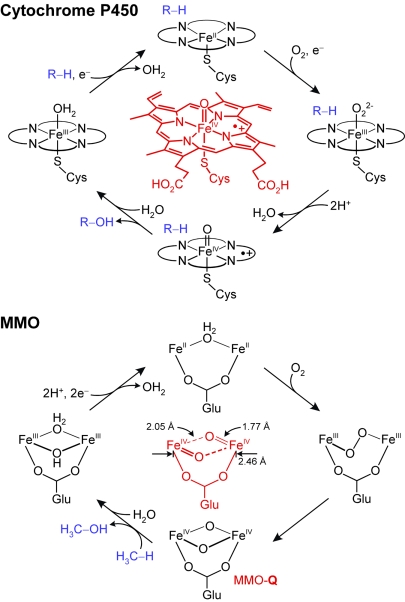
Molecular oxygen (O2) is the principal oxidant used by aerobic organisms to carry out a wide range of metabolic reactions and transformations. The enzymes involved in these processes use a rich variety of different active sites to activate O2, such as heme cofactors, mono- and binuclear nonheme iron centers, mono- and binuclear copper complexes, and heteronuclear heme iron/copper clusters (1). Typically, oxygen is activated in a tightly controlled manner to ensure that the formation of the key reactive species in the corresponding catalytic cycle occurs only when the target substrate is present, thereby suppressing potentially harmful side reactions in the enzyme active site (2). A particularly well studied example of an enzyme that follows this general strategy is provided by cytochrome P450 (3). As outlined in Fig. 1Upper, binding of substrate to the heme-containing active site of cytochrome P450 causes dissociation of the FeIII-bound water ligand, thereby triggering the reduction of the iron center to the reactive FeII state. The FeII species binds and activates O2 to generate an iron(IV)-oxo porphyrin radical intermediate. This highly potent oxidant then proceeds to incorporate one of the O atoms derived from molecular oxygen into a specific C H bond of the substrate, in a process that yields the oxidized product and restores the resting FeIII state of the enzyme. High-valent intermediates have also been identified in the catalytic cycles of an increasing number of nonheme diiron enzymes (1, 4), such as methane monooxygenase (MMO) (2, 5). Found in methanotropic bacteria, MMO catalyzes the chemically challenging conversion of methane to methanol (Fig. 1 Lower), thereby reducing the amount of this greenhouse gas that is being released into the atmosphere by nearly 1 billion tons per year (6). Detailed kinetic and spectroscopic studies (2, 7) have revealed the transient formation of at least six intermediates in the catalytic cycle of MMO, among which the intermediate Q (MMO-Q) is the key oxidizing species. On the basis of extended x-ray absorption fine structure (EXAFS) and Mössbauer spectroscopic studies (6), MMO-Q was described as possessing an unprecedented [FeIV2(μ-O)2] diamond core. In support of this model for the active-site structure of MMO-Q, the work of Xue et al. (8) in this issue of PNAS provides experimental evidence for the chemical viability of a [FeIV2(μ-O)2] diamond core in diiron complexes that possess biologically relevant supporting ligands.
H bond of the substrate, in a process that yields the oxidized product and restores the resting FeIII state of the enzyme. High-valent intermediates have also been identified in the catalytic cycles of an increasing number of nonheme diiron enzymes (1, 4), such as methane monooxygenase (MMO) (2, 5). Found in methanotropic bacteria, MMO catalyzes the chemically challenging conversion of methane to methanol (Fig. 1 Lower), thereby reducing the amount of this greenhouse gas that is being released into the atmosphere by nearly 1 billion tons per year (6). Detailed kinetic and spectroscopic studies (2, 7) have revealed the transient formation of at least six intermediates in the catalytic cycle of MMO, among which the intermediate Q (MMO-Q) is the key oxidizing species. On the basis of extended x-ray absorption fine structure (EXAFS) and Mössbauer spectroscopic studies (6), MMO-Q was described as possessing an unprecedented [FeIV2(μ-O)2] diamond core. In support of this model for the active-site structure of MMO-Q, the work of Xue et al. (8) in this issue of PNAS provides experimental evidence for the chemical viability of a [FeIV2(μ-O)2] diamond core in diiron complexes that possess biologically relevant supporting ligands.
