Abstract
Although the ecological effects of invasions often become obvious soon after introduced species become established, more gradual effects may take years to manifest and can thus require long-term data for quantification. We analyzed an 8-year record of stable isotope data on Argentine ants (Linepithema humile) from southern California to infer how the trophic position of this widespread invasive species changes over time as native ant species are displaced. We couple this longitudinal analysis with a biregional comparison of stable isotope data (δ15N) on ants from Argentina (native range) and California (introduced range) to quantify (i) how the trophic position of L. humile differs between native and introduced populations, and (ii) how relative trophic position as estimated by δ15N values of Argentine ants compare with those of other ants at the same site. Both long-term and biregional comparisons indicate that the Argentine ant's relative trophic position is reduced at sites with a longer history of occupation. Over the course of 8 years, the relative trophic position of L. humile remained high at the leading edge of an invasion front but declined, on average, behind the front as native ants disappeared. Relative to native populations, where L. humile is among the most carnivorous of ants, Argentine ants from California occupied lower trophic positions. These results support the hypothesis that Argentine ants shift their diet after establishment as a result of resource depletion and increasing reliance on plant-based resources, especially honeydew-producing Hemiptera. Our results demonstrate the value of long-term and biregional data in uncovering ecological effects of invasions.
Keywords: biological invasions, stable isotopes, food webs
Biological invasions threaten biodiversity and drain economic resources. Despite the importance of species introductions, the short-term and small-scale nature of most invasive species research is a recognized limitation of this field (1). Additionally, confounding environmental factors can obscure links between the spread of invasive species and decline of natives (2). Spatiotemporal fluctuations in the population sizes of introduced species (3) that result from changes in resource use or availability (4) can greatly alter the extent to which invaders disrupt ecosystems. Because a better understanding of such variation will inform both ecological theory and management strategies, there is an urgent need for long-term studies as well as for research that investigates ecological interactions in the native ranges of introduced species.
One underappreciated source of variation regarding introduced species concerns dietary flexibility and shifts in trophic position between native and introduced populations. This form of ecological plasticity may enhance invasion success in a number of ways. Species capable of extracting required nutrients from multiple trophic levels might establish in a broader range of environments compared with more specialized consumers. Furthermore, theory predicts diminishing biomass at higher trophic levels, suggesting that species feeding at lower trophic levels might attain greater abundance (5).
In this study we examine the direct effects of Argentine ant (Linepithema humile) invasions on native ant diversity and then quantify how dietary flexibility in this widespread invader affects spatiotemporal variation in its trophic position. Invasive ants provide an ideal system to test how trophic flexibility contributes to invasion success. With many species introductions, it can be difficult to separate the effects of the invader from covarying factors, such as habitat disturbance, that might also negatively affect natives (2, 6). Because Argentine ants aggressively displace above-ground foraging native ants (7), changes in the diet of L. humile that occur during and just after invasion can be linked to native ant displacement. Second, invasive ants, such as L. humile, are highly omnivorous and frequently form nonspecialized associations with honeydew-producing Hemiptera (7–10). Greater use of honeydew and other plant-based resources in introduced populations might result in a decrease in trophic position (relative to that of native populations), such that invasive ants would persist at higher densities than if they were acting as carnivores (7, 11, 12). Similar arguments have been proposed to explain the great abundance of ants in tropical rainforest canopies (13). Although the exploitation of honeydew and other plant-based resources might subsidize invasive ants at high densities, few data are available to evaluate this hypothesis.
An important obstacle in this area of research concerns the quantification of ant diets. Incomplete dietary information greatly hinders an understanding of the community-wide effects of ant invasions. Invasive ants forage extensively on liquids (11, 14, 15), so observational data on diets are of limited use, because the exact composition of consumed or stored liquids remains unknown (e.g., the relative proportions of nectar, honeydew, and hemolymph). For these reasons, we use stable isotope analysis, an approach of demonstrated value in quantifying trophic relationships in ants (13, 16, 17).
