Abstract
The product of the UL11 gene of HSV-1 is a small, membrane-bound tegument protein with features that are conserved among all herpesviruses. For all viruses examined, mutants lacking this protein (or its homolog) have budding defects and accumulate capsids in the cytoplasm of the infected cell. UL11 binds to the cytoplasmic faces of host membranes via N-terminal myristate and nearby palmitate moieties. These fatty-acid modifications are typical of proteins that localize to detergent-resistant membranes (DRMs), and the experiments described here revealed that a small amount (~10%) of UL11 retains the ability to float in sucrose gradients following treatment of cells with Triton X-100. However, mutants lacking sequences previously shown to be involved in the trafficking of UL11 from the plasma membrane (LI and acidic cluster motifs) were found to have a dramatically increased association with DRMs. These findings emphasize the dynamic properties of this poorly-understood but conserved tegument protein.
Keywords: UL11, Lipid Raft, DRM, Herpes Simplex, Acidic Cluster, Dileucine, Myristate, Palmitate
Introduction
The UL11 protein of herpes simplex virus (HSV) is necessary for the efficient production of virions in cell cultures (Baines and Roizman, 1992; Fulmer et al., 2007; MacLean et al., 1992). This small, 96-amino-acid molecule is thought to be made on cytoplasmic ribosomes, where it is cotranslationally modified with myristate on its N-terminal glycine following removal of the initiator methionine (MacLean et al., 1989; Resh, 1999). UL11 subsequently binds to the cytoplasmic faces of cellular membranes and becomes palmitylated on at least one of the three cysteines located near the amino terminus (Baines et al., 1995; Loomis et al., 2001). Modifications with myristate and palmitate are needed for the accumulation of UL11 on Golgi-derived membranes (Bowzard et al., 2000; Loomis et al., 2001), the site of maturation budding where capsids acquire their envelopes (Mettenleiter, 2004). Approximately 700 molecules of UL11 are packaged into the virion (Loomis et al., 2006), and these are thought to extend from the membrane into the tegument, the region located between the membrane and the capsid (Mettenleiter, 2004). Mutants having large deletions in the UL11-coding sequence exhibit an accumulation of unenveloped capsids in the cytoplasm (Baines and Roizman, 1992; Fulmer et al., 2007; MacLean et al., 1992). Moreover, all herpesviruses encode a homolog of UL11, and in those cases where this gene has been disrupted, replication defects and cytoplasmic capsid accumulations also result (Britt et al., 2004; Kopp et al., 2003; Kopp et al., 2004; Schimmer and Neubauer, 2003; Silva et al., 2003; Silva et al., 2005). However, in contrast to human cytomegalovirus, where null mutants are completely defective for the release of extracellular virions, HSV mutants have been reported to be reduced at most ~1000 fold, perhaps due to redundancy. Alternatively, this could be due to incomplete removal of the UL11-coding sequence to avoid the overlap with the UL12 gene, thereby leaving large portions of the N-terminus of UL11 intact (Baines and Roizman, 1992; Fulmer et al., 2007; MacLean et al., 1992).
Although UL11 mutants have defects in maturation budding, the actual function of this protein is unknown. Insight was provided by the discovery of an interaction between UL11 and UL16 (Loomis et al., 2003; Vittone et al., 2005), a tegument protein that has been reported to be associated with capsids (Meckes, Jr. and Wills, 2007; Oshima et al., 1998). This led to a model that is reminiscent of the function of viral matrix proteins in which UL11 links capsids (via UL16) to host membranes to promote the budding process (Loomis et al., 2003). However, it is clear that this view is overly simplistic because UL11 mutants have been found that are incorporated into virions, even though they lack the LI (leucine-isoleucine) and acidic cluster (AC) motifs needed for the interaction with UL16 (Loomis et al., 2006). These motifs are particularly interesting because they are important for the recovery of UL11 from the plasma membrane back to internal membranes. That is, when either motif is absent, a portion of UL11 accumulates at the cell periphery (Loomis et al., 2001). Moreover, chimeras that have foreign acidic clusters (from Nef or furin) do not accumulate on the plasma membrane and are packaged, even though they do not interact with UL16 (Loomis et al., 2001; Loomis et al., 2003; Loomis et al., 2006). Based on these observations, another model for the function of UL11 in virus assembly can be imagined in which passage through a particular trafficking pathway is needed (Loomis et al., 2006). This might enable the recruitment of other proteins (virus or host encoded) from the plasma membrane, or enable posttranslational modifications of UL11 that are essential for budding.
In light of the apparent importance of membrane trafficking for the function of UL11, the ability of this tegument protein to associate with detergent-resistant membranes (DRMs) was examined. DRMs are microdomains within cellular membranes that are enriched in cholesterol and sphingolipids, yielding “platforms” that are thought to be important for several functions including signal transduction, cytoskeletal organization, and pathogen entry and exit (Anderson and Jacobson, 2002; Chazal and Gerlier, 2003; Helms and Zurzolo, 2004; Lichtenberg et al., 2005; Ono and Freed, 2005; Pike, 2004; Simons and Vaz, 2004). DRMs are insoluble in nonionic detergents such as Triton X-100 (TX-100), conditions that disrupt non-DRM membranes (Lichtenberg et al., 2005; Simons and Ikonen, 1997; Vitetta et al., 1973). Because of this property, DRMs can be released by adding TX-100 and purified by flotation in sucrose gradients, enabling the resident proteins to be analyzed. Proteins that are dually modified with myristate and palmitate are typically associated with DRMs (Pike, 2004; Resh, 1999; Simons and Vaz, 2004), and therefore, we predicted that UL11 would be, too. The results described below suggest that a population of UL11 molecules traffick through DRMs under the control of the LI and acidic cluster motifs.
