Abstract
The phenomenon of spaced (longer intertrial interval) compared with massed (shorter intertrial interval) training leading to better long-term habituation and associative learning is well documented. However, the effects of intertrial intervals on response habituation to repeated stress exposures have not been previously examined. The present experiments found that massed (six 30-min exposures of 95 dB white noise in 6 hr) and spaced (one 30-min exposure daily for 6 days) noise exposures led to similar habituation of plasma corticosterone and ACTH responses, heart rate, and core body temperature after the 6th exposure in male Sprague–Dawley rats. However, these habituated responses were not retained in the massed group on a similar noise re-exposure 48 hr later, compared with the spaced group. The habituated responses found in the massed group after the 6 noise exposures were not due to differential hearing threshold shifts, as examined with modifications of the acoustic startle reflex. These data indicate that relatively short interstressor intervals impair long-term stress adaptation. This series of studies supports the idea of distinct short- and long-term habituation processes to stress responsiveness.
Keywords: ACTH, audiogenic stress, corticosterone, telemetry
Habituation is defined as a decrease in the strength of a response after repeated presentations of the same stimulus (Groves & Thompson, 1970; Thompson & Spencer, 1966). These decrements in responding are not mediated by motor fatigue or adaptation at the level of sensory receptors, but are likely to involve central neural plastic processes (Carew & Kandel, 1973). The rate and extent of habituation can be affected by several parameters, including interstimulus intervals (Christoffersen, 1997; Groves & Thompson, 1970), which are the focus of the present experiments. In general, spaced (longer intertrial interval) stimulus exposures have been shown to lead to more complete and durable (long-term) habituation than massed (shorter intertrial intervals) presentations. Short-term habituation is considered to be a weakening of a response over minutes to hours, whereas long-term habituation is normally observed over relatively longer time periods of days, weeks, or even months (Christoffersen, 1997; Davis, 1970). Effects of length of intertrial intervals on habituation of several reflexive behavioral responses have been examined for over 50 years in several species. Spaced stimulus presentations have led to more complete and durable habituation compared with massed presentations in the siphon withdrawal reflex in Aplysia, startle responses in rats, mopping responses in chaffinches toward predatory birds, proboscis extension reflex in honeybees, and withdrawal responses to ground taps in Caenorhabditis elegans (Carew, Pinsker, & Kandel, 1972; Davis, 1970; Hinde, 1954; Menzel, Manz, Menzel, & Greggers, 2001; Rose & Rankin, 2001). A similar phenomenon also has been observed in the context of associative learning and memory. Spaced learning episodes have been shown to lead to better memory retention than massed training in maze learning and spatial memory tasks in rodents (Commins, Cunningham, Harvey, & Walsh, 2003; Goodrick, 1973) and in human learning and memory tests (Keppel, 1967; Willingham, 2002). One important characteristic of the distinction between massed and spaced stimulus presentations on associative and nonassociative plasticity is the observation that, whereas both spaced and massed exposures usually produce equivalent short-term habituation/memory, spaced stimulus exposures usually lead to better long-term habituation/retention. These observations have provided the basis for a study of the differential central mechanisms underlying short-term versus long-term associative/nonassociative plasticity.
With regard to stress, it has been suggested that habituation of neuroendocrine and autonomic responses may involve phenomenologically similar processes to habituation of behavioral responses to repeated stimulation (De Boer, Koopmans, Slangen, & Van Der Gugten, 1990; Natelson et al., 1988; Pitman, Ottenweller, & Natelson, 1988). Adrenocortical hormones routinely are used as primary indicators of an acute stress response, specifically plasma levels of ACTH and corticosterone (CORT; Endroczi, 1983; Levine, 2000; Selye, 1956). Indeed, these endocrine measures are often reported to habituate to repeated homotypic (same) stressor exposures (Armario, Castellanos, & Balasch, 1984; Bhatnagar, Huber, Nowak, & Trotter, 2002; Campeau, Dolan, Akil, & Watson, 2002; Cole et al., 2000; De Boer et al., 1990; Masini, Sauer, White, Day, & Campeau, 2006; Natelson et al., 1988). Interestingly, there has been very little empirical work reported with regard to interstressor intervals and their effects on stress habituation. Surprisingly, in one study, longer interstressor intervals were reported to weaken habituation (De Boer et al., 1990). Other than this report, there have been few attempts to distinguish the putative differential effects of interstressor intervals on endocrine or autonomic responses normally associated with stress. Such knowledge could likewise be employed to begin to evaluate the neural mechanisms associated with this important form of adaptive synaptic plasticity.
The present studies were designed to examine the effects of interstressor interval length on neuroendocrine (CORT and ACTH), autonomic (heart rate and core body temperature), and behavioral responses to repeated loud noise exposures. We hypothesized that at least some of these measures would habituate to a greater extent in rats that were exposed to a longer interstressor interval (30 min of noise once/day for 6 days) than rats exposed to a shorter interstressor interval (30 min of noise once/hr in 6 hr), following the classical pattern of habituation found with reflexive behaviors. In addition, it was of further interest to determine whether these different interstressor intervals would also produce relatively equivalent short-term habituation, as reported with other reflexive behaviors. It was also of interest to determine the extent to which the multiple responses measured would covary with stress exposure, which is an experimental question that has been addressed rarely with regard to stressful situations. Our results strongly suggest that relatively short interstressor intervals produce weaker and less durable habituation of endocrine and autonomic responses, while displaying equivalent short-term habituation, as compared with a longer interstressor interval. Importantly, most of the responses measured displayed a similar pattern of variation over repeated stimulus exposures, further suggesting that a central state of stress regulates multiple response systems simultaneously. Some of these data have been presented in abstract form (Campeau, Masini, Srinidhi, & Day, 2006; Masini, Day, & Campeau, 2005).
Experiment 1
Experiment 1 examined whether repeated exposures to loud noise given at different intertrial intervals led to habituation of endocrine responses both short term (immediately after sixth exposure) and long term (2 days after sixth exposure). CORT and ACTH responses were examined after the sixth exposure given in the same day (massed group) or after the sixth exposure when only one exposure was given per day (spaced group). Endocrine responses were also examined after an additional test exposure to noise given 48 hr after the sixth exposure.
Materials and Method
Subjects
Thirty-two male Sprague–Dawley rats (Harlan, Indianapolis, IN) weighing 200–225 g and approximately 2 months old were used. They were housed in a dedicated colony facility and initially grouped 4 to 5 in clear polycarbonate cages (48 × 27 × 20 cm) containing wood shavings and covered with wire lids providing food (rat chow [Harlan Teklad, Chow 8640; Madison, WI]) and water ad libitum. Rats were housed for a period of 7 days after arrival from the supplier before any experimental manipulations were conducted. They were kept on a controlled light–dark cycle (lights on at 7 a.m. and off at 7 p.m.), under constant humidity and temperature conditions. All procedures were performed between 8 a.m. and 4 p.m. to reduce variability due to normal circadian hormonal variations. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Colorado and conformed to the National Institutes of Health (1986) Guide for the Care and Use of Laboratory Animals.
Noise apparatus
The acoustic chambers used in this experiment consisted of ventilated double wooden (2.54 cm plywood board) chambers, with the outer chamber lined internally with 2.54 cm insulation (Celotex, Suffolk, England). The internal dimensions of the inner box were 59.69 cm (w) × 38.10 cm (d) × 38.10 cm (h), which allowed placement of a polycarbonate rat home cage. Each chamber is fitted with a single 15.24 cm × 22.86 cm Optimus speaker (#12-1769-120 W RMS [Radio Shack, Fort Worth, TX) in the middle of the ceiling. Lighting was provided by a fluorescent lamp (15 W) located in the upper left corner of the chamber. Noise was produced by a General Radio (#1381 [Concord, MA]) solid-state random-noise generator with the bandwidth set at 2 Hz–50 kHz. The output of the noise generator was amplified (Pyramid Studio Pro #PA-600× [Brooklyn, NY]) and fed to the speakers. The speaker characteristics allowed relatively flat delivery between 20 and 27,000 Hz, rolling off quickly (20 dB/octave) at both ends. Noise intensity was measured by placing a Radio Shack Realistic Sound Level Meter (A scale; #33-2050) in the rat's home cage at several locations and taking an average of the different readings. The ambient/quiet noise level inside the chamber was approximately 60 dBA and approximately 55 dBA in the rat colony.
Procedure
Seven days after arrival from the supplier, the rats were single-housed in 46 × 25 × 22 cm clear polycarbonate cages with wire lids and divided into four groups: spaced (n = 8), massed (n = 8), acute (n = 8), and no noise (n = 8). The rats were all preexposed to the acoustic chambers for 10 min/day for 4 days prior to their initial noise exposure. The rats in their individual home cages were carefully transported down a hallway on a cart and put in the acoustic chambers that were in a separate room. After 10 min in the acoustic chamber, the rats were then gently removed from their cages and handled for 1 min each. After this acclimation period, the spaced group rats in their home cages were transported down the hallway and placed in the acoustic chambers for 30 min and then were exposed to 95 dBA noise for 30 min; they were then removed from the chamber and taken back to the animal colony. The choice of this time point was dictated by our previous findings that indicated peak CORT and ACTH release in responses to loud noise (Burow, Day, & Campeau, 2005). The acute and no-noise groups were also put in acoustic chambers for 60 min but were not exposed to the noise. These groups were put in the chambers once a day for 6 days. On the 6th day, the massed group was exposed to six 30-min 95 dBA noise exposures, with a 30-min interpresentation interval (6 hr overall session). After the sixth exposure of noise for the spaced and massed groups (and sixth placement in the acoustic chamber for the acute and no-noise groups), blood was sampled from half the rats in each group via tail nicks. Only half of the rats had blood taken to examine whether the tail blood collection itself would affect the later noise test results. All rats were then returned to the colony room.