We combine longitudinal and biregional comparisons (i) to track changes in nitrogen isotopic ratios of Argentine ants over time (i.e., as they actively displace native ants), and (ii) to compare the trophic position of L. humile between its native and introduced ranges. To measure changes in Argentine ant trophic position and native ant diversity during the course of an invasion, we sampled an active invasion front over an 8-year period. We predict that the trophic level of resources assimilated by L. humile will drop if the process of invasion either (i) depletes resources from high trophic levels (e.g., the consumption of native ants and other arthropod predators), or (ii) leads to increased exploitation of plant-based resources. Alternatively, a downward shift in trophic position would not be expected if resources exploited by Argentine ants remained stable through time, or if plant-based resources, such as honeydew, were lacking. We also perform a biregional comparison of L. humile trophic position between multiple native and introduced populations to test whether L. humile shifts its trophic position between Argentina and California. These comparisons also yield a wealth of information about the ant communities and the arthropod food webs in both ranges.
Results
Temporal Shifts in Trophic Position.
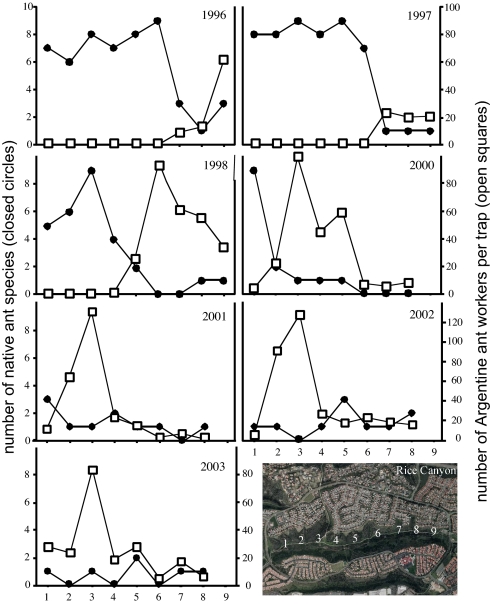
Annual sampling of the Rice Canyon invasion front revealed the steady westward expansion of Argentine ants from the eastern end of the canyon starting in 1996 (Fig. 1). Each year after 1996, L. humile occurred at sampling stations progressively farther west until 2001, when it reached the western end of the canyon. The number of L. humile workers captured in pitfall traps each year reached its maximum at sampling stations just behind the invasion front (Fig. 1). Argentine ants displaced native ants as soon as they entered Rice Canyon (Fig. 1). Over the 8-year period of sampling, for example, 7.5 ± 0.4 native ant species were captured at sampling stations without Argentine ants (i.e., before invasion), whereas only 1.1 ± 0.1 species were captured at stations with Argentine ants (i.e., after invasion). In Rice Canyon as a whole, the number of native ant species dropped from 23 before 1996 to just 2 by 2003 (Table 1).
Fig. 1.
Displacement of native ants by Argentine ants over an 8-year period in Rice Canyon, San Diego County, California. Sampling stations (1–9) are shown on the x axis. Open squares, number of Argentine ant workers captured per pitfall trap at each sampling station; closed circles, number of native ant species captured in pitfall traps at each sampling station. The photograph (Bottom Right) shows an aerial view of Rice Canyon (coastal sage scrub surrounded by residential development) and the approximate location of the center of each sampling station. Image provided by Google Earth.
Table 1.
Native ant species detected in Rice Canyon, San Diego County, California
| Genus and species | Before invasion (1996) | After invasion (2003) |
|---|---|---|
| Dorymyrmex bicolor | X | |
| Dorymyrmex insanus | X | |
| Tapinoma sessile | X | |
| Forelius mccooki | X | |
| Brachymyrmex depilis | X | |
| Paratrechina cf. terricola | X | |
| Camponotus fragilis | X | |
| Camponotus yogi | X | |
| Prenolepis imparis | X | |
| Stenamma diecki | X | |
| Pheidole hyatti | X | |
| Pheidole vistana | X | |
| Pheidole clementensis | X | |
| Crematogaster mormonum | X | |
| Crematogaster californica | X | |
| Solenopsis xyloni | X | |
| Solenopsis molesta | X | X |
| Temnothorax andrei | X | X |
| Messor andrei | X | |
| Cyphomyrmex wheeleri | X | |
| Neivamyrmex californicus | X | |
| Neivamyrmex nigrescens | X | |
| Pseudomyrmex apache | X |
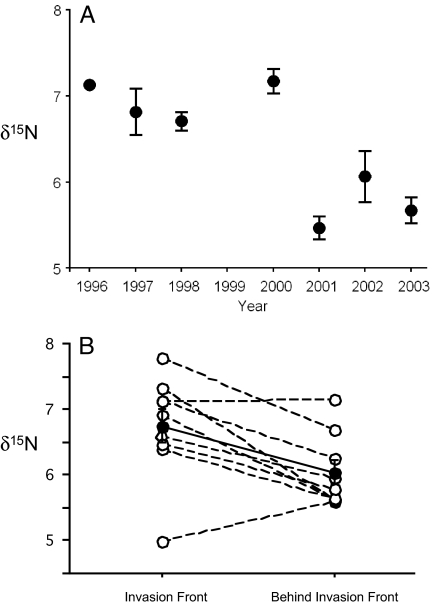
As L. humile spread through Rice Canyon and displaced resident ants (Fig. 1), its average δ15N value decreased over the 8-year period of investigation (Fig. 2A). For seven of the nine fixed sampling stations, the δ15N value for the first year that L. humile was recorded at a station (i.e., at the leading edge of the invasion front) exceeded that of the mean δ15N value for that station in all subsequent years (i.e., after the displacement of native ants) (paired t test: t8 = 3.07, P = 0.0154) (Fig. 2B).
