Abstract
Loss of imprinting (LOI) of the insulin-like growth factor-II gene (IGF2), leading to abnormal activation of the normally silent maternal allele, is a common human epigenetic population variant associated with a 5-fold increased frequency of colorectal neoplasia. Here, we show first that LOI leads specifically to increased expression of proliferation-related genes in mouse intestinal crypts. Surprisingly, LOI(+) mice also have enhanced sensitivity to IGF-II signaling, not simply increased IGF-II levels, because in vivo blockade with NVP-AEW541, a specific inhibitor of the IGF-II signaling receptor, showed reduction of proliferation-related gene expression to levels half that seen in LOI(−) mice. Signal transduction assays in microfluidic chips confirmed this enhanced sensitivity with marked augmentation of Akt/PKB signaling in LOI(+) cells at low doses of IGF-II, which was reduced in the presence of the inhibitor to levels below those found in LOI(−) cells, and was associated with increased expression of the IGF1 and insulin receptor genes. We exploited this increased IGF-II sensitivity to develop an in vivo chemopreventive strategy using the azoxymethane (AOM) mutagenesis model. LOI(+) mice treated with AOM showed a 60% increase in premalignant aberrant crypt foci (ACF) formation over LOI(−) mice. In vivo IGF-II blockade with NVP-AEW541 abrogated this effect, reducing ACF to a level 30% lower even than found in exposed LOI(−) mice. Thus, LOI increases cancer risk in a counterintuitive way, by increasing the sensitivity of the IGF-II signaling pathway itself, providing a previously undescribed epigenetic chemoprevention strategy in which cells with LOI are “IGF-II addicted” and undergo reduced tumorigenesis in the colon upon IGF-II pathway blockade.
Keywords: Akt, cancer, chemoprevention, epigenetics, signal transduction
IGF-II is an important autocrine and paracrine growth factor in development and cancer, signaling primarily through the insulin-like growth factor-I receptor (IGF1R), a transmembrane receptor tyrosine kinase (1). Activation of IGF1R leads to autophosphorylation of the receptor and activation of signaling cascades including the IRS-1/PI3K/AKT and GRB2/Ras/ERK pathways (2). Previous studies have revealed importance of Akt for cell cycle regulation, with sustained activity implicated in growth factor-mediated transition through G1 (3, 4) and resistance to apoptosis (5). IGF-II is overexpressed in a wide variety of malignancies, including colorectal cancer (CRC) (reviewed in ref. 6). Genomic imprinting is an epigenetic modification in the gamete or zygote that leads to relative silencing of a specific parental allele in somatic cells of the offspring. Loss of imprinting (LOI) of the insulin-like growth factor II gene (IGF2) is defined as aberrant expression of the normally silent maternally inherited allele, which we and others have found is associated with a 5-fold increased frequency of intestinal neoplasia in humans and a 5-fold increased frequency of first degree relatives with CRC, suggesting that LOI of IGF2 contributes substantially to the population risk of CRC (7, 8). The precise effect of LOI of IGF2 on cell signaling is unknown although it is thought to be due to an excess of IGF-II ligand (9, 10).
Previously, we developed a concurrent epigenetic-genetic model of intestinal neoplasia (11), crossing female mice with a deletion of the differentially methylated region (DMR) of H19 as well as H19 itself (12), with male mice harboring a mutation in the adenomatous polyposis coli (Apc) gene (Min mice) (13). Maternal transmission of the DMR deletion leads to aberrant activation of the maternal Igf2 allele and LOI, a two-fold increased expression of Igf2 in the intestine, and a 1.8- to 2.5-fold increase in the frequency of intestinal adenomas in LOI(+) Min double heterozygotes, as well as an increase in the progenitor cell (crypt) compartment (11). This increase in crypt compartment could be due to increased proliferation, decreased apoptosis, altered differentiation, or cell kinetics.
To gain insight into the mechanism for the increase in the progenitor cell compartment in these mice, we first performed laser capture microdissection of intestinal crypts, comparing gene expression profiles of LOI(+) and LOI(−) littermates, identifying a specific and significant increase in proliferation-related genes. We then treated mice in vivo with NVP-AEW541, an ATP-competitive pyrrolo[2,3-d]pyrimidine derivative that specifically inhibits IGF1R over the related insulin receptor (14). In this way, we could test for the effects of IGF-II signaling specifically, regardless of any other effects that deletion of the Igf2 regulatory sequences or H19 might have. These experiments demonstrated a surprising enhanced sensitivity of gene expression to IGF-II signaling blockade in LOI(+) mice, compared with LOI(−) mice. We thus performed direct analysis of the magnitude and dynamics of signaling activity in LOI(+) and LOI(−) cells on a single cell level in vitro, demonstrating enhanced sensitivity with marked augmentation of Akt/PKB signaling in LOI(+) cells at low doses of IGF-II, which was reduced in the presence of the inhibitor to the levels below those found in LOI(−) cells. Finally, we exploited this discovery to develop a previously undescribed chemopreventive strategy in mice using the chemical carcinogen azoxymethane (AOM), which, unlike the Min/LOI model, targets the colon and the exposure can be timed postnatally (15). This model is also more like the human situation, because in the LOI/Min model both the Apc mutation and double dose of Igf2 events are congenital, whereas LOI in humans seems to increase risk by changing the progenitor cell population in apparently normal tissue before adenoma formation. We found that LOI increases the formation of premalignant aberrant crypt foci (ACF) after AOM treatment, and that mice with LOI show enhanced sensitivity to IGF-II blockade with NVP-AEW541 in preventing ACF formation. Thus, using diverse experimental approaches, these data provide strong support for the idea that LOI enhances pathway sensitivity to IGF2-II signaling both in vivo and in vitro, and they provide a window into a new therapeutic strategy in which the epigenetic alteration (LOI) is exploited rather than reversed, i.e., one takes advantage of alterations in the signaling pathway itself for chemoprevention.
