Abstract
The synaptic vesicle membrane protein synaptotagmin (tagmin) is essential for fast, calcium-dependent, neurotransmitter release and is likely to be the calcium sensor for exocytosis, because of its many calcium-dependent properties. Polyphosphoinositides are needed for exocytosis, but it has not been known why. We now provide a possible connection between these observations with the finding that the C2B domain of tagmin I binds phosphatidylinositol-4,5-bisphosphate (PIns-4,5-P2), its isomer phosphatidylinositol-3,4-bisphosphate and phosphatidylinositol-3,4,5-trisphosphate (PIns-3,4,5-P3). Calcium ions switch the specificity of this binding from PIns-3,4,5-P3 (at calcium concentrations found in resting nerve terminals) to PIns-4,5-P2 (at concentration of calcium required for transmitter release). Inositol polyphosphates, known blockers of neurotransmitter release, inhibit the binding of both PIns-4,5-P2 and PIns-3,4,5-P3 to tagmin. Our findings imply that tagmin may operate as a bimodal calcium sensor, switching bound lipids during exocytosis. This connection to polyphosphoinositides, compounds whose levels are physiologically regulated, could be important for long-term memory and learning.
Keywords: neurotransmitter release, exocytosis
A central event of synaptic transmission is the release of neurotransmitters from synaptic vesicles at the active zones of the presynaptic nerve terminal. This process is tightly coupled to the increase of intracellular calcium as response to the arrival of a nerve impulse, which triggers the activation of the synaptic vesicle fusion machinery (1, 2). Attractive candidates for a calcium sensor in regulated exocytosis are the members of the synaptotagmin (tagmin) family, composed of highly conserved integral membrane proteins with a broad distribution in neuronal and nonneuronal tissues (3, 4). In the nervous and neuroendocrine systems, tagmins are localized to synaptic vesicles and secretory granules (4). The majority of the protein is cytoplasmic and contains two internal repeats that have homology to the C2 regulatory region found in protein kinase C, phospholipase A2, rabphillin, and, recently, in lipid kinases (5–7). The first of these two domains (C2A), whose three-dimensional structure was recently solved (8, 9), binds calcium and acidic phospholipids in a ternary complex with a concentration dependence and specificity for calcium resembling that of neurotransmitter release (10–12). In addition the C2A domain binds syntaxin, a presynaptic membrane protein (13), in a calcium-dependent manner (14, 15). The second C2 homology domain (C2B) has been shown to interact with a soluble N-ethylmaleimide fusion protein (NSF) attachment protein (β-SNAP) (16) and inositol polyphosphates (InsPP) (17) and is responsible for calcium-dependent tagmin dimerization (18, 19). Furthermore the complex of tagmin with β-SNAP is able to recruit NSF (20) and to assemble a large particle containing α-SNAP and SNAP receptor proteins, which is likely to be involved in the docking and fusion process at the nerve terminal (16). In addition to the biochemical data, genetic evidence in mammals (21), Drosophila (22, 23), and Caenorhabditis elegans (24) strongly indicates that tagmin is the major calcium sensor for synchronous neurotransmitter release in neurons.
Although the primary role of proteins in exocytosis is indisputable, several experimental approaches suggests that lipids, in particular the highly phosphorylated metabolites of phosphatidylinositol, also play a fundamental role in this process. In chromaffin and pheochromocytoma PC12 cells, calcium-stimulated secretion is blocked by disruption of the PIns-4,5-bisphosphate (PIns-4,5-P2) pool with PIns-specific phospholipase (25) or with anti-PIns-4,5-P2 antibodies (26). Furthermore, three cytosolic proteins involved in phosphoinositide metabolism (27), namely PIns transfer protein (28), PIns 4-kinase (29), and PIns-4P 5-kinase (26) are required for calcium-dependent secretion, but the molecular basis for polyphosphoinositides involvement in this process was unclear.
Here we provide biochemical evidence for a direct linkage between calcium-sensing and polyphosphoinositides with the observation that the C2B domain of tagmin 1 shows a specific and stoichiometrical binding to PIns-4,5-P2, its natural isomer PIns-3,4-bisphosphate (PIns-3,4-P2), and phosphatidylinositol-3,4,5-trisphosphate (PIns-3,4,5-P3). Calcium ions at physiological concentrations switch the specificity of this interaction, thus suggesting that the complex of tagmin and polyphosphoinositides constitutes a possible bimodal calcium sensor for regulated exocytosis.
