Abstract
The muscle-specific microRNAs, miR-1 and miR-133, play important roles in muscle growth and differentiation. Here, we show that the MEF2 transcription factor, an essential regulator of muscle development, directly activates transcription of a bicistronic primary transcript encoding miR-1-2 and 133a-1 via an intragenic muscle-specific enhancer located between the miR-1-2 and 133a-1 coding regions. This MEF2-dependent enhancer is activated in the linear heart tube during mouse embryogenesis and thereafter controls transcription throughout the atrial and ventricular chambers of the heart. MEF2 together with MyoD also regulates the miR-1-2/-133a-1 intragenic enhancer in the somite myotomes and in all skeletal muscle fibers during embryogenesis and adulthood. A similar muscle-specific intragenic enhancer controls transcription of the miR-1-1/-133a-2 locus. These findings reveal a common architecture of regulatory elements associated with the miR-1/-133 genes and underscore the central role of MEF2 as a regulator of the transcriptional and posttranscriptional pathways that control cardiac and skeletal muscle development.
Keywords: striated muscle, heart development, transcriptional regulation
Muscle development is accompanied by the transcriptional activation of large sets of structural genes encoding components of the contractile apparatus, enzymes, receptors, and ion channels. The myocyte enhancer factor-2 (MEF2) transcription factor is required for cardiac and skeletal muscle development in organisms ranging from fruit flies to mammals (1). MEF2 activates muscle gene expression in combination with other transcription factors that are often cell type-restricted and signal-responsive. In skeletal muscle, for example, MEF2 activates transcription synergistically with members of the MyoD family of muscle-specific bHLH proteins (2).
Recent studies have revealed a previously unrecognized layer of muscle gene regulation in which small, noncoding RNAs, known as microRNAs (miRNAs), modulate growth, differentiation, and morphogenesis of muscle cells (3). MiRNAs, which are ≈22 nt in length, act as negative regulators of gene expression by promoting mRNA degradation and/or inhibiting mRNA translation through sequence-specific interactions with the 3′ UTRs of target mRNAs (4). MiRNAs have been implicated in diverse biological processes including cell proliferation, apoptosis, tissue morphogenesis, tumorigenesis, and heart disease.
miRNA biogenesis is initiated when primary miRNA precursors (pri-miRNAs), ranging in length from a few hundred to thousands of nucleotides, are transcribed by RNA polymerase II. Pri-miRNAs contain stem–loop structures that are recognized and cleaved by a “microprocessor complex” composed of the RNase III enzyme Drosha and its protein partner DGCR8 (5). The resulting pre-miRNA stem–loop is then transported to the cytoplasm by Exportin 5, where it is processed into an imperfect RNA duplex by the RNase Dicer and its protein partners (5). The mature miRNA in the duplex is assembled into an RNA-induced silencing complex (RISC), which directs miRNA binding to the 3′ UTR sequences of target mRNAs.
Three pairs of related muscle-specific miRNAs (miR-1-1/133a-2, miR-1-2/133a-1, and miR-206/133b), which are transcribed as bicistronic transcripts on different chromosomes, have been shown to play multiple roles in the control of muscle growth and differentiation. miR-1 inhibits cardiac growth by repressing the expression of the Hand2 transcription factor, a positive regulator of cardiac growth (6, 7), and promotes myoblast differentiation as a consequence of its repressive influence on histone deacetylase 4, a transcriptional repressor of myogenesis (8). miR-133, in contrast, enhances myoblast proliferation by repressing expression of serum response factor (SRF), an essential activator of myogenesis (8), and miR-206 reinforces the muscle differentiation program by inhibiting the expression of DNA polymerase and the inhibitory HLH protein Id, which functions as a negative regulator of MyoD (9).
In the course of analyzing miRNA functions in the adult heart (10, 11), we noted that miR-1-1/133a-2 and miR-1-2/133a-1 were down-regulated in the hearts of mice lacking MEF2 expression. Consistent with these findings, a MEF2-dependent enhancer upstream of the miR-1-1/133a-2 locus has been shown to regulate cardiac and skeletal muscle expression in vivo (7). Here, we show that MEF2 also activates transcription of the bicistronic precursor RNA encoding miR-1-2 and miR-133a-1 via an intragenic muscle-specific enhancer. A similar muscle-specific enhancer is located within the miR-1-1/133a-2 gene. We conclude that MEF2 regulates muscle gene expression at transcriptional and posttranscriptional levels by governing the expression of muscle structural genes and miRNA genes, respectively.