Results
LOI-Related Increase in Proliferation-Related Gene Expression Is Differentially Sensitive to IGF1R Inhibition.
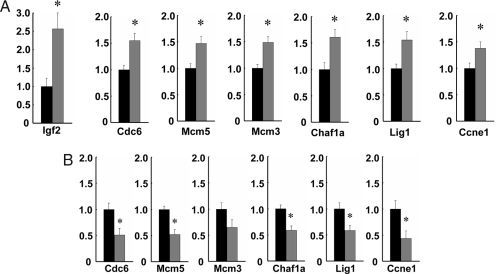
We had shown earlier that LOI leads to an increase in the progenitor cell compartment in crypt cells of Min mice (11), but the mechanism was unknown. We therefore sought to determine what changes in gene expression occur in gastrointestinal epithelial progenitor cells in LOI mice. We measured gene expression in laser capture microdissected crypts, comparing 8,000 crypts from each of three LOI(+) and three LOI(−) mice on microarrays. Two hundred eighty-three genes showed increased expression, and 109 genes showed decreased expression (supporting information (SI) Table 1). GO annotation showed a striking overrepresentation of genes showing increased expression involved with DNA replication (P < 10−15), DNA metabolism (P < 10−15), cell cycle (P < 10−9), and cell proliferation (P < 10−9) (SI Table 2), consistent with our earlier observations of increased progenitor cells in LOI(+) mice (11). These results were confirmed by real-time quantitative RT-PCR analysis of 15,000 additional crypts microdissected from an additional 12 LOI(+) and 9 LOI(−) mice (Fig. 1A). We also confirmed the expected doubling of Igf2 mRNA levels in LOI(+) mice (Fig. 1A). The top ranking genes showing altered expression with LOI included: Cdc6, 1.55-fold (P = 0.003), an essential licensing factor leading to initiation of DNA replication and onset of S-phase (16, 17); Mcm5, 1.47-fold (P = 0.007) and Mcm3, 1.49-fold (P = 0.002), both required for DNA replication at early S-phase (18, 19); Chaf1a, 1.61-fold (P = 0.009), which assembles the histone octamer onto replicating DNA (20); Lig1, 1.54-fold (P = 0.008), DNA ligase involved in joining Okazaki fragments during DNA replication (21); and Ccne1, 1.38-fold (P = 0.04), which stimulates replication complex assembly by cooperating with Cdc6 (22) (Fig. 1A, SI Table 3).
Fig. 1.
Gene expression levels in microdissected intestinal crypts. Quantitative real-time PCR was performed on laser capture microdissected intestinal crypts from 12 LOI(+) and 9 LOI(−) mice. Expression was normalized to β-actin, and the expression level in LOI(+) samples (gray) relative to LOI(−) samples (black) is shown. The bars indicate standard error. (A) Among up-regulated genes in the top ranking GO-annotation categories (DNA replication/cell cycle genes listed in SI Table 3), six genes were validated, and all of the genes showed a statistically significant difference in LOI(+) compared with LOI(−) crypts: Cdc6, 1.55-fold (P = 0.003); Mcm5, 1.47-fold (P = 0.007); Mcm3, 1.49-fold (P = 0.002); Chaf1a, 1.61-fold (P = 0.009); Lig1, 1.54-fold (P = 0.008); and Ccne1, 1.38-fold (P = 0.04). In addition, Igf2 was up-regulated 2.54-fold (P = 0.002) in LOI(+) LCM-dissected crypts. (B) Receptor inhibition by NVP-AEW541 had a differential effect on proliferation-related gene expression in LOI(+) crypts. Analysis was by quantitative real-time PCR of laser capture microdissected intestinal crypts from four LOI(+) and four LOI(−) mice treated with NVP-AEW541 for 3 weeks. Reduction in gene expression in microdissected crypts of LOI(+) mice (gray bars) relative to LOI(−) mice (black bars, normalized to 1.0): Cdc6, 0.49-fold (P = 0.048); Mcm5, 0.48-fold (P = 0.007); Mcm3, 0.65-fold (P = 0.1); Chaf1a, 0.42-fold (P = 0.010); Lig1, 0.42-fold (P = 0.029); and Ccne1, 0.57-fold (P = 0.030). Asterisks indicate significant difference between LOI(+) and LOI(−).