Results
Flotation analysis of UL11-GFP
Initially, the ability of UL11 to localize to DRMs in the absence of other viral proteins was examined using a UL11-GFP fusion protein. This construct has been studied extensively and appears to behave identically to the untagged protein in all assays used (Loomis et al., 2001; Loomis et al., 2003; Loomis et al., 2006). Using metabolic labeling, membrane binding was determined in the absence of detergent. Labeling and immunoprecipitations allow an increased sensitivity, reproducibility, and ability to be quantitative over a wider range of expression levels compared to Western blots. As expected from previous studies of UL11-GFP (Loomis et al., 2001), about 80% of the protein was found to float in the absence of detergent, indicating that it was stably membrane bound (Fig. 1A and 1B). After treatment with TX-100, only about 10% of the protein could float into the top three fractions, and this represents the DRM-associated molecules present during 2.5 h of continuous radiolabeling. Although low, this amount was above background levels, as determined by flotation in the presence of 0.5% SDS to solubilize all membranes (Fig. 1A and 1B). While this is not a statistically significant difference from TX-100 (Student T-test, P = 0.10), if the definition of floating protein is restricted to the top two fractions (thereby eliminating the possibility of contamination from the large amount of underlying material that does not float), then a dramatic increase in statistical significance is seen (P = 0.003). As will be seen below, mutants that disrupt the trafficking of UL11 result in dramatic differences in DRM localization, even with the less restrictive definition, and hence, it was used for the remainder of the experiments.
FIG. 1.
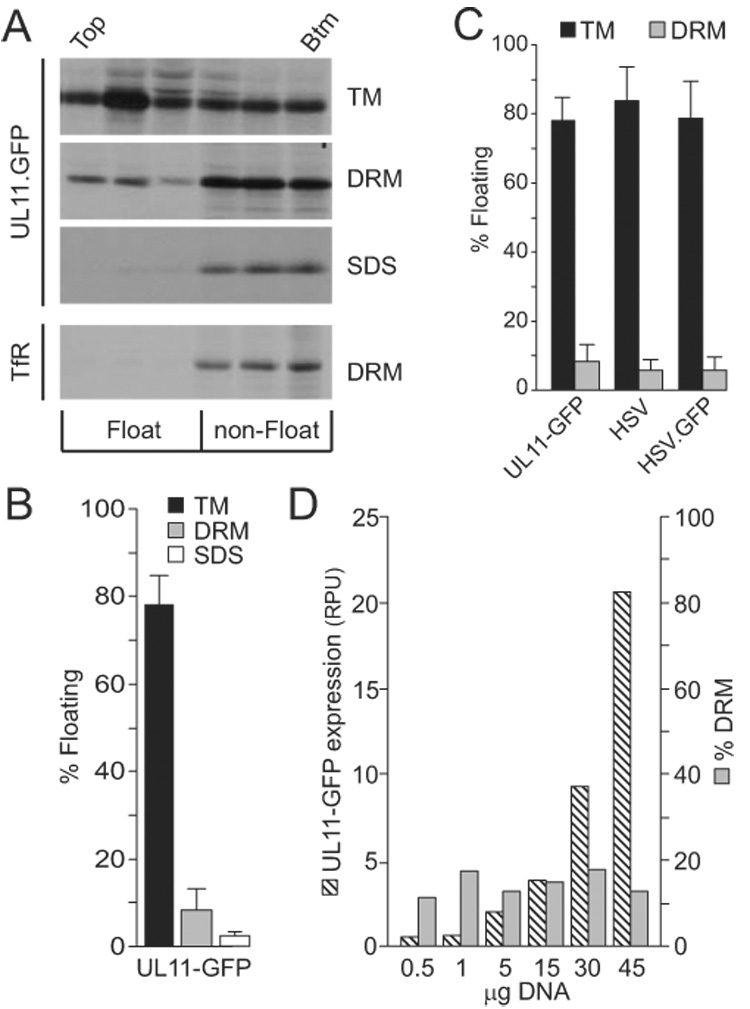
Flotation analysis of UL11. (A) A7 cells transfected with a UL11-GFP expression vector were metabolically labeled for 2.5 h and osmotically disrupted, as described in the text. Cytoplasmic membranes were treated with nothing (TM, total membranes), 0.5% TX-100 (DRM, detergent-resistant membranes), or 0.5% SDS (negative control). The ability of UL11-GFP to float to the upper fractions of sucrose step gradients during centrifugation was examined, and representative autoradiograms, obtained following immunoprecipitation and SDS-PAGE analysis, are shown. As a control for DRM disruption, endogenous transferrin receptor (TfR) was monitored in one experiment following radiolabeling, TX-100 treatment, and flotation. The tops and bottoms of the gradients are indicated. (B) Phosphorimager analysis was used to quantitate the flotation results, which are shown as the percentage of floating protein (top three fractions) relative to the total protein (all fractions). The averages of four experiments are shown, along with the standard deviations. (C) Flotation assays were used to compare the membrane-binding properties of radiolabeled UL11-GFP produced in transfected cells with untagged UL11 and UL11-GFP produced by wild-type and recombinant viruses (HSV and HSV.GFP, respectively). Cells were labeled for the final 2.5 h of infection, harvested, and floated as described in the text. The averages from at least four experiments are shown, along with the standard deviations. (D) To examine the saturability of DRMs, cells were transfected with increasing amounts of plasmid DNA, metabolically labeled, and subsequently analyzed for UL11-GFP expression levels (hatched bars; RPU = relative phosphorimager units) and DRM localization (grey bars). A repeat of this experiment gave comparable results.
