Abstract
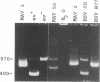
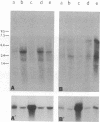
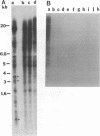
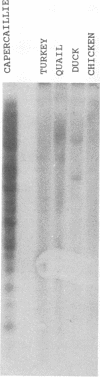
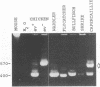
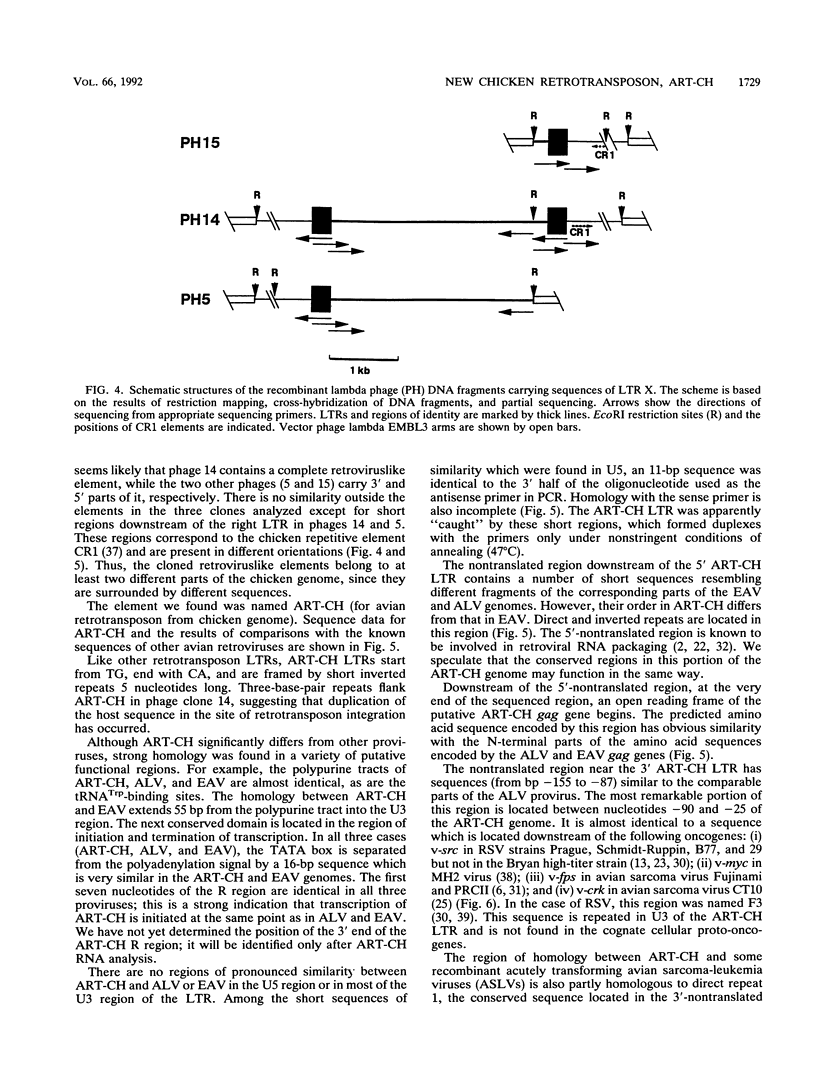
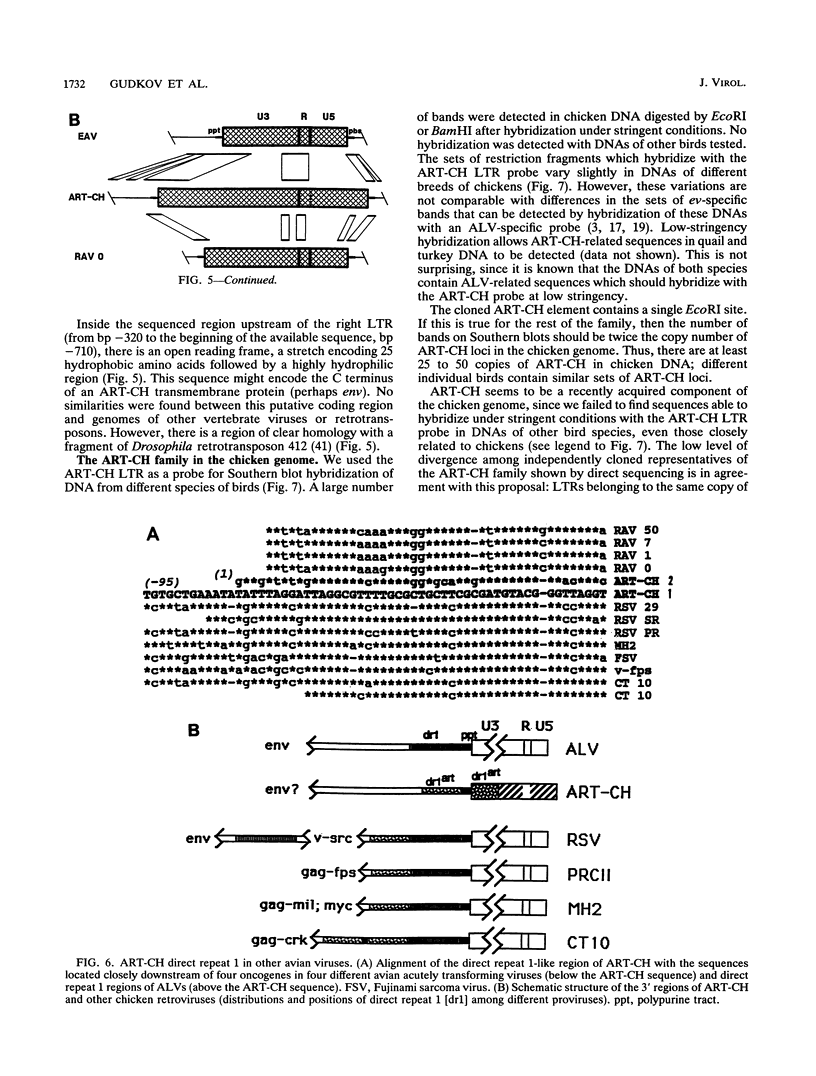
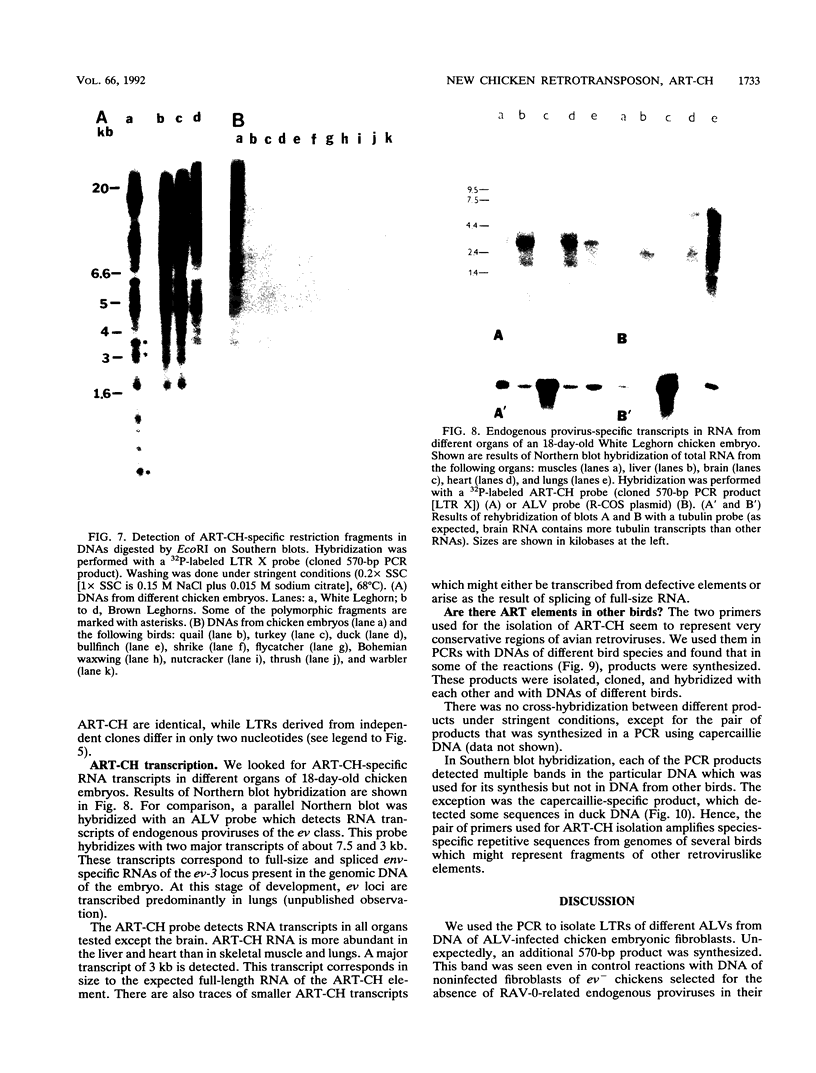
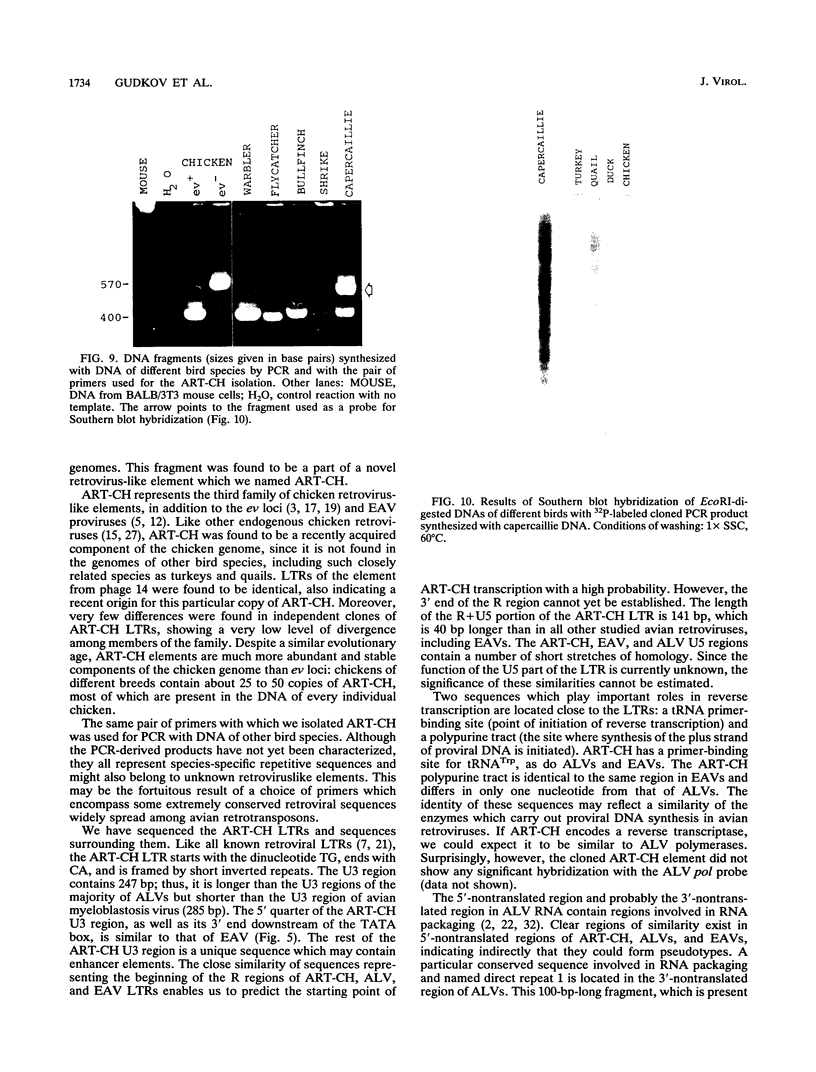
A 3' region of a previously unknown retroviruslike element named ART-CH (avian retrotransposon from chicken genome) was obtained in the course of polymerase chain reaction-mediated cloning of avian leukosis virus long terminal repeats (LTRs) from DNAs of infected chicken cells. About 50 copies of ART-CH are present in the genome of chickens of different breeds. ART-CH is not found in DNA of quails, ducks, turkeys, or several other birds tested. The ART-CH element is about 3 kb in size, including 388 bp LTRs. The major class of ART-CH-specific RNA, also 3 kb in size, is detected in various organs of chickens. An ART-CH polypurine tract, a tRNA(Trp)-binding site, regions around the TATA box and polyadenylation signal, and the beginning of the putative gag gene strongly resemble the corresponding regions of avian leukosis viruses and EAV, the two described classes of chicken retroviruses. An open reading frame capable of encoding a polypeptide with a putative transmembrane domain is located upstream of the right ART-CH LTR. This sequence, as well as the U3 and U5 regions of the ART-CH LTR, has no obvious similarities with the corresponding parts of other known vertebrate retroviruses and retrotransposons. A short sequence upstream of the right LTR of ART-CH is very similar to sequences which flank the 3' ends of the oncogenes v-src, v-myc, v-fps, and v-crk in four different recombinant avian retroviruses and which are absent from the genomes of other studied avian retroviruses. Thus, ART-CH is a new endogenous chicken provirus that may participate in the formation of recombinant oncogenic retroviruses.