MATERIALS AND METHODS
Liposome Preparation and Binding Assay.
Liposomes (175 μg of lipid per ml) were made from pure 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (PC) or PC and 1% (wt/wt) of 1-stearoyl-2-arachidonoie-sn-glycero-3-phospho-d-myo-inositol (PIns), PIns-4-phosphate (PIns-4-P), PIns-4,5-P2, 1,2-dipalmitoyl-sn-glycero-3-phospho-d-myo-inositol-3,4-bisphosphate (PIns-3,4-P2), 1,2-dipalmitoyl-sn-glycero-3-phospho-d-myo-inositol-3,4,5-trisphosphate (PIns-3,4,5-P3); 1 μCi/ml (1 Ci = 37 GBq) of 1,2-dipalmitoyl-sn-glycero-3-phospho-[methyl-3H]choline (66 Ci/mmol, Amersham) was added as a tracer. Lipids were purchased from Avanti Polar Lipids (PC and PIns), Boehringer Mannheim (PIns-4-P and PIns-4,5-P2), or Matreya (Pleasant Gap, PA; PIns-3,4-P2). PIns-3,4,5-P3 was synthesized as described before (30) and its identity was confirmed by 1H-NMR and 31P-NMR. Lipids were dried by a gentle flow of argon, dissolved in 200 μl of 100% ethanol, and then kept under vacuum for 30 min at room temperature. The homogeneous lipid film was then resuspended in 20 mM Hepes·KOH (pH 7.6), 100 mM KCl, and 0.2 mM DTT (buffer A) by vigorous stirring for 10 min, after overlaying the top of the solution with argon. Liposomes were prepared either by sonication (12) followed either by liposome purification on Sephadex G-25 M (Pharmacia) or by extrusion (31). Large aggregates were eliminated by centrifugation at 15,000 × g for 10 min. Purified glutathione S-transferase (GST)-fusion proteins containing the cytoplasmic domain of tagmin 1 (aa 79–421; 15 μg) or GST alone (8 μg) were immobilized on 20 μl 50% glutathione-agarose beads (Sigma). The beads were then washed twice with 500 μl of the same buffer containing 1.0 mM free Mg2+ and either 2 mM EGTA or 100 μM free calcium buffered with 2 mM EGTA. For the experiment shown in Fig. 2, PC liposomes (350 μg of lipid per ml) containing either 1% (wt/wt) of PIns-4,5-P2 and 0.1 μCi/ml of 1-palmitoyl-2-[1-14C]palmitoyl-sn-glycero-3-phosphocholine (55 mCi/mmol, Amersham) or 1% (wt/wt) of PIns-3,4,5-P3 and 1 μCi/ml of 1,2-dipalmitoyl-sn-glycero-3-phospho-[methyl-3H]choline (66 Ci/mmol, Amersham) were prepared as above. The two liposome populations were premixed at a 1:1 ratio (final lipid concentration 175 μg/ml), and free Ca2+ concentrations ranging from 1 nM to 300 μM in the presence of 0.5 mM free Mg2+ were obtained by adding the suitable amount of CaCl2 and MgCl2 to 2 mM EGTA in buffer A. Subsequently, 100 μl of 3H-labeled liposomes was added and the beads were incubated for 30 min at room temperature with vigorous shaking. Beads were centrifuged at 3000 × g for 2 min and washed three times with buffer A containing 1.0 mM free Mg2+ and the corresponding amount of free Ca2+. The bound liposomes were solubilized with 0.3 ml of 10% SDS and radioactivity associated with the pellet was then determined by scintillation counting. Data shown are the average of three independent experiments ± SD.
Figure 2.
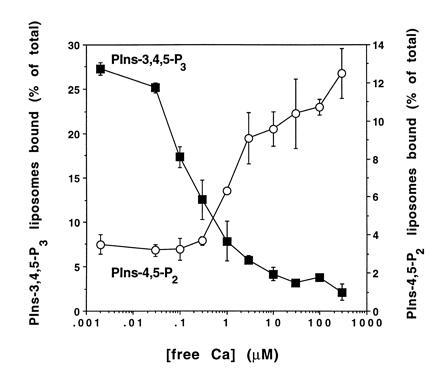
Calcium dependency of PIns-4,5-P2 and PIns-3,4,5-P3 binding to tagmin. GST-tagmin beads were simultaneously incubated with two populations of PC liposomes either containing PIns-4,5-P2 or PIns-3,4,5-P3 at variable Ca2+ concentrations (○, PIns-4,5-P2; ▪, PIns-3,4,5-P3). Liposome binding to tagmin and its domains was determined as described in Fig. 1.