We also treated four LOI(+) and four LOI(−) mice with NVP-AEW541 to inhibit IGF-II signaling, at a dose of 50 mg/kg by oral gavage daily for 3 weeks (twice daily except daily on weekends). NVP-AEW541 is an ATP-competitive inhibitor of IGF1R that blocks signaling at the IGF1 receptor, which mediates IGF-II signaling (14). We confirmed that NVP-AEW541 blocks IGF-II at IGF1R in vitro (SI Fig. 4). Interestingly, NVP-AEW541 had a dramatic effect on expression of proliferation-related genes in LOI(+) crypts, with reduction to levels even lower than those seen in LOI(−) crypts (five of six genes statistically significant): Cdc6, 0.49-fold (P = 0.048); Mcm5, 0.48-fold (P = 0.007); Mcm3, 0.65-fold (P = 0.1); Chaf1a, 0.42-fold (P = 0.010); Lig1, 0.42-fold (P = 0.029); and Ccne1, 0.57-fold (P = 0.030)(Fig. 1B). Thus, LOI-induced changes in proliferation-related gene expression were mediated, at least in part, through IGF-II signaling itself. The drug-induced decrease in the expression of proliferation-related genes did not occur simply due simply to changes in numbers of proliferating crypt cells, because there were approximately the same number of cells in this short term treatment (data not shown).
These results imply that LOI causes a specific alteration in replication-associated gene expression in intestinal epithelium. Nevertheless, we did observe increased expression of some genes not associated with DNA replication per se. For example, Card11 (1.44-fold, P = 0.04; SI Fig. 5) is an antiapoptotic gene acting through phosphorylation of BCL10 and induction of NF-κB (23). We also analyzed expression of Msi1 by real-time PCR, as the encoded progenitor cell marker Musashi-1 showed increased immunostaining in our previous study. Expression of Msi1 was also significantly increased (1.49-fold, P = 0.01, SI Fig. 5), supporting a pleiotropic mechanism for IGF-II in LOI. In addition, several genes showed down regulation in LOI(+) crypts (SI Table 1), including p21 (0.55-fold, P = 0.007, SI Fig. 5), an inhibitor of cell cycle progression (24).
We also asked whether Wnt signaling is activated in LOI(+) intestinal crypts, because it was reported that IGF-II caused relocation of β-catenin to the nucleus in vitro and activated transcription of target genes of the β-catenin/TCF4 complex (25). However, among 36 target genes of Wnt/β-catenin signaling (26), only Tiam1, a Wnt-responsive Rac GTPase activator (27), showed a P value <0.0001, and the other 35 genes did not show significant differences between LOI(−) and LOI(+) crypts (SI Table 4). Furthermore, no significant increase was detected in real-time RT-PCR of Tiam1 (1.16-fold, P = 0.5), or in the well known target gene Axin2 (28, 29) (1.19-fold, P = 0.4) (SI Fig. 5). Thus, activation of Wnt signaling does not seem to be involved in the increase of progenitor cells in LOI(+) crypts.
Enhanced Sensitivity of the IGF2 Signaling Network in LOI.
The in vivo experiments described above raise a very interesting question. Do LOI(+) cells have differential sensitivity to IGF-II and the NVP-AEW541? To determine whether cells from LOI(+) mice exhibited differential sensitivity to these agents and to investigate the molecular underpinnings of the cell response, we performed a high throughput signal transduction assay based on an immunostaining automation device comprising microfluidic chambers housing multiple cells (30). An advantage of the microfluidic chip is that all of the cells can be cultured simultaneously in the same chip and under internally controlled conditions, with precise determination of the cell microenvironment over the time of the experiment and subsequent analysis, allowing a much larger number of measurements than would be possible by conventional means. The device was constructed within a monolithic two-layer PDMS chip sealed with a glass coverslip, with defined media delivery controlled by a multiplexed system of valves. We examined signaling of Akt/PKB and Erk2, two canonical signaling pathways activated by IGF-II, having derived for this purpose mouse embryo fibroblast (MEF) lines from LOI(+) and LOI(−) embryos. Live LOI(+) and LOI(−) cells were stimulated with varying doses of IGF-II, for varying periods of time, fixed, and processed for Akt/PKB measurements, with all steps performed within the chip.
For each cell type, IGF-II concentration, and time point, at least 200 individual cellular measurements were obtained by digital imaging and analysis, providing ample information for statistically significant evaluation of both the average response and cell–cell variability. The results were consistent in chip-to-chip variation analysis, with two chips used for each cell line. As a read-out we used immunostaining of the nuclear phosphorylated Akt (Ser-473) because of its nuclear activity in regulation of FOXO (29) and other proteins controlling cell cycle progression, as well as possible interactions with modifiers of histone modulation (31).
IGF-II triggered a transient Akt activation signal (peak at 10–40 min followed by a return to the baseline within 90 min) in LOI(−) cells (Fig. 2A) at all concentrations tested (400, 800, and 1,600 ng/ml), comparable with levels used to support mouse fetal liver hematopoietic stem cells (500–1,000 ng/ml) (32). In contrast, when subjected to the lowest (400 ng/ml) IGF-II concentration, LOI(+) cells showed markedly sustained Akt activation (> 120 min), which increased steadily over time after stimulation (Fig. 2A). At higher IGF-II doses, the Akt signal in LOI(+) cells became progressively more transient and less pronounced (Fig. 2A). Furthermore, if NVP-AEW541 was added alongside IGF-II, the Akt activation was inhibited to the baseline levels in LOI(−) cells and significantly below the baseline in LOI(+) cells (Fig. 2B). Signaling differences in Erk2 between LOI(−) and LOI(+), although statistically significant, were very small compared with the effect of LOI on Akt activation (Fig. 2C), suggesting that Akt has a particularly important role in IGF2 response in these cells. These results demonstrate that Akt response in LOI(+) cells has enhanced sensitivity to IGF2 at lower doses as well as hypersensitivity to IGF1R inhibition.