Forty-eight hours after the sixth noise exposure, the rats were returned to the acoustic chambers. The spaced, massed, and acute noise groups received a 30-min 95 dBA noise exposure test. The no-noise group was exposed to the acoustic chambers for 30 min. Immediately after the 30-min noise (or chamber exposure) test, the rats were rapidly decapitated and trunk blood collected.
Tail blood collection
Immediately after the sixth noise exposure (or no noise for the acute and no-noise groups), half of the rats from each group were gently restrained using a clean towel. Then a small incision was made in the lateral tail vein with the corner of a razor blade. Approximately 300 μl of blood was collected, using hematocrit capillaries, and deposited into 0.5 mL microcentrifuge tubes containing EDTA on ice. The whole procedure lasted less than 3 min from removal to return of the rat to its home cage. Blood was collected into ice-chilled tubes containing EDTA (20 mg/ml). Blood samples were centrifuged at 2,000 rpm for 10 min, the plasma pipetted into 0.5 ml Ependorf microcentrifuge tubes, and stored at −80 °C until assayed.
CORT enzyme linked immunosorbent assays
The CORT assay was performed according to the manufacturer's instructions (kit #901-097; AssayDesigns, Ann Arbor, MI). Plasma plus steroid displacement reagent (10 μl) in the standard buffer was used. Levels were then quantified on a BioTek Elx808 microplate reader and calculated against a standard curve generated concurrently.
ACTH assay
ACTH was measured with a ACTH IRMA kit (DiaSorin, Stillwater, MN) according to the manufacturer's protocol. For the plasma collected from tailnicks, 100 μl of plasma plus 100 μl of buffer were used. The sensitivity of the assay ranged from 1.5 to 1400 pg/ml. All samples from the experiment were measured simultaneously to reduce interassay variability.
Data analysis
Values for CORT and ACTH were statistically analyzed with analyses of variance (ANOVAs). Tukey's honestly significant difference (HSD) multiple means comparisons were used to analyze post hoc differences (p < .05). CORT and ACTH responses on the test day were also examined for differences between rats that had and had not previously had blood taken via tail nicks.
Results
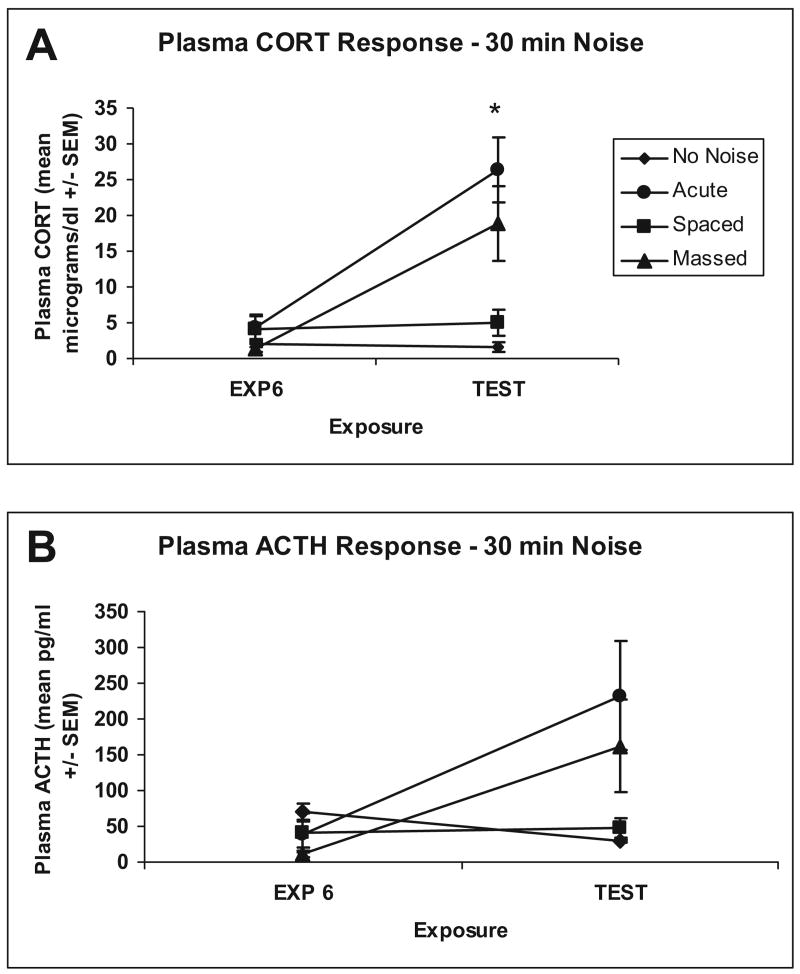
ANOVA revealed no significant differences between groups for CORT response, F(3, 11) = 0.938, p = .455, or ACTH response, F(3, 12) = 1.974, p = .172, after the sixth noise exposure, suggesting near complete and equivalent habituation of both the massed and spaced groups. On the test day, 2 days after the sixth exposure, ANOVA revealed significant differences between the groups for CORT, F(3, 28) = 10.401, p = .0001, and ACTH, F(3, 28) = 3.627, p = .025. Post hoc comparisons revealed that the main effects were due mostly to differences between the acute and massed groups compared with the no-noise and spaced groups, respectively (Tukey's HSD; ps < .05), as shown in Figure 1 (Panel A). The ACTH results were similar, with the massed group being more intermediate, no different from the acute or the spaced groups, whereas the spaced groups showed reliably lower ACTH than the acute group, indicating more complete habituation; see Figure 1 (Panel B).
Figure 1.
Graphs show mean (± SEM) plasma levels of corticosterone (CORT; Panel A) and ACTH (Panel B) for no-noise (n = 4), acute (n = 4), spaced (n = 4), and massed (n = 4) groups after sixth exposure to 30 min of 95 dBA noise (space and massed) or no-noise (acute and no-noise) groups; EXP 6. Graph also shows mean (± SEM) plasma levels of CORT (Panel A) and ACTH (Panel B) for no-noise (n = 8), acute (n = 8), spaced (n = 8), and massed (n = 8) groups after 30 min 95 dBA noise test; TEST. *Significant difference between massed and spaced groups (p < .05).
No significant differences between CORT and ACTH values of rats that had and had not had tail blood taken after the sixth exposure on the test day were found: F(3, 24) = 0.173, p = .913 (CORT), and F(3, 24) = 0.754, p = .531 (ACTH). Overall, these results suggest that the two interstressor intervals produced equivalent habituation after six consecutive exposures, but the longer interstressor interval (24 hr) was associated with more durable and complete long-term habituation 48 hr after the last stressor exposure.
Discussion
The results of Experiment 1 suggest a reliably weaker long-term habituation of the hypothalamic–pituitary–adrenal (HPA) axis produced by relatively massed (once/hr for 6 hr) compared with spaced (once/day for 6 days) noise exposures, as indexed by plasma concentrations of CORT and ACTH. After the sixth exposure, no differences between the groups on these indices were observed, indicating significant and similar habituation (relatively short term for the massed group) in the massed and spaced groups. Both groups showed plasma CORT and ACTH levels similar to the control no-noise group, suggesting relatively complete habituation of these measures. On retest (seventh exposure) with the same noise 48 hr later, the spaced group still exhibited near complete habituation of HPA axis hormones, with levels similar to the control no-noise group. However, the massed group exhibited elevated HPA axis hormone levels more similar to rats that were exposed to noise for the first time (acute group). The massed group thus did not retain the apparent habituated response displayed 2 days earlier.
Experiment 2
Experiment 2 was conducted to determine whether the results of Experiment 1 were influenced by associative factors. Because the massed group received all the noise trials in 1 day, they were not moved in and out of the acoustic chambers as many times as the spaced group that received noise exposures over 6 days and thus taken in and out of the acoustic chambers only for the noise exposures, which might have provided an associative cue leading to more complete long-term habituation. This potentially could have influenced rates of habituation between the spaced and massed groups. In Experiment 2, all of the rats remained in the acoustic chambers for the duration of the entire experiment (8 days). This replication reduced the possibility that clear associative factors were responsible for putative differential habituation, whether short or longer term.
Materials and Method
Subjects
Thirty-two male Sprague–Dawley rats (Harlan, Indianapolis, IN) weighing 200–225 g and approximately 2 months old were used. They were housed in the same manner as rats in Experiment 1. Seven days after arrival from the supplier, the rats were single-housed in 46 × 25 × 22 cm clear polycarbonate cages with wire lids and divided into four groups: spaced (n = 8), massed (n = 8), acute (n = 8), and no noise (n = 8). The rats were then placed in acoustic chambers for the entire experiment. The acoustic chambers were kept on a controlled light–dark cycle (lights on at 7 a.m. and off at 7 p.m.), under constant humidity and temperature conditions.
Procedure
Experiment 2 was conducted as a replication of Experiment 1 with the following exceptions: All rats remained in the acoustic testing chambers for the entire experimental period and all rats received tail nicks on Day 6. All rats were sacrificed (rapid decapitation) 48 hr later, after the 30-min noise (or no-noise) exposure as in Experiment 1. CORT and ACTH assays were conducted as in Experiment 1, and the data were analyzed in the same manner.