Micellar Phosphoinositides Binding Assay.
For the determination of the apparent affinity constant and the total number of binding sites for PIns-4,5-P2 on tagmin, 1,2-dioleoy-sn-glycero-3-phospho-d-myo[2-3H(N)]inositol-4,5-bisphosphate (7 Ci/mmol; New England Nuclear) and unlabeled PIns-4,5-P2 were mixed and dried with a gentle flow of argon. The lipid film was then dissolved in 200 μl of 100% ethanol and the trace amounts of solvent were eliminated under vacuum. PIns-4,5-P2 was solubilized at a final concentration of 20 μM (20 Ci/mol) in buffer A containing 0.8% (wt/vol) octyl β-d-glucopyranoside (OG) in the presence of 2 mM EGTA and sonicated as above. For the experiments shown in Fig. 4 C and D, native tagmin (5 μg per sample) was immunoprecipitated from OG membrane extracts of bovine brain cortex (16) with an antitagmin monoclonal antibody (32) covalently coupled to protein G-Sepharose Fast Flow (Pharmacia) (33). After overnight incubation at 4°C, the beads were washed extensively with buffer A containing 0.5 M NaCl and 0.8% OG and then were rinsed in buffer A containing 0.8% OG. Monomeric PIns-4,5-P2 in detergent micelles were mixed with immunopurified native tagmin or immobilized GST-tagmin (6 μg) or control beads, incubated 30 min at 4°C in the presence of 2 mM EGTA, and then analyzed as described in Fig. 1. For Fig. 4D, beads were analyzed by SDS/PAGE and the proteins were stained with Coomassie blue R. Competition experiments were carried out by incubating GST-tagmin-containing beads with saturable amount of [3H]PIns-4,5-P2 (6 μM) premixed with increasing concentrations of inositol 1,2,3,4,5,6-hexakisphosphate (InsP6) ranging from 1 nM to 1 mM in buffer A containing 0.8% OG and 2 mM EGTA for 30 min at 4°C. After washing with the same buffer, the amount of radioactivity was determined and results expressed as a percentage of the binding in absence of InsP6.
Figure 4.
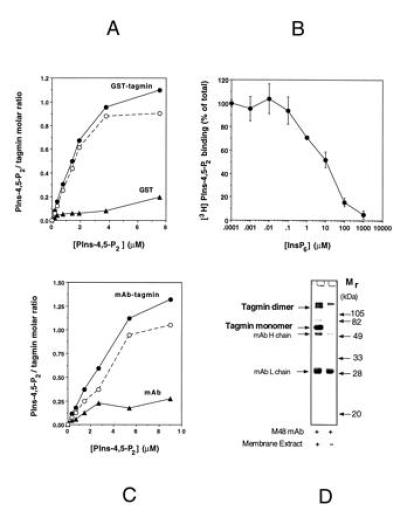
The binding of PIns-4,5-P2 to recombinant and native tagmin is saturable and is competed by InsP6. (A) GST-tagmin (•) or GST alone (○) was incubated at increasing concentration of radioactive PIns-4,5-P2 in detergent (OG) micelles in the presence of 2 mM EGTA. Samples were analyzed as described in Fig. 1. (A) Tagmin/PIns-4,5-P2 molar ratio as function of the micellar PIns-4,5-P2 concentration. Dashed line represents the PIns-4,5-P2 specifically associated with tagmin. Parallel experiments using Triton X-100 [0.02% (wt/vol)] gave the same results (not shown). (B) GST-tagmin was incubated in the presence of saturable amount of PIns-4,5-P2 (6 μM) with increasing amount of InsP6 in the presence of 2 mM EGTA. (C) Immunoprecipitated native tagmin (•) or antitagmin antibody alone (○) were incubated with radioactive PIns-4,5-P2 in detergent micelles in the presence of 2 mM EGTA. Dashed line represents the PIns-4,5-P2 specifically associated with tagmin. (D) SDS/PAGE profile of the immunopurified native tagmin used in C. In addition to the tagmin monomer with an apparent molecular weight of 65 kDa, an SDS-resistant tagmin dimer is also visible (37).