Fig. 2.
Single cell analysis of Akt activation by IGF2 in LOI(+) and LOI(−) cells. (A) Akt activation by IGF2. Akt/PKB activation was assayed by single cell immunocytochemistry with an antibody to phosphorylated Akt (Ser-473), in a monolithic two-layer PDMS chip sealed with a glass coverslip, with defined media delivery controlled by a multiplexed system of valves. Live LOI(+) and LOI(−) MEF cells were stimulated within the microfluidic chips with varying doses of IGF2, with measurements at multiple time points at each IGF2 concentration. The y axis shows the ratio of nuclear to background fluorescence. For each cell type, IGF2 concentration, and time point, at least 200 individual cellular measurements were obtained by digital imaging and analysis. LOI(+) but not LOI(−) showed a sustained signaling response to low dose IGF2. Standard error bars are not visible behind symbols at this scale. (B) Inhibition of Akt activation by NVP-AEW541. The cells were assayed as in A at 60 min after coincubation with 400 ng/ml IGF2 and 3 μM NVP-AEW541 and compared with the unstimulated control. Each bar is based on measurements of >400 cells. Whereas NVP-AEW541 inhibited Akt activation to the baseline levels in LOI(−) cells, the inhibitor inhibited Akt activation significantly below the baseline in LOI(+) cells. Error bars show SDs. Asterisk indicates statistical difference vs. LOI(+) control (t test, P < 0.001). (C) Single cell analysis of Erk activation by IGF2 in LOI(+) and LOI(−) MEF cells. Erk2 activation was assayed by single cell immunocytochemistry within microfluidic chips using an antibody to phosphorylated Erk2 from Upstate (Charlottesville, VA). LOI(+) cells (red) and LOI(−) cells (black) were exposed to 400 ng/ml IGF2 for indicated times. The y axis shows the ratio of nuclear to background fluorescence normalized to the maximum level achieved in the LOI(+) cells. Error bars represent SD. Standard error bars are completely subsumed by the symbols at this scale. (D) Gene expression levels in mouse embryonic fibroblasts. Quantitative real-time PCR was performed on LOI(+) and LOI(−) MEF cells, with expression normalized to transferrin receptor expression. The expression level in LOI(+) samples (black) relative to LOI(−) samples (white) is shown. The bars indicate standard error. Both Igf1R and Insr show significant differences in expression: Igf1R, 1.93-fold (P = 0.05); Igf2R 0.75-fold (P = 0.33); and Insr, 2.19-fold (P = 0.05).
One potential mechanism of this hypersensitivity might be based on differential expression of the components of the underlying signaling network, e.g., the IGF1 receptor, which is the primary signaling receptor for IGF2, or members of the insulin receptor family sensitive to IGF2 (33). We therefore analyzed the expression of Igf1r, Igf2r, whose protein product is a sink for IGF2, and Insr, in MEFs showing the altered signaling response. Strikingly, we found a doubling of Igf1r expression and Insr in LOI(+)cells (Fig. 2D). Although, the reasons for altered expression of Igf1r and Insr are not clear at this point, this change in the expression of these receptors provides an intriguing model for alterations in signaling sensitivity in LOI.
LOI Increases Premalignant Lesion Formation in the AOM/LOI Model, Which Shows Enhanced Sensitivity to IGF2 Signaling Inhibition.
Based on these results, we were interested to know whether IGF2 signaling inhibition would inhibit in vivo carcinogenesis, or even show an enhanced chemopreventive effect. Treatment with NVP-AEW541 requires twice daily gavage, and Min mice develop lesions over a longer period than was practical for use of this drug. In addition, a limitation of the Min model is that it does not reflect the human situation, in which LOI occurs in normal cells before the Apc mutation is present (34). We therefore turned to the azoxymethane (AOM) model in which the carcinogen is administered postnatally, and premalignant lesions termed aberrant crypt foci (ACF) appear 5 weeks after the first dose. An additional advantage is that the AOM is a widely studied rodent colon cancer model (15, 35).
Eight LOI(+)and 14 LOI(−) mice were given AOM i.p. weekly for 3 weeks and killed at 5 weeks after the first dose; then, ACFs were scored as described in ref. 35. Histologic examination of colons from AOM-treated mice confirmed the presence of ACFs, with hyperproliferative features including increased mitosis, crypt enlargement and crypt disarray (SI Fig. 6 A and B). LOI(+) mice showed 19.8 ± 2.2 ACF per colon, compared with 12.4 ± 0.9 ACF per colon in LOI(−) mice, a 60% increase (P = 0.002; Fig. 3A).
Fig. 3.

