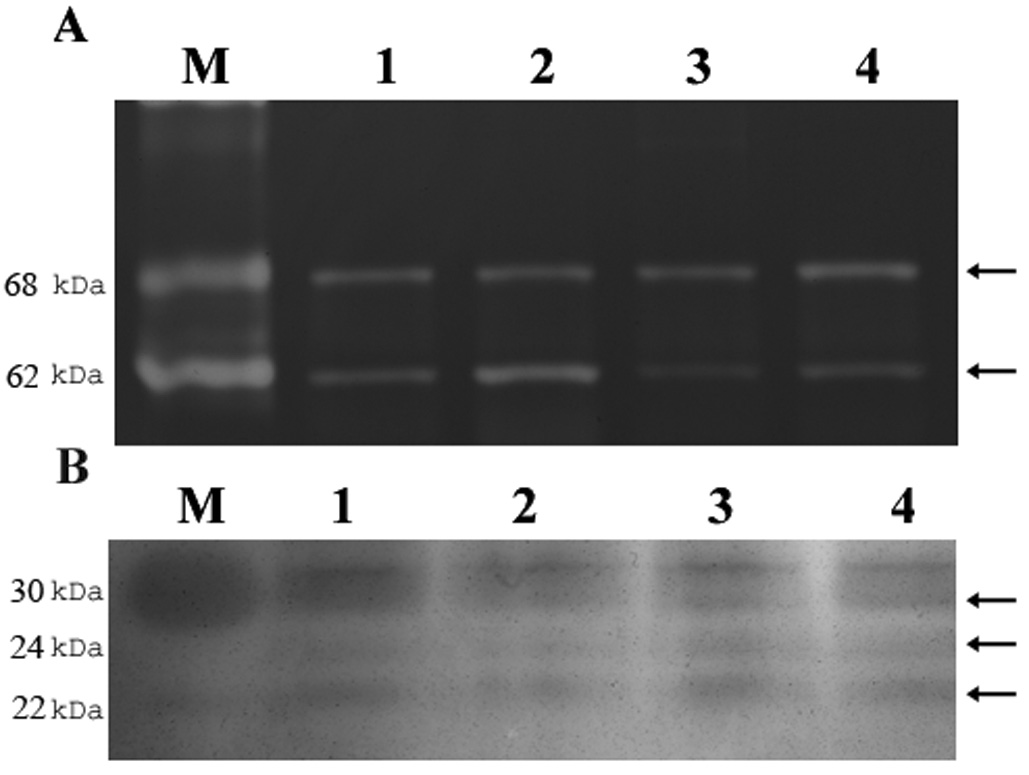
Freshly isolated RPE sheets from ERKOα (lane 2), ERKOβ (lane 4) and their respective wildtype littermates (lanes 1 and 3) were subjected to zymography (A) and reverse zymography (B) as described in methods. Both pro (upper arrowhead, 68kDa) and active MMP-2 (lower arrowhead, 62kDa) increased in ERKOα RPE sheets compared to littermate controls and to ERKOβ sheets. TIMP-1 (upper arrowhead, 30kDa), 3 (middle band, 24kDa) or 2 (lower arrowhead, 28kDa) did not differ. n=2 eyes/group. The experiment was repeated two times on separate mice. M=standard.
E2 treatment increases MMP-2 activity only in the presence of ERβ
A physiologic concentration of E2 (1nM) induced an increase in MMP-2 activity in wildtype littermates (Figure 2C, +p<0.05 compared to vehicle) and ERKOα cell lines (Figure 2F, +p<0.05). This occurred in a dose dependent manner (data not shown).
Figure 2. E2 regulates MMP-2 activity before and after oxidant injury in mouse RPE cell lines only in the presence of ERβ.