Figure 1.
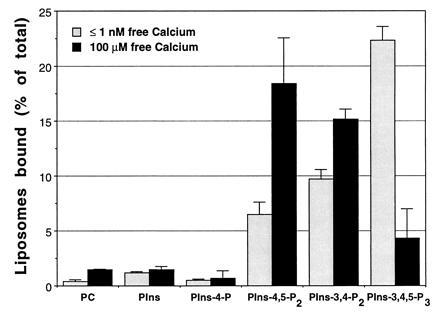
Tagmin binds specifically to polyphosphoinositide-containing liposomes in a calcium-dependent manner. GST-tagmin was incubated with liposomes containing pure PC or PC together with distinct polyphosphoinositides (as indicated) in the absence (shaded bars, 2 mM EGTA) or presence of calcium ions (solid bars, 100 μM free Ca2+). Lipid binding was quantified by liquid scintillation counting of the radioactive PC used as tracer and expressed as percent of total radioactivity used. Specific binding was calculated by subtracting the nonspecific lipid interaction of GST (2.4 ± 0.7%) from individual samples. Similar results were obtained both with small and large unilamellar vesicles, thus suggesting that size and curvature of the liposome do not influence the binding.
RESULTS AND DISCUSSION
Tagmin is known to bind the soluble inositol polyphosphates (InsPP) InsP4, InsP5, and InsP6 (17). To test if the InsPP binding site on tagmin can also bind corresponding lipid phosphoinositides, beads containing GST linked to tagmin I (residues 79–421) were incubated with liposomes containing PC and different inositol phospholipids [1% (wt/wt)] at close to the physiological concentration of free Mg2+ (0.5–1 mM). Small unilamellar vesicles made of pure PC do not bind tagmin in the absence or presence of calcium (11, 12). The inclusion of PIns or PIns-4-P does not result in binding of liposomes to tagmin, either in the presence (100 μM) or in the absence of free calcium (Fig. 1A). The naturally occurring phosphatidylinositol bisphosphates, PIns-4,5-P2 or PIns-3,4-P2, only moderately increase the interaction of tagmin with the liposomes in the absence of free calcium (Fig. 1A). However, under the same conditions, PIns-3,4,5-P3 strongly promotes the binding of liposomes to tagmin (Fig. 1).
Calcium (100 μM) switches the specificity of these interactions. Binding of tagmin to PIns-3,4,5-P3-containing liposomes is greatly reduced, while tagmin now binds efficiently to both PIns-4,5-P2 and PIns-3,4-P2-containing liposomes (Fig. 1). Similar results were obtained by using native tagmin immunopurified from brain cortex (not shown), thus indicating that the conclusions obtained with the GST-tagmin have a general validity.
Fig. 2 shows the dependence of PIns-4,5-P2 and PIns-3,4,5-P3 binding on the concentration of free calcium. PIns-4,5-P2 and PIns-3,4,5-P3 were incorporated into separate liposome populations, labeled either with a [14C] or with a [3H]PC tracer. Binding of each vesicle type to tagmin-containing beads was determined in a mixed incubation as a function of free calcium concentration. Tagmin loses the capacity to bind PIns-3,4,5-P3 between about 0.1 and 1 μM free calcium. It gains the capacity to bind PIns-4,5-P2 progressively above about 1 μM free calcium with maximum binding reached only in the 100 μM range. The calcium dependency of the switch of specificity was not significantly affected by altering the concentration of PIns-4,5-P2 or PIns-3,4,5-P3 in the liposome (data not shown). However, magnesium (0.5–1.0 mM) was required. In the absence of Mg2+, the calcium dependency of the binding of tagmin to PIns-4,5-P2-containing liposomes was less pronounced, as was the calcium-dependent reduction of the interaction of PIns-3,4,5-P3 with tagmin (data not shown).