Two additional experiments provided evidence for the association of wild-type UL11 with DRMs. In the first, the flotation property of UL11-GFP was compared with endogenous transferrin receptor (TfR) present in the transfected cells. TfR is a membrane-bound but DRM-excluded protein, and when TX-100 was present, the amount of floating material was found to be only 25% that of UL11-GFP, a difference that was similar to that seen for UL11-GFP when comparing SDS and TX-100 treated samples (Fig. 1A and 1B). Second, when DRMs were disrupted with the cholesterol chelating drug methyl-β-cyclodextrin (MβCD; 10mM), the amount of WT UL11-GFP localized to DRMs decreased ~20%. While this is not a complete abolishment of DRM-bound UL11, not all DRM resident proteins are completely sensitive to MβCD (Sugawara et al., 2007).
To ascertain whether the association of UL11-GFP with DRMs depends on its level of expression, increasing amounts of the expression vector were transfected into A7 cells. If the capacity of the cell to create DRMs was saturable, then the amount of UL11-GFP floating would plateau as expression continued to increase, and consequently, the percentage of DRM-associated protein would decrease. This was not found. Instead, the percentage of DRM-associated protein remained constant over a 20-fold range of expression (Fig. 1D). Thus, any variation in UL11-GFP expression between experiments appears to be unimportant for the studies below. Nevertheless, attempts were made to keep expression-levels equal by transfecting consistent amounts of DNA (15µg) for each construct in each experiment.
Because UL11 has been shown to interact with other HSV tegument proteins (Farnsworth et al., 2007; Loomis et al., 2003; Vittone et al., 2005) and such interactions may alter the localization of UL11, the membrane association of UL11-GFP during an HSV infection was examined. To this extent, a recombinant virus was created to express the fusion protein. For the flotation analyses, A7 cells were infected with the wild-type or recombinant virus at a multiplicity of infection of 10, radiolabeled for the final 2.5 h of infection, and osmotically disrupted at early (10–12 h) or late (18–20 h) times post-infection. In all cases, the flotation properties for virus-encoded UL11 and UL11-GFP were similar to that of transfected-only UL11-GFP (Fig. 1C). These results demonstrate that other viral proteins do not influence the DRM distribution of UL11; nor does the attachment of GFP (which by itself did not float; data not shown). Given this, all subsequent experiments were performed in the absence of all other viral proteins and in the context of a GFP fusion protein.
Specificity of the UL11 antibody
It was of concern that one or more of the mutants used in these studies (Fig. 2A) would lack epitopes needed for efficient recognition by the previously-described, UL11-specific antibody employed here (Loomis et al., 2003). Therefore, the ability of this antibody to immunoprecipitate the various UL11-GFP derivatives was compared to that of an anti-GFP antibody. All constructs were immunoprecipitated with equal efficiency using either antibody (Fig. 2B).
FIG. 2.
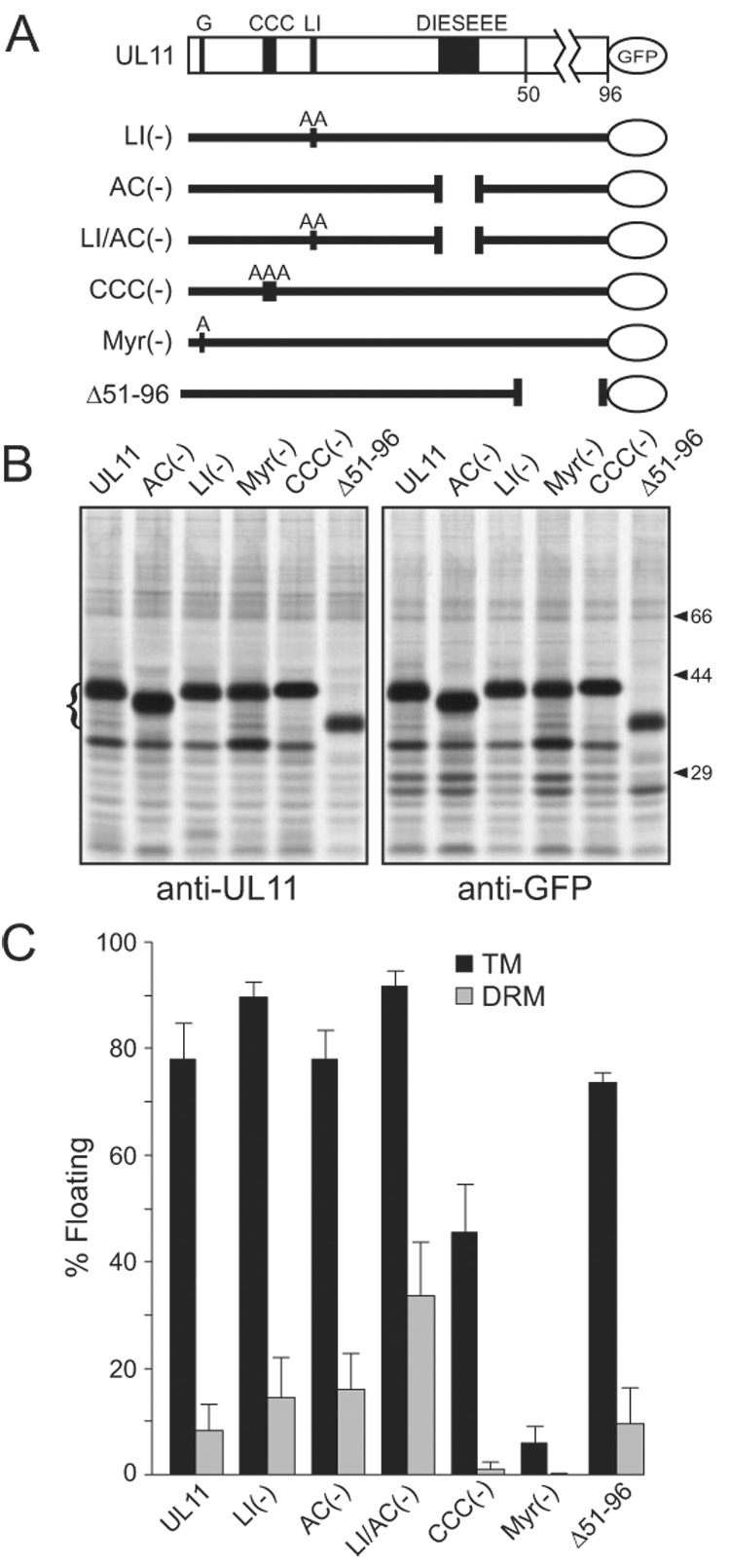