Fig. 2.
Trophic ecology of Argentine ants at an active invasion front. (A) Mean (±SE) δ15N values for Argentine ants invading Rice Canyon from 1996 to 2003. For each year, δ15N values are averaged over sampling stations at which Argentine ants were present. (B) For each of the nine sampling stations in Rice Canyon, the δ15N value for the first year that L. humile was recorded at a sampling station (i.e., at the leading edge of the invasion front) vs. the mean δ15N value for that station in all subsequent years (i.e., after the displacement of native ants). The solid line and closed circles represent the mean (±SE) values across all sample stations.
Biregional Comparisons of Trophic Ecology.
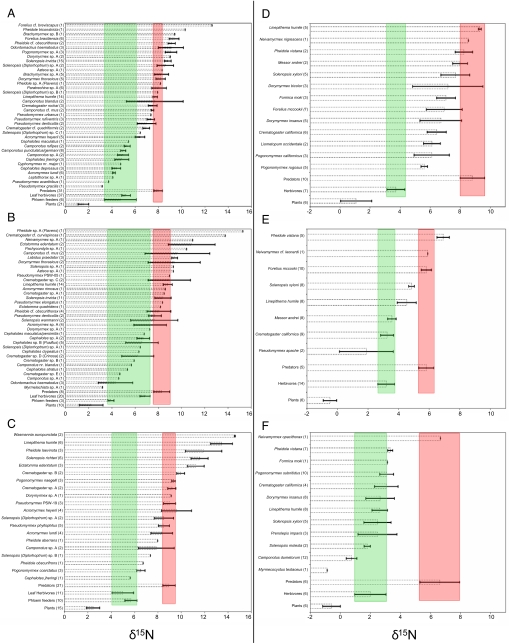
At our six study sites in Argentina and California, δ15N values for ants as a whole encompassed a broad range of values (Fig. 3), suggesting that ants at each site occupy several trophic levels. Minimum to maximum spans of δ15N are as follows: 7.8‰ at Herradura, 6.4‰ at Ocampo, 5.9‰ at Otamendi, 3.7‰ at Sweetwater, 5‰ at Elliot, and 7.3‰ at Torrey Pines. These ecosystems (especially those in Argentina) include several ant species with δ15N values overlapping those of herbivores and many ant species with δ15N values similar to or higher than those of predacious, non-ant arthropods (Fig. 3).
Fig. 3.
Mean (±SE) δ15N values of ants, plants, and herbivorous and predacious arthropods at three native range sites in Argentina [(A) Ocampo, (B) Herradura, and (C) Otamendi)] and three introduced range sites in California [(D) Sweetwater, (E) Elliot, and (F) Torrey Pines]. Numbers in parentheses indicate the number of replicates (colonies) for each species. Names in parentheses indicate species groups. Shading indicates δ15N values of herbivorous (green, left vertical bars) and predacious (red, right vertical bars) arthropods at each site.