Results
Down-Regulation of miR-1/133 in Hearts from MEF2 Mutant Mice.
In the course of studying the functions of MEF2 and miRNAs in the adult heart (10, 11), we noted that the miR-1-1/133a-2 and miR-1-2/133a-1 miRNA pairs were down-regulated in hearts of mice lacking MEF2C and MEF2D (Fig. 1). miR-1-2 and miR-133a-1 are transcribed from a bicistronic miRNA precursor on the antisense strand of the Mindbomb (Mib) gene on mouse chromosome 18. Similarly, miR-1-1 and miR-133a-2 are transcribed together on mouse chromosome 2. However, little is known about the structures of primary transcripts from which these miRNAs are derived, and there are conflicting reports as to whether miR-1-2 and miR-133a-1 are independently regulated (8, 12).
Fig. 1.
Down-regulation of miR-1 and 133 in hearts of MEF2 mutant mice. Mef2c and Mef2d were deleted in the heart by breeding Mef2cloxP/−; Mef2dloxP/loxP mice to Nkx2.5-Cre transgenic mice (KO). RNA was isolated from hearts of these mutant mice, which are viable, and WT littermates, and expression of the indicated miRNAs was detected by real-time PCR.
Analysis of miR-1-2 and 133a-1 pri-miRNAs.
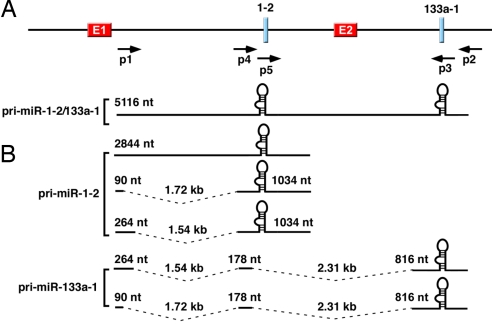
Because MEF2 has not been shown to regulate miR-1-2/133a-1, we focused on its transcriptional regulation and began by mapping the 5′ transcriptional start site and 3′ end of the bicistronic pri-miRNA by 5′- and 3′-RACE by using mouse embryonic (E10–E12.5) RACE-ready cDNA. In the RACE reactions, we used primers specific to the miR-1-2 and 133a-1 stem–loop sequences for primary amplification (p3, p5, and their reverse complements in Fig. 2A). Cloning and sequencing of the RACE products revealed three RACE products containing miR-1-2 stem–loop sequences and two products containing miR-133a-1 stem–loop sequences (Fig. 2B). Two of the miR-1-2 RACE products and both of the miR-133a-1 products aligned discontinuously along the genome, indicating that they were derived from spliced primary transcripts (Fig. 2B). All of the RACE transcripts were mapped to the same 5′ start site, which is located ≈2.1 kb upstream of the genomic sequence predicted to encode the miR-1-2 stem–loop and 4.4 kb upstream of the predicted miR-133a-1 stem–loop sequence. Inspection of the genomic sequence revealed a canonical TATA box (TATAAA) 30 nt upstream of the 5′ terminus and a canonical polyadenylation signal (AATAAA) 20 nt upstream of the 3′ terminus of pri-miR-133a-1 transcripts [supporting information (SI) Fig. 7]. None of the pri-miR-133a-1 transcripts that we were able to detect contained miR-1-2 stem–loop sequences, suggesting that these sequences are rapidly spliced out of the common pri-miR-1-2/133a-1 precursor before processing by Drosha. The presence of conserved splice donor and acceptor sites at the junctions of each exon supports this conclusion (data not shown). Thus, it is likely that RNA polymerase II transcribes a common pri-miRNA precursor from the miR-1-2/miR-133a-1 locus (Fig. 2A), and subsequent alternative splicing generates different primary transcripts that serve as substrates of Drosha processing to generate pre-miR-1-2 and pre-miR-133a-1 stem–loops (Fig. 2B).
Fig. 2.