Inhibition of azoxymethane (AOM)-induced aberrant crypt foci (ACF) by NVP-AEW541. (A) ACF formation in the colon was induced by AOM i.p. injection, and treatment with NVP-AEW541 was by gastric gavage. Each ACF was formed of one to four aberrant crypts, and the number of ACF (# of ACF), the number of total aberrant crypts (# of AC), and the average number of aberrant crypts per ACF were measured. LOI(+) mice (gray bars) formed 1.60-fold more ACF than LOI(−) mice (black bars) (P = 0.002). Similarly, LOI(+) mice formed 1.65-fold more AC than LOI(−) mice (P = 0.01). LOI(−) mice treated with AOM and NVP-AEW541 (pink bars), showed no significant reduction of ACF over LOI(−) mice treated with AOM injection alone (P = 0.5). However, LOI(+) mice treated with AOM and NVP-AEW541 (azure bars) showed a 61% decrease in ACF compared with LOI(+) mice treated with AOM alone (P = 0.0002), and a 37% decrease compared with LOI(−) mice treated with AOM alone (P = 0.007). Similarly, LOI(+) mice treated with AOM and NVP-AEW541 showed a 64% decrease in AC from LOI(+) mice treated with AOM alone (P = 0.0002), and a 40% reduction compared with LOI(−) mice treated with AOM alone (P = 0.003). (B) The number of ACF and the number of total aberrant crypts (AC) were corrected by colon surface area (cm2). LOI(+) mice (gray bars) formed 1.59-fold more ACF (P = 0.004) and 1.63-fold more AC than LOI(−) mice (black bars) (P = 0.002). LOI(−) mice treated with AOM and NVP-AEW541 (pink bars), showed no reduction of ACF over LOI(−) mice treated with AOM injection alone (P = 0.5). However, LOI(+) mice treated with AOM and NVP-AEW541 (azure bars) showed a 56% decrease in ACF compared with LOI(+) mice treated with AOM alone (P = 0.0008), and a 30% decrease compared with LOI(−) mice treated with AOM alone (P = 0.05). Similarly, LOI(+) mice treated with AOM and NVP-AEW541 showed a 60% decrease in AC from LOI(+) mice treated with AOM alone (P = 0.03), and a 33% reduction compared with LOI(−) mice treated with AOM alone (P = 0.02). Asterisks indicate significant difference between LOI(+) and LOI(−), and between NVP-AEW541 treatment and no treatment for a given epigenotype.
We then similarly exposed an additional nine LOI(+) mice and nine LOI(−) control littermates to AOM, adding treatment with NVP-AEW541 to inhibit IGF-II signaling, at a dose of 50 mg/kg by oral gavage daily for 6 weeks (twice daily except daily on weekends), starting 1 week before AOM administration. LOI(−) mice showed no difference in ACF formation after NVP-AEW541 drug treatment (11.3 ± 1.6, N.S.; Fig. 3A). Surprisingly, LOI(+) mice showed a striking reduction in AOM-induced ACF formation after NVP-AEW541 treatment (7.8 ± 1.2, P = 0.0002; Fig. 3A), significantly lower even than that seen in LOI(−) AOM-treated mice (P = 0.007).
Because LOI also leads to an increase in birth weight and therefore potentially in the size of the colon, we also normalized the number of ACFs to colon surface area. We found a similar increase in ACFs in LOI(+) mice, 59% (P = 0.004), and a similar decrease in LOI(+) mice treated with the inhibitor, 56% (P = 0.0008), but there was no decrease in ACFs with inhibitor in LOI(−) mice (Fig. 3B). Thus, LOI of Igf2 increased the sensitivity to AOM through an IGF1R-dependent mechanism, and LOI(+) mice were more sensitive to the effects of IGF1R blockade than were LOI(−) mice.
An additional intriguing finding in AOM-treated LOI(+) mice was cystically dilated crypts lined by enlarged cells with atypical nuclei and containing necrotic debris, that were reminiscent of sessile serrated adenomas (SSAs) seen in the human colon (SI Fig. 6 C and D). SSAs also show crypt dilatation in association with cytologic atypia and are currently of immense interest for their recently recognized association with colorectal cancer (36). It will thus be of interest to determine whether SSAs are associated with LOI in the human population.
Discussion
In this article, we present evidence that LOI increases proliferation-related gene expression in epithelial progenitor cells, and surprisingly in vivo blockade of the IGF-II signaling receptor with NVP-AEW541 causes enhanced inhibition of signaling in LOI(+) mice. We confirmed that LOI(+) cells are inherently more sensitive to IGF-II signaling in single cell signal transduction experiments. This in vitro analysis showed a marked augmentation of Akt/PKB signaling in LOI(+) cells at low doses of IGF-II, which was reduced in the presence of the inhibitor to the levels below those found in LOI(−) cells. One potential mechanism for increased IGF-II sensitivity is up-regulation of the signaling receptors in LOI(+) cells, which would be consistent with our data. The exact reason for an increase in the receptor levels are at present not clear, and further exploration of the changes in the receptor expression in vivo remains done. However, this finding lends further support to the importance of the IGF-II signaling cascade in cancer (37). We then exploited this increased sensitivity to IGF-II signaling to develop an in vivo chemopreventive strategy using a previously undescribed LOI/AOM mouse model. LOI increases the number of premalignant lesions in mice exposed to AOM, and blockade of IGF-II at its signaling receptor reduced the numbers of ACF significantly below even that of AOM-treated LOI(−) mice.