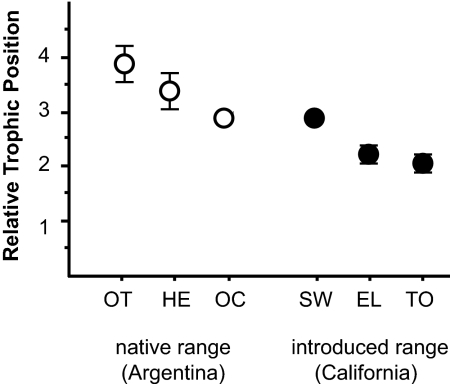
At all native range sites, L. humile had δ15N values equal to or higher than those of syntopic predatory arthropods, whereas at all introduced range sites, L. humile had δ15N values equal to or lower than those of syntopic predatory arthropods (Fig. 3). Moreover, Argentine ants from California had a lower trophic position compared with those from Argentina (nested ANOVA: F1,46 = 14.01, P < 0.001) (Fig. 4). Significant differences in δ15N also exist among sites (F4,46 = 3.38, P < 0.05). Tukey comparisons indicate that the trophic position of Argentine ants at Torrey Pines is lower than that of L. humile from both Herradura and Otamendi, and that the trophic position of Argentine ants from Elliot is lower than that of L. humile from Herradura. In addition to ant diversities that were two to three times higher than those of California sites, Argentina sites included a preponderance of ant species with δ15N values similar to or higher than those of predacious, non-ant arthropods. At Herradura, the genus Ectatomma and the army ants Neivamyrmex and Labidus had the highest δ15N values; these patterns are consistent with the known predatory behavior of these genera. Many ant species at Ocampo also had δ15N values overlapping those of predacious non-ant arthropods, with a few genera (Forelius, Pheidole, Odontomachus, and Pogonomyrmex) having δ15N values 1.5–3 ‰ higher than the mean δ15N for non-ant predators. Although Odontomachus is highly predatory, Forelius and Pheidole probably obtain protein through both predation and scavenging. Pogonomyrmex, although typically a seed-harvesting genus, also collects insect prey (18). The species at Ocampo had a δ15N of 9.1 ‰, which is 3.3 ‰ higher than the δ15N of a homogenized sample of their seeds (5.8 ‰). This suggests that a high value of δ15N may not always result from a predatory diet and reinforces the importance of direct observations and natural history information in trophic ecology research.
Fig. 4.
Mean (±SE) relative trophic position of Argentine ants from three native range sites in Argentina [Ocampo (OC), Herradura (HE), and Otamendi (OT)] and three introduced range sites in California [Torrey Pines (TP), Elliot (EL), and Sweetwater (SW)]. The number of Argentine ant nests sampled at each location is reported in Fig. 3.
Within native range sites, the relative ranking of δ15N values of Argentine ants vs. those of other ants appears influenced by the number of predatory ant species present. At Herradura and Ocampo, for example, the mean δ15N for L. humile ranks near the middle of the δ15N values of other ants, with genera such as Neivamyrmex, Ectatomma, and Labidus ranking higher than L. humile (Fig. 3 a and b). At Otamendi, a more southerly site and one where L. humile had a relatively high mean δ15N value (Fig. 3c), ants in several key predacious genera (e.g., Labidus, Odontomachus) do not occur (with the exception of Neivamyrmex, which we did not find) (19).
At the other end of the spectrum, native range sites (especially Herradura and Ocampo) also include numerous ant species with δ15N values similar to those of syntopic herbivorous insects. Examples of such ants include twig and tree nesting Cephalotes, Crematogaster, Camponotus, Myrmelachista, and Pseudomyrmex. Although some of these genera also occur at our California sites, Herradura and Ocampo included numerous taxa strongly to wholly associated with perennial vegetation. Such behavior is nearly absent among ants at our California sites (the only exceptions being Liometopum occidentale and Pseudomyrmex apache).
Discussion
An understanding of the community-wide effects of invasions requires detailed information about trophic ecology. We use longitudinal and biregional comparisons (i) to track changes in the trophic position of Argentine ant invasions over time and (ii) to compare ant communities in multiple food webs in Argentina and California. At an active invasion front in southern California, we found that the relative trophic position of L. humile declines over time simultaneously with the displacement of native ants. Moreover, introduced populations of L. humile have a lower trophic position, on average, compared with native populations. These results support the hypotheses that dietary flexibility and reduction in trophic level can contribute to invasion success.
By measuring annual changes in the number of native ant species during the invasion of Rice Canyon, we obtained a chronological record of native ant displacement. Sampling stations ahead of the invasion front always captured 6–10 native ant species, but as Argentine ants moved progressively through the canyon, native ants declined sharply right at the invasion front and remained low thereafter (Fig. 1). As Argentine ants advanced, the only native ants that remained were Temnothorax andrei and Solenopsis molesta (Table 1), two small hypogeic species that appear resilient to L. humile (2, 20, 21).