Fig. 1.
Principal steps in the O2 activation and substrate (R H) oxidation mechanisms used by cytochrome P450 (Upper) and MMO (Lower), along with schematic representations of the corresponding key oxidizing species (shown in red). In the cytochrome P450 catalytic cycle, binding of substrate to the resting enzyme triggers the reduction of the FeIII center of the heme cofactor to the reactive FeII state, which binds and activates O2 to generate a high-valent iron(IV)-oxo porphyrin radical intermediate that is capable of oxidizing a wide range of organic substrates (a notable exception being methane, the native substrate of MMO). In the MMO catalytic cycle, the diiron(III) cluster of the resting enzyme is reduced by two electrons to yield the reactive diiron(II) species, which binds O2 to generate a peroxodiiron(III) intermediate. Further reduction of the O2 moiety in this species yields the oxodiiron(IV) key oxidizing intermediate (MMO-Q), which converts the substrate methane to methanol.
H) oxidation mechanisms used by cytochrome P450 (Upper) and MMO (Lower), along with schematic representations of the corresponding key oxidizing species (shown in red). In the cytochrome P450 catalytic cycle, binding of substrate to the resting enzyme triggers the reduction of the FeIII center of the heme cofactor to the reactive FeII state, which binds and activates O2 to generate a high-valent iron(IV)-oxo porphyrin radical intermediate that is capable of oxidizing a wide range of organic substrates (a notable exception being methane, the native substrate of MMO). In the MMO catalytic cycle, the diiron(III) cluster of the resting enzyme is reduced by two electrons to yield the reactive diiron(II) species, which binds O2 to generate a peroxodiiron(III) intermediate. Further reduction of the O2 moiety in this species yields the oxodiiron(IV) key oxidizing intermediate (MMO-Q), which converts the substrate methane to methanol.
Synthetic model complexes can contribute significantly to a detailed understanding of the geometric, electronic, and spectroscopic properties, as well as the reactivity, of a given metalloenzyme active site. Consequently, considerable effort has been made by synthetic chemists to prepare viable models for the putative reaction intermediates in the catalytic cycles of O2-activating enzymes. Although in the case of MMO these efforts culminated in a number of structurally characterized model complexes that closely mimic the geometric and spectroscopic properties of several intermediates participating in the catalytic cycle of the enzyme (4, 9), a synthetic precedent for the [FeIV2(μ-O)2] diamond core proposed for MMO-Q had been lacking prior to the work of Xue et al. (8). For this reason, relatively little is currently known about the geometric and electronic structures of this powerful oxidant. A detailed analysis of EXAFS and Mössbauer spectroscopic data by Que, Münck, and coworkers (6) revealed that MMO-Q is best described as a strongly exchange-coupled diiron(IV) species with an Fe···Fe distance of 2.46 Å and pairs of short and long Fe O bonds of 1.77 and 2.05 Å, respectively (Fig. 1 Lower), consistent with an [FeIV2(μ-O)2] diamond core. However, because EXAFS data only reflect the presence of at least two single-atom oxygen bridges, other core structures for MMO-Q are also conceivable (4). Because of this uncertainty, as well as the intriguing structural, electronic, and reactivity properties of MMO-Q, the generation of a viable active-site model for this species has been a subject of considerable interest among computational chemists (4, 10). However, no consensus has yet been reached regarding the core structure of MMO-Q, which is probably not surprising considering that at present the geometric constraints imposed by experimental data on viable active-site models of this species are rather loose.
O bonds of 1.77 and 2.05 Å, respectively (Fig. 1 Lower), consistent with an [FeIV2(μ-O)2] diamond core. However, because EXAFS data only reflect the presence of at least two single-atom oxygen bridges, other core structures for MMO-Q are also conceivable (4). Because of this uncertainty, as well as the intriguing structural, electronic, and reactivity properties of MMO-Q, the generation of a viable active-site model for this species has been a subject of considerable interest among computational chemists (4, 10). However, no consensus has yet been reached regarding the core structure of MMO-Q, which is probably not surprising considering that at present the geometric constraints imposed by experimental data on viable active-site models of this species are rather loose.