The miR-1-2/133a-1 gene and primary transcripts encoding miR-1-2 and miR-133a-1. (A) The genomic structure of the mouse miR-1-2/133a-1 gene is shown. The premiR-1-2 stem–loop is 2.5 kb upstream of the premiR-133a-1. Locations of enhancer 1 (E1) and the 330-bp intragenic enhancer (E2) are shown. Primers used in the RACE and RT-PCR are also shown. The proposed structure of the primary transcript containing stem–loop sequences of both miRs is shown. This transcript was not detected in RT-PCR. (B) Structures of primary transcripts of miR-1-2 and miR-133a-1 mapped by 5′- and 3′-RACE. (Upper) Three transcripts were identified for pri-miR-1-2, and two of them were spliced internally. (Lower) Two transcripts were identified for pri-miR-133a-1. Both transcripts were spliced and did not contain premiR-1-2 sequences.
Identification of an Intragenic miR-1-2/133a-1 Enhancer.
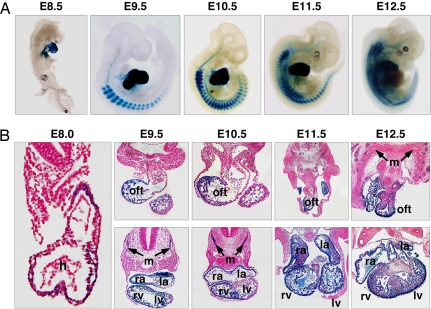
Sequence analysis of genomic DNA encompassing the miR-1-2/133a-1 locus revealed an evolutionarily conserved sequence (CTATTTTAG) resembling the MEF2 consensus binding site [CTA(A/T)4TAR] in the genomic region between the miR-1-2 and 133a-1 coding regions (SI Fig. 8). To test whether this MEF2-like binding site might direct muscle-specific expression, we fused the 2.5-kb intragenic region to the hsp68 basal promoter upstream of a lacZ reporter gene and generated stable lines of transgenic mice. Robust lacZ expression from this transgene was seen in the heart as early as E8.5 and persisted throughout the heart from embryogenesis to adulthood (Fig. 3A and SI Fig. 9). Within the heart, lacZ was expressed in the outflow tract region and all four cardiac chambers at all stages analyzed. At E12.5, lacZ was expressed in endocardium, myocardium, and epicardium, but not in endocardial cushions (Fig. 3B). The expression pattern of the intragenic enhancer in the embryonic heart differs from that of the upstream enhancer (enhancer 1 in Fig. 2A), which directs expression in the ventricular chambers and outflow tract but not in the atria (7). In developing skeletal muscle cells, the miR-1-2/133a-1 intragenic enhancer directed robust lacZ expression in the somite myotomes and, later, throughout the skeletal musculature irrespective of fiber type (Fig. 3 and SI Fig. 9).
Fig. 3.
Muscle-specific expression of the intragenic miR-1-2/133a-1 enhancer. (A) β-Galactosidase staining was performed on embryos from staged mating of a stable transgenic line bearing the 2.5-kb hsp68-lacZ construct. LacZ expression in the heart is observed as early as E8.5. LacZ is also expressed in somites starting from E9.5. At E11.5, lacZ expression is observed in body wall musculature. (B) Transverse sections revealed lacZ expression in the outflow tract and four chambers of the heart as well as in somites. Embryos from the stable transgenic line were stained for β-galactosidase activity, sectioned, and counterstained with Nuclear Fast red. h, heart; oft, outflow tract; ra, right atrium; la, left atrium; rv, right ventricle; lv, left ventricle; m, somite myotomes.
Delineation of the miR-1-2/133a-1 Intragenic Enhancer.
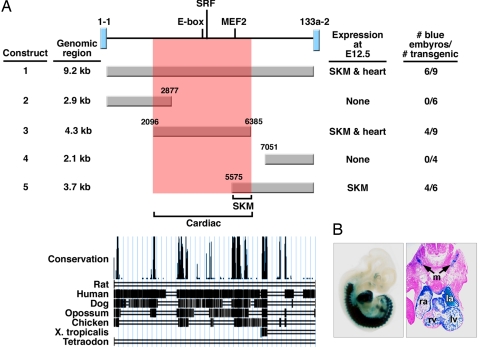
To delineate the regulatory elements within the intragenic enhancer, we created a series of deletions and assayed their expression in F0 transgenic embryos at E12.5 (Fig. 4). These studies showed that a 330-bp fragment was sufficient to direct cardiac and skeletal muscle expression (construct 5, Fig. 4 and SI Fig. 10). Deletion of the 330-bp region in the context of the 2.5-kb enhancer fragment abolished expression in both heart and somites (construct 6, Fig. 4 and SI Fig. 10), indicating that this fragment is necessary and sufficient for cardiac and skeletal muscle expression. This region shows high cross-species conservation in vertebrates (Fig. 4).