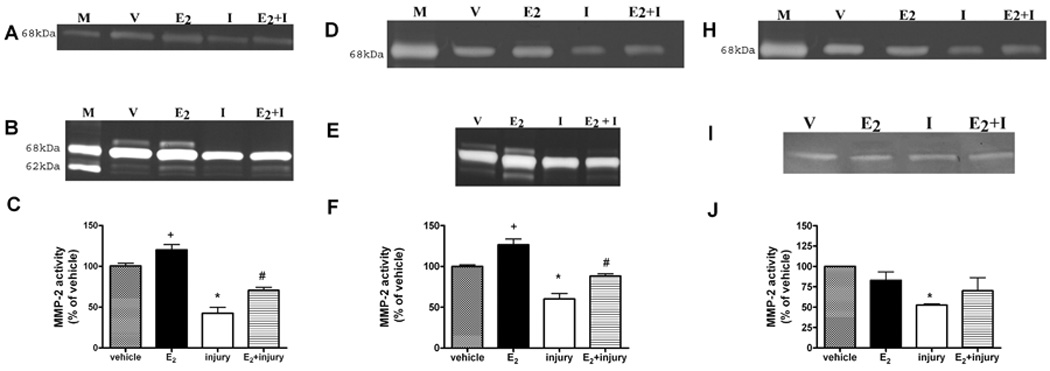
Representative zymograms of wildtype (A and B). ERKOα (D and E) and ERKOβ (H and I) RPE cell lines treated with 17β-estradiol (E2,1nM) for 24 hours before and after oxidant injury with MPO/H2O2 (I, injury). Supernatants were collected and electrophoresed on zymogram gels as described in methods. The upper zymograms (A, D and H) were loaded to visualize the pro-MMP-2 bands. The lower zymograms (B, E and I) were loaded to visualize the active MMP-2 bands. MMP-2 activity was measured by densitometry and % of vehicle control (v) determined. Data are graphed as mean ± SEM of the active band, + * p<0.05 compared to vehicle, # p<0.05 injury compared to E2 + injury, n=3. There was no protection against oxidant injury induced decreased MMP-2 activity in the absence of ERβ (ERKOβ cells). n=4. M=standard
Transient oxidant injury decreases MMP-2 and is prevented with E2 treatment only in the presence of ERβ
Transient oxidant injury decreased pro and active MMP-2 in cells isolated from wildtype littermates (Figure 2C, * p<0.05). A similar decrease was seen for RPE cell lines isolated from ERKOα and ERKOβ mice (Fig 2F and J *p<0.05 compared to vehicle). Pretreatment of wildtype and ERKOα cells with physiologic concentrations of 17β-estradiol 24 hours prior to the injury protocol partially prevented the decrease of oxidant induced –downregulation of MMP-2 (Figure 2, #p<0.05 injury compared to E2+ injury alone) Treatment with ICI, a complete ER antagonist, completely blocked the effect of E2, for all molecules suggesting an ER-dependent mechanism (data not shown).
E2 Regulation of TIMP-2 and MMP-14 before and after oxidant injury
Since the trimolecular complex of proMMP-2, MMP-14 and TIMP-2 is critical for MMP-2 activation in RPE cells (Elliot et al., 2006), it was surprising that E2 did not increase either TIMP-2 or MMP-14 activity in cells isolated from wildtype littermates or knockouts (Figure 3 and Figure 4). This data was confirmed for TIMP-2 using the Biotrak ELISA kit.
Figure 3. TIMP-2 activity is partially protected against oxidant injury induced downregulation by E2 only in RPE cell lines containing ERβ.
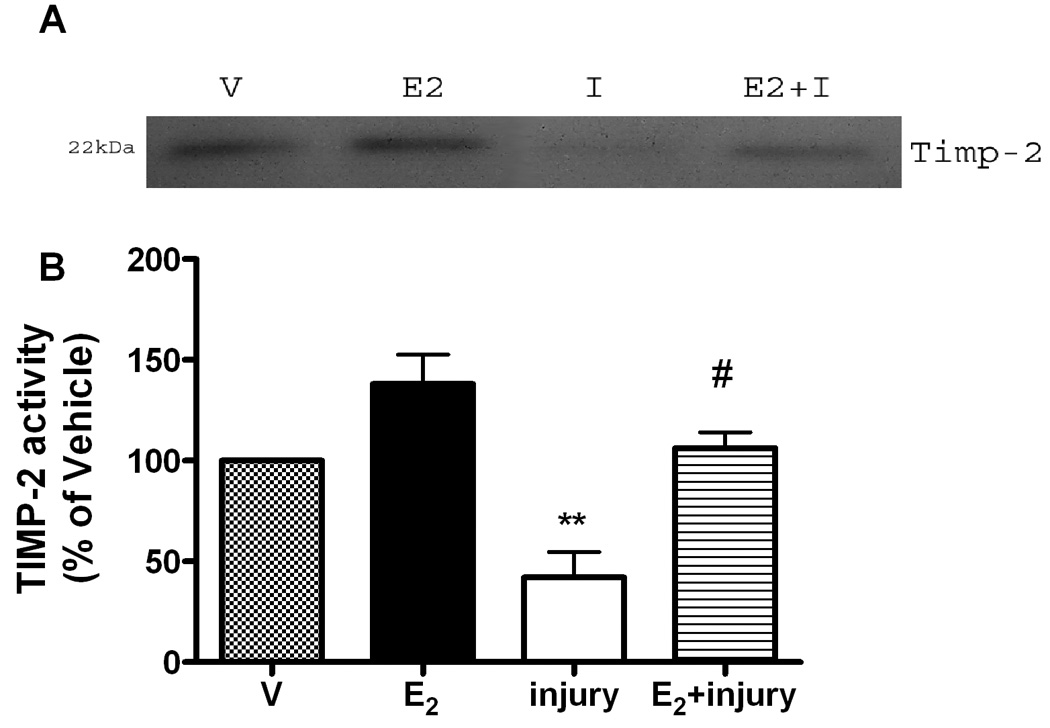
ERKOα, ERKOβ and wildtype (data not shown) RPE cells were treated with E2 (1nM) for 24 hours before and after oxidant injury with MPO/H2O2 (I, injury) as described in methods. TIMP-2 activity (22kDa) was assessed on reverse zymography gels (inset), and densitometry performed. Shown is the data derived from ERKOα cell lines,**p<0.001 compared to vehicle (V), ##p<0.001 injury compared to injury +E2,
Figure 4. Injury-induced decrease of MMP-14 activity is prevented by E2 only in RPE cell lines containing ERβ.