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. E., Rathjen P. D., Stanway C. A., Fulton S. M., Malim M. H., Wilson W., Ogden J., King L., Kingsman S. M., Kingsman A. J. Complete nucleotide sequence of a mouse VL30 retro-element. Mol Cell Biol. 1988 Aug;8(8):2989–2998. doi: 10.1128/mcb.8.8.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff R., Linial M. Specificity of retroviral RNA packaging. J Virol. 1991 Jan;65(1):71–80. doi: 10.1128/jvi.65.1.71-80.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrin S. M. Endogenous viral genes of the White Leghorn chicken: common site of residence and sites associated with specific phenotypes of viral gene expression. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5941–5945. doi: 10.1073/pnas.75.12.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizub D., Katz R. A., Skalka A. M. Nucleotide sequence of noncoding regions in Rous-associated virus-2: comparisons delineate conserved regions important in replication and oncogenesis. J Virol. 1984 Feb;49(2):557–565. doi: 10.1128/jvi.49.2.557-565.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce-Jacino M. T., Resnick R., Faras A. J. Structural and functional characterization of the unusually short long terminal repeats and their adjacent regions of a novel endogenous avian retrovirus. Virology. 1989 Nov;173(1):157–166. doi: 10.1016/0042-6822(89)90231-6. [DOI] [PubMed] [Google Scholar]
- Carlberg K., Chamberlin M. E., Beemon K. The avian sarcoma virus PRCII lacks 1020 nucleotides of the fps transforming gene. Virology. 1984 May;135(1):157–167. doi: 10.1016/0042-6822(84)90126-0. [DOI] [PubMed] [Google Scholar]