As signaling through the Akt pathway has been implicated in cell cycle regulation, enhanced protein synthesis and antiapoptotic cytoprotection, these results may help to explain why LOI has a strong biological effect at the low overall IGF-II levels found in the intestine. The increased sensitivity to IGF-II at low dose could also help shed more light on the relationship between receptor-mediated signaling and cell growth. How does a group of dividing and differentiating cells in a developing epithelial tissue sense when it has reached the right critical number of similarly differentiated cells? Our results suggest that both normal and LOI(−) cells might respond to increasing IGF-II with signaling of progressively lower magnitude and duration. One can therefore envision a scenario in which proliferating cells are more sensitive to this ligand at low density of IGF-II secreting cells, with a relatively low accumulated IGF-II. A gradual increase in signaling cell density leads to a build-up of the local interstitial concentration of IGF-II causing a reduction in cell proliferation, providing an important check on growth control as the tissue reaches a critical size. However, because of enhanced sensitivity of LOI(+) cells at lower doses of IGF-II at the initial stages of the differentiated tissue expansion, the differentiated tissue compartment might increase more rapidly, leading to relatively stable foci of increased tissue mass. This scenario is distinct from the commonly accepted view that LOI simply leads to an enhanced local build-up of IGF-II, which enhances cell proliferation with cells as sensitive to IGF-II as their LOI(−) counterparts.
The abrogation of AOM-induced aberrant crypt foci by an IGF-II signaling receptor inhibitor also suggests a chemopreventive strategy for CRC in patients with LOI. This approach could have a significant public health impact, because 5–10% of the population shows this epigenetic alteration (7, 8), although other compounds would likely need to be developed for maximum specificity and safety. Nevertheless the results argue for a fundamentally different approach for cancer mortality reduction, compared with screening for the presence of early tumors. In cardiovascular disease prevention, there has been a shift in emphasis toward pharmacologically mediated risk reduction, even (and preferably) in those patients with no apparent end organ disease at all (38). Thus, the general population might eventually be screened for LOI, and pharmacological intervention might reduce those at high risk to average or even reduced risk of colon cancer. These results do not exclude other potentially important mechanisms by which LOI might affect tumor development, including other signaling pathways or interchromosomal interactions (39) or a role for H19 itself (40), but they provide one potentially important avenue for cancer prevention.
The data also support the “epigenetic progenitor model,” which argues that there is a polyclonal change in the numbers and states of progenitor cells that arises before the genetic mutation, and increases the risk of cancer when a mutation occurs stochastically (41). Thus, the importance of LOI has been confirmed in a second animal model, and a plausible mechanism for this progenitor cell expansion has been offered, namely increased IGF-II sensitivity in LOI(+) cells, leading to increased proliferation of progenitor cells. We suggest that LOI is a paradigm for other epigenetic changes in apparently normal cells of patients at risk of cancer, and that an intensive effort should be undertaken to identify other epigenetic abnormalities in imprinting, DNA methylation, or chromatin that may distinguish non-tumor cells of cancer patients, or patients at risk of cancer, from those of non-cancer patients.
Materials and Methods
Mouse Strains.
Mice with C56BL/6J background carrying a deletion in the H19 gene (3 kb) and 10 kb of the upstream region including the differentially methylated region (DMR) regulating IGF-II silencing were obtained from S. Tilghman (Princeton University, Princeton, NJ) (12), and we maintained the strain by breeding female wild-type C56BL/6J and male H19+/−, keeping the maternal Igf2 allele silenced. We obtained the H19+/− mice in 2001 and maintained the strain by breeding female wild-type C56BL/6J and male H19+/− three times per year. In experimental crosses, mice with biallelic Igf2 expression and control littermates were isolated by crossing female H19+/− with male wild-type C56BL/6J mice. Mice were genotyped by PCR, which identified an 847-bp product for the wild-type allele and a 1,000-bp product for the mutant allele, using the following primers: H19-F, TCC CCT CGC CTA GTC TGG AAG CA; Mutant-F, GAA CTG TTC GCC AGG CTC AAG; Common-R, ACA GCA GAC AGC AAG GGG AGG GT. Because of known strain variation in progression of these lesions, we chose to treat littermate controls with and without LOI, in which the dams were heterozygous for a deletion of the H19 differentially methylated region (DMR); inheritance of a maternal allele lacking the DMR leads to activation of the normally silent allele of Igf2 [LOI(+)], whereas inheritance of a wild-type maternal allele leads to normal imprinting [LOI(−)].
Microarray Analysis.
To detect gene expression changes in intestinal progenitor cells, laser capture microdissection (LCM) was performed to isolate intestinal crypt cells. Microarray analysis was performed on these samples. The detailed methods are described in SI Materials and Methods.
Microfluidic Chamber Assays.
The immunostaining automation device consists of a monolithic two-layer PDMS chip sealed with a glass coverslip, fabricated using techniques described in ref. 42. Multiplexing valves were actuated by pressure lines connected to off-device solenoid valves. Details are described in SI Materials and Methods.
Azoxymethane and NVP-AEW541 Administration in Vivo.