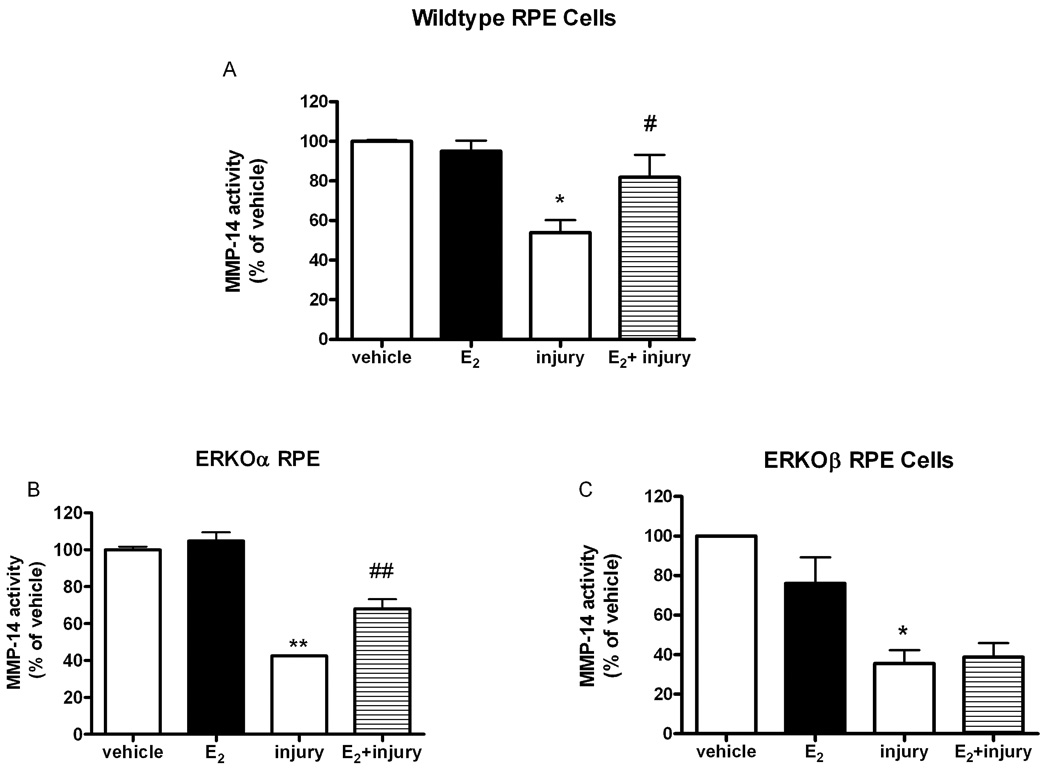
Mouse wildtype (A) ERKOα (B) and ERKOβ (C) RPE cells were treated with 17β-estradiol (E2,1nM) for 24 hours prior to MPO/H2O2 treatment (I, injury). MMP-14 was determined with an activity kit as described in methods and data were normalized to the protein concentrations of each sample, *p<0.05 compared to vehicle (V), # p<0.05, ## p<0.001 injury compared to E2+injury, n=5 wildtype, n=3 ERKOα, n=4 ERKOβ. Data are expressed as percent of vehicle treatment.
Following oxidant injury, both TIMP-2 (Figure 3) and MMP-14 (Figure 4) activity decreased in all cell lines similar to that shown for MMP-2 activity. In addition, E2 treatment partially prevented the oxidant induced decrease of TIMP-2 activity (Figure 3, ##p<0.001 injury compared to injury +E2) and MMP-14 (Figure 4A and B, # p<0.05, ## p<0.001 injury compared to E2+injury). Activity of TIMP-2 as measured by the biotrak kit was consistent with reverse zymography data. Injury decreased TIMP-2 activity to 86% of vehicle, while E2 treatment was 145%±14% over vehicle treatment.