- Chen H. R., Barker W. C. Nucleotide sequences of the retroviral long terminal repeats and their adjacent regions. Nucleic Acids Res. 1984 Feb 24;12(4):1767–1778. doi: 10.1093/nar/12.4.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernov M. V., Tikhonenko A. T., Zaitsevskaia T. E., Denisova R. A., Obukh I. B. V genome kur, svobodnykh ot éndogennykh provirusov leikozo-sarkomnogo kompleksa ptits, prisutstvuiu posledovatel'nosti, otdalenno rodstvennye virusu sarcomy Rausa. Mol Gen Mikrobiol Virusol. 1987 Mar;(3):6–11. [PubMed] [Google Scholar]
- Dunwiddie C. T., Resnick R., Boyce-Jacino M., Alegre J. N., Faras A. J. Molecular cloning and characterization of gag-, pol-, and env-related gene sequences in the ev- chicken. J Virol. 1986 Sep;59(3):669–675. doi: 10.1128/jvi.59.3.669-675.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie C., Faras A. J. Presence of retrovirus reverse transcriptase-related gene sequences in avian cells lacking endogenous avian leukosis viruses. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5097–5101. doi: 10.1073/pnas.82.15.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A., Wang L. H., Hanafusa T., Hanafusa H. Partial nucleotide sequence of Rous sarcoma virus-29 provides evidence that the original Rous sarcoma virus was replication defective. J Virol. 1985 Sep;55(3):728–735. doi: 10.1128/jvi.55.3.728-735.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., Defeo D., Shih T. Y., Gonda M. A., Young H. A., Tsuchida N., Lowy D. R., Scolnick E. M. The p21 src genes of Harvey and Kirsten sarcoma viruses originate from divergent members of a family of normal vertebrate genes. Nature. 1981 Aug 6;292(5823):506–511. doi: 10.1038/292506a0. [DOI] [PubMed] [Google Scholar]
- Frisby D. P., Weiss R. A., Roussel M., Stehelin D. The distribution of endogenous chicken retrovirus sequences in the DNA of galliform birds does not coincide with avian phylogenetic relationships. Cell. 1979 Jul;17(3):623–634. doi: 10.1016/0092-8674(79)90270-8. [DOI] [PubMed] [Google Scholar]