Fig. 4.
Delineation of a miR-1-2/133a-1 muscle-specific enhancer. Summary of transgenic constructs used to delineate the intragenic miR-1-2/133a-1 enhancer. Fractions of F0 transgenic embryos showing cardiac and skeletal muscle (SKM) expression at E12.5 are indicated in the right column. Construct 1 is identical to the construct used in Fig. 3. Evolutionary conservation of the 2.5-kb fragment is shown at the bottom.
A MEF2 Site Is Required for Activity of the miR-1-2/133a-1 Intragenic Enhancer.
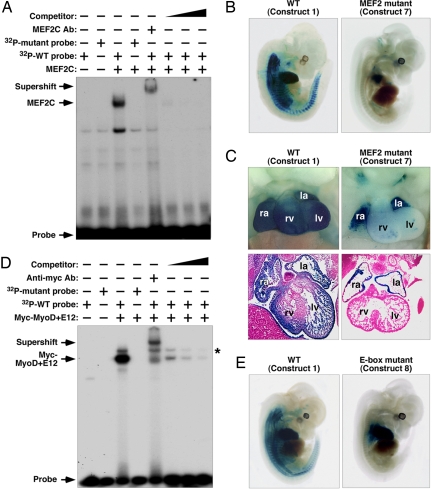
The MEF2-like-binding site in the 330-bp minimal intragenic enhancer could bind MEF2C in gel mobility shift assays with extracts of COS cells transfected with myc-MEF2C expression plasmid, but not with extracts from cells transfected with a myc-expression plasmid alone (Fig. 5A). Mutant probe did not form a DNA–protein complex with MEF2C protein. The DNA–protein complex was competed with cognate unlabeled oligonucleotide competitor in a dose-dependent manner, and could be supershifted by an anti-MEF2C antibody.
Fig. 5.
Analysis of the MEF2 site and E-box in the miR-1-2/133a-1 enhancer. (A) Binding of MEF2C to the MEF2-binding site in the minimal enhancer by gel mobility shift assay. A 32P-labeled oligonucleotide probe containing the MEF2-binding site and total-cell extract from COS-1 cells transfected with a MEF2C expression plasmid formed a DNA–protein complex in the assay. A 32P-labeled oligonucleotide probe containing a mutated MEF2-binding site did not form a DNA–protein complex with the MEF2C protein. The complex was supershifted by using a MEF2C-specific antibody, and unlabeled WT oligonucleotide containing the MEF2-binding site competed for binding. (B) Mutation of the MEF2-binding site in the 2.5-kb enhancer abolished expression in somites at E12.5. (C) Heart and transverse sections showed loss of ventricular chamber expression in the MEF2 mutant embryos. Expression in the atrial chambers was not affected. ra, right atrium; la, left atrium; rv, right ventricle; lv, left ventricle. (D) Binding of MyoD and E12 complex to the E-box binding site in the minimal enhancer element by gel mobility shift assay. A 32P-labeled oligonucleotide containing the E-box-binding site and total-cell extract from COS-1 cells transfected with myc-tagged MyoD and E12 expression plasmids formed a DNA–protein complex in the assay. Mutant E-box probe did not form a DNA–protein complex with MyoD and E12. The complex was supershifted by using a Myc-specific antibody and unlabeled WT oligonucleotide containing the E-box binding site competed for binding. The asterisk represents nonspecific binding. (E) Mutation of the E-box-binding site in the 2.5-kb enhancer abolished expression in somites and ventral myoblasts at E12.5. Expression in the heart was not affected by the E-box mutation.
A mutation of the MEF2 site in the context of the 2.5-kb enhancer (construct 7 in Fig. 4) abolished skeletal muscle expression in nine F0 transgenic embryos analyzed at E12.5 (Fig. 5B). LacZ expression was also dramatically reduced in ventricular myocardium but not in the atria of these embryos (Fig. 5C).
Skeletal Muscle Expression of the Intragenic Enhancer Requires an E-Box.