Transfection studies
To determine if the E2-mediated increase in MMP-2 activity was due to an increase in transcriptional activation of the MMP-2 promoter, RPE cell lines were transfected with an MMP-2 promoter construct linked to luciferase. Luciferase increased in the presence of physiologic concentrations of E2 in the wildtype cells (Figure 5A, *p<0.05). This was an ER-dependent response since ICI blocked the effect (Figure 5A, *p<0.05). Importantly, luciferase response was diminished when cells were transfected with a mutant reporter plasmid containing a deletion at an Sp1 site (1789) and treated with E2 (# p<0.05) compared to the transfection with the full length plasmid (1790). As expected E2 treatment of ERKOβ cells (Figure 5C) did not elicit an increase in luciferase activity. In contrast E2 stimulated an ER-dependent increase of luciferease activity in ERKOα cells (Figure 5B, *p<0.05), although the response was diminished compared to that of the wildtype cells.
Figure 5. E2 stimulates transcriptional activation of the MMP-2 promoter only in the presence of an Sp1 site and ERβ.
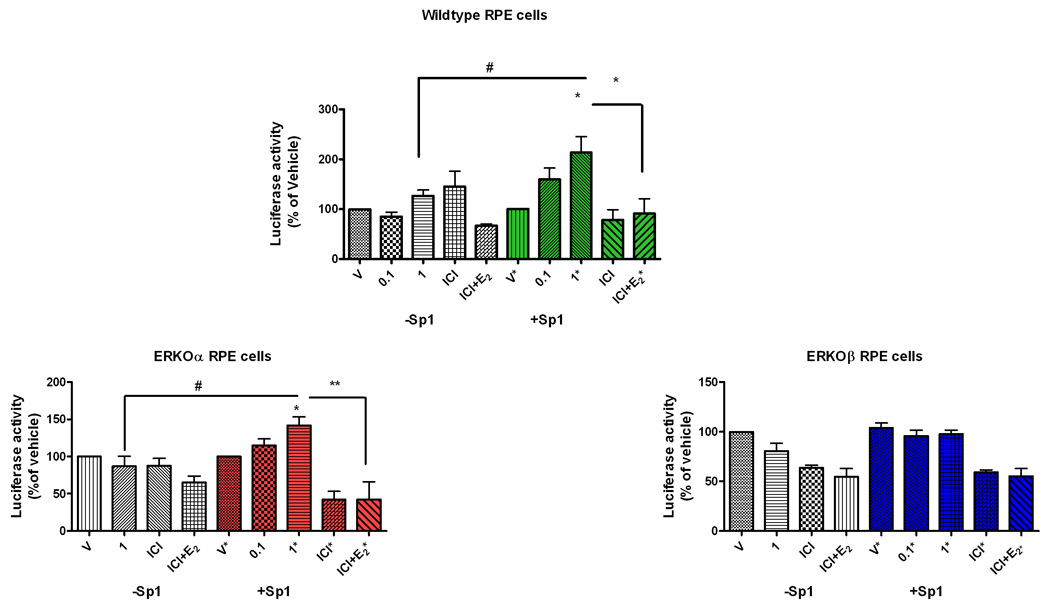
Wildtype (A), ERKOα(B), and ERKOβ (C) RPE cells were co-transfected with MMP-2-promoter-luciferase reporter gene constructs containing an Sp1 site (+Sp1) or a deletion removing the Sp1 site (−Sp1) and β-gal. Cells were exposed to either vehicle (V) or 17β-estradiol (E2, 0.1and 1nM) or ICI (1uM) or ICI+E2 (1nM) for 24 hours and cell lysates collected. Data are the ratio of luciferase activity/β-gal expressed as percentage of vehicle control (v). Shown are means ± SEM of cell lysates collected from triplicate wells for each treatment. *p < 0.05, compared to vehicle +p<0.05 1nm E2 compared to ICI+E2, #p < 0.05 compared to same treatment using different constructs, n = 3 individual collections.
Discussion
MMPs and their tissue inhibitors play a central role in the pathogenesis of deposit accumulation in multiple disorders such as renal disease and atherosclerosis (Belkhiri et al., 1997;Dollery and Libby, 2006;Peten et al., 1992). Aging and AMD eyes accumulate extracellular deposits under the RPE and within Bruch’s membrane, and are in part due to dysregulation of ECM. dysregulation of MMP-2 activity To date, there is little consensus on the exact pathophysiologic mechanisms responsible for early AMD, although oxidant injury of the RPE appears to play an important role. We have shown in vitro and in vivo that oxidant injury induces a decrease of MMP-2 activity which is most likely due to changes in the trimolecular complex (Elliot et al., 2006;Marin-Castano et al., 2005;Marin-Castano et al., 2006).