- Gudkov A. V., Korec E., Chernov M. V., Tikhonenko A. T., Obukh I. B., Hlozánek I. Genetic structure of the endogenous proviruses and expression of the gag gene in Brown Leghorn chickens. Folia Biol (Praha) 1986;32(1):65–72. [PubMed] [Google Scholar]
- Gudkov A. V., Obukh I. B., Serov S. M., Naroditsky B. S. Variety of endogenous proviruses in the genomes of chickens of different breeds. J Gen Virol. 1981 Nov;57(Pt 1):85–94. doi: 10.1099/0022-1317-57-1-85. [DOI] [PubMed] [Google Scholar]
- Hauber J., Nelböck-Hochstetter P., Feldmann H. Nucleotide sequence and characteristics of a Ty element from yeast. Nucleic Acids Res. 1985 Apr 25;13(8):2745–2758. doi: 10.1093/nar/13.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H., Toyoshima K., Bishop J. M., Varmus H. E. Organization of the endogenous proviruses of chickens: implications for origin and expression. Virology. 1981 Jan 15;108(1):189–207. doi: 10.1016/0042-6822(81)90538-9. [DOI] [PubMed] [Google Scholar]
- Humphries E. H., Danhof M. L., Hlozanek I. Characterization of endogenous viral loci in five lines of white Leghorn chickens. Virology. 1984 May;135(1):125–138. doi: 10.1016/0042-6822(84)90123-5. [DOI] [PubMed] [Google Scholar]
- Katz R. A., Terry R. W., Skalka A. M. A conserved cis-acting sequence in the 5' leader of avian sarcoma virus RNA is required for packaging. J Virol. 1986 Jul;59(1):163–167. doi: 10.1128/jvi.59.1.163-167.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner T. L., Hanafusa H. DNA sequence of the Bryan high-titer strain of Rous sarcoma virus: extent of env deletion and possible genealogical relationship with other viral strains. J Virol. 1984 Feb;49(2):549–556. doi: 10.1128/jvi.49.2.549-556.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B. J., Hamaguchi M., Hanafusa H. A novel viral oncogene with structural similarity to phospholipase C. Nature. 1988 Mar 17;332(6161):272–275. doi: 10.1038/332272a0. [DOI] [PubMed] [Google Scholar]