Seven-week-old LOI(+) and LOI(−) littermate controls were treated with AOM at 10 mg/kg body weight i.p. once per week for 3 weeks and killed 5 weeks after the first dose of AOM. The entire colon was resected from mice after laparotomy, flushed with PBS, filled with 10% buffered formalin (Sigma) for 1 min, opened longitudinally, fixed flat between filter paper in formalin at 4°C overnight, rinsed with PBS, and stained with 0.2% methylene blue in saline. The number of ACF per colon and the number of aberrant crypts per ACF were scored by light microscopy as described in ref. 35. NVP-AEW541 (50 mg/kg) was administered by oral gavage, beginning 1 week before AOM treatment, and NVP-AEW541 was administered 7 days/week, twice a day for 5 weekdays and once a day for 2 weekend days, for 6 weeks until killing. For in vivo analysis of inhibitory effect of NVP-AEW541, the drug was administered by oral gavage 7 days/week, twice a day for 5 weekdays and once a day for 2 weekend days, for 3 weeks until killing. LCM was performed to isolate intestinal crypt cells from fresh frozen intestines as described above. Approximately 1,000 crypts were isolated from each of four LOI(+) and four LOI(−) mice, and RNA were collected by using the RNeasy Kit (Qiagen). Gene expression levels were quantified by using SYBR Green PCR Core Reagents and an ABI Prism 7700 Sequence Detection System.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Yulan Piao, Dawn Nines, Jason Clardy, and Yong Qian for technical assistance and Mitsuo Oshimura for technical advice. This work was supported by National Institutes of Health (NIH) Grant CA65145 (to A.P.F.), by the Swedish Cancer Research Foundation (R.O.), and in part by the NIH Intramural Research Program (M.S.H.K. and D.L.L.).
Footnotes
Conflict of interest statement: R.A. and M.A.P. (Novartis, Inc.) have a proprietary interest in NVP-AEW541. The Johns Hopkins University and the National Institutes of Health have filed a patent on data presented in this manuscript.
Data deposition: The microarray data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GEO) Database, www.ncbi.nlm.nih.gov/geo (accession no. GSE8583).
This article contains supporting information online at www.pnas.org/cgi/content/full/0710359105/DC1.
References
- 1.Lamonerie T, Lavialle C, Haddada H, Brison O. Int J Cancer. 1995;61:587–592. doi: 10.1002/ijc.2910610425. [DOI] [PubMed] [Google Scholar]
- 2.Foulstone E, Prince S, Zaccheo O, Burns JL, Harper J, Jacobs C, Church D, Hassan AB. J Pathol. 2005;205:145–153. doi: 10.1002/path.1712. [DOI] [PubMed] [Google Scholar]
- 3.Jones SM, Klinghoffer R, Prestwich GD, Toker A, Kazlauskas A. Curr Biol. 1999;9:512–521. doi: 10.1016/s0960-9822(99)80235-8. [DOI] [PubMed] [Google Scholar]
- 4.Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K, Lee JH, Ciarallo S, Catzavelos C, Beniston R, et al. Nat Med. 2002;8:1153–1160. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- 5.Downward J. Semin Cell Dev Biol. 2004;15:177–182. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Feinberg AP, Tycko B. Nat Rev Cancer. 2004;4:143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 7.Cui H, Cruz-Correa M, Giardiello FM, Hutcheon DF, Kafonek DR, Brandenburg S, Wu Y, He X, Powe NR, Feinberg AP. Science. 2003;299:1753–1755. doi: 10.1126/science.1080902. [DOI] [PubMed] [Google Scholar]
- 8.Woodson K, Flood A, Green L, Tangrea JA, Hanson J, Cash B, Schatzkin A, Schoenfeld P. J Natl Cancer Inst. 2004;96:407–410. doi: 10.1093/jnci/djh042. [DOI] [PubMed] [Google Scholar]
- 9.Kaneda A, Feinberg AP. Cancer Res. 2005;65:11236–11240. doi: 10.1158/0008-5472.CAN-05-2959. [DOI] [PubMed] [Google Scholar]
- 10.Ohlsson R. Novartis Found Symp. 2004;262:108–121. [PubMed] [Google Scholar]
- 11.Sakatani T, Kaneda A, Iacobuzio-Donahue CA, Carter MG, de Boom Witzel S, Okano H, Ko MS, Ohlsson R, Longo DL, Feinberg AP. Science. 2005;307:1976–1978. doi: 10.1126/science.1108080. [DOI] [PubMed] [Google Scholar]
- 12.Leighton PA, Ingram RS, Eggenschwiler J, Efstratiadis A, Tilghman SM. Nature. 1995;375:34–39. doi: 10.1038/375034a0. [DOI] [PubMed] [Google Scholar]