The 330-bp enhancer region also contained a conserved E-box (CANNTG) (SI Fig. 8), the consensus binding site for the MyoD family of bHLH transcription factors (2). Cell extracts expressing myc-tagged MyoD and its dimerization partner E12 bound to the E-box probe but not to a probe in which the E-box was mutated (Fig. 5D). Binding by MyoD/E12 was competed by the cognate unlabeled sequence in a dose-dependent manner, and the MyoD/E12-DNA complex was supershifted with anti-myc antibody.
A mutation of the E-box site abolished lacZ expression in the somites and body wall musculature at E12.5 but did not affect cardiac expression (Fig. 4, construct 8, and Fig. 5E). Thus, the E-box in the miR-1-2/133a-1 intragenic enhancer is necessary for expression in skeletal muscle but dispensable for cardiac expression.
Identification of a Muscle-Specific Enhancer Within the miR-1-1/133a-2 Locus.
In light of the responsiveness of miR-1-1 and miR-133a-2 to MEF2 (Fig. 1) and the presence of a muscle-specific enhancer within the miR-1-2/133a-1 locus, we analyzed the genomic DNA between the miR-1-1 and miR-133a-2 coding regions for a similar enhancer. We identified a MEF2-like site and an E-box as well as a CArG box, the binding site for SRF, in this region of the miR-1-2/133a-1 locus (Fig. 6A). Moreover, the 9.2 kb of intragenic DNA separating the miR-1-1 and miR-133a-2 coding regions directed robust expression specifically in the atrial and ventricular chambers of the heart and skeletal muscle (Fig. 6 A and B). Deletion mutations localized the enhancer activity to a 4.3-kb DNA fragment encompassing the MEF2-like site and E-box, as well as a CArG box. We conclude that the miR-1-2/133a-1 and miR-1-1/133a-2 genes contain similar architectures of regulatory elements that confer cardiac and skeletal muscle specificity.
Fig. 6.
Delineation of an intragenic miR-1-1/133a-2 muscle-specific enhancer. (A) Summary of transgenic constructs used to delineate the intragenic miR-1-1/133a-2 enhancer. Fractions of F0 transgenic embryos showing cardiac and skeletal muscle (SKM) expression at E12.5 are indicated in the right column. (B) Representative transgenic embryo showing lacZ expression from construct 1 and transverse section revealing lacZ expression in the outflow tract and four chambers of the heart as well as in somites. ra, right atrium; la, left atrium; rv, right ventricle; lv, left ventricle; m, somite myotomes.
Discussion
The results of this study show that MEF2 directly regulates the expression of muscle-specific miRNAs through a muscle-specific enhancer in an intron separating the miR-1-2 and miR-133a-1 coding regions. These findings, together with those of previous studies (7, 13, 14), demonstrate that MEF2 exerts its control over the programs for muscle development through direct and indirect mechanisms by coordinating the regulation of mRNAs and miRNAs, which act through a plethora of downstream targets to modulate cell phenotypes.
Control of the Intragenic miR-1-2/miR-133a-1 Enhancer by MEF2.
MEF2C expression is initiated in the cardiac crescent at E7.75, and Mef2c null mice die at approximately E9.5 because of cardiac looping defects (14, 15). Therefore, it is likely that MEF2C controls early cardiac expression of the miR-1-2/133a-1 intragenic enhancer. Although MEF2 factors are expressed in all four chambers of the developing heart, activity of the miR-1-2/133a-1 intragenic enhancer in the atria was unaffected by mutation of the MEF2 site in the enhancer, suggesting the existence of additional atrial activators of the enhancer.
Within the skeletal muscle lineage, the miR-1-2/133a-1 enhancer is strongly activated in the somite myotomes by E9.5. Skeletal muscle expression of the enhancer by MEF2 required an E-box-binding site for myogenic bHLH proteins, consistent with the known cooperativity between MEF2 and MyoD in activation of the skeletal muscle gene program (2). These findings are consistent with ChIP experiments, which showed binding of MyoD and myogenin in the region of the miR-1-2/133a-1 intragenic enhancer, although the functional significance of these sites was not addressed (12).
Multiple Muscle-Specific Enhancers for the miR-1-2/miR-133a-1 Locus.
Muscle genes are commonly controlled by multiple, independent cis-regulatory elements that cooperate to generate the complete developmental expression pattern of the gene (16, 17). Multiple enhancers expand the regulatory potential and allow for fine-tuning of temporal-spatial control of gene expression. Redundancy of enhancer activity may also provide a means of reinforcing the expression patterns of critical genes during development.