Results
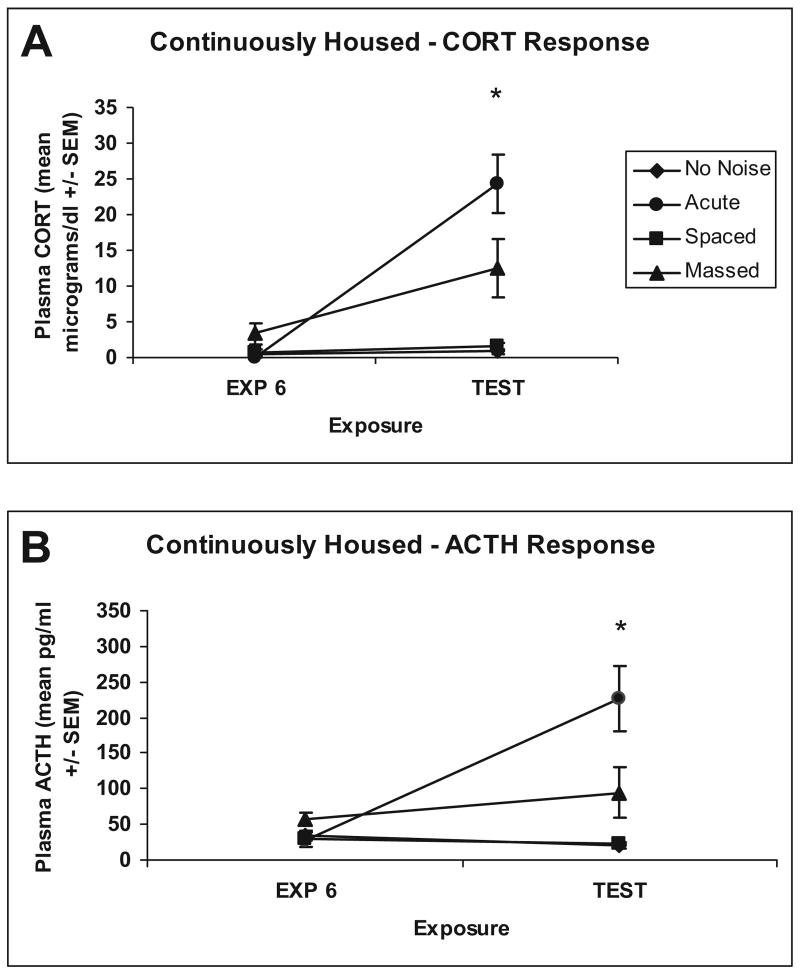
Experiment 2 replicated Experiment 1 with the exception of the movement of animals from the animal colony to the testing chambers. Plasma CORT and ACTH responses were determined from blood taken after the sixth exposure to noise or no noise in all of the rats because Experiment 1 determined that the tail nick procedure did not affect the subsequent CORT and ACTH determination. Trunk blood was also taken after the additional noise exposure test. ANOVA revealed a significant difference between groups for CORT response after the sixth noise exposure, F(3, 28) = 3.656, p = .024. Post hoc differences were found between the massed and no-noise groups (Tukey's HSD; p = .031). ANOVA revealed no significant differences between groups for ACTH response, F(3, 27) = 2.691, p = .066, after the sixth noise exposure. On the test day, 48 hr after the sixth exposure, ANOVA revealed significant differences between the groups for CORT, F(3, 28) = 14.893, p = .0001, and ACTH, F(3, 28) = 11.864, p = .0001. Post hoc comparisons revealed that the main effects were due mostly to differences between the no-noise and massed groups, acute and massed groups, acute and spaced groups, and acute and no-noise groups, respectively for CORT (Tukey's HSD; ps < .04), as shown in Figure 2 (Panel A). The ACTH results were similar, with the massed group being more intermediate, different from the acute and the spaced groups, and the spaced groups showed reliably lower ACTH than the acute and massed groups (Tukey's HSD; ps < .05), indicating more complete habituation; see Figure 2 (Panel B).
Figure 2.
Graphs show mean (± SEM) plasma levels of corticosterone (CORT; Panel A) and ACTH (Panel B) for no-noise (n = 8), acute (n = 8), spaced (n = 8), and massed (n = 8) groups after sixth exposure to 30 min of 95 dBA noise (space and massed) or no-noise (acute and no-noise) groups; EXP 6. Graph also shows mean (± SEM) plasma levels of CORT (Panel A) and ACTH (Panel B) for no-noise (n = 8), acute (n = 8), spaced (n = 8), and massed (n = 8) groups after 30-min 95-dBA noise test; TEST. *Significant difference between massed and spaced groups (p < .05).
Discussion
The results of this experiment again strongly suggest that repeated stressor exposures lead to relatively equivalent habituation after six consecutive exposures, but that more spaced exposures produce more complete long-term habituation, even in the absence of clear associative cues. The spaced noise-exposed rats exhibited levels of CORT and ACTH similar to the no-noise control groups after the sixth and seventh (test) exposures to loud noise, indicating nearly complete and durable habituation. The massed noise-exposed rats displayed habituated CORT and ACTH after the sixth exposure to levels comparable to the spaced noise-exposed rats, but had significantly higher levels than the spaced and no-noise groups after the test exposure. The results of this study again suggest much weaker and less durable habituation in the massed group, even when their level of short-term habituation was very similar to the spaced group.
Experiment 3
Experiment 3 was conducted to determine whether exposure to six 30-min 95 dBA noise exposures received in 1 day, as with the massed group in Experiments 1 and 2, led to reversible hearing threshold shifts that would explain why plasma CORT levels after the sixth exposure were similar to levels in no-noise rats but were higher on the 48-hr test day because of recovery from such temporary shifts. Experiment 3 was designed to examine deficits in prepulse inhibition of an acoustic startle response to determine whether the massed group shows hearing threshold shifts immediately after the six noise exposures. Although we have previously shown that rats' hearing thresholds are not affected by multiple noise presentations using auditory-evoked brainstem potentials measurements (Campeau et al., 2002), the possibility that higher density (massed) noise presentations could temporarily increase hearing thresholds certainly has precedents (Clark, 1991). Furthermore, instead of ABPs, prepulse inhibition of the acoustic startle reflex was chosen because it has been shown to be sensitive to threshold shifts (see below) and the acoustic stimuli employed (white noise) were nearly identical to the characteristics of the audiogenic stressor employed; this is important given recent reports showing very specific auditory impairments in the bands of noise preexposed (Turner et al., 2006). A separate group of rats was used to show that a relatively short session of more intense noise (115 dBA) readily produces threshold shifts measured with prepulse inhibition of the acoustic startle reflex, as reported previously (Chen & Fechter, 2003; Chen & Zhao, in press; Rybalko & Syka, 2005).
Materials and Method
Subjects
Twenty-four Sprague–Dawley rats (Harlan, Indianapolis, IN) weighing 320–340 g at the time of testing were used. The rats were housed and fed as described in Experiment 1. All testing in this experiment was conducted between 9 a.m. and 12 p.m.
Acoustic startle test
Startle testing was performed in eight identical ventilated isolation chambers (Hamilton-Kinder LLC, San Diego, CA), and illuminated by 8-W bulbs located in the ceiling of the chambers. Each startle chamber contained a rat holder (19.05 × 9.14 × 10.67 cm acrylic cage) held in place on a platform load cell that detects cage displacement. Startle amplitude was defined as the maximum load cell output (amplified and digitized) during the first 200 ms after the startle stimulus onset. The startle stimuli (pulses) were 50-ms (rise–decay: 1 ms) bursts of white noise at intensities of 105 dBA (SPL; A scale). Background noise (white noise) of 60 dBA was delivered throughout the startle test. The auditory prepulse stimuli consisted of 20-ms bursts of white noise (rise–decay: 1 ms) at intensities of 62, 65, 70, or 80 dB, presented 50 ms before the pulse stimuli (auditory prepulse trials). A 400-lux, 20-ms flash of light, provided by arrays of 6 × 20 diodes on each side of the cage (rise–decay: 100 μs) each located 3 cm from the cage and extending the entire height of the cage, served as a visual prepulse presented 50 ms before the pulse (visual prepulse trial). For the startle test, the rats were placed in the holders, and 5 min later, the first of ten 105 dBA pulses was presented at a fixed 20-s interstimulus interval (ISI). These initial startle pulses produce some startle habituation, which reduce variability and stabilize baseline startle amplitudes, but they were not used in the estimation of startle modification. Twenty seconds later, the first of 60 additional test trials was presented, at a 20-s ISI, consisting of pulse alone stimuli or prepulse (auditory or visual prepulses) + pulse trials. There were 10 occurrences of each trial type in a quasi-random sequence.
Procedure
Approximately 2 months after arrival from the supplier, the rats were assigned to groups for two separate startle tests. The first test was massed noise exposures (n = 8) versus no-noise preexposure (n = 8) test. The rats were transported in their home cages to the noise chambers described in Experiment 1. The massed group received six 30-min 95 dBA noise exposures, and then no noise for 30 min 6 times. The no-noise preexposure group was placed in the noise chambers for the same amount of time but was not exposed to noise. Immediately after the session, the rats were put in the acoustic startle chambers, and startle testing was conducted as described above.
Separate rats were used for the second startle test. This test examined the effects of 115 dBA noise preexposure (n = 4) versus no-noise preexposure (n = 4). The rats were transported in their home cages to the noise chambers described in Experiment 1. The 115 dBA noise group rats were exposed to 115 dBA noise for 40 min. The no-noise preexposure group rats were placed in the noise chambers for the same amount of time but were not exposed to the noise. Immediately after the session, the rats were put in the acoustic startle chambers, and startle testing was conducted as described above.
Data analysis
Startle responses were analyzed using repeated measures ANOVA (Greenhouse–Geisser) with trial type (prepulse stimuli) as the repeated measure and group as the between-subjects factor (p < .05). Tukey's HSD tests were used to analyze post hoc mean differences (p < .05). Linear contrasts were used to evaluate the prepulse intensity dependency of the startle inhibition obtained (p < .05).