Tagmin contains two C2 domains homologous to the calcium and acidic phospholipid binding domains of protein kinase C (5, 34). The more N-terminal C2 domain (C2A) binds 1,2-dioleoy-sn-glycero-3-phospho-l-serine (PS) [25% (wt/wt)] in a calcium-dependent manner with a Kd of 3–6 μM (11, 12). The more C-terminal C2 domain (C2B) binds InsPP (17, 35). Beads containing bacterially expressed GST-C2A or GST-C2B domains of tagmin were incubated with liposomes containing 1% (wt/wt) PIns-4,5-P2 or PIns-3,4,5-P3. The PIns-4,5-P2 and the PIns-3,4,5-P3-directed binding of liposomes both occur with the C2B domain of tagmin, with an efficiency similar to or higher than the full-length tagmin cytoplasmic domain (Fig. 3 B and C). While the interaction of the C2B domain with PIns-3,4,5-P3 is reduced by calcium, as for the entire cytoplasmic domain, the interaction of the C2B domain with PIns-4,5-P2 is strong even in the absence of calcium, and only weakly enhanced by calcium, implying cooperativity between the two C2 domains as suggested (36). While the C2A domain is known to bind liposomes containing PS in a calcium-dependent manner (11, 12), the C2B domain binds PS-containing liposomes weakly and in a calcium-independent manner (17, 36) and this is confirmed in our study, further establishing the specificity of the binding to polyphosphoinositides we report (Fig. 3A).
Figure 3.
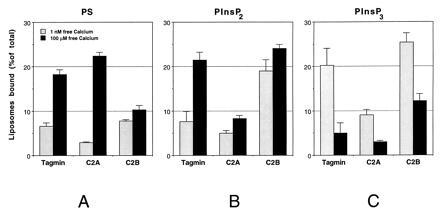
The polyphosphoinositides binding site on tagmin is localized to the C2B domain. GST fusion proteins containing tagmin or its N-terminal (C2A; aa 96–265; 10 μg) or the C-terminal (C2B; aa 248–421; 10 μg) domains were incubated with liposomes containing PC and 25% (wt/wt) PS (A), 1% (wt/wt) PIns-4,5-P2 (B), or 1% (wt/wt) PIns-3,4,5-P3 (C) (shaded bars, 2 mM EGTA; solid bars, 100 μM free Ca2+). Liposome binding to tagmin and its domains was determined as described in Fig. 1.
The interaction of both tagmin and C2B with PIns-4,5-P2 and PIns-3,4,5-P3-containing liposomes is competitively inhibited by InsP6 (not shown), suggesting that all three compounds bind to the same site in the C2B domain. InsP6 does not affect the calcium-dependent interaction of PS-containing liposomes with tagmin or its C2A domain (not shown). In summary, the C2B domain appears to preferentially interact with polyphosphoinositides; however the C2A domain may have some binding activity. Full calcium sensitivity seems to require both the C2A and C2B domains.
The binding of individual molecules of PIns-4,5-P2 (as distinct from liposomes containing PIns-4,5-P2) to tagmin could be measured directly because of the availability of radiolabeled PIns-4,5-P2. Radiolabeled PIns-4,5-P2 was dispersed in OG micelles using a vast excess of detergent to ensure that there would be at most one molecule of PIns-4,5-P2 per detergent micelle. At saturation almost exactly 1 mol of PIns-4,5-P2 was bound per mol of tagmin (Fig. 4A), with an apparent Kd of about 1 μM. Unlike the binding of tagmin to PIns-4,5-P2 contained in lipid bilayer vesicles, the binding of micellar PIns-4,5-P2 monomers was independent of calcium. The explanation of this is unclear. InsP6 competes with this binding, with a Ki of about 10 μM (Fig. 4B). Full-length native tagmin, immunopurified from brain cortex with an antibody directed against its first C2 homology domain (32) (Fig. 4D), shows the saturable and stoichiometric binding properties for PIns-4,5-P2 (Fig. 4C) as was observed with the recombinant protein. InsP6 inhibits the interaction of micellar PIns-4,5-P2 monomers with the native protein, but less efficiently than observed with the recombinant tagmin (Ki ≥ 100 μM; not shown).
The intracellular concentration of InsPP is estimated to be in the low micromolar range (38), which is unlikely to interfere in vivo with the binding of tagmin to the polyphosphoinositides described here. Presynaptic injection of higher concentrations of InsPP causes a reversible blockade of neurotransmitter release (39). We previously suggested that this inhibition could be due to inhibition of the binding of β-SNAP to the C2B domain of tagmin as demonstrated in vitro (16). The new findings suggest that InsPP could also act by competing for the binding of tagmin to PIns-4,5-P2 and/or PIns-3,4,5-P3.