DRM association of UL11 mutants. (A) Diagram of UL11-GFP and the mutants that were analyzed. The motifs of interest are shown: G, myristylation site; CCC, palmitylation site; LI, di-leucine-like; DIESEEE, acidic cluster (AC). Sites of alanine substitutions are indicated. (B) To examine the reactivity of the mutants to anti-UL11 and anti-GFP sera, transfected cells were metabolically labeled and immunoprecipitated proteins were analyzed by SDS-PAGE. The positions of the UL11-GFP species are indicated with a bracket to the left. Positions of markers (in kDa) are indicated to the right. (C) Constructs depicted in panel A were analyzed for their ability to float in the absence (TM) and presence (DRM) of 0.5% TX-100. Each construct was analyzed a minimum of three times.
Role of fatty acid-modification
Based on studies of other proteins, dual modification with myristate and palmitate is predicted to be essential for DRM targeting of UL11 (Pike, 2004; Resh, 1999; Simons and Vaz, 2004). To test this, mutants that lack these modifications were analyzed. Mutant Myr(—), which lacks the site for myristylation and therefore fails to reach membranes where palmitylation occurs (Greaves and Chamberlain, 2007; Linder and Deschenes, 2007; Resh, 1999), behaved as expected and was not associated with any membranes, including DRMs (Fig. 2C). Likewise, mutant CCC(—), which is myristylated but lacks sites for palmitylation (Loomis et al., 2001), would be expected to retain some capacity to associate with membranes but not to be associated with DRMs, and that is what was found. Even though 45% of the CCC(—) molecules were able to float in the absence of TX-100, only 1% was DRM-associated (Fig. 2C), which is equivalent to the background levels seen for wild type when SDS was used (Fig. 1B). These data support the hypothesis that myristate alone is capable of directing UL11 to membranes, whereas both palmitate and myristate are required for the targeting of UL11 to DRMs.
To attempt rescue of the Myr(—) and CCC(—) mutants into DRMs, previously-constructed chimeras (Loomis et al., 2001) were examined that have the first 10 amino acids of the v-Src or Fyn proteins added to the N-terminus of UL11-GFP (mutants sUL11 and fUL11, respectively; Fig. 3). Attachment of these peptides precludes myristylation of the N-terminal glycine of UL11, however, both foreign sequences have their own site for this modification (Resh, 1999). In addition, each has characteristics that increase membrane-binding. The v-Src peptide has three basic residues that interact with acidic phospholipids on the cytoplasmic faces of membranes (Resh, 1999). In addition to basic residues, the Fyn peptide has two cysteine residues that can be palmitylated (Alland et al., 1994; Resh, 1999; Shenoy-Scaria et al., 1994). The membrane-binding properties of these two chimeras (i.e., without other alterations to UL11) were examined first. As expected, sUL11 and fUL11 were about 80% membrane associated in the absence of detergent (Fig. 3A and 3B, respectively). When the membranes were treated with TX-100, sUL11 was found to be associated with DRMs to an extent similar to wild-type UL11-GFP. The ability of this chimera to target DRMs is apparently due to palmitylation of one or more of the UL11 cysteines because when these were eliminated in mutant sCCC(—), DRM association dropped to background levels. In contrast, the association of fUL11 with DRMs was increased ~3-fold compared to UL11-GFP, perhaps due to palmitylation of the extra cysteine residues present in the Fyn peptide. However, even in this case, the cysteines in UL11 appear to contribute to DRM association because when these were eliminated in mutant fCCC(-), levels dropped to that of wild-type UL11-GFP.
FIG. 3.