Results
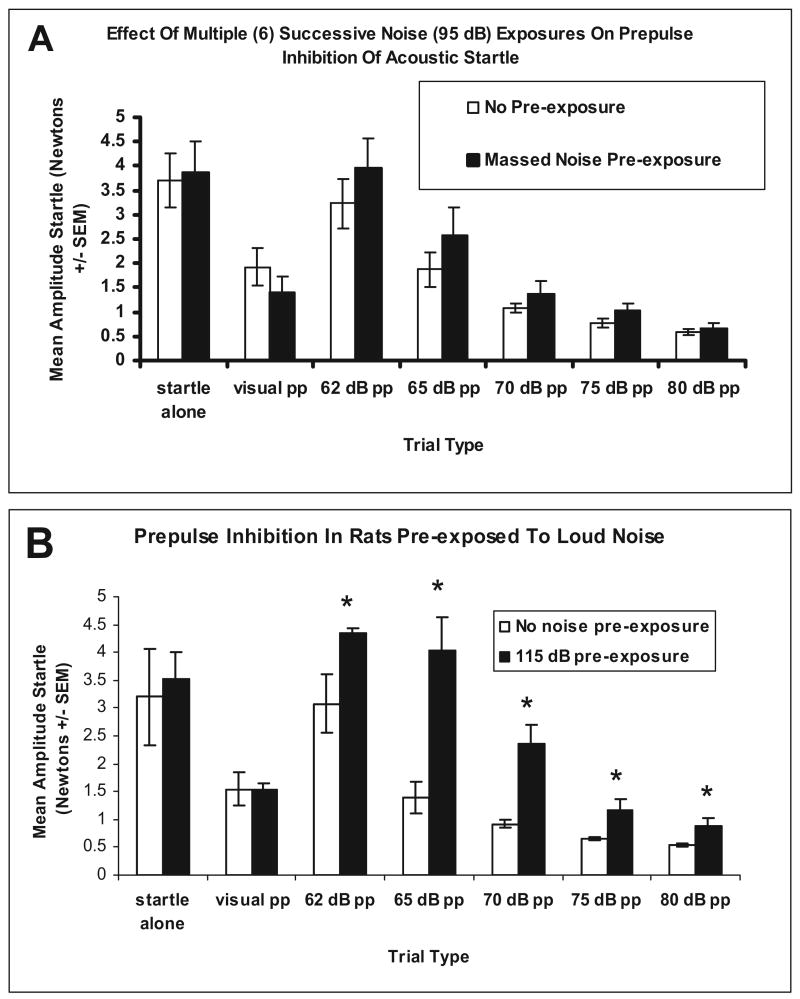
In Experiment 3, startle responses after the massed noise test procedure were examined to determine whether this noise exposure protocol, through temporary hearing threshold shifts, was responsible for the apparent short-term habituation of the massed groups of Experiments 1 and 2. Prepulse inhibition of the startle responses of the 95 dBA massed group compared with the no-noise preexposure group revealed a significant trial (prepulse intensity) effect, without additional interactions with groups, indicating equivalent auditory prepulse functions in noise preexposed or non-preexposed rats. A significant linear within-subject contrast was found for the increasing intensities of noise prepulses, F(1, 14) = 6.157, p = .026, indicating increasing inhibition of the acoustic startle reflex by increasing prepulse intensities, as shown in Figure 3 (Panel A). A prepulse light stimulus significantly inhibited the startle response, F(1, 14) = 68.095, p = .001, but no differences between noise preexposed or non-preexposed rats, F(1, 14) = 0.081, p = .780, were found.
Figure 3.
Graphs show prepulse inhibition results of Experiment 3. Panel A depicts prepulse inhibition results after six massed exposures to 30 min of 95 dBA noise (n = 8) or no preexposure to noise (n = 8). No statistically significant group differences were found. Panel B depicts prepulse inhibition results after 40 min of 115 dBA noise (n = 4) or no preexposure to noise (n = 4). *Significant differences between groups (Tukey HSD; p < .05). pp = prepulse.
Experiment 3 also examined prepulse inhibition after a 40-min exposure to a 115 dBA noise compared with a no-noise exposure to show that the prepulse inhibition test can detect hearing threshold shifts. Prepulse inhibition of the startle responses of the 115 dBA group compared with the no-noise preexposure group revealed a significant trial effect, F(1, 6) = 25.718, p = .0001, and group effects, F(1, 6) = 10.224, p = .019, but no Trial × Group interaction, F(1, 6) = 3.186, p = .075, indicating that this preexposure effect led to significant prepulse deficits (higher auditory thresholds by approximately 10 dB); see Figure 3 (Panel B). A prepulse light stimulus significantly inhibited the startle response, F(1, 6) = 15.010, p = .008, but no differences between the two groups were revealed, F(1, 6) = 0.073, p = .797, suggesting that the threshold shifts found were modality-specific. These results thus suggest that although prepulse inhibition of the acoustic startle can detect reliable and modality-specific hearing threshold shifts in response to a relatively short but intense noise (40 min, 115 dBA) exposure, no reliable threshold shifts are produced by repeated moderate (6 × 30 min, 95 dBA) noise exposures.
Discussion
One explanation for the relatively short-term CORT and ACTH habituation seen in the massed noise-exposed group after the sixth exposure could be that the protocol of giving the massed rats six exposures of 95 dBA noise (noise on for 30 min and then off for 30 min 6 times in 1 day) led to reversible short-term hearing threshold shifts that reduced the ability of the rats to detect and respond accordingly to the last noise exposure, but this temporary shift had resolved 48 hr later, which then allowed rats in this group to respond to the test noise again, in a fashion more similar to the acute group. And although it has been found that rats exposed to a 105 dBA noise for 30 min per day for 8 days exhibit no hearing loss as measured by changes in auditory brainstem evoked potentials (Campeau et al., 2002), these tests were carried out with very different auditory stimuli. Experiment 3 was designed to test the effects of the massed noise exposure on the hearing thresholds of rats to stimuli similar to the loud noise stimulus using prepulse inhibition. It was found that the effect of the massed presentations of 95 dBA on the prepulse inhibition of startle using 62, 65, 70, 75, and 80 dBA prepulse stimuli was similar to a non-preexposed noise group. This suggests that the massed noise exposure protocol did not reversibly shift the auditory thresholds of the short intertrial interval (massed) noise-treated rats. In a separate set of rats, however, prepulse inhibition was tested after a single 40-min exposure to a 115 dBA white noise to show that very significant threshold shifts can be measured with the present prepulse inhibition of acoustic startle protocol. This led to 10–15 dB threshold shifts, which was selective to the auditory modality, as the visual prepulses were equally effective compared with the control (no-noise) rats. The weaker and less durable habituation of HPA axis indices observed in the massed noise-exposed groups of Experiments 1 and 2, therefore, cannot easily be explained by auditory threshold shifts.
Experiment 4
Experiment 4 examined heart rate, temperature, and behavioral activity in response to massed or spaced presentations of 95 dBA noise using telemetry. Experiments 1 and 2 examined endocrine responses to relatively short and long interstimulus interval presentations of noise. Negative feedback effects of glucocorticoids on HPA axis activity can influence the endocrine responses and might have been responsible for an apparent short-term reduction of HPA axis activity in the massed group but recovery during the 48-hr test. Additional measures that should not be influenced to a significant extent by glucocorticoid feedback were thus employed to examine the short- and long-term adaptations found with spaced and massed noise presentations. These same subjects were also tested for possible hearing threshold shifts using additional prepulse modulation of the acoustic startle reflex, including noise gap detection and noise increment potentiation of acoustic startle. These additional startle tests were included on the basis of the findings that different brain regions are associated with disruptions of these different prepulse manipulations (Bowen, Lin, Taylor, & Ison, 2003; Ison, O'Connor, Bowen, & Bocirnea, 1991; Kelly, Rooney, & Phillips, 1996). These manipulations therefore increased the number of assessed putative brain regions that might display loud noise-induced threshold shifts, although there is no guarantee that all putative sensitive regions were considered.
All groups were tested in this study to evaluate the possibility that noise exposure might differentially affect hearing thresholds, especially in the spaced and massed noise preexposed rats.
Materials and Method
Subjects
Sixteen Sprague–Dawley rats (Harlan, Indianapolis, IN) weighing 250–300 g at the time of testing were used. The rats were housed and fed as described in Experiment 1. All testing in this experiment was conducted between 9 a.m. and 2 p.m.
Surgery
E-mitter (Mini Mitter, Sunny River, OR) monitors were surgically implanted under halothane anesthesia and aseptic conditions. Rats' abdominal and thoracic areas were shaved and a 1.5-cm midline incision (below the diaphragm) was made to implant an E-mitter Transponder (E4000) in the abdominal cavity. A 1-cm incision was made over the right clavicle, through which the negative lead of the E-mitter was threaded subcutaneously (10 G trocar), and stitched to the muscle layer using stainless steel thread (to achieve greater area of conductance for the lead tip). The same was done on the left side, but the incision was made just anterior to the diaphragm, where the positive E-mitter lead was attached to muscle layer. This achieved a 45–60° angle in the transverse plane of the heart necessary to accurately measure heart rate. The abdominal muscle was then closed with absorbable (3-0) thread, and the skin incisions closed with silk thread (3-0). On recovery from anesthesia, the rats were returned to single housing home cages for 7 days prior to any experimental manipulations.