Binding of PIns-4,5-P2 and PIns-3,4,5-P3 to tagmin is highly specific, as shown by (i) lack of tagmin binding to liposomes containing PIns and PIns-4-P; (ii) saturable binding of PIns-4,5-P2 to tagmin with 1:1 stoichiometry; (iii) the competitive inhibition of the above binding reactions by the soluble inositol derivative InsP6; and (iv) the switch in specificity from PIns-3,4,5-P3 to PIns-4,5-P2 as a function of free calcium ion.
As the free calcium concentration is raised from resting (basal) levels (≤30 nM) to about between 0.1 and 10 μM, tagmin switches its specificity from PIns-3,4,5-P3 to PIns-4,5-P2. Many proteins of diverse function are known to contain C2 domains (5–7) or are known to bind InsPP (35) and we would suggest that they, too, may have switchable lipid binding specificity, although the switch may be thrown by mechanisms other than calcium binding.
What might the polyphosphoinositide binding to tagmin and the calcium-dependent switch in its specificity mean for the mechanism of exocytosis? A PIns-specific transfer protein, a PIns 4-kinase (synthesizing PIns-4-P), a PIns-4-P 5-kinase (synthesizing PIns-4,5-P2) and PIns-4,5-P2 or PIns-3,4,5-P3 are required for fusion to be triggered by calcium (26, 28, 29). In addition, calcium at concentrations below the threshold for exocytosis (1–10 μM) is required for an ATP-dependent “priming” phase (40). Exocytosis is triggered when free calcium is above a threshold of about 20 μM (41), the same range at which tagmin binds PS and has switched from binding PIns-3,4,5-P3 to PIns-4,5-P2 (Fig. 2). Our data can now explain the requirement for polyphosphoinositide synthesis and for calcium in the priming step. The C2B domain of tagmin could be bound to PIns-3,4,5-P3 at basal calcium, representing a resting (off) conformation. An increase of calcium in the low micromolar range, as would be typically experienced by synaptic vesicles docked in the active zone, but not near a Ca2+-channel (42, 43), would release the C2B domain from PIns-3,4,5-P3 and trigger the binding to PIns-4,5-P2 representing a fusion-competent “on” conformation. This switch (and the binding to PS) could be important in the rapid triggering of exocytosis that occurs after calcium levels rise. The need for PIns-specific transfer protein, PIns 4-kinase and PIns-4-P 5-kinase activity during priming (26, 28, 29) could thus be explained by the need to maintain a pool of PIns-4,5-P2 to be available to bind the C2B domain when it is released from PIns-3,4,5-P3.
How would this model fit into the synaptic vesicle cycle? If the PIns-3,4,5-P3 pool were on synaptic vesicles and PIns-4,5-P2 pool were localized at the presynaptic membrane active zones, then vesicles at a distance from the membrane, sensing a small rise in calcium, could be mobilized to dock during that calcium wave, switching from PIns-3,4,5-P3 to PIns-4,5-P2. These vesicles would be competent to fuse rapidly at the next wave, when they will again experience a rise in local calcium concentration, now to an even higher level. This would create an assembly line for docking and fusion.
While the rate of fusion of synaptic vesicles in nerve endings is half-maximal at 200 μM free calcium, increasing about three orders of magnitude between 20–300 μM Ca2+ concentrations, the extent of calcium-triggered fusion is almost completely independent of calcium over the same range, provided calcium is raised above a threshold of about 20 μM (41). At 20 μM free Ca2+ a complete fusion event take only 50 msec, slow for a synapse, but fast for almost all other cell types. This threshold correlates well with the calcium concentration required for the C2A domain to bind PS (11, 12) and for C2B to switch from PIns-3,4,5-P3 to PIns-4,5-P2 (Fig. 2). In addition to these lipid-protein interactions, tagmin has been reported to bind the t-SNARE syntaxin (14, 15) and to self-associate in the high calcium range when magnesium is present at physiological level (18, 19). All these interactions may equally well contribute to switching fusion “on” as response to an intracellular calcium rise. This view of the regulation of exocytosis, from the perspective of tagmin, remains to be convincingly integrated with the core machinery that is generally important in docking and fusion, such as SNAP receptors (33), α- and β-SNAP (44), NSF (20). However, the C2B domain of tagmin is known to bind β-SNAP and NSF in a Ca2+-independent fashion and assemble with them and α-SNAP and SNARE, into a putative docking and fusion particle for exocytosis (16). A well-defined, reconstituted calcium-dependent fusion reaction in a liposomal system will be needed to allow a coherent view to emerge in which the significance of the various binding reactions can be better understood.