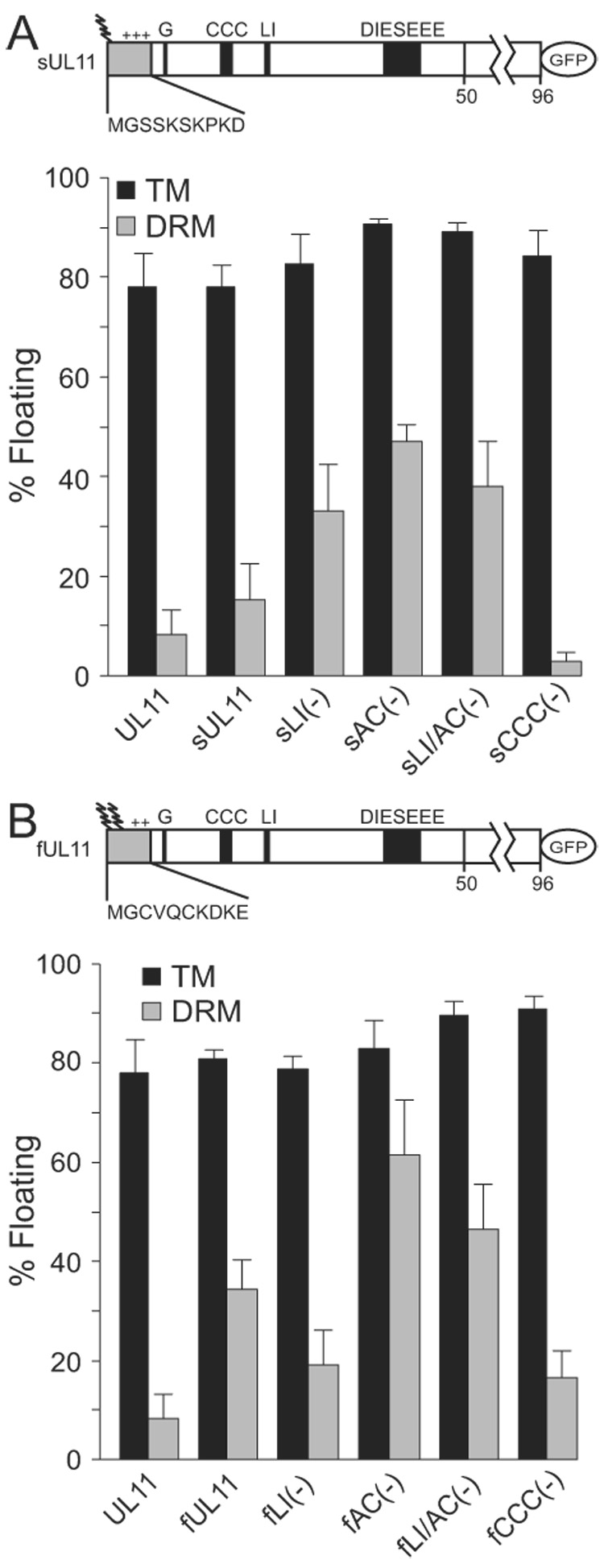
Analyses of N-terminal UL11 chimeras. N-terminal extensions corresponding to the first 10 amino acids of (A) Src or (B) Fyn were attached to UL11-GFP, as represented by the shaded boxes in the diagrams. Wavey lines denote fatty acid modifications and “+” indicates basic residues. These N-terminal chimeras and the indicated mutants (defined in Fig 2A) were analyzed for their ability to float in the absence (TM) and presence (DRM) of 0.5% TX-100. Each construct was anlayzed a minimum of three times
Sequences that control levels of DRM association
Although myristate and palmitate are essential for the accumulation of UL11-GFP within DRMs, it is possible that other parts of the protein actually control its trafficking through these membrane locations (e.g., by enabling interactions with cellular factors to enable endocytosis). If so, these functions must reside in the first half of UL11 because mutant Δ51–96, which lacks the second half, was indistinguishable from wild type in the flotation assays (Fig. 2C). The obvious elements to examine were the LI and the acidic cluster motifs. To analyze these motifs, previously characterized mutants lacking either the leucine-isoleucine motif [mutant LI(-), changed to alanines] or the acidic cluster motif [mutant AC(-), deletion of the seven-residue cluster] were used (Loomis et al., 2001; Loomis et al., 2003). Additionally, a third variant of UL11, which combines both mutations, was used (mutant LI/AC(-); Fig. 2A). Elimination of either of these motifs alone from UL11-GFP had small enhancing effects on DRM association, but when both motifs were missing, a striking 3-fold increase occurred (Fig. 2C). These results are consistent with the hypothesis that either motif is sufficient to enable UL11 to exit DRMs, perhaps through either of two different pathways. This model is reminiscent of the dual-pathway trafficking found for the Nef protein of HIV in which LL and acidic cluster motifs are separately utilized for downregulation of CD4 (the virus receptor) and the major histocompatibility complex class I, respectively (Doms and Trono, 2000; Roeth and Collins, 2006).
The LI and acidic cluster mutants of UL11-GFP were also examined in the context of the chimeras. Addition of the v-Src peptide appears to make UL11 more sensitive to removal of the trafficking motifs in that enhanced DRM association occurred even with the single mutations (Fig. 3A). In contrast, DRM association of the Fyn chimera appears to be controlled primarily by the acidic cluster. That is, removal of the acidic cluster (alone or in combination with the LI substitution) resulted in an increase, whereas removal of the LI resulted in at most a small reduction in DRM accumulation. Collectively, these complex results suggest that the foreign sequences may interfere with the normal trafficking properties of UL11, possibly by altering the conformation and hence the ability of the LI and acidic cluster motifs to be properly recognized by the sorting machinery.