- Mount S. M., Rubin G. M. Complete nucleotide sequence of the Drosophila transposable element copia: homology between copia and retroviral proteins. Mol Cell Biol. 1985 Jul;5(7):1630–1638. doi: 10.1128/mcb.5.7.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick R. M., Boyce-Jacino M. T., Fu Q., Faras A. J. Phylogenetic distribution of the novel avian endogenous provirus family EAV-0. J Virol. 1990 Oct;64(10):4640–4653. doi: 10.1128/jvi.64.10.4640-4653.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovigatti U. G., Astrin S. M. Avian endogenous viral genes. Curr Top Microbiol Immunol. 1983;103:1–21. doi: 10.1007/978-3-642-68943-7_1. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Vass W. C., Howk R. S., Duesberg P. H. Defective retrovirus-like 30S RNA species of rat and mouse cells are infectious if packaged by type C helper virus. J Virol. 1979 Mar;29(3):964–972. doi: 10.1128/jvi.29.3.964-972.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa H. Nucleotide sequence of Fujinami sarcoma virus: evolutionary relationship of its transforming gene with transforming genes of other sarcoma viruses. Cell. 1982 Oct;30(3):787–795. doi: 10.1016/0092-8674(82)90283-5. [DOI] [PubMed] [Google Scholar]
- Sorge J., Ricci W., Hughes S. H. cis-Acting RNA packaging locus in the 115-nucleotide direct repeat of Rous sarcoma virus. J Virol. 1983 Dec;48(3):667–675. doi: 10.1128/jvi.48.3.667-675.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P., Gridley T., Jaenisch R. Retroviruses and insertional mutagenesis in mice: proviral integration at the Mov 34 locus leads to early embryonic death. Genes Dev. 1987 Jun;1(4):366–375. doi: 10.1101/gad.1.4.366. [DOI] [PubMed] [Google Scholar]