Acknowledgments
We thank R. Scheller for the kind gift of tagmin constructs and the tagmin-specific monoclonal antibody, and M. Craighead, J. A. McNew, M. A. Stamnes, G. Stenbeck, and T. Weber for critical reading the manuscript. This work was supported by National Institutes of Health grants to J.E.R. and G.D.P., and by the Mathers Charitable Foundation. Fellowship support came from European Molecular Biology Organization (G.S.)
Footnotes
Abbreviations: InsP6, inositol 1,2,3,4,5,6-hexakisphosphate; InsPP, inositol polyphosphates; OG, octyl-β-d-glucopyranoside; PC, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine; PIns, phosphatidyl inositol or 1-stearoyl-2-arachidonoyl-sn-glycero-3-phospho-d-myo-inositol; PIns-4,5-P2, PIns-4,5-bisphosphate; PIns-3,4,5-P3, 1,2-dipalmitoyl-sn-glycero-3-phospho-d-myo-inositol-3,4,5-trisphosphate; tagmin, synaptotagmin I; SNAP, soluble NSF attachment protein; PIns-4-P, PIns-4-phosphate; GST, glutathione S-transferase; PS, 1,2-dioleoy-sn-glycero-3-phospho-l-serine; NSF, N-ethylmaleimide fusion protein; SNARE, SNAP receptor.
References
- 1.Rothman J E. Nature (London) 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 2.Rothman J E, Wieland F T. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- 3.Südhof T C. Nature (London) 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 4.Littleton J T, Bellen H J. Trends Neurosci. 1995;18:177–183. doi: 10.1016/0166-2236(95)93898-8. [DOI] [PubMed] [Google Scholar]
- 5.Newton A C. Curr Biol. 1995;5:973–976. doi: 10.1016/s0960-9822(95)00191-6. [DOI] [PubMed] [Google Scholar]
- 6.Virbasius J V, Guilherme A, Czech M P. J Biol Chem. 1996;271:13304–13307. doi: 10.1074/jbc.271.23.13304. [DOI] [PubMed] [Google Scholar]
- 7.Molz L, Chen Y-W, Hirano M, Williams L T. J Biol Chem. 1996;271:13892–13899. doi: 10.1074/jbc.271.23.13892. [DOI] [PubMed] [Google Scholar]
- 8.Sutton R B, Davletov B A, Berghuis A M, Südhof T C, Sprang S R. Cell. 1995;80:929–938. doi: 10.1016/0092-8674(95)90296-1. [DOI] [PubMed] [Google Scholar]
- 9.Shao X, Davletov B A, Suttun R B, Südhof T C, Rizo J. Science. 1996;273:248–251. doi: 10.1126/science.273.5272.248. [DOI] [PubMed] [Google Scholar]
- 10.Brose N, Petrenko A G, Südhof T C, Jahn R. Science. 1992;256:1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- 11.Davletov B A, Südhof T C. J Biol Chem. 1993;268:26386–26390. [PubMed] [Google Scholar]
- 12.Chapman E R, Jahn R. J Biol Chem. 1994;269:5735–5741. [PubMed] [Google Scholar]
- 13.Bennett M K, Calakos N, Scheller R H. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Ullrich B, Zhang J Z, Anderson R G, Brose N, Südhof T C. Nature (London) 1995;375:594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- 15.Chapman E R, Hanson P I, An S, Jahn R. J Biol Chem. 1995;270:23667–23671. doi: 10.1074/jbc.270.40.23667. [DOI] [PubMed] [Google Scholar]
- 16.Schiavo G, Gmachl M J, Stenbeck G, Söllner T H, Rothman J E. Nature (London) 1995;378:733–736. doi: 10.1038/378733a0. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda M, Aruga J, Niinobe M, Aimoto S, Mikoshiba K. J Biol Chem. 1994;269:29206–29211. [PubMed] [Google Scholar]
- 18.Chapman E R, An S, Edwardson J M, Jahn R. J Biol Chem. 1996;270:5844–5849. doi: 10.1074/jbc.271.10.5844. [DOI] [PubMed] [Google Scholar]
- 19.Sugita S, Hata Y, Südhof T C. J Biol Chem. 1996;271:1262–1265. doi: 10.1074/jbc.271.3.1262. [DOI] [PubMed] [Google Scholar]