Argentine ants peaked in local abundance at sampling stations just behind the leading edge of the invasion front (Fig. 1). This pattern, an initial wave of high density followed by lower numbers of individuals through time, is a common feature of invasions (3) and may result from a reduction in resources exploited by the invader. This may also be a consequence of the unicolonial colony structure typical of introduced populations of this species (22); Argentine ants can redistribute workers and nests closer to areas where resources (23) or competitive interactions are highest. Even though the density of L. humile declined over time, native ant diversity failed to rebound during the course of the study. As predicted, the trophic position of L. humile at the leading edge of the invasion front exceeded that behind the front (Fig. 2). During the course of the invasion, ants at the front of the invasion presumably displaced native ants through a combination of competition and predation (18, 24, 25). Observations of L. humile raiding the nests of native ant species such as Pogonomyrmex subnitidus, Messor andrei, and Solenopsis xyloni support the contention that native ants may be eliminated by consumption (18). Behind the front, however, with almost no native ants to compete with or to consume, Argentine ants appeared to acquire N from lower trophic levels. Interannual differences in mean δ15N values (Fig. 2B) may have also reflect prevailing environmental conditions. The sampling period included winters that were abnormally wet (1997–1998) and dry (2001–2002), which may have influenced the availability of resources such as arthropod prey.
The biregional comparison of the Argentine ant's trophic position further supports the proposition that dietary flexibility and exploitation of plant-based resources could contribute to invasion success. As a whole, the omnivorous ant community in Argentina exhibits a more enriched nitrogen signature compared with that of the Californian ant community. This disparity may reflect the greater availability of arthropod carrion in the more productive Argentine subtropics (26). The Argentine ant's ability to achieve high densities in the relatively less-productive scrub environments of California is a testament to its dietary flexibility and perhaps a consequence of the loss of native ants with which L. humile competes. Relative to introduced populations, native populations of L. humile occupy a higher trophic position (Fig. 4) and appear to obtain much of their N from animal-based resources. Although several ant genera exceed L. humile in their δ15N values, the mean δ15N values for Argentine ants overlapped the range of δ15N values for predacious non-ant arthropods (Fig. 3). This pattern suggests that the primary input of N into Argentine ant colonies is protein from predation and scavenging, rather than from plant-based N from nectar or hemipteran honeydew.
Compared with the findings of Davidson et al. (13) for tropical rainforest canopy ants, the ants of floodplain woodlands in Argentina span a similar range of δ15N values and include many presumed omnivores (Fig. 3). However, the ground-foraging ant communities from Argentina are skewed toward animal-based N (Fig. 3), whereas many canopy ants appear to obtain N predominantly from plant-based sources (13). This disparity points to key differences between the competitive environments of epigeic ants in subtropical woodlands and arboreal ants in tropical rainforests (see also ref. 27). The extensive overlap in δ15N values between L. humile and other ants suggests that competition for animal-based protein is fierce, a contention supported by LeBrun et al. (28).
This study found support for two mechanisms that favor invasion success: (i) flexible patterns of resource use and (ii) a shift to a lower trophic position in new environments. The proximate causes of these dietary changes are unclear. In California, honeydew-producing insects may be more abundant (e.g., in citrus agroecosystems) or more accessible as a result of depauperate arboreal ant faunas (Fig. 3). Alternatively, a drop in trophic position might result from the depletion of high-trophic level resources from invaded areas. Although arthropod surveys from California show that many predacious arthropods (especially spiders and carabid beetles) remain abundant in areas invaded by Argentine ants (29–31), dramatic declines in native ant abundance might represent the depletion of an important high-trophic-level resource. Future work that focuses on teasing apart these alternative, but not mutually exclusive, hypotheses for the pattern reported here may provide insight into the mechanisms behind shifts in trophic position in this species.
Materials and Methods
Stable Isotope Analysis.
Stable isotope analysis measures the relative abundance of naturally occurring, stable (i.e., nonradioactive) forms of biologically relevant elements, such as nitrogen (N). This method allows the tracking of nutrients and energy through food webs and can thus clarify trophic relationships that are otherwise difficult to quantify (32–35). A general result of isotope studies is that consumers are usually enriched relative to their prey (i.e., predators have a higher 15N/14N ratio). Studies on insects generally report a δ15N enrichment of 2–3‰ per trophic level (16, 36–40), although this value can vary depending on the organism, its age, or the quality of the food resource (40–42).