We also reported that aged female or ovariectomized estrogen deficient B6 mice were more susceptible to sub-retinal deposits than males most likely due to dysregulation in matrix turnover (Cousins et al., 2003). In addition, in vitro E2 administration increased human RPE cell MMP-2 activity (Marin-Castano et al., 2003). Estrogens regulate MMP-2 activity not only in RPE cells, but multiple other cell types such as smooth muscle cells, glomerular mesangial cells and skin fibroblasts (Gilliver et al., 2007;Karl et al., 2006;Marin-Castano et al., 2003;Wingrove et al., 1998). Therefore our goal in this study was to determine whether estrogen can prevent oxidant injury induced ECM dysregulation. To further investigate the mechanisms and the subtype specificity involved in oxidant injury induced ECM dysregulation and the E2-mediated regulation of the trimolecular complex, we isolated mouse RPE cell lines from mice lacking either ERα or ERβ or their wildtype littermates. These cell lines retained their in vivo epithelial and RPE markers as noted by immunofluorescent staining pattern for ZO-1 and mRNA and protein expression of RPE65 respectively (Hamel et al., 1993). In addition, expression of ezrin was documented on their apical surface (Catanuto et al., 2007).
To begin our studies, we assessed baseline MMP-2 activity in the absence or presence of ERβ. We found that the presence of ERβ (ERKOα) alone led to a higher baseline MMP-2 activity compared to the presence of ERα (ERKOβ) alone, suggesting a positive regulatory effect by ERβ and an inhibitory effect of ERα on MMP-2 activity. This appears contrary to many cell types where ERβ exhibits an inhibitory effect on ERα-mediated gene expression (Matthews et al., 2006). In this investigation we confirmed that physiological doses of E2 increased mouse RPE MMP-2 activity similar to what has been shown in human RPE (Marin-Castano et al., 2003). However, we also found a selective ER subtype specificity for MMP-2 activity regulation. RPE cell lines without ERβ did not respond to E2 stimulation of MMP-2 activity. There are multiple reports of estrogen’s effects via ERα on matrix components in non-reproductive cell types and tissues in vitro, including glomerular mesangial cells, smooth muscle cells, and intact arteries (Elliot et al., 2006;Potier et al., 2002;Wingrove et al., 1998). To our knowledge this is the first study to address the importance of ERβ in regulating MMP-2 activity in the eye or any other organ.
In contrast, E2 stimulation of RPE cells did not increase MMP-14 and TIMP-2 activity. Although unexpected, this response is certainly cell type specific since E2 has been shown to regulate glomerular TIMP-2 from TGFβ transgenic mice and induce MMP-14 in osteoblasts (Blush et al., 2004;Liao et al., 2004). Surprisingly there was a modest increase of MMP-14 transcriptional activation and protein expression after E2 treatment (data not shown). It is possible that E2 acting through an ER-independent mechanism, regulates MMP-14 protein. These studies are beyond the scope of this paper however and are the subject of ongoing investigations.
Oxidant injury decreased activity of all three components of the trimolecular complex in mouse RPE cell lines. As we showed for human cells, this did not appear to be due to cell death and/or reduction of protein synthesis since the total protein extracted from all culture conditions was approximately the same (Elliot et al., 2006). Importantly, E2 preincubation partially prevented the decrease of the trimolecular complex but only if ERβ was present. These data suggest that E2 protection preserves proMMP-2, TIMP-2 and MMP-14 in the necessary ratio to allow for MMP-2 activation and that ERβ plays a central role. Partial protection and not full restoration of these molecules to baseline levels may reflect oxidatative damage to ERs and/or transcription factors. It is well established that cysteines present in zinc finger transcription factors (ER, Sp1, Egr-1) are susceptible to oxidation leading to either loss or enhancement of function (Webster et al., 2001). This has been shown to occur in breast cancer and in aging (Liang et al., 1998;Mattana et al., 1998;Quong et al., 2002).
MMP-2 activity regulation is complex in that it can be controlled at multiple levels (Corcoran et al., 1996). Prevention against oxidant mediated injury induced decrease of MMP-2 can occur by at least two mechanisms. One, an indirect effect on the trimolecular complex whereby all the components are present in the necessary ratio as discussed above. The other is a direct effect on the MMP-2 promoter. Therefore we utilized MMP-2 reporter constructs in transient transfection studies and found that E2 administration increased luciferase activity in a dose dependent manner. This was ER dependent since treatment with the complete ER antagonist ICI blocked the increase. Importantly luciferase activity only increased when ERβ was present confirming our results on MMP-2 activity discussed above.