Zhao et al. (7) described an enhancer upstream of the miR-1-2/133a-1 locus that also required MyoD for activity in the skeletal muscle lineage. However, in contrast to the requisite role of MEF2 in activation of the intragenic enhancer within the heart, this upstream enhancer relies on SRF for cardiac expression. The upstream and intragenic miR-1-2/133a-1 enhancers also differ with respect to their expression patterns in the heart. Whereas the intragenic enhancer is highly active in the atrial and ventricular chambers, the upstream enhancer is active only in ventricular myocardium (7). Another enhancer located ≈50 kb upstream of the gene was reported to direct cardiac and skeletal muscle expression in transgenic Xenopus laevis (8), but its temporal-spatial expression pattern during mouse embryogenesis has not been reported.
Like the miR-1-2/133a-1 locus, the miR-1-1/miR-133a-2 locus is controlled by multiple muscle-specific regulatory elements. Our results show that a cardiac and skeletal muscle-specific enhancer, containing putative binding sites for MEF2 and myogenic bHLH proteins, lies between the miR-1-1 and miR-133a-2 coding regions. Although we have not analyzed the functions of these sites through mutagenesis, a MyoD and myogenin-binding site was identified in the genomic region of this enhancer by ChIP analyses (12). Similarly, a cardiac and skeletal muscle-specific enhancer upstream of the gene serves as a direct target of SRF in the heart and MEF2 in skeletal muscle (7), and another enhancer was identified ≈50 kb upstream of the miR-1-1 gene (8). Thus, the miR-1-1/miR-133a-2 and miR-1-2/miR-133a-1 genes employ a common architecture of enhancers and upstream regulators to control muscle-specific gene expression.
Implications.
miR-1 and miR-133 play opposing roles in the control of muscle cell proliferation and differentiation (8). miR-1 inhibits myoblast proliferation, which favors entry into the differentiation pathway, whereas miR-133 promotes myoblast growth, which suppresses differentiation. It seems paradoxical that two miRNAs expressed from the same locus under control of common cis-regulatory elements would counteract each other. Perhaps additional regulatory mechanisms, such as differing stabilities of miR-1 and miR-133 as well as different regulation of their target mRNAs and signaling inputs differentially modulate the actions of these miRNAs in the muscle differentiation pathway downstream of MEF2.
In addition to its central role in the control of muscle development, MEF2 modulates the growth, gene expression patterns, and functions of cardiac and skeletal muscle in response to activity and extracellular signaling (1, 18). MEF2 promotes cardiac hypertrophy in response to increased workload or excessive neurohumoral signaling, and miR-133 has been implicated in this process (18, 19). In skeletal muscle, MEF2 drives the slow myofiber gene program in response to motor innervation and exercise (20). In each of these settings, the actions of MEF2 are governed by a variety of signaling systems and the signal-dependent association of MEF2 with class II HDACs. Thus, manipulating the signaling pathways that control MEF2 activity provides possibilities for modulating the expression of miR-1 and miR-133 and the regulatory programs they govern in striated muscle.
Materials and Methods
RNA Analyses.
A 5′- and 3′-RACE was performed by using mouse embryo (10–12 days) FirstChoice RACE-ready cDNA kit (Ambion). RNA was isolated by using TRIzol (Invitrogen) and treated with Turbo RNase-free DNase (Ambion) before the reverse-transcription step. RT-PCR and real-time PCR analyses were performed as described (20). Methods and primer sequences are described in SI Materials and Methods.
Generation and Analysis of Transgenic Mice and Knockout Mice.
Transgenes were generated by cloning DNA fragments into the hsp68 basal promoter upstream of a LacZ reporter gene (21). Transgenic mice were generated, and lacZ staining was analyzed as described (21). See SI Materials and Methods for details.
Mef2c−/−, Mef2c, and Mef2d conditional knockout mice were described (14, 22, 23). Cardiac-specific deletion of Mef2c and Mef2d was achieved by using Nkx2.5-Cre transgenic mice expressing Cre recombinase under the Nkx2.5 basal promoter and cardiac enhancer (24).
Electrophoretic Mobility Shift Assays.
Details of electrophoretic mobility shift assays can be found in SI Materials and Methods.
Site-Directed Mutagenesis.