Measurement of heart rate, body temperature, and activity
Responses were measured simultaneously following transport and placement of the rat's home cage over the ER-4000 Energizer receivers (Mini Mitter), which were located in the acoustic chambers. The receivers captured these responses from the E-mitter transponder, without additional manipulations. Behavioral (total activity counts) and physiologic (heart rate in beats per minute and temperature in degrees Celsius) responses were sampled at 1-min intervals. The associated VitalView Data Acquisition Software (version 4.1) digitized and saved data for off-line analyses. The measures analyzed were changes (delta) in heart rate and temperature during and for 30 min after the 95 dBA noise exposure. Changes were computed for each rat by subtracting the average of each 1-min sample during the 30-min noise exposure and the 30-min postnoise duration, with the average of the lowest 2 sample values in the 10-min period immediately prior to noise/no-noise exposure. Total behavioral activity counts reflected all horizontal and vertical movements, as well as vigorous grooming. Heart rate and temperature changes and behavioral activity were examined in 5-min blocks of time by averaging the five 1-min samples for each subject.
Acoustic startle test
Acoustic startle testing was conducted as described above in Experiment 3 with the following exceptions. In addition to prepulse inhibition trials, noise increments and auditory gap trials were included. Noise increment stimuli consisted of bursts of white noise (rise–decay: 1 ms) at intensities of 62, 64, or 68 dBA, presented 10 ms before the pulse and ending with the presentation of the pulses (auditory increment trials). The auditory gap stimuli consisted of either 4-, 8-, or 16-ms gaps in the background white noise (reduction of noise from 60 to 52 dBA, which was the ambient noise intensity in the acoustic chamber) presented 75 ms before the pulse stimuli (auditory gap trials). These parameters were derived from Bowen et al. (2003). For the startle test, the rats were placed in the holders, and 5 min later, the first of ten 105 dBA pulses was presented at a fixed 20-s ISI. These initial startle pulses produced some startle habituation, which reduces variability and stabilizes baseline startle amplitudes, but they were not used in the estimation of startle modification. Twenty seconds later, the first of 120 additional test trials was presented, at a 20-s ISI, consisting of pulse alone stimuli or prepulse (auditory and visual prepulses and increments, or gaps) + pulse trials. There were 10 occurrences of each trial type in a quasi-random sequence, with the restriction that each trial type occurred in every 15-trial block.
Procedure
Seven days after surgery, the rats were tested with the acoustic startle test (Day 0 Startle) and placed into groups on the basis of the startle amplitude to counterbalance different baseline startle amplitudes between groups. The resultant groups—acute noise (n = 4), no noise (n = 4), massed noise (n = 4), and spaced noise (n = 4)—therefore had statistically similar baseline startle amplitudes. The rats were then acclimated to the acoustic chambers by transporting the rats to the experimental room and placing their home cages into the acoustic chambers for 20 min/day for 4 days. On the 4th acclimation day, the rats were put in the acoustic chambers for 1 hr and their heart rate, body temperature, and activity were recorded. Twenty-four hours later, acute, no-noise, and spaced group rats were again placed in the acoustic chambers and heart rate, body temperature, and activity were recorded for 1.5 hr/day for 6 days. During these sessions, the spaced group rats received a 30-min 95 dBA noise exposure 30 min after the rats were placed in the chambers. The acute and no-noise groups did not receive noise. After acclimation to the acoustic chambers for 4 days, the massed group rats were placed in the chambers and after 30 min were exposed to the first of six 95 dBA noise exposures followed by 30-min no-noise exposures. Heart rate, body temperature, and activity were recorded during this session. Immediately after the sixth noise exposure (or no noise for the acute and no-noise groups), the rats were tested in the startle apparatus (Exposure 6 Startle). The experimental protocol was designed such that the sixth noise exposure occurred on the same day for all groups to avoid any potential daily variations. Two days later, all the rats were put in the acoustic chambers again and after 30 min, a 30-min 95 dBA noise test was given to the acute, massed, and spaced group rats. Heart rate, body temperature, and activity were again recorded during this test. The next day, all the rats received a final startle test (Final Startle). Following the experiment, the rats were euthanized (rapid decapitation) and the sensors surgically removed and visually inspected for defects and placement.
Data analysis
Heart rate and temperature changes (delta) were computed for each rat for exposure (first, sixth, and seventh—48-hr test) by subtracting from the average of the lowest two sample values in the 10-min period immediately prior to noise/no-noise exposure the average of each 1-min sample during the 30-min noise exposure and 30-min postnoise duration. Repeated measures ANOVAs with trials (twelve 5-min blocks) and exposures (1, 6, and test) as within-subjects factors and group as the between-subjects factor (p < .05) were used to analyze temperature and heart rate changes. In addition, heart rate and temperature changes were examined separately for each exposure using repeated measures ANOVAs with trials (twelve 5-min blocks) as the repeated measure and group as the between-subjects factor (p < .05). Data presentation (Figure 4) and analyses on Exposures 1 and 6 grouped the results of the no-noise and acute noise conditions because the acute noise rats were not exposed to noise at those times. Activity counts were analyzed in 5-min blocks during and after noise or no noise. These counts were analyzed with repeated measures ANOVAs as described for heart rate and temperature changes. Significant interaction effects on the separate exposure analyses were followed by Tukey's HSD to analyze the source of the group differences (p < .05).
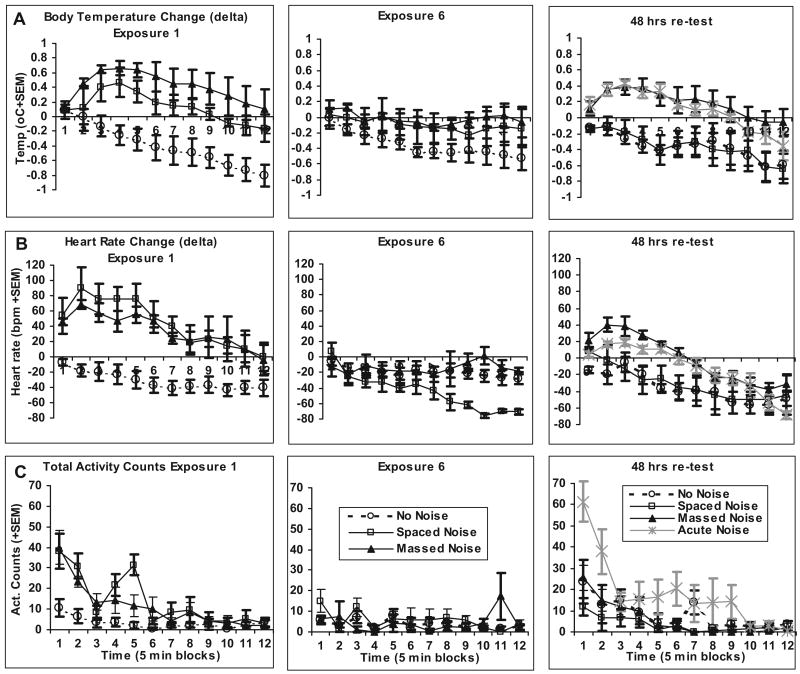
Figure 4.
A. Graphs show temperature (degrees Celsius) changes from baseline (±1 SEM) during (Trials 1–6) and after (Trials 7–12) noise exposures. Trials are 5-min blocks of time. The left panel depicts Exposure 1, during which acute (n = 4) and no-noise (n = 4) groups did not receive noise and were therefore combined, and the massed (n = 4) and spaced (n = 4) groups were exposed to 30 min of 95 dBA noise. The middle panel depicts Exposure 6, during which again the acute and no-noise groups were combined and received no noise, and the massed and spaced groups were exposed to 30 min of 95 dBA noise for the sixth time. The right panel depicts the 30-min 95 dBA noise test 48 ht after the sixth exposure. The acute group received 30 min of 95 dBA noise for the first time. B. Graphs show heart rate (beats per minute) changes from baseline (±1 SEM) during (Trials 1–6) and after (Trials 7–12) noise exposures. The left, middle, and right panels depict Exposures 1, 6, and 7 (48-hr test after the sixth exposure), respectively. C. Graphs show total behavior counts (± SEM) during (Trials 1–6) and after (Trials 7–12) noise exposures. The left, middle, and right panels depict Exposures 1, 6, and 7 (48-hr test after the sixth exposure), respectively.
Startle responses were analyzed using repeated measures ANOVAs with trial type (prepulse stimuli, noise gaps, and noise increments) and days as repeated measures and group (no noise, acute, massed, and spaced) as the between-subjects factor (p < .05). Tukey's HSD tests were used to analyze post hoc mean differences (p < .05). Linear contrasts were used to evaluate the prepulse and increment intensity dependency of the auditory startle inhibition obtained (p < .05). Graphs are expressed in percentage of startle response after noise gap or noise increment divided by baseline startle response.
Results
In Experiment 4, rats were exposed to the massed or spaced presentations of noise as previously described in Experiment 1 and telemetric sensors recorded heart rate, temperature, and behavioral activity. An analysis of baseline values for core body temperatures prior to noise/no noise on the first, sixth, and seventh exposures indicated no group differences on any of these exposures: highest F(2, 15) = 1.58, p = .24. A repeated measures analysis of body temperature changes during these exposures revealed several main within-subjects effects and interactions with groups, but importantly, a triple interaction, between exposure (first, sixth, and seventh test), time (twelve 5-min blocks), and groups (no-noise, acute, spaced, and massed noise conditions), F(66, 220) = 2.056, p < .005. As shown in Figure 4, this interaction represented a general decline in body temperature over the course of all sessions, combined with group differences within each session to the different noise exposures. Temperature change analysis on the first exposure revealed a reliable group, F(2, 13) = 12.01, p = .001, and Time × Group interaction, F(22, 143) = 1.74, p = .028, mainly due to reliable and similar hyperthermia in both noise-exposed groups compared with control rats (Tukey's HSD; p < .024). Significant habituation of the hyperthermic component on the sixth noise exposure was indicated by lack of significant differences between groups, F(2, 13) = 1.998, p = .175, or Group × Time interaction, F(22, 143) = 1.035, p = .427. However, the last (test) exposure produced a return of the hyperthermic response in the massed group, as indicated by a reliable group effect, F(3, 10) = 5.571, p = .016, although the Group × Time interaction did not reach significance, F(33, 110) = 0.929, p = .582, suggesting that the group differences were similar throughout the noise exposure and recovery phases. The post hoc comparisons indicated that the massed and acute groups were similar, but the massed group was different from both the spaced and no-noise groups, respectively (Tukey's HSD; ps' .046), whereas the spaced group was not different from the no-noise control group.