Sp1 has been shown to be important for transcription of the MMP-2 and MMP-14 genes (Alfonso-Jaume et al., 2004;Harendza et al., 2003). Therefore we utilized an MMP-2 promoter construct containing a common functional polymorphism (−1306C → T) leading to a deletion of an Sp1 site (Price et al., 2001). In RPE cell lines, transient transfection studies showed decreased baseline promoter activity when the deletion was present compared to the intact promoter. This has been noted previously for breast cancer cell lines (Harendza et al., 2003). Importantly, the E2 mediated luciferase increase was abolished when the Sp1 site was deleted from the promoter construct. Activated ERs can mediate gene expression either by direct DNA binding in the promoter regions of E2 responsive genes or through tethering to other transcription factors such as Sp1 (Bjornstrom and Sjoberg, 2005;Matthews and Gustafsson, 2003). The MMP-2 promoter contains three potential half–palandromic EREs and a Sp1 site where liganded ERs can bind (Harendza et al., 2003;Wingrove et al., 1998). Recent data suggest that in some cell types the additive effect of binding to these sites not the physical interaction of ER and Sp1 on the promoter leads to upregulation of genes (Saville et al., 2000).
ER-Sp1 complexes are responsible for activation of an increasing number of genes such as LDL-R, cyclin D1 and possibly MMP-14 (Bjornstrom and Sjoberg, 2005). There is evidence for differential activation of ERα-Sp1 and Erβ-Sp1 complexes in the presence of selective estrogen receptor modulators (Saville et al., 2000) although this is cell type and promoter specific. It has been proposed that Sp1 expression is upregulated by E2, as a predicted ERE exists in the reported human Sp1 promoter (Nicolas et al., 2001). However other data suggests that ER-dependent estrogen stimulated p42/44 MAPK leads to increased Sp1 protein (Bjornstrom and Sjoberg, 2005). In RPE cell lines the presence of the Sp1 site is clearly necessary for the E2 induced increase in MMP-2 promoter activity. Whether this is due to ER binding to Sp1, E2 induction of Sp1 or both has not been addressed in these studies. However this is the first report to our knowledge of transcription factors important for MMP-2 regulation in RPE cells and the retina.
In summary we have shown that E2 through ERβ activation regulates MMP-2 activity in mouse RPE cell lines. Non-lethal oxidant injury leads to downregulation of MMP-2 activity. This is partially prevented by E2 through prevention of downregulation of the trimolecular complex necessary for MMP-2 activation. E2 exerts a direct effect on the MMP-2 promoter which requires the presence of an Sp1 site. These studies suggest that E2 may have a protective effect against oxidant-induced matrix dysregulation, which could lead to deposit formation prevalent in early AMD. Using molecules which specifically target ERβ may therefore offer therapeutic benefit.
Acknowledgements
This work was supported in part by National Institutes of Health National Eye Institute Grant RO1 EY14477-04 (SJE MK, and SWC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Fine SL, et al. Age-related macular degeneration. N.Engl.J.Med. 2000;342:483–492. doi: 10.1056/NEJM200002173420707. [DOI] [PubMed] [Google Scholar]
- Friedman DS, et al. Prevalence of age-related macular degeneration in the United States. Arch.Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- Berger JW, et al. Age-related macular degeneration. St. Louis: Mosby; 1999. Ref Type: Book, Whole. [Google Scholar]
- Green WR. Histopathology of age-related macular degeneration. Mol.Vis. 1999;5:27. [PubMed] [Google Scholar]
- Corcoran ML, et al. MMP-2: expression, activation and inhibition. Enzyme Protein. 1996;49:7–19. doi: 10.1159/000468613. [DOI] [PubMed] [Google Scholar]
- Nagase H, et al. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc.Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, et al. The extracellular matrix of human retinal pigment epithelial cells in vivo and its synthesis in vitro. Invest Ophthalmol Vis.Sci. 1986;27:1615–1621. [PubMed] [Google Scholar]
- Ahir A, et al. Expression of metalloproteinases from human retinal pigment epithelial cells and their effects on the hydraulic conductivity of Bruch's membrane. Invest Ophthalmol.Vis.Sci. 2002;43:458–465. [PubMed] [Google Scholar]