- 13.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Echeverria C, Pearson MA, Marti A, Meyer T, Mestan J, Zimmermann J, Gao J, Brueggen J, Capraro HG, Cozens R, et al. Cancer Cell. 2004;5:231–239. doi: 10.1016/s1535-6108(04)00051-0. [DOI] [PubMed] [Google Scholar]
- 15.Bissahoyo A, Pearsall RS, Hanlon K, Amann V, Hicks D, Godfrey VL, Threadgill DW. Toxicol Sci. 2005;88:340–345. doi: 10.1093/toxsci/kfi313. [DOI] [PubMed] [Google Scholar]
- 16.Dutta A, Bell SP. Annu Rev Cell Dev Biol. 1997;13:293–332. doi: 10.1146/annurev.cellbio.13.1.293. [DOI] [PubMed] [Google Scholar]
- 17.Coleman TR, Carpenter PB, Dunphy WG. Cell. 1996;87:53–63. doi: 10.1016/s0092-8674(00)81322-7. [DOI] [PubMed] [Google Scholar]
- 18.Chong JP, Mahbubani HM, Khoo CY, Blow JJ. Nature. 1995;375:418–421. doi: 10.1038/375418a0. [DOI] [PubMed] [Google Scholar]
- 19.Madine MA, Khoo CY, Mills AD, Laskey RA. Nature. 1995;375:421–424. doi: 10.1038/375421a0. [DOI] [PubMed] [Google Scholar]
- 20.Smith S, Stillman B. Cell. 1989;58:15–25. doi: 10.1016/0092-8674(89)90398-x. [DOI] [PubMed] [Google Scholar]
- 21.Tomkinson AE, Mackey ZB. Mutat Res. 1998;407:1–9. doi: 10.1016/s0921-8777(97)00050-5. [DOI] [PubMed] [Google Scholar]
- 22.Coverley D, Laman H, Laskey RA. Nat Cell Biol. 2002;4:523–528. doi: 10.1038/ncb813. [DOI] [PubMed] [Google Scholar]
- 23.Narayan P, Holt B, Tosti R, Kane LP. Mol Cell Biol. 2006;26:2327–2336. doi: 10.1128/MCB.26.6.2327-2336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gartel AL, Serfas MS, Tyner AL. Proc Soc Exp Biol Med. 1996;213:138–149. doi: 10.3181/00379727-213-44046. [DOI] [PubMed] [Google Scholar]
- 25.Morali OG, Delmas V, Moore R, Jeanney C, Thiery JP, Larue L. Oncogene. 2001;20:4942–4950. doi: 10.1038/sj.onc.1204660. [DOI] [PubMed] [Google Scholar]
- 26.Gordon MD, Nusse R. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 27.Malliri A, Rygiel TP, van der Kammen RA, Song JY, Engers R, Hurlstone AF, Clevers H, Collard JG. J Biol Chem. 2006;281:543–548. doi: 10.1074/jbc.M507582200. [DOI] [PubMed] [Google Scholar]
- 28.Yan D, Wiesmann M, Rohan M, Chan V, Jefferson AB, Guo L, Sakamoto D, Caothien RH, Fuller JH, Reinhard C, et al. Proc Natl Acad Sci USA. 2001;98:14973–14978. doi: 10.1073/pnas.261574498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogt PK, Jiang H, Aoki M. Cell Cycle. 2005;4:908–913. doi: 10.4161/cc.4.7.1796. [DOI] [PubMed] [Google Scholar]
- 30.Wang CJ, Cheong R, Levchenko A. Proceeding of 10th International Conference on Miniaturized Systems for Chemistry and Life Sciences; 2006. (microTAS2006) [Google Scholar]
- 31.Gao H, Yu Z, Bi D, Jiang L, Cui Y, Sun J, Ma R. Mol Cell Biochem. 2007;305:35–44. doi: 10.1007/s11010-007-9525-3. [DOI] [PubMed] [Google Scholar]
- 32.Zhang CC, Lodish HF. Blood. 2004;103:2513–2521. doi: 10.1182/blood-2003-08-2955. [DOI] [PubMed] [Google Scholar]
- 33.Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, Goldfine ID, Belfiore A, Vigneri R. Mol Cell Biol. 1999;19:3278–3288. doi: 10.1128/mcb.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui H, Horon IL, Ohlsson R, Hamilton SR, Feinberg AP. Nat Med. 1998;4:1276–1280. doi: 10.1038/3260. [DOI] [PubMed] [Google Scholar]
- 35.Bird RP. Cancer Lett. 1987;37:147–151. doi: 10.1016/0304-3835(87)90157-1. [DOI] [PubMed] [Google Scholar]
- 36.Snover DC, Jass JR, Fenoglio-Preiser C, Batts KP. Am J Clin Pathol. 2005;124:380–391. doi: 10.1309/V2EP-TPLJ-RB3F-GHJL. [DOI] [PubMed] [Google Scholar]
- 37.LeRoith D, Helman L. Cancer Cell. 2004;5:201–202. doi: 10.1016/s1535-6108(04)00054-6. [DOI] [PubMed] [Google Scholar]
- 38.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 39.Ling JQ, Li T, Hu JF, Vu TH, Chen HL, Qiu XW, Cherry AM, Hoffman AR. Science. 2006;312:269–272. doi: 10.1126/science.1123191. [DOI] [PubMed] [Google Scholar]
- 40.Gabory A, Ripoche MA, Yoshimizu T, Dandolo L. Cytogenet Genome Res. 2006;113:188–193. doi: 10.1159/000090831. [DOI] [PubMed] [Google Scholar]
- 41.Feinberg AP, Ohlsson R, Henikoff S. Nat Rev Genet. 2006;7:21–33. doi: 10.1038/nrg1748. [DOI] [PubMed] [Google Scholar]
- 42.Unger MA, Chou HP, Thorsen T, Scherer A, Quake SR. Science. 2000;288:113–116. doi: 10.1126/science.288.5463.113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.