Discussion
The experiments described here demonstrate that the association of UL11 with DRMs requires the addition of both myristate and palmitate. This is consistent with UL11 of HSV-2 (Koshizuka et al., 2007); however, this report extends our understanding by demonstrating the importance of the LI and acidic cluster motifs to further regulate membrane association. The increase in DRM accumulation seen when both the LI and the AC motifs were removed could be due to either enhanced entry or inhibited exit from DRMs; however, the latter seems more reasonable for two reasons. First, the combination of myristate and palmitate appear to function as “enter” signals for DRMs (Pike, 2004; Resh, 1999; Simons and Vaz, 2004). Second, acidic clusters and di-leucine-like motifs are well established signals involved in exit and the recovery of various proteins off the plasma membrane (Bonifacino and Traub, 2003; Heilker et al., 1999), and previous studies have shown that removal of either motif in the context of a CD4-UL11 chimera results in accumulation of the mutant on the cell surface (Loomis et al., 2001). While it would be very interesting to examine the above chimeras and mutant alleles in the context of the virus, this is not possible due to the overlap of UL11 with the essential UL12 gene. Moreover, a complete knock-out of the gene is not possible either, and this is why the deletion viruses that have been created retain most of the described motifs, possibly explaining the “mild” decrease in viral titer (~1000-fold).
Given these data, why does UL11 traffic to the plasma membrane and DRMs? At least three possibilities can be imagined. First, there might not be any function for UL11 on the plasma membrane, and a recovery mechanism may be needed merely to return this protein to the site of budding whenever it happens to escape to the cell periphery. The problem with this model is that it does not explain why UL11 contains two recycling motifs (Loomis et al., 2001), only one of which is needed for packaging (Loomis et al., 2006). In light of the similarity with the Nef protein (mentioned above), a second model emerges in which UL11 travels to the plasma membrane and back to internal membranes for one or more specific purposes (e.g., to downregulate a host protein, to acquire a modification, or to bring another protein to the site of budding). This could be the sole purpose for UL11, or it could be a function that is needed in addition to bridging connections between capsids and membranes. A third possibility is that UL11 travels to the plasma membrane to promote the cell-to-cell spread of infection, either by enabling the egress of vesicle-enclosed virions to the cell surface or by promoting a direct interaction of the capsid (via UL16) with the plasma membrane. Further studies will be needed to determine which (if any) of these models is correct.
Materials and Methods
Cells and viruses
Vero and A7 melanoma cells were grown in Dulbeccos Modified Eagle’s Medium (DMEM, Invitrogen) supplemented with 5% fetal bovine serum (FBS), penicillin, and streptomycin (Gibco, 15140–148). Confluent Vero cells were infected with the KOS strain of HSV-1 (Smith, 1964) in DMEM supplemented with 2% FBS, 25mM HEPES buffer, glutamine (0.3ug/ml), penicillin, and streptomycin. A recombinant virus expressing UL11-GFP was created by homologous recombination. Sequences from upstream and downstream of UL11 (350 bp each) were PCR amplified from the viral genome and ligated to the 5’ and 3’ termini of UL11-gfp, respectively. The composite DNA fragment was inserted into the pSP72 vector (Promega) and then transfected into A7 cells, which were infected 16 h later with the KOS strain of HSV-1. Recombinant virus was selected by five rounds of plaque purification. The resulting virus was confirmed by a combination of PCR analyses using primers that flank the the UL11-gfp coding sequence (yielding a larger product than untagged UL11) and the failure to express the wild-type, untagged UL11, as determined by Western blotting and radiolabeling/immunoprecipitating for UL11. The recombinant was analyzed for specific infectivity and plaque size, as well as localization and kinetics of UL11-GFP expression. All characteristics examined were undiminished compared the parental virus (data not shown).
Antibodies
UL11-specific antibodies were developed in rabbits and have been described previously (Loomis et al., 2003). GFP-specific antibodies, produced by Cocalico Biologicals, Inc., were obtained from rabbits injected with purified His6-GFP. The specificity of this antibody was tested in both immunoblot and immunoprecipitation assays (this study; data not shown). Transferrin receptor antibodies were purchased from BD Pharmingen (555534).
Membrane flotation
DRMs were isolated by using a slight modification of an established protocol (Spearman et al., 1997). Briefly, the calcium phosphate method was used to transfect human melanoma (A7) cells with a plasmid containing UL11-gfp, derived using the KOS strain of HSV-1 (Loomis et al., 2001). After 16–18 h, the cells were radiolabeled with an L-[35S]methionine-cysteine mix (75 µCi/ml, >1,000 Ci/mmol) for 2.5 h, scraped off the plates, and washed in cold PBS. After pelleting, the cells were resuspended and swollen in hypotonic lysis buffer (10 mM Tris-HCl, pH 7.4, 0.2 mM MgCl2) on ice for 30 min. They were lysed at 4°C by 35 strokes with a dounce homogenizer and then centrifuged at low speed to remove unbroken cells and nuclei. Post-nuclear supernatants were split into two equal aliquots, one untreated (for total membranes) and the other adjusted to a concentration of 0.5% TX-100 (for DRMs). In some experiments, samples were adjusted to 0.5% SDS to disrupt all membranes (negative control). After incubation on ice for 30 min, the samples were mixed with 65% (w/w) sucrose (58% final, 2.0 ml total), placed in the bottom of a Beckman SW55 Ti tube, and sequentially overlaid with 2.0 ml of 45% and 1.0 ml of 2.5% sucrose. Sucrose solutions were made in NTE buffer (10 mM Tris-HCl, pH 7.4, 100 mM NaCl, 1 mM EDTA). The samples were centrifuged for 18 h at 200,000 × g and 4°C in a Beckman ultracentrifuge, and six equal-volume fractions were collected from the top. UL11-GFP was immunoprecipitated using rabbit anti-UL11 antibodies (Loomis et al., 2003), separated by SDS-PAGE, and quantitated by phosphorimager analysis.