- Eichler W, et al. Modulation of matrix metalloproteinase and TIMP-1 expression by cytokines in human RPE cells. Invest Ophthalmol.Vis.Sci. 2002;43:2767–2773. [PubMed] [Google Scholar]
- Marin-Castano ME, et al. Regulation of estrogen receptors and MMP-2 expression by estrogens in human retinal pigment epithelium. Invest Ophthalmol.Vis.Sci. 2003;44:50–59. doi: 10.1167/iovs.01-1276. [DOI] [PubMed] [Google Scholar]
- Padgett LC, et al. Matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 in the retinal pigment epithelium and interphotoreceptor matrix: vectorial secretion and regulation. Exp.Eye Res. 1997;64:927–938. doi: 10.1006/exer.1997.0287. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Seiki M. MT1-MMP: an enzyme with multidimensional regulation. Trends Biochem.Sci. 2004;29:285–289. doi: 10.1016/j.tibs.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Marin-Castano ME, et al. Nonlethal oxidant injury to human retinal pigment epithelium cells causes cell membrane blebbing but decreased MMP-2 activity. Invest Ophthalmol.Vis.Sci. 2005;46:3331–3340. doi: 10.1167/iovs.04-1224. [DOI] [PubMed] [Google Scholar]
- Elliot S, et al. Retinal Pigment Epithelium Protection From Oxidant-mediated Loss of MMP-2 Activation Requires Both MMP-14 and TIMP-2. Invest Ophthalmol.Vis.Sci. 2006;47:1697–1702. doi: 10.1167/iovs.05-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potier M, et al. Expression and regulation of estrogen receptors in mesangial cells: influence on matrix metalloproteinase-9. J Am.Soc.Nephrol. 2001;12:241–251. doi: 10.1681/ASN.V122241. [DOI] [PubMed] [Google Scholar]
- Lei J, et al. Serum-stimulated alpha 1 type IV collagen gene transcription is mediated by TGF-beta and inhibited by estradiol. Am.J.Physiol. 1998;274:F252–F258. doi: 10.1152/ajprenal.1998.274.2.F252. [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- Greco B, et al. Coexpression of ER beta with ER alpha and progestin receptor proteins in the female rat forebrain: effects of estradiol treatment. Endocrinology. 2001;142:5172–5181. doi: 10.1210/endo.142.12.8560. [DOI] [PubMed] [Google Scholar]
- Mor G, et al. The role of the Fas/Fas ligand system in estrogen-induced thymic alteration. Am J Reprod.Immunol. 2001;46:298–307. doi: 10.1034/j.1600-0897.2001.d01-16.x. [DOI] [PubMed] [Google Scholar]
- Espinosa-Heidmann DG, et al. Gender and estrogen supplementation increases severity of experimental choroidal neovascularization. Exp.Eye Res. 2005;80:413–423. doi: 10.1016/j.exer.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Catanuto P, et al. Immortalization of Mouse Retinal Pigmented Epithelial Cell Lines: A New Tool to Further Age Related Macular Degeneration Research; Annual Meeting of the Association for Research in Vision and Opthalmology; 2007. Ref Type: Abstract. [Google Scholar]
- Fontijn R, et al. Maintenance of vascular endothelial cell-specific properties after immortalization with an amphotrophic replication-deficient retrovirus containing human papilloma virus 16 E6/E7 DNA. Exp.Cell Res. 1995;216:199–207. doi: 10.1006/excr.1995.1025. [DOI] [PubMed] [Google Scholar]
- Cousins SW, et al. Female gender, estrogen loss, and Sub-RPE deposit formation in aged mice. Invest Ophthalmol.Vis.Sci. 2003;44:1221–1229. doi: 10.1167/iovs.02-0285. [DOI] [PubMed] [Google Scholar]
- Karl M, et al. Autocrine activation of the local insulin-like growth factor I system is up-regulated by estrogen receptor (ER)-independent estrogen actions and accounts for decreased ER expression in type 2 diabetic mesangial cells. Endocrinology. 2005;146:889–900. doi: 10.1210/en.2004-1121. [DOI] [PubMed] [Google Scholar]
- Belkhiri A, et al. Increased expression of activated matrix metalloproteinase-2 by human endothelial cells after sublethal H2O2 exposure. Lab Invest. 1997;77:533–539. [PubMed] [Google Scholar]
- Dollery CM, Libby P. Atherosclerosis and proteinase activation. Cardiovascular Research. 2006;69:625–635. doi: 10.1016/j.cardiores.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Peten EP, et al. Age-related changes in alpha 1- and alpha 2-chain type IV collagen mRNAs in adult mouse glomeruli: competitive PCR. Am J Physiol. 1992;263:F951–F957. doi: 10.1152/ajprenal.1992.263.5.F951. [DOI] [PubMed] [Google Scholar]
- Marin-Castano ME, et al. Repetitive nonlethal oxidant injury to retinal pigment epithelium decreased extracellular matrix turnover in vitro and induced sub-RPE deposits in vivo. Invest Ophthalmol.Vis.Sci. 2006;47:4098–4112. doi: 10.1167/iovs.05-1230. [DOI] [PubMed] [Google Scholar]