Analysis of the heart rate response changes revealed a similar pattern of results. However, baseline heart rates differed on the first and third exposures because of higher heart rate in the spaced group on the first exposure and higher heart rate in the acute group on the last exposure (ps ≤ .015). These differences, however, did not preclude the measurements of significant tachycardic components in these groups, as revealed by an overall repeated measures analysis of heart rate changes during and after noise/no noise on the first, sixth, and seventh (test) exposures with a triple Group × Time × Exposure interaction, F(66, 220) = 2.098, p < .001. Heart rate change analysis on the first exposure revealed a reliable group effect, F(2, 12) = 14.463, p = .001, and a Time × Group interaction, F(22, 132) = 2.463, p = .001, mainly because of reliable and similar tachycardia in both noise-exposed groups compared with control rats (Tukey HSD; p < .004). Significant habituation of the tachycardic component on the sixth noise exposure was revealed by lack of significant differences between groups, especially during the initial six trial blocks of noise exposures: group effect, F(2, 12) = 0.92, p = .418, and the Time × Group interaction, F(10, 60) = 1.067, p = .399. A moderate tachycardic component was again observed on the 48-hr test noise exposure in the acute and massed groups: group effect, F(3, 11) = 3.708, p = .046, and Time × Group interaction, F(33, 121) = 1.613, p = .033, which led, especially during the initial six trial blocks of noise exposures—group effect, F(2, 11) = 8.548, p = .003—to significant differences between the no-noise and spaced groups compared with the massed group (Tukey's HSD; ps < .048). These results indicated that massed and spaced noise exposure produces similar short-term habituation of tachycardic and hyperthermic responses, but this short-term habituation does not translate into long-term changes following massed noise exposures.
Analyses of activity counts during similar phases of noise/no-noise exposures revealed a significant Time × Exposure × Group interaction effect, F(66, 264) = 2.49, p < .001. Significant group differences in activity counts were observed on the first exposure, F(2, 13) = 26.537, p < .001, because of reliably more activity in the two noise groups, as compared with the control no-noise group. This hyperactive response habituated by the sixth exposure in the two noise groups as revealed by lack of group, F(2, 13) = 1.047, p = .379, and Time × Group interaction effects, F(22, 143) = 1.532, p = .072. Group differences returned on the test exposure, F(3, 12) = 3.801, p = .040, and Time × Group interaction, F(33, 132) = 2.554, p < .001; however, this was mainly due to a difference between the spaced and acute noise groups (Tukey's HSD; p = .037). Analysis of overall locomotor activity on the first six blocks of time during the test noise exposure indicated similar results.
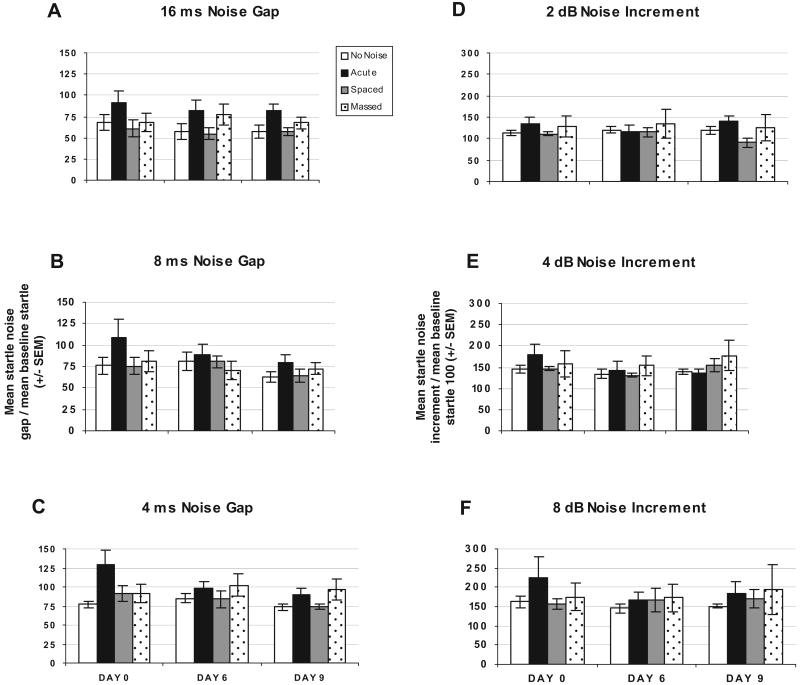
Acoustic startle responses were tested to determine whether massed versus spaced exposures to noise altered startle amplitude, prepulse inhibition, noise gap detection, or noise increments detection that can be used to evaluate hearing impairments. Analysis of 105 dBA startle-alone trials on Days 0, 6, and 9 revealed no significant day, group, or interaction effects.
Prepulse inhibition of the startle responses between groups revealed a significant trial effect, F(1, 12) = 103.222, p = .0001, and Trial × Day effect, F(1, 12) = 3.597, p = .017, but no group effect, F(3, 12) = 0.106, p = .955. Significant linear within-subject contrasts were found, suggesting that noise prepulses, F(1, 12) = 157.611, p = .0001, led to significant startle inhibition (data not shown).
Noise gap inhibition of the startle responses between groups revealed a significant trial effect, F(1, 12) = 35.139, p = .0001, but no group effect, F(3, 12) = 0.164, p = .919. Significant linear within-subject contrasts were found, suggesting that noise gaps, F(1, 12) = 53.364, p = .0001, led to significant startle inhibition, as shown in Figure 5 (Panels A–C).
Figure 5.
Graphs show percentage of baseline startle to noise gap stimuli for Days 0, 6, and 9 for no-noise (n = 4), acute (n = 4), spaced (n = 4), and massed (n = 4) groups. Panel A depicts percentage of baseline startle to 16-ms noise gap stimuli. Panel B depicts percentage of baseline startle to 8-ms noise gap stimuli. Panel C depicts percentage of baseline startle to 4-ms noise gap stimuli. Graphs show percentage of baseline startle to noise increment stimuli for Days 0, 6, and 9 for no-noise (n = 4), acute (n = 4), spaced (n = 4), and massed (n = 4) groups. Panel D depicts percentage of baseline startle to 2 dBA 10-ms noise increment stimuli. Panel E depicts percentage of baseline startle to 4 dBA 10-ms noise increment stimuli. Panel F depicts percentage of baseline startle to 8 dBA 10-ms light noise increment stimuli. pp = prepulse.
Noise increment potentiation of the startle responses between groups revealed a significant trial effect, F(1, 12) = 150.252, p = .0001, but no group effect, F(3, 12) = 0.330, p = .804. Significant linear within-subject contrasts were found, suggesting that noise increments, F(1, 12) = 465.377, p = .0001, led to significant startle potentiation, as depicted in Figure 5 (Panels D–F).
Discussion
This experiment examined autonomic and behavioral responses to loud noise presented in a similar manner as in the previous experiments using telemetry. The first noise exposure led to reliable increases in heart rate, core body temperature, and total behavioral activity, which decreased both during noise exposure and further following noise termination. These responses had different dynamic profiles, with behavioral activity peaking within the first 5-min block, but also showing signs of within-session habituation; tachycardia was also observed in the first 5-min block of noise exposure, but peaked in the second 5-min block, with some attenuation prior to noise termination; and the hyperthermic response was slower to rise, and peaked in the third to fourth 5-min block and also showed signs of return to baseline prior to noise termination. It should be noted that the behavioral responses to loud noise are likely biphasic, in that the initial activity evoked by noise is followed by immobility toward the end of the session (Campeau & Watson, 1997); however, because activity in the control group was already very low because of the testing in the subjective night time of the rats, this noise-induced immobility was impossible to distinguish from the quiet state of the control rats in the present study. It should further be noted that autonomic measures in the control group drifted downward in most tests, indicating that transport of the animals from the colony to the test room, despite acclimation, induced some tachycardic and hyperthermic reactions that had not completely subsided at the beginning of the noise/quiet exposures. To maximize the changes in heart rate and body temperature, we therefore decided to employ the two lowest “baseline” values that were recorded within the 10-min period immediately prior to noise/quiet exposure; even with this manipulation, however, the autonomic changes reported are likely underestimates of maximum changes, and this appears to have influenced all groups to a similar extent. It is important to note that both massed and spaced noise-exposed groups showed a significantly greater change from baseline (for heart rate and body temperature) or total activity compared with the no-noise control rats, without reliably differing from each other on the first noise exposure.
Results from the sixth noise exposure indicated that both the spaced and massed groups showed habituated heart rate, body temperature, and behavioral responses to the noise compared with those of the first noise exposure. In addition, none of the experimental or control groups differed significantly from each other. These results therefore strongly suggest that the similar habituation observed during the sixth noise exposure between the massed and spaced noise-exposed rats of Experiments 1 and 2 cannot be entirely explained by feedback inhibition of the endocrine responses measured. During the final noise exposure (48 hr after the sixth exposure), the massed noise-exposed group exhibited changes from baseline heart rate and temperature similar to the acute group (that received noise for the first time), whereas the spaced noise-exposed group exhibited habituated responses similar to those of the group having never been exposed to noise. The amplitude of the acute group's heart rate response was somewhat lower than that observed during the first exposure of the massed and spaced noise-exposed groups; this was due in large part to a significantly higher basal heart rate level in the acute group on the test exposure day, which reduced the apparent change in heart rate induced by noise exposure. The massed noise-exposed group did not differ in total behavioral activity on the test exposure, but this was due mostly to an unexpected and relatively high behavioral activity in the control no-noise group, at least compared with the prior quiet exposures (see Figure 4). Therefore, the autonomic and behavioral results closely mimic the endocrine responses measured in Experiments 1 and 2, and weakens the possibility that the endocrine results observed in the initial studies could be solely explained by feedback inhibitory processes initiated by CORT release during the massed noise exposures.