Transferrin receptor and methyl-β-cyclodextrin treatment
To examine membrane localization of the endogenous transferrin receptor (TfR), confluent monolayers of A7 cells were radiolabeled with an L-[35S]methionine-cysteine mix (300 µCi/ml, >1,000 Ci/mmol) for 3 h. Following labeling, cells were lysed, TX-100 treated, and floated as before. TfR was immunoprecipitated from fractions using anti-TfR antibodies, separated by SDS-PAGE, and quantitated by phosphorimager analysis. Methyl-β-cyclodextrin (Sigma, C4555) was added to A7 cells expressing either wild-type or mutant UL11-GFP during the final hour of a 2.5 h label (75 µCi/ml, >1,000 Ci/mmol). DRM localized UL11 was then immunoprecipitated and examined as before.
Acknowledgments
Special thanks are extended to Amy Harper for her assistance in producing the anti-GFP antibody, Joshua Loomis for help with the recombinant virus construction, and Carol Wilson for constructing some of the expression vectors. Additional thanks go to David Meckes, Jake Marsh, Kevin O’Regan, and Michael Murphy for helpful discussions and comments on the manuscript. This work was supported by NIH grants to J.W.W. (CA47482, AI071286) and R.J.C. (CA42460).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References List
- Alland L, Peseckis SM, Atherton RE, Berthiaume L, Resh MD. Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. J. Biol. Chem. 1994;269(24):16701–16705. [PubMed] [Google Scholar]
- Anderson RG, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296(5574):1821–1825. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- Baines JD, Jacob RJ, Simmerman L, Roizman B. The herpes simplex virus 1 UL11 proteins are associated with cytoplasmic and nuclear membranes and with nuclear bodies of infected cells. J. Virol. 1995;69(2):825–833. doi: 10.1128/jvi.69.2.825-833.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines JD, Roizman B. The UL11 gene of herpes simplex virus 1 encodes a function that facilitates nucleocapsid envelopment and egress from cells. J. Virol. 1992;66(8):5168–5174. doi: 10.1128/jvi.66.8.5168-5174.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Bowzard JB, Visalli RJ, Wilson CB, Loomis JS, Callahan EM, Courtney RJ, Wills JW. Membrane targeting properties of a herpesvirus tegument protein-retrovirus Gag chimera. J. Virol. 2000;74(18):8692–8699. doi: 10.1128/jvi.74.18.8692-8699.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt WJ, Jarvis M, Seo JY, Drummond D, Nelson J. Rapid genetic engineering of human cytomegalovirus by using a lambda phage linear recombination system: demonstration that pp28 (UL99) is essential for production of infectious virus. J. Virol. 2004;78(1):539–543. doi: 10.1128/JVI.78.1.539-543.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazal N, Gerlier D. Virus entry, assembly, budding, and membrane rafts. Microbiol. Mol. Biol. Rev. 2003;67(2):226–237. doi: 10.1128/MMBR.67.2.226-237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms RW, Trono D. The plasma membrane as a combat zone in the HIV battlefield. Genes Dev. 2000;14(21):2677–2688. doi: 10.1101/gad.833300. [DOI] [PubMed] [Google Scholar]
- Farnsworth A, Wisner TW, Johnson DC. Cytoplasmic residues of herpes simplex virus glycoprotein gE required for secondary envelopment and binding of tegument proteins VP22 and UL11 to gE and gD. J. Virol. 2007;81(1):319–331. doi: 10.1128/JVI.01842-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulmer PA, Melancon JM, Baines JD, Kousoulas KG. The UL20 protein functions precede and are required for UL11 functions in herpes simplex virus type-1 (HSV-1) cytoplasmic virion envelopment. J. Virol. 2007 doi: 10.1128/JVI.02201-06. JVI.02201-06v1 Available online- [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves J, Chamberlain LH. Palmitoylation-dependent protein sorting. J. Cell Biol. 2007;176(3):249–254. doi: 10.1083/jcb.200610151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilker R, Spiess M, Crottet P. Recognition of sorting signals by clathrin adaptors. Bioessays. 1999;21(7):558–567. doi: 10.1002/(SICI)1521-1878(199907)21:7<558::AID-BIES4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Helms JB, Zurzolo C. Lipids as targeting signals: lipid rafts and intracellular trafficking. Traffic. 2004;5(4):247–254. doi: 10.1111/j.1600-0854.2004.0181.x. [DOI] [PubMed] [Google Scholar]
- Kopp M, Granzow H, Fuchs W, Klupp B, Mettenleiter TC. Simultaneous deletion of pseudorabies virus tegument protein UL11 and glycoprotein M severely impairs secondary envelopment. J. Virol. 2004;78(6):3024–3034. doi: 10.1128/JVI.78.6.3024-3034.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp M, Granzow H, Fuchs W, Klupp BG, Mundt E, Karger A, Mettenleiter TC. The pseudorabies virus UL11 protein is a virion component involved in secondary envelopment in the cytoplasm. J. Virol. 2003;77(9):5339–5351. doi: 10.1128/JVI.77.9.5339-5351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshizuka T, Kawaguchi Y, Nozawa N, Mori I, Nishiyama Y. Herpes simplex virus protein UL11 but not UL51 is associated with lipid rafts. Virus Genes. 2007 doi: 10.1007/s11262-007-0156-2. [DOI] [PubMed] [Google Scholar]