Temporal Shifts in Trophic Position.
To determine whether the δ15N values of L. humile change as it displaces native ants, we measured isotopic ratios of Argentine ants over an 8-year period at an active invasion front in Rice Canyon (32°38′35′′ N, 117°01′15′′ W), a 78-ha coastal sage scrub site in Chula Vista, San Diego County, California (2). We used pitfall traps to sample ants along a transect oriented perpendicular to the front. The transect contained nine fixed sampling stations (each with five traps) placed every 120 m. Sampling took place in August/September each year between 1996 and 2003 (except for 1999, when no sampling occurred). Traps consisted of glass jars (6 cm in diameter) half full of ethylene glycol; traps were left open for 5 days during each sampling period. We stored all samples in 95% ethanol from collection until processing. Tillberg et al. (43) demonstrated that ethanol storage does not affect the δ15N signature of ants. For each sampling station and year, we determined the δ15N of a sample that consisted of heads, thoraces, and legs of 10–15 L. humile workers, such that sample dry masses weighed ≈1,500 μg. We weighed all samples into tin capsules and used a Mettler & Toledo microbalance to estimate mass. Analysis of nitrogen isotopic ratios was performed at the University of California, Davis, Stable Isotope Facility using a Europa Hydra 20/20 continuous-flow isotope ratio mass spectrometer.
To test whether the δ15N values of Argentine ants changed as the invasion proceeded, we compared the δ15N value for the first year that L. humile was recorded at a sampling station (i.e., at the leading edge of the invasion front) with the mean δ15N value for that station in all subsequent years (i.e., after the displacement of native ants). For the nine fixed sampling stations, we used a paired t test to compare δ15N values of Argentine ants at the front vs. those behind. This data set is unsuitable for repeated-measures ANOVA as a result of a missing year of data (1999) and the unfortunate loss of several samples during processing.
Biregional Comparisons of Trophic Ecology.
We next analyzed stable isotope data on ants from six study sites in Argentina (native range) and California (introduced range) to quantify (i) how the trophic position of L. humile differs between native and introduced populations, and (ii) how the δ15N values of Argentine ants compare with those of other syntopic ants. Sites in Argentina include three woodland/pasture locations along the Parana and Paraguay Rivers: (i) Reserva Natural Otamendi, Buenos Aires Province (34°13′30″ S, 58°54′00″ W), (ii) 6 km E Villa Ocampo, Santa Fe Province (28°30′30″ S, 59°16′20″ W), and (iii) 5 km E Herradura, Formosa Province (26°31′05″ S, 58°16′50″ W). Previous research at these sites (referred to as Otamendi, Ocampo, and Herradura) demonstrates that Argentine ants coexist with a diverse assemblage of native ants (28, 44). Sites in California include three scrub sites in San Diego County, California: (i) Otay-Sweetwater Unit of the San Diego National Wildlife Refuge (32°43′45″ N, 116°56′30″ W), (ii) University of California Elliot Chaparral Reserve (32°53′30″ N, 117°06′10″ W), and (iii) Torrey Pines State Reserve Extension (32°56′25″ N, 117°14′55″ W). Each of these sites (referred to as Sweetwater, Elliot, and Torrey Pines) varies in the extent of invasion by Argentine ants (2, 21, 45, 46). Sweetwater and Elliot contain large tracts of uninvaded scrub; L. humile is restricted either to riparian corridors (Sweetwater) or a Eucalyptus grove (Elliot). At Torrey Pines, a coastal site, Argentine ants occur nearly throughout the 100-ha reserve, and native ants are restricted to pockets of dry habitat unsuitable for Argentine ants (21).
At each site, we collected ants and other arthropods live into empty vials [with the exception of army ants (Neivamyrmex), which were collected into 95% ethanol (see ref. 43)]. For non-ant arthropods, we collected multiple herbivore and predator taxa. Herbivores included leaf feeders, such as larval Lepidoptera and adult Chrysomelidae, as well as phloem feeding Hemiptera. Predators were represented by spiders (Araneae: Lycosidae, Salticidae), scorpions, assassin bugs (Reduviidae), mantids, and beetles (Coccinellidae, Carabidae). We froze live arthropods and then dried them in a low-temperature oven at 45°C to 60°C. Finally, we collected leaves from common grasses, forbs, shrubs, and trees at each site to estimate the basal N-values of the primary producers in the above ground arthropod food web. Plant specimens were placed in sealed paper envelopes and then dried. After drying, all arthropod and plant samples were held in a sealed container with desiccant until processing.