- 20.Wilson D W, Wilcox C A, Flynn G C, Chen E, Kuang W J, Henzel W J, Block M R, Ullrich A, Rothman J E. Nature (London) 1989;339:355–359. doi: 10.1038/339355a0. [DOI] [PubMed] [Google Scholar]
- 21.Geppert M, Goda Y, Hammer R E, Li C, Rosahl T W, Stevens C F, Südhof T C. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 22.Littleton J T, Stern M, Perin M, Bellen H J. Proc Natl Acad Sci USA. 1994;91:10888–10892. doi: 10.1073/pnas.91.23.10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiAntonio A, Schwarz T L. Neuron. 1994;12:909–920. doi: 10.1016/0896-6273(94)90342-5. [DOI] [PubMed] [Google Scholar]
- 24.Nonet M L, Grundahl K, Meyer B J, Rand J B. Cell. 1993;73:1291–1305. doi: 10.1016/0092-8674(93)90357-v. [DOI] [PubMed] [Google Scholar]
- 25.Eberhard D A, Cooper C L, Low M G, Holz R W. Biochem J. 1990;268:15–25. doi: 10.1042/bj2680015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hay J C, Fisette P L, Jenkins G H, Fukami K, Takenawa T, Anderson R A, Martin T F. Nature (London) 1995;374:173–177. doi: 10.1038/374173a0. [DOI] [PubMed] [Google Scholar]
- 27.De Camilli P, Emr S D, McPherson P S, Novick P. Science. 1996;271:1533–1539. doi: 10.1126/science.271.5255.1533. [DOI] [PubMed] [Google Scholar]
- 28.Hay J C, Martin T F. Nature (London) 1993;366:572–575. doi: 10.1038/366572a0. [DOI] [PubMed] [Google Scholar]
- 29.Wiedemann C, Schafer T, Burger M M. EMBO J. 1996;15:2094–2101. [PMC free article] [PubMed] [Google Scholar]
- 30.Gu, Q.-M. & Prestwich, G. D. (1996) J. Org. Chem., in press.
- 31.Duzgunes N, Wilschut J. Methods Enzymol. 1993;220:3–14. doi: 10.1016/0076-6879(93)20069-f. [DOI] [PubMed] [Google Scholar]
- 32.Matthew W D, Tsavaler L, Reichardt L F. J Cell Biol. 1981;91:257–269. doi: 10.1083/jcb.91.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Söllner T, Whiteheart S W, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman J E. Nature (London) 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 34.Perin M S, Fried V A, Mignery G A, Jahn R, Südhof T C. Nature (London) 1990;345:260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- 35.Fukuda M, Kojima T, Aruga J, Niinobe M, Mikoshiba K. J Biol Chem. 1995;270:26523–26527. doi: 10.1074/jbc.270.44.26523. [DOI] [PubMed] [Google Scholar]
- 36.Damer C K, Creutz C E. J Biol Chem. 1994;269:31115–31123. [PubMed] [Google Scholar]
- 37.Li C, Davletov B A, Südhof T C. J Biol Chem. 1995;270:24898–24902. doi: 10.1074/jbc.270.42.24898. [DOI] [PubMed] [Google Scholar]
- 38.Majerus P W. Annu Rev Biochem. 1992;61:225–250. doi: 10.1146/annurev.bi.61.070192.001301. [DOI] [PubMed] [Google Scholar]
- 39.Llinas R, Sugimori M, Lang E J, Morita M, Fukuda M, Niinobe M, Mikoshiba K. Proc Natl Acad Sci USA. 1994;91:12990–12993. doi: 10.1073/pnas.91.26.12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bittner M A, Holz R W. J Biol Chem. 1992;267:16219–16225. [PubMed] [Google Scholar]
- 41.Heidelberger R, Heinemann C, Neher E, Matthews G. Nature (London) 1994;371:513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- 42.Cheek T R, O’Sullivan A J, Moreton R B, Berridge M J, Burgoyne R D. FEBS Lett. 1989;247:429–434. doi: 10.1016/0014-5793(89)81385-7. [DOI] [PubMed] [Google Scholar]
- 43.Augustine G J, Neher E. J Physiol (London) 1992;450:247–271. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whiteheart S W, Griff I C, Brunner M, Clary D O, Mayer T, Buhrow S A, Rothman J E. Nature (London) 1993;362:353–355. doi: 10.1038/362353a0. [DOI] [PubMed] [Google Scholar]