Using electrochemical methods, Xue et al. (8) have succeeded in preparing the first example of a synthetic complex possessing an [FeIV2(μ-O)2] diamond core. Analysis of Mössbauer and EXAFS spectroscopic data revealed that the two FeIV centers in this complex are antiferromagnetically coupled and provided Fe···Fe and Fe O bond distances of 2.73 and 1.78 Å, respectively. Intriguingly, in their preliminary reactivity studies, the authors noted that this complex is 100-fold less effective in carrying out H-atom abstraction than a closely related mononuclear species featuring an FeIV
O bond distances of 2.73 and 1.78 Å, respectively. Intriguingly, in their preliminary reactivity studies, the authors noted that this complex is 100-fold less effective in carrying out H-atom abstraction than a closely related mononuclear species featuring an FeIV O unit. Considering that MMO-Q is capable of oxidizing methane, and thus constitutes an oxidant superior to any mononuclear oxoiron(IV) complex studied to date (11–13), this finding has some important implications with respect to the geometric and electronic structures of the enzyme intermediate. In particular, Xue et al. note that although their complex possesses two low-spin iron(IV) centers, MMO-Q contains a pair of high-spin iron(IV) ions, which is expected to increase the reactivity of the enzyme active site for H-atom abstraction (14). Nonetheless, the authors' results may also indicate that the [FeIV2(μ-O)2] diamond core of MMO-Q must in fact be further activated for it to become a sufficiently powerful oxidant to convert methane to methanol (15). This notion would be consistent with Nature's general strategy of protecting enzyme active sites by unleashing the full power of potentially harmful oxidants only in the presence of
O unit. Considering that MMO-Q is capable of oxidizing methane, and thus constitutes an oxidant superior to any mononuclear oxoiron(IV) complex studied to date (11–13), this finding has some important implications with respect to the geometric and electronic structures of the enzyme intermediate. In particular, Xue et al. note that although their complex possesses two low-spin iron(IV) centers, MMO-Q contains a pair of high-spin iron(IV) ions, which is expected to increase the reactivity of the enzyme active site for H-atom abstraction (14). Nonetheless, the authors' results may also indicate that the [FeIV2(μ-O)2] diamond core of MMO-Q must in fact be further activated for it to become a sufficiently powerful oxidant to convert methane to methanol (15). This notion would be consistent with Nature's general strategy of protecting enzyme active sites by unleashing the full power of potentially harmful oxidants only in the presence of
High-valent iron-oxo species are well suited for catalyzing a wide range of technologically important oxidations.
substrate, as is beautifully exemplified by cytochrome P450 (see above and Fig. 1 Upper).
Another important aspect of the work of Xue et al. (8) is that it provides a suitable basis for the pursuit of a number of different research avenues pertaining to the mechanism of O2 activation and substrate oxidation by diiron centers. First, carrying out detailed studies of the reactivity of this model complex will provide invaluable information regarding the mechanism by which enzyme intermediates possessing an [FeIV2(μ-O)2] diamond core oxidize organic substrates. This information will also be valuable for testing predictions made using computational tools; good agreement between theoretically predicted and experimentally observed outcomes of chemical reactions would lend credence to the use of a given computational tool for investigating the structure and reactivity of MMO-Q at the theoretical level (4, 10). Second, the authors' synthetic strategy should allow for the preparation of [FeIV2(μ-O)2] model complexes that more closely mimic the electronic properties of MMO-Q, thereby affording an excellent means for testing the hypothesis that complexes possessing a pair of exchange-coupled, high-spin iron(IV) centers are more reactive than their low-spin counterparts in H-atom abstraction (14). Lastly, given the tremendous oxidation power of MMO-Q, which is Nature's only species known to date that is capable of converting methane to methanol, synthetic complexes possessing an [FeIV2(μ-O)2] diamond core may also represent promising candidates for the advancement of green oxidation technology. Indeed, research carried out by Collins and coworkers (16–18) over the last two decades has revealed that high-valent iron-oxo species are well suited for catalyzing a wide range of technologically important oxidations, such as the rapid bleaching of water-soluble dyes, the decolorization of pulp mill effluents, and the complete remediation of chlorophenol persistent pollutants.