Individual ant samples consisted of heads, thoraces, and legs of 10–15 workers from the same colony, such that sample masses were ≈1,500 μg. When possible, we sampled ants from at least five colonies per site per species to incorporate intercolony variation in trophic level (43). Individual samples of non-ant arthropods were homogenized and weighed to ≈1,500 μg per sample. Each plant sample was immersed in liquid N and ground into a fine powder using a mortar and pestle and weighed to ≈3,000 μg. To obtain isotopic ratios, all samples were weighed and processed as described above under Temporal Shifts in Trophic Position.
To estimate the relative trophic position of L. humile, we used a single-isotope two food-source general mixing model (47); this statistic incorporates isotopic data from herbivorous and carnivorous arthropods from each site. First, we established a site-specific level of nitrogen enrichment, or trophic step (ΔN), rather than using a mean trophic step from the literature. We then calculated the relative proportion of dietary inputs to the ants from trophic levels X−1 (ρ1) and X (ρ2), where ρ1 + ρ2 = 1.
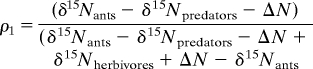 |
Using these proportions, we estimate trophic position (TP) as follows:
where TPherbivores = 2 and TPpredators = 3.
To analyze differences in trophic position between native and introduced ranges and among sites within each range, we performed a nested ANOVA with site nested within range. After ANOVA, we used Tukey comparisons to test for differences among sites.
ACKNOWLEDGMENTS.
A. Wild, S. Cover, P. Ward, and J. Longino provided invaluable advice on the identification of ant specimens. I. C. Quilmes provided inspiration in Argentina. This research was supported by National Science Foundation Grants NSF-INT 0305773 (to D.A.H. and A.V.S.) and NSF-DEB 0516452 (to A.V.S. and C.V.T.) and by U.S. Department of Agriculture Award USDA-NRI 2006-35302-17255 (to D.A.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Strayer DL, Eviner V. T., Jeschke JM, Pace ML. Trends Ecol Evol. 2006;21:645–651. doi: 10.1016/j.tree.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Suarez AV, Bolger DT, Case TJ. Ecology. 1998;79:2041–2056. [Google Scholar]
- 3.Simberloff D, Gibbons L. Biol Invas. 2004;6:161–172. [Google Scholar]
- 4.Dwyer G, Morris WF. Am Nat. 2006;167:165–176. doi: 10.1086/498944. [DOI] [PubMed] [Google Scholar]
- 5.Elton C. Animal Ecology. London: Sidgwick & Jackson; 1927. [Google Scholar]
- 6.King JR, Tschinkel WR. J Anim Ecol. 2006;75:1370–1378. doi: 10.1111/j.1365-2656.2006.01161.x. [DOI] [PubMed] [Google Scholar]
- 7.Holway DA, Lach L, Suarez AV, Tsutsui ND, Case TJ. Annu Rev Ecol Syst. 2002;33:181–233. [Google Scholar]
- 8.Helms KR, Vinson SB. Ecology. 2002;83:2425–2438. [Google Scholar]
- 9.Lach L. Ann Mo Bot Gard. 2003;90:91–108. [Google Scholar]
- 10.Ness JH, Bronstein IL. Biol Invas. 2004;6:445–461. [Google Scholar]
- 11.Tennant LE, Porter SD. J Ent. 1991;26:450–465. [Google Scholar]
- 12.Davidson DW. Ecol Ent. 1998;23:484–490. [Google Scholar]
- 13.Davidson DW, Cook SC, Snelling RR, Chua TH. Science. 2003;300:969–972. doi: 10.1126/science.1082074. [DOI] [PubMed] [Google Scholar]