Mutagenesis of the MEF2 site and E-box was achieved by using the overlap extension method as described (25). Same mutations were introduced within each site as those used in electrophoretic mobility shift assays (SI Materials and Methods). Mutant fragments were cloned into the transgenic expression vector.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Dr. E. van Rooij for insightful comments on the manuscript, A. Tizenor for graphics, J. Brown for editorial assistance, and Drs. W. Klein and J. Martin for comments on the manuscript. N.L. was supported by a grant from the American Heart Association. Work in the laboratory of E.N.O. was supported by grants from the National Institutes of Health, the Donald W. Reynolds Cardiovascular Clinical Research Center, and the Robert A. Welch Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710558105/DC1.
References
- 1.Potthoff MJ, Olson EN. MEF2: A central regulator of diverse developmental programs. Development. 2007;134:4131–4140. doi: 10.1242/dev.008367. [DOI] [PubMed] [Google Scholar]
- 2.Molkentin JD, Olson EN. Combinatorial control of muscle development by basic helix–loop–helix and MADS-box transcription factors. Proc Natl Acad Sci USA. 1996;93:9366–9373. doi: 10.1073/pnas.93.18.9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Rooij E, Olson EN. MicroRNAs: Powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest. 2007;117:2369–2376. doi: 10.1172/JCI33099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Y, et al. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1–2. Cell. 2007;129:303–317. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436:214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 8.Chen JF, et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HK, et al. Muscle-specific microRNA miR-206 promotes muscle differentiation. J Cell Biol. 2006;174:677–687. doi: 10.1083/jcb.200603008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Rooij E, et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA. 2006;103:18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Rooij E, et al. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 12.Rao PK, et al. Myogenic factors that regulate expression of muscle-specific microRNAs. Proc Natl Acad Sci USA. 2006;103:8721–8726. doi: 10.1073/pnas.0602831103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Potthoff MJ, et al. Regulation of skeletal muscle sarcomere integrity and postnatal muscle function by Mef2c. Mol Cell Biol. 2007 doi: 10.1128/MCB.01187-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Q, Schwarz J, Bucana C, Olson EN. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science. 1997;276:1404–1407. doi: 10.1126/science.276.5317.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edmondson DG, Lyons GE, Martin JF, Olson EN. Mef2 gene expression marks the cardiac and skeletal muscle lineages during mouse embryogenesis. Development. 1994;120:1251–1263. doi: 10.1242/dev.120.5.1251. [DOI] [PubMed] [Google Scholar]
- 16.Firulli AB, Olson EN. Modular regulation of muscle gene transcription: A mechanism for muscle cell diversity. Trends Genet. 1997;13:364–369. doi: 10.1016/s0168-9525(97)01171-2. [DOI] [PubMed] [Google Scholar]
- 17.Kelly RG, Buckingham ME. Modular regulation of the MLC1F/3F gene and striated muscle diversity. Microsc Res Tech. 2000;50:510–521. doi: 10.1002/1097-0029(20000915)50:6<510::AID-JEMT8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 18.McKinsey TA, Zhang CL, Olson EN. Signaling chromatin to make muscle. Curr Opin Cell Biol. 2002;14:763–772. doi: 10.1016/s0955-0674(02)00389-7. [DOI] [PubMed] [Google Scholar]
- 19.Care A, et al. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 20.Potthoff MJ, et al. Histone deacetylase degradation and MEF2 activation promote the formation of slow-twitch myofibers. J Clin Invest. 2007;117:2459–2467. doi: 10.1172/JCI31960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kothary R, et al. Inducible expression of an hsp68-lacZ hybrid gene in transgenic mice. Development. 1989;105:707–714. doi: 10.1242/dev.105.4.707. [DOI] [PubMed] [Google Scholar]
- 22.Arnold MA, et al. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev Cell. 2007;12:377–389. doi: 10.1016/j.devcel.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y, et al. The MEF2D transcription factor mediates stress-dependent cardiac remodeling. J Clin Invest. 2007 doi: 10.1172/JCI33255. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McFadden DG, et al. The Hand1 and Hand2 transcription factors regulate expansion of the embryonic cardiac ventricles in a gene dosage-dependent manner. Development. 2005;132:189–201. doi: 10.1242/dev.01562. [DOI] [PubMed] [Google Scholar]
- 25.Wang DZ, et al. The Mef2c gene is a direct transcriptional target of myogenic bHLH and MEF2 proteins during skeletal muscle development. Development. 2001;128:4623–4633. doi: 10.1242/dev.128.22.4623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.