Footnotes
The author declares no conflict of interest.
See companion article on page 20713.
References
- 1.Holm RH, Kennepohl P, Solomon EI. Chem Rev. 1996;96:2239–2314. doi: 10.1021/cr9500390. [DOI] [PubMed] [Google Scholar]
- 2.Kovaleva EG, Neibergall MB, Chakrabarty S, Lipscomb JD. Acc Chem Res. 2007;40:475–483. doi: 10.1021/ar700052v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denisov IG, Makris TM, Sligar SG, Schlichting I. Chem Rev. 2005;105:2253–2277. doi: 10.1021/cr0307143. [DOI] [PubMed] [Google Scholar]
- 4.Solomon EI, Brunold TC, Davis MI, Kemsley JN, Lee SK, Lehnert N, Neese F, Skulan AJ, Yang YS, Zhou J. Chem Rev. 2000;100:235–349. doi: 10.1021/cr9900275. [DOI] [PubMed] [Google Scholar]
- 5.Feig AL, Lippard SJ. Chem Rev. 1994;94:759–805. [Google Scholar]
- 6.Shu LJ, Nesheim JC, Kauffmann K, Münck E, Lipscomb JD, Que L., Jr Science. 1997;275:515–518. doi: 10.1126/science.275.5299.515. [DOI] [PubMed] [Google Scholar]
- 7.Lee SK, Fox BG, Froland WA, Lipscomb JD, Münck E. J Am Chem Soc. 1993;115:6450–6451. [Google Scholar]
- 8.Xue G, Wang D, De Hont R, Fiedler AT, Shan X, Münck E, Que L., Jr Proc Natl Acad Sci USA. 2007;104:20713–20718. doi: 10.1073/pnas.0708516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tshuva EY, Lippard SJ. Chem Rev. 2004;104:987–1011. doi: 10.1021/cr020622y. [DOI] [PubMed] [Google Scholar]
- 10.Rinaldo D, Philipp DM, Lippard SJ, Friesner RA. J Am Chem Soc. 2007;129:3135–3147. doi: 10.1021/ja0654074. and references cited. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costas M, Mehn MP, Jensen MP, Que L., Jr Chem Rev. 2004;104:939–986. doi: 10.1021/cr020628n. [DOI] [PubMed] [Google Scholar]
- 12.Kaizer J, Klinker EJ, Oh NY, Rohde JU, Song WJ, Stubna A, Kim J, Münck E, Nam W, Que L., Jr J Am Chem Soc. 2004;126:472–473. doi: 10.1021/ja037288n. [DOI] [PubMed] [Google Scholar]
- 13.Que L., Jr Acc Chem Res. 2007;40:493–500. doi: 10.1021/ar700024g. [DOI] [PubMed] [Google Scholar]
- 14.Shaik S, Hirao H, Kumar D. Acc Chem Res. 2007;40:532–542. doi: 10.1021/ar600042c. [DOI] [PubMed] [Google Scholar]
- 15.Siegbahn PEM, Crabtree RH. J Am Chem Soc. 1997;119:3103–3113. [Google Scholar]
- 16.Collins TJ. Acc Chem Res. 1994;27:279–285. [Google Scholar]
- 17.Collins TJ. Acc Chem Res. 2002;35:782–790. doi: 10.1021/ar010079s. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh A, de Oliveira FT, Yano T, Nishioka T, Beach ES, Kinoshita I, Münck E, Ryabov AD, Horwitz CP, Collins TJ. J Am Chem Soc. 2005;127:2505–2513. doi: 10.1021/ja0460458. [DOI] [PubMed] [Google Scholar]