- Spence S. E., Gilbert D. J., Swing D. A., Copeland N. G., Jenkins N. A. Spontaneous germ line virus infection and retroviral insertional mutagenesis in eighteen transgenic Srev lines of mice. Mol Cell Biol. 1989 Jan;9(1):177–184. doi: 10.1128/mcb.9.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoye J. P., Coffin J. M. The four classes of endogenous murine leukemia virus: structural relationships and potential for recombination. J Virol. 1987 Sep;61(9):2659–2669. doi: 10.1128/jvi.61.9.2659-2669.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoye J. P., Fenner S., Greenoak G. E., Moran C., Coffin J. M. Role of endogenous retroviruses as mutagens: the hairless mutation of mice. Cell. 1988 Jul 29;54(3):383–391. doi: 10.1016/0092-8674(88)90201-2. [DOI] [PubMed] [Google Scholar]
- Stumph W. E., Hodgson C. P., Tsai M. J., O'Malley B. W. Genomic structure and possible retroviral origin of the chicken CR1 repetitive DNA sequence family. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6667–6671. doi: 10.1073/pnas.81.21.6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutrave P., Jansen H. W., Bister K., Rapp U. R. 3'-Terminal region of avian carcinoma virus MH2 shares sequence elements with avian sarcoma viruses Y73 and SR-A. J Virol. 1984 Nov;52(2):703–705. doi: 10.1128/jvi.52.2.703-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Tereba A. Asymmetric chromosomal distribution of endogenous retrovirus loci in chickens and mice. Curr Top Microbiol Immunol. 1983;107:29–50. doi: 10.1007/978-3-642-69075-4_2. [DOI] [PubMed] [Google Scholar]
- Yuki S., Inouye S., Ishimaru S., Saigo K. Nucleotide sequence characterization of a Drosophila retrotransposon, 412. Eur J Biochem. 1986 Jul 15;158(2):403–410. doi: 10.1111/j.1432-1033.1986.tb09767.x. [DOI] [PubMed] [Google Scholar]