Experiment 4 further examined modulation of the acoustic startle responses of rats exposed to massed versus spaced 95 dBA noise with additional prepulse manipulations. Gap detection and startle amplitude increases are reported to be mediated by different brain regions than prepulse inhibition of acoustic startle (Bowen et al., 2003; Ison, 1982; Ison et al., 1991; Ison, Taylor, Bowen, & Schwarzkopf, 1997; Kelly et al., 1996; Turner et al., 2006). For instance, the prepulse facilitation of the acoustic startle obtained with very short lead time (10 ms or less) stimuli such as used in the present study is argued to be mediated either directly at the cochlea or at cochlear nuclei (Ison et al., 1997). On the other end, gap detection appears to rely importantly on cortical processing (Bowen et al., 2003; Kelly et al., 1996). These might provide additional, although not necessarily exhaustive, plastic sites that could be influenced by repeated loud noise exposures.
Comparisons of acoustic startle amplitudes and their modulation by various prepulse manipulations before and after multiple massed or spaced noise exposures, compared with relatively quiet conditions, again revealed no reliable changes in auditory functions in this study. The mean amplitude of startle alone to a 105 dB startle stimulus was examined for the three tests, and no group differences were found in rats preexposed to loud noise or not. As indicated in Experiment 3, even large threshold shifts do not reliably modify the startle reflex to a loud startling stimulus, so the startle results of Experiment 4 were consistent with those of Experiment 3. Prepulse inhibition again revealed no regulation by prior loud noise preexposures in that all rats exhibited equivalent inhibition of the startle response during the three tests with the different levels of auditory or visual prepulses tested. Interestingly, a significant Trial × Days effect was found, suggesting that inhibition of the startle response improved over the three startle tests, but no group or interaction effects indicated no group differences. Gap detection, like prepulse inhibition, significantly reduces startle amplitudes with increasing gap length, and this was not different in any of the groups given repeated noise preexposures compared with quiet conditions. Similarly, the enhancement of startle amplitudes produced by very short prepulse–pulse intervals (10 ms) was equivalent whether or not rats were pretreated with repeated loud noise exposures. These results again strongly suggest that the massed (short interval) noise exposures did not lead to measurable changes in the rats' auditory thresholds, and the endocrine results found are unlikely to be explained by peripheral or more central auditory function regulation. It is still conceivable, however, that regions sensitive to loud noise influences were missed with the above manipulations, but such more centrally plastic changes might be more closely associated with habituation-related plasticity per se.
General Discussion
The present studies examined the effects of two different interstimulus intervals on habituation to repeated stress exposures. As expected from studies of behavioral habituation (Carew et al., 1972; Davis, 1970; Hinde, 1954; Menzel et al., 2001; Rose & Rankin, 2001), massed exposures to moderately stressful, long-duration (30 min) white noises produced evidence of weaker, less durable habituation compared with an equal number of relatively spaced exposures. In addition, these differences were observed even if the earlier (Postexposure 6) measures of habituation were similar between the spaced and massed groups. And with the exception of the behavioral responses, the endocrine and autonomic measures varied with remarkable similarity in response to the repeated noise exposures in the different experimental and control groups. This finding may have important implications for the underlying neural basis of the brain state evoked by stressful situations and the regulation or modulation of multiple response systems by these regions.
The results of Experiment 1, which examined HPA axis hormones (CORT and ACTH), support the hypothesis that massed exposure to stress stimuli produces weaker and less durable habituation compared with a more spaced exposure regimen, as reported in a number of studies of behavioral response habituation (see Christoffersen, 1997, for a review). There were some treatment differences, however, between the spaced and massed groups of Experiment 1 that may have led to the observed differences. Importantly, the spaced group rats were transported to and from the animal colony to the testing room and apparatus 6 times (six daily exposures), but the massed group received all the exposures in 1 day, without removal from the testing apparatus. The better retention of habituation in the spaced group could possibly be explained by stronger associative learning provided by an association of the transport and apparatus transfer cues with exposure to loud noises, cues that were minimally available in the massed group. To test this possibility, Experiment 2 was designed such that all the rats were kept in the acoustic chambers for the entire duration of the experiment (8 days). The rats only left the chambers to be blood sampled after the sixth exposure, which was similar for all groups. The results of Experiment 2 were nearly identical to those of Experiment 1. Thus, the associative cues provided by transport and experimental apparatus placement in the spaced noise-exposed rats do not provide a likely explanation for the observed group differences, although diffuse circadian cues could still be invoked (light–dark cycle, endogenous hormone levels).
Experiments 3 and 4 examined startle responses and confirmed that the massed noise presentation protocol did not alter the rats' ability to detect prepulses at the intensities employed. The startle response amplitudes did not differ from rats that were not preexposed to noise. This suggests that the massed noise exposure protocol did not reversibly shift the auditory thresholds of the short intertrial interval (massed) noise-treated rats. In addition, evidence was provided to show that a 115 dBA white noise can produce 10–15 dB threshold shifts. These data suggest that the weaker and less durable habituation of HPA axis indices observed in the massed noise-exposed groups of Experiments 1 and 2 are not easily explained by auditory threshold shifts.
Another possible explanation for the apparent short-term, but weaker long-term habituation of endocrine responses found in the massed groups of Experiments 1 and 2 was that the initial stress-induced release of CORT produced negative feedback that inhibited CORT and ACTH responses to the last noise exposure. Such inhibitory feedback might have reduced ACTH and CORT release on the last massed exposure, without concomitant neural habituation; however, 48 hr later, this inhibition would have subsided and allowed the observation of higher noise-induced endocrine release compared with the spaced noise-exposed group. Experiment 4 examined this possibility by measuring habituation of autonomic and behavioral responses that should be minimally affected by direct endocrine feedback inhibition effects. Similar to HPA axis indices, stress, including loud noise, elicits reliable heart rate and core body temperature regulation (Overton et al., 1991; Silva, 2005). These responses do habituate in response to repeated stress (Stamp & Herbert, 1999; McDougall, Paull, Widdop, & Lawrence, 2000; Carter, Pinnock, & Herbert, 2004). The important question was whether these autonomic and behavioral responses to repeated loud noises would mimic the endocrine responses observed in Experiments 1 and 2. Using telemetry, heart rate, core body temperature, and total behavioral activity measures, the present study found that these measures did indeed mimic the endocrine results. The results thus strongly suggest that the similar habituation observed during the sixth noise exposure between the massed and spaced noise-exposed rats of Experiments 1 and 2 cannot be explained entirely by feedback inhibition of the endocrine responses measured.
Overall, the results of the present experiments suggest that habituation to an audiogenic stressor is, to a significant extent, influenced by interstressor intervals. A 24-hr interval produces relatively long-lasting habituation of multiple responses; however, a 1-hr interstressor interval gives rise to relatively short-term habituation, but much weaker long-term, durable habituation. Aside from reproducing a phenomenon that was classically observed with reflexive behavioral responses to the domains of endocrine and autonomic responses and much longer duration stimuli, these results strongly point to different forms of plasticity initiated by short- versus long-interval stress exposures. This observation provides an important tool in linking the underlying neural circuits and cellular mechanisms specifically associated with long-term habituation to repeated stress exposures. One question posed by these results is whether there exists an optimal interstressor duration, deviations from which (longer or shorter) would weaken habituation to stress, and, therefore, exaggerate the long-term impact of each stress exposure. In addition, the duration of optimally produced long-term plastic changes is unknown with regard to stress. These issues remain to be empirically determined. Answers to these questions would have some direct important clinical implications and applications, given the association of repeated or chronic stress with several human mood disorders (Brown, Bifulco, & Harris, 1987; Kessler, 1997; McEwen, 2004). Optimal intervals between stress events may help people cope better with future stress events, thus reducing the overall impact of repeated stress exposures. The use of exposure therapies to reduce phobic, panic, and obsessive syndromes could further benefit from the definition of optimal exposure intervals. The present results are thus laying a foundation for a further exploration of the underlying brain circuits and molecular mechanisms that are responsible for the short- and long-term reductions of the impact of stress on health.
Acknowledgments
This work was supported by National Institute of Mental Health Grant R01 MH065327 to Serge Campeau.