- Lichtenberg D, Goni FM, Heerklotz H. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem. Sci. 2005;30(8):430–436. doi: 10.1016/j.tibs.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat. Rev. Mol. Cell Biol. 2007;8(1):74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- Loomis JS, Bowzard JB, Courtney RJ, Wills JW. Intracellular trafficking of the UL11 tegument protein of herpes simplex virus type 1. J. Virol. 2001;75(24):12209–12219. doi: 10.1128/JVI.75.24.12209-12219.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis JS, Courtney RJ, Wills JW. Binding partners for the UL11 tegument protein of herpes simplex virus type 1. J. Virol. 2003;77(21):11417–11424. doi: 10.1128/JVI.77.21.11417-11424.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis JS, Courtney RJ, Wills JW. Packaging determinants in the UL11 tegument protein of herpes simplex virus type 1. J. Virol. 2006;80(21):10534–10541. doi: 10.1128/JVI.01172-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean CA, Clark B, McGeoch DJ. Gene UL11 of herpes simplex virus type 1 encodes a virion protein which is myristylated. J. Gen. Virol. 1989;70:3147–3157. doi: 10.1099/0022-1317-70-12-3147. [DOI] [PubMed] [Google Scholar]
- MacLean CA, Dolan A, Jamieson FE, McGeoch DJ. The myristylated virion proteins of herpes simplex virus type 1: investigation of their role in the virus life cycle. J. Gen. Virol. 1992;73:539–547. doi: 10.1099/0022-1317-73-3-539. [DOI] [PubMed] [Google Scholar]
- Meckes DG, Jr, Wills JW. Dynamic Interactions of the UL16 Tegument Protein with the Capsid of Herpes Simplex Virus. J. Virol. 2007 doi: 10.1128/JVI.01306-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter TC. Budding events in herpesvirus morphogenesis. Virus Res. 2004;106(2):167–180. doi: 10.1016/j.virusres.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Ono A, Freed EO. Role of lipid rafts in virus replication. Adv. Virus Res. 2005;64:311–358. doi: 10.1016/S0065-3527(05)64010-9. [DOI] [PubMed] [Google Scholar]
- Oshima S, Daikoku T, Shibata S, Yamada H, Goshima F, Nishiyama Y. Characterization of the UL16 gene product of herpes simplex virus type 2. Arch. Virol. 1998;143(5):863–880. doi: 10.1007/s007050050338. [DOI] [PubMed] [Google Scholar]
- Pike LJ. Lipid rafts: heterogeneity on the high seas. Biochem. J. 2004;378(Pt 2):281–292. doi: 10.1042/BJ20031672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh MD. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta. 1999;1451(1):1–16. doi: 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- Roeth JF, Collins KL. Human immunodeficiency virus type 1 Nef: adapting to intracellular trafficking pathways. Microbiol. Mol. Biol. Rev. 2006;70(2):548–563. doi: 10.1128/MMBR.00042-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmer C, Neubauer A. The equine herpesvirus 1 UL11 gene product localizes to the trans-Golgi network and is involved in cell-to-cell spread. Virology. 2003;308(1):23–36. doi: 10.1016/s0042-6822(02)00060-0. [DOI] [PubMed] [Google Scholar]
- Shenoy-Scaria AM, Dietzen DJ, Kwong J, Link DC, Lublin DM. Cysteine3 of Src family protein tyrosine kinase determines palmitoylation and localization in caveolae. J. Cell Biol. 1994;126(2):353–363. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MC, Schroer J, Shenk T. Human cytomegalovirus cell-to-cell spread in the absence of an essential assembly protein. Proc. Natl. Acad. Sci. U. S. A. 2005;102(6):2081–2086. doi: 10.1073/pnas.0409597102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MC, Yu QC, Enquist L, Shenk T. Human cytomegalovirus UL99-encoded pp28 is required for the cytoplasmic envelopment of tegument-associated capsids. J. Virol. 2003;77(19):10594–10605. doi: 10.1128/JVI.77.19.10594-10605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387(6633):569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simons K, Vaz WL. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. 269–295. [DOI] [PubMed] [Google Scholar]
- Smith KO. Relationship between the envelope and the infectivity of herpes simplex virus. Proc. Soc. Exp. Biol. Med. 1964;115:814–816. doi: 10.3181/00379727-115-29045. [DOI] [PubMed] [Google Scholar]
- Spearman P, Horton R, Ratner L, Kuli-Zade I. Membrane binding of human immunodeficiency virus type 1 matrix protein in vivo supports a conformational myristyl switch mechanism. J. Virol. 1997;71(9):6582–6592. doi: 10.1128/jvi.71.9.6582-6592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara Y, Nishii H, Takahashi T, Yamauchi J, Mizuno N, Tago K, Itoh H. The lipid raft proteins flotillins/reggies interact with Galphaq and are involved in Gq-mediated p38 mitogen-activated protein kinase activation through tyrosine kinase. Cell Signal. 2007;19(6):1301–1308. doi: 10.1016/j.cellsig.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Vitetta ES, Boyse EA, Uhr JW. Isolation and characterization of a molecular complex containing Thy-1 antigen from the surface of murine thymocytes and T cells. Eur. J. Immunol. 1973;3(7):446–453. doi: 10.1002/eji.1830030714. [DOI] [PubMed] [Google Scholar]
- Vittone V, Diefenbach E, Triffett D, Douglas MW, Cunningham AL, Diefenbach RJ. Determination of interactions between tegument proteins of herpes simplex virus type 1. J. Virol. 2005;79(15):9566–9571. doi: 10.1128/JVI.79.15.9566-9571.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