- Gilliver SC, et al. The hormonal regulation of cutaneous wound healing. Clinics in Dermatology. 2007;25:56–62. doi: 10.1016/j.clindermatol.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Karl M, et al. Differential effects of continuous and intermittent 17beta-estradiol replacement and tamoxifen therapy on the prevention of glomerulosclerosis: modulation of the mesangial cell phenotype in vivo. Am.J.Pathol. 2006;169:351–361. doi: 10.2353/ajpath.2006.051255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingrove CS, et al. 17beta-oestradiol enhances release of matrix metalloproteinase-2 from human vascular smooth muscle cells. Biochim.Biophys.Acta. 1998;1406:169–174. doi: 10.1016/s0925-4439(97)00097-5. [DOI] [PubMed] [Google Scholar]
- Hamel CP, et al. Molecular cloning and expression of RPE65, a novel retinal pigment epithelium-specific microsomal protein that is post-transcriptionally regulated in vitro. J Biol.Chem. 1993;268:15751–15757. [PubMed] [Google Scholar]
- Matthews J, et al. ER{beta} modulates ER{alpha}-mediated transcriptional activation by altering the recruitment of c-Fos and c-Jun to estrogen responsive promoters. Mol.Endocrinol. 2006;20:534–543. doi: 10.1210/me.2005-0140. [DOI] [PubMed] [Google Scholar]
- Potier M, et al. Estrogen-related abnormalities in glomerulosclerosis-prone mice: reduced mesangial cell estrogen receptor expression and prosclerotic response to estrogens. Am.J.Pathol. 2002;160:1877–1885. doi: 10.1016/S0002-9440(10)61134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blush J, et al. Estradiol reverses renal injury in Alb/TGF-beta1 transgenic mice. Kidney Int. 2004;66:2148–2154. doi: 10.1111/j.1523-1755.2004.66005.x. [DOI] [PubMed] [Google Scholar]
- Liao EY, et al. Membrane-type matrix metalloproteinase-1 (MT1-MMP) is down-regulated in estrogen-deficient rat osteoblast in vivo. J Endocrinol Invest. 2004;27:1–5. doi: 10.1007/BF03350902. [DOI] [PubMed] [Google Scholar]
- Webster KA, et al. Oxidation of zinc finger transcription factors: physiological consequences. Antioxid.Redox.Signal. 2001;3:535–548. doi: 10.1089/15230860152542916. [DOI] [PubMed] [Google Scholar]
- Liang X, et al. Oxidant stress impaired DNA-binding of estrogen receptor from human breast cancer. Mol.Cell Endocrinol. 1988;146:151–161. doi: 10.1016/s0303-7207(98)00161-0. [DOI] [PubMed] [Google Scholar]
- Mattana J, et al. Oxidation of the mesangial matrix metalloproteinase-2 impairs gelatinolytic activity. Inflammation. 1998;22:269–276. doi: 10.1023/a:1022396015294. [DOI] [PubMed] [Google Scholar]
- Quong J, et al. Age-dependent changes in breast cancer hormone receptors and oxidant stress markers. Breast Cancer Res.Treat. 2002;76:221–236. doi: 10.1023/a:1020886801674. [DOI] [PubMed] [Google Scholar]
- Alfonso-Jaume MA, et al. Co-operative interactions between NFAT (nuclear factor of activated T cells) c1 and the zinc finger transcription factors Sp1/Sp3 and Egr-1 regulate MT1-MMP (membrane type 1 matrix metalloproteinase) transcription by glomerular mesangial cells. Biochem.J. 2004;380:735–747. doi: 10.1042/BJ20031281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harendza S, et al. Linked common polymorphisms in the gelatinase a promoter are associated with diminished transcriptional response to estrogen and genetic fitness. J.Biol.Chem. 2003;278:20490–20499. doi: 10.1074/jbc.M211536200. [DOI] [PubMed] [Google Scholar]
- Price SJ, et al. Identification of novel, functional genetic variants in the human matrix metalloproteinase-2 gene: role of Sp1 in allele-specific transcriptional regulation. J.Biol.Chem. 2001;276:7549–7558. doi: 10.1074/jbc.M010242200. [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol.Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol.Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- Saville B, et al. Ligand-, cell-, and estrogen receptor subtype (alpha/beta)-dependent activation at GC-rich (Sp1) promoter elements. J.Biol.Chem. 2000;275:5379–5387. doi: 10.1074/jbc.275.8.5379. [DOI] [PubMed] [Google Scholar]
- Nicolas M, et al. Cloning and characterization of the 5′-flanking region of the human transcription factor Sp1 gene. J Biol.Chem. 2001;276:22126–22132. doi: 10.1074/jbc.M010740200. [DOI] [PubMed] [Google Scholar]