References
- Armario A, Castellanos JM, Balasch J. Adaptation of anterior pituitary hormones to chronic noise stress in male rats. Behavioral and Neural Biology. 1984;41:71–76. doi: 10.1016/s0163-1047(84)90745-3. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Huber R, Nowak N, Trotter P. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. Journal of Neuroendocrinology. 2002;14:403–410. doi: 10.1046/j.0007-1331.2002.00792.x. [DOI] [PubMed] [Google Scholar]
- Bowen GP, Lin D, Taylor MK, Ison JR. Auditory cortex lesions in the rat impair both temporal acuity and noise increment thresholds, revealing a common neural substrate. Cerebral Cortex. 2003;13:815–822. doi: 10.1093/cercor/13.8.815. [DOI] [PubMed] [Google Scholar]
- Brown GW, Bifulco A, Harris TO. Life events, vulnerability and onset of depression: Some refinements. British Journal of Psychiatry. 1987;150:30–42. doi: 10.1192/bjp.150.1.30. [DOI] [PubMed] [Google Scholar]
- Burow A, Day HEW, Campeau S. A detailed characterization of loud noise stress: Intensity analysis of hypothalamo–pituitary–adrenocortical axis and brain activation. Brain Research. 2005;1062:63–73. doi: 10.1016/j.brainres.2005.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Dolan D, Akil H, Watson SJ. C-fos mRNA induction in acute and chronic audiogenic stress: Possible role of the orbitofrontal cortex in habituation. Stress. 2002;5:121–130. doi: 10.1080/10253890290027895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Masini CV, Srinidhi SK, Day HEW. Long-term adaptation of body temperature, heart rate, and behavioral responses to repeated loud noise is impaired by relatively short inter-trial intervals in rats [Abstract]. Presented at the annual meeting of the Society for Neuroscience (Program No. 595); 2006. Oct, Retrieved from http://sfn.scholarone.com. [Google Scholar]
- Campeau S, Watson SJ. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. Journal of Neuroendocrinology. 1997;9:577–588. doi: 10.1046/j.1365-2826.1997.00593.x. [DOI] [PubMed] [Google Scholar]
- Carew TJ, Kandel ER. Acquisition and retention of long-term habituation in Aplysia. Science. 1973 December 14;182:1158–1160. doi: 10.1126/science.182.4117.1158. [DOI] [PubMed] [Google Scholar]
- Carew TJ, Pinsker HM, Kandel ER. Long-term habituation of a defensive withdrawal reflex in Aplysia. Science. 1972 January 28;175:451–454. doi: 10.1126/science.175.4020.451. [DOI] [PubMed] [Google Scholar]
- Carter RN, Pinnock SB, Herbert J. Does the amygdala modulate adaptation to repeated stress? Neuroscience. 2004;126:9–19. doi: 10.1016/j.neuroscience.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Chen GD, Fechter LD. The relationship between noise-induced hearing loss and hair cell loss in rats. Hearing Research. 2003;177:81–90. doi: 10.1016/s0378-5955(02)00802-x. [DOI] [PubMed] [Google Scholar]
- Chen GD, Zhoa HB. Effects of intense noise exposure on the outer hair cell plasma membrane fluidity. Hearing Research. doi: 10.1016/j.heares.2006.06.007. in press. [DOI] [PubMed] [Google Scholar]
- Christoffersen GRJ. Habituation: Events in the history of its characterization and linkage to synaptic depression. A new proposed kinetic criterion for its identification. Progress in Neurobiology. 1997;53:45–66. doi: 10.1016/s0301-0082(97)00031-2. [DOI] [PubMed] [Google Scholar]
- Clark WW. Recent studies of temporary threshold shift (TTS) and permanent threshold shift (PTS) in animals. Journal of the Acoustical Society of America. 1991;90:155–163. doi: 10.1121/1.401309. [DOI] [PubMed] [Google Scholar]
- Cole MA, Kalman BA, Pace TWW, Topczewski R, Lowrey MJ, Spencer RL. Selective blockade of the mineralocorticoid receptor impairs hypothalamic–pituitary–adrenal axis expression of habituation. Journal of Neuroendocrinology. 2000;12:1034–1042. doi: 10.1046/j.1365-2826.2000.00555.x. [DOI] [PubMed] [Google Scholar]
- Commins S, Cunningham L, Harvey D, Walsh D. Massed but not spaced training impairs spatial memory. Behavioral Brain Research. 2003;139:215–223. doi: 10.1016/s0166-4328(02)00270-x. [DOI] [PubMed] [Google Scholar]
- Davis M. Effects of interstimulus interval length and variability on startle-response habituation in the rat. Journal of Comparative and Physiological Psychology. 1970;72:177–192. doi: 10.1037/h0029472. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Koopmans SJ, Slangen JL, Van Der Gugten J. Plasma catecholamine, corticosterone, and glucose responses to repeated stress in rats: Effect of interstressor interval length. Physiology & Behavior. 1990;47:1117–1124. doi: 10.1016/0031-9384(90)90361-7. [DOI] [PubMed] [Google Scholar]
- Endroczi E. Limbic system, pituitary–adrenal axis, and adaptive behavior. In: Selye H, editor. Selye's guide to stress research. Vol. 2. Scarborough, Ontario: Van Nostrand Reinhold; 1983. pp. 249–270. [Google Scholar]
- Goodrick CL. Maze learning of mature-young and aged rats as a function of distributed practices. Journal of Experimental Psychology. 1973;98:344–349. doi: 10.1037/h0034421. [DOI] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: A dual-process theory. Psychological Review. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Hinde RA. Factors governing the changes in strength of a partially inborn response, as shown by the mobbing behavior of the chaffinch (Fringilla coelebs): II. The waning of the response. Proceedings of the Royal Society of London. 1954;142:331–358. doi: 10.1098/rspb.1954.0029. [DOI] [PubMed] [Google Scholar]
- Ison JR. Temporal acuity in auditory function in the rat: Reflex inhibition by brief gaps in noise. Journal of Comparative and Physiological Psychology. 1982;96:945–954. [PubMed] [Google Scholar]
- Ison JR, O'Connor K, Bowen GP, Bocirnea A. Temporal resolution of gaps in noise by the rat is lost with functional decortication. Behavioral Neuroscience. 1991;105:33–40. doi: 10.1037//0735-7044.105.1.33. [DOI] [PubMed] [Google Scholar]
- Ison JR, Taylor MK, Bowen GP, Schwarzkopf SB. Facilitation and inhibition of the acoustic startle reflex in the rat after momentary increase in background noise level. Behavioral Neuroscience. 1997;111:1335–1352. doi: 10.1037//0735-7044.111.6.1335. [DOI] [PubMed] [Google Scholar]
- Kelly JB, Rooney BJ, Phillips DP. Effects of bilateral auditory cortical lesions on gap-detection thresholds in the ferret (Mustela putorius) Behavioral Neuroscience. 1996;110:542–550. doi: 10.1037//0735-7044.110.3.542. [DOI] [PubMed] [Google Scholar]
- Keppel G. A reconsideration of the extinction-recovery theory. Journal of Verbal Learning and Behavior. 1967;6:476–486. [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annual Reviews in Psychology. 1997;48:1912–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Levine S. Influence of psychological variables on the activity of the hypothalamic–pituitary–adrenal axis. European Journal of Pharmacology. 2000;405:149–160. doi: 10.1016/s0014-2999(00)00548-3. [DOI] [PubMed] [Google Scholar]
- Masini CV, Day HEW, Campeau S. Long-term adaptation of corticosterone response to repeated loud noise is impaired by relatively short inter-trial intervals [Abstract]. Presented at the Neuroendocrinology Workshop; San Diego, CA. 2005. Jun, [Google Scholar]
- Masini CV, Sauer S, White J, Day HEW, Campeau S. Non-associative defensive responses of rats to ferret odor. Physiology & Behavior. 2006;87:72–81. doi: 10.1016/j.physbeh.2005.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SJ, Paull JR, Widdop RE, Lawrence AJ. Restraint stress: Differential cardiovascular responses in Wistar–Kyoto and spontaneously hypertensive rats. Hypertension. 2000;35:126–129. doi: 10.1161/01.hyp.35.1.126. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Annals of the New York Academy of Sciences. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- Menzel R, Manz G, Menzel R, Greggers U. Massed and spaced learning in honeybees: The role of CS, US, the intertrial interval, and the test interval. Learning & Memory. 2001;8:198–208. doi: 10.1101/lm.40001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natelson BH, Ottenweller JE, Cook JA, Pitman D, McCarty R, Tapp WN. Effect of stressor intensity on habituation of the adrenocortical stress response. Physiology & Behavior. 1988;43:41–46. doi: 10.1016/0031-9384(88)90096-0. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Guide for the care and use of laboratory animals (DHEW Publication No. 86–23) Washington, DC: U.S. Government Printing Office; 1986. [Google Scholar]
- Overton JM, Kregel KC, Davis-Gorman G, Seals DR, Tipton CM, Fisher LA. Effects of exercise training on responses to central injection of CRF and noise stress. Physiology & Behavior. 1991;49:93–98. doi: 10.1016/0031-9384(91)90237-i. [DOI] [PubMed] [Google Scholar]
- Pitman DL, Ottenweller JE, Natelson BH. Plasma corticosterone levels during presentation of two intensities of restraint stress: Chronic stress and habituation. Physiology & Behavior. 1988;43:47–55. doi: 10.1016/0031-9384(88)90097-2. [DOI] [PubMed] [Google Scholar]
- Rose JK, Rankin CH. Analysis of habituation in Caenorhabditis elegans. Learning & Memory. 2001;8:63–69. doi: 10.1101/lm.37801. [DOI] [PubMed] [Google Scholar]
- Rybalko N, Syka J. Effect of noise exposure on gap detection in rats. Hearing Research. 2005;200:63–72. doi: 10.1016/j.heares.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Selye H. The stress of life. New York: McGraw-Hill; 1956. [Google Scholar]
- Silva JE. Thermogenic mechanisms and their hormonal regulation. Physiological Reviews. 2005;86:435–464. doi: 10.1152/physrev.00009.2005. [DOI] [PubMed] [Google Scholar]
- Stamp JA, Herbert J. Multiple immediate-early gene expression during physiological and endocrine adaptation to repeated stress. Neuroscience. 1999;94:1313–1322. doi: 10.1016/s0306-4522(99)00368-1. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: A model phenomenon for the study of neuronal substrates of behavior. Psychological Review. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM. Gap detection deficits in rats with tinnitus: A potential novel screening tool. Behavioral Neuroscience. 2006;120:188–195. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- Willingham DT. Allocating student study time: “Massed versus “distributed” practice. American Educator. 2002 Summer; Retrieved March 9, 2006 from http://www.aft.org/pubs-reports/american_educator.html.