- 14.Markin GP. J Econ Ent. 1970;63:740–744. [Google Scholar]
- 15.Human KG, Weiss S, Weiss A, Sandler B, Gordon DM. Environ Ent. 1998;27:822–833. [Google Scholar]
- 16.Mooney KA, Tillberg CV. Ecology. 2005;86:1225–1235. [Google Scholar]
- 17.Bluthgen N, Gebauer G, Fiedler K. Oecologia. 2003;137:426–435. doi: 10.1007/s00442-003-1347-8. [DOI] [PubMed] [Google Scholar]
- 18.Zee J, Holway DA. Insectes Soc. 2006;53:161–167. [Google Scholar]
- 19.Fuentes MB, Cuezzo FC, DiIorio OR. G Italiano Ent. 1998;9:97–98. [Google Scholar]
- 20.Holways DA. Biol Conserv. 2005;121:561–567. [Google Scholar]
- 21.Glenn S, Holway DA. Biol Invas. 2007 in press. [Google Scholar]
- 22.Tsutsui ND, Suarez AV, Holway DA, Case TJ. Proc Natl Acad Sci USA. 2000;97:5948–5953. doi: 10.1073/pnas.100110397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holway DA, Case TJ. Anim Behav. 2000;59:433–441. doi: 10.1006/anbe.1999.1329. [DOI] [PubMed] [Google Scholar]
- 24.Human KG, Gordon DM. Oecologia. 1996;105:405–412. doi: 10.1007/BF00328744. [DOI] [PubMed] [Google Scholar]
- 25.Holway DA. Ecology. 1999;80:238–251. [Google Scholar]
- 26.Loveland TR, Reed BC, Brown JF, Ohlen DO, Zhu Z, Yang L, Merchant J. Int J Remote Sens. 1998;21:1303–1330. [Google Scholar]
- 27.Yanoviak SP, Kaspari M. Oikos. 2000;89:259–266. [Google Scholar]
- 28.LeBrun EG, Tillberg CV, Suarez AV, Folgarait PJ, Smith CR, Holway DA. Ecology. 2007;88:63–75. doi: 10.1890/0012-9658(2007)88[63:aesocb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Human KG, Gordon DM. Conserv Biol. 1997;11:1242–1248. [Google Scholar]
- 30.Holway DA. Oecologia. 1998;116:252–258. doi: 10.1007/s004420050586. [DOI] [PubMed] [Google Scholar]
- 31.Bolger DT, Suarez AV, Crooks KR, Morrison SA, Case TJ. Ecol Appl. 2000;10:1230–1248. [Google Scholar]
- 32.Stapp P, Polis GA, Pinero FS. Nature. 1999;401:467–469. [Google Scholar]
- 33.Eggers T, Jones TH. Trends Ecol Evol. 2000;15:265–266. doi: 10.1016/s0169-5347(00)01877-2. [DOI] [PubMed] [Google Scholar]
- 34.Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP. Annu Rev Ecol Syst. 2002;33:507–559. [Google Scholar]
- 35.Edwards MS, Turner TF. Ecol Rest. 2003;21:49. [Google Scholar]
- 36.DeNiro MJ, Epstein S. Geochim Cosmochim Acta. 1981;45:341–351. [Google Scholar]
- 37.Minagawa M, Wada E. Geochim Cosmochim Acta. 1984;48:1135–1140. [Google Scholar]
- 38.Owens NJP. Adv Mar Biol. 1987;24:389–451. [Google Scholar]
- 39.Cabana G, Rasmussen JB. Nature. 1994;372:255–257. [Google Scholar]
- 40.McCutchan JH, Lewis WM, Kendall C, McGrath CC. Oikos. 2003;102:378–390. [Google Scholar]
- 41.Oelbermann K, Scheu S. Oecologia. 2002;130:337–344. doi: 10.1007/s004420100813. [DOI] [PubMed] [Google Scholar]
- 42.Vanderklift MA, Ponsard S. Oecologia. 2003;136:169–182. doi: 10.1007/s00442-003-1270-z. [DOI] [PubMed] [Google Scholar]
- 43.Tillberg CV, McCarthy DP, Dolezal AG, Suarez AV. Insectes Soc. 2006;53:65–69. [Google Scholar]
- 44.Suarez AV, Tsutsui ND, Holway DA, Case TJ. Biol Invas. 1999;1:43–53. [Google Scholar]
- 45.Holway DA. Biol Conserv. 2005;121:561–567. [Google Scholar]
- 46.Menke SB, Holway DA. J Anim Ecol. 2006;75:368–376. doi: 10.1111/j.1365-2656.2006.01056.x. [DOI] [PubMed] [Google Scholar]
- 47.Post DM. Ecology. 2002;83:703–718. [Google Scholar]