Abstract
Auditory fear memory is thought to be maintained by fear conditioning-induced potentiation of synaptic efficacy, which involves enhanced expression of surface AMPA receptor (AMPAR) at excitatory synapses in the lateral amygdala (LA). Depotentiation, reversal of conditioning-induced potentiation, has been proposed as a cellular mechanism for fear extinction; however, a direct link between depotentiation and extinction has not yet been tested. To address this issue, we applied both ex vivo and in vivo approaches to rats in which fear memory had been consolidated. A unique form of depotentiation reversed conditioning-induced potentiation at thalamic input synapses onto the LA (T-LA synapses) ex vivo. Extinction returned the enhanced T-LA synaptic efficacy observed in conditioned rats to baseline and occluded the depotentiation. Consistently, extinction reversed conditioning-induced enhancement of surface expression of AMPAR subunits in LA synaptosomal preparations. A GluR2-derived peptide that blocks regulated AMPAR endocytosis inhibited depotentiation, and microinjection of a cell-permeable form of the peptide into the LA attenuated extinction. Our results are consistent with the use of depotentiation to weaken potentiated synaptic inputs onto the LA during extinction and provide strong evidence that AMPAR removal at excitatory synapses in the LA underlies extinction.
Keywords: lateral amygdala, fear conditioning, AMPA receptor, endocytosis
The cortical and thalamic input synapses onto the lateral amygdala (LA) (C-LA and T-LA synapses, respectively) carry auditory information from the auditory cortex and auditory thalamus onto the LA, respectively (1). Long-term potentiation (LTP; an in vitro model of memory) (2)-like changes in these pathways are thought to underlie both the encoding and consolidation of auditory fear memory (3–8). The results of a recent study suggest that long-term retention of conditioning-induced potentiation at excitatory synapses in the LA is a critical requirement for consolidated fear memory within the LA (7, 9). Also, LTP requiring the synaptic delivery of AMPA receptors (AMPARs) at excitatory synapses in the LA appears to be necessary for establishing consolidated fear memory (6, 8, 10). Conditioning-induced potentiation and auditory fear memory encoded in the LA have been shown to be consolidated within 24 h after fear conditioning (5, 7, 11). Moreover, auditory fear memory appears to be maintained in the LA across the adult lifetime of rats (12). Thus, consolidation of auditory fear memory encoded in the LA is rapid and localized, unlike hippocampus-dependent memory, which involves slow and distributed consolidation processes (13).
In the present study, we tested the hypothesis that depotentiation of conditioning-induced potentiation at excitatory synapses in the LA underlies extinction of consolidated fear memory. Synaptic weights were monitored ex vivo by using whole-cell (or field potential) recordings in amygdala slices prepared from behavior-trained rats.
Results
Extinction of Consolidated Fear Memory Results in Apparent Reversal of Conditioning-Induced Potentiation.
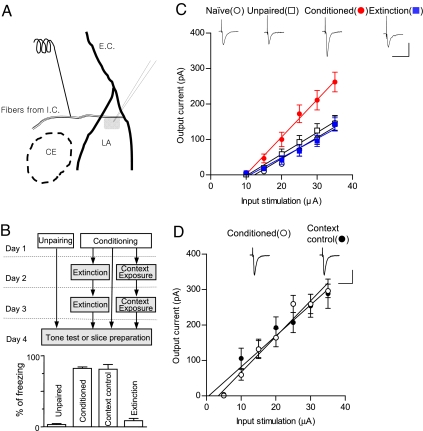
To determine whether T-LA synaptic efficacy is reversed with conditioning and extinction, the input–output relationships for the excitatory postsynaptic current (EPSC) amplitude as a function of afferent fiber stimulus intensity were compared in four groups: naïve, unpaired, conditioned, and extinction. The slopes of the linear fits to the data points of the input–output relationship obtained in each neuron were averaged within each group. EPSCs were potentiated in the conditioned group and reduced to near the baseline level in the extinction group compared with naïve and unpaired groups (naïve, 5.94 ± 0.92 pA/μA; unpaired, 6.11 ± 0.95 pA/μA; conditioned, 10.52 ± 1.11 pA/μA; extinction, 5.39 ± 0.70 pA/μA). ANOVA indicated a main effect of group (F3,70 = 6.689, P < 0.001), with post hoc tests confirming that the slope of the input–output curve was significantly steeper in the conditioned group than in the other three groups (P < 0.01 for the three pairs, Newman–Keuls posttest; Fig. 1C), and that the slope of the input–output curve in the extinction group did not differ significantly from that in the unpaired and naïve groups (P > 0.05 for all designated pairs, Newman–Keuls posttest). The lack of potentiation in unpaired groups means that encoding of contextual fear memory, which was also present in unpaired groups, was not responsible for the potentiation observed in conditioned groups. We also compared decay time constants of EPSCs with input stimulations of 35 μA (naïve, 5.94 ± 0.69 ms; unpaired, 5.86 ± 0.39 ms; conditioned, 6.72 ± 0.71 ms; extinction, 5.90 ± 0.55 ms) and series resistances of whole-cell recordings (naïve, 15.15 ± 0.94 MΩ; unpaired, 16.44 ± 0.93 MΩ; conditioned, 15.51 ± 0.63 MΩ; extinction, 15.07 ± 0.63 MΩ) between the four groups and did not detect any significant differences (F3,70 = 0.4833, P > 0.6 for decay time, P > 0.05 for all designated pairs, Newman–Keuls posttest; F3,70 = 0.5955, P > 0.6 for series resistance, P > 0.05 for all designated pairs, Newman–Keuls posttest). These results show that neither slow NMDA responses nor altered recording conditions account for the observed results. To rule out the possibility that the reversal of conditioning-induced potentiation was caused merely by exposure to the extinction chambers, conditioning-induced potentiation was compared between fear-conditioned groups and context controls in which conditioned rats were placed in the extinction chambers for an equivalent period but were not exposed to any tones. There was no significant difference between these two groups (conditioned, 10.09 ± 0.87 pA/μA; extinction-context controls, 8.74 ± 1.06 pA/μA; P > 0.05, unpaired t test; Fig. 1D), showing that the observed reversal was specific to extinguishing tone stimuli. Collectively, our data show that the extinction of consolidated fear memory results in apparent reversal of conditioning-induced potentiation at T-LA synapses.
Fig. 1.
Extinction-induced reversal of conditioning-induced potentiation. (A) Schematic illustration of a brain slice containing amygdala. A stimulating electrode was placed in the fibers from the internal capsule. The location of the recorded neurons in the LA is shaded. LA, lateral nucleus; CE, central nucleus; I.C., internal capsule; E.C., external capsule. (B) (Upper) The behavioral procedure for the experiments shown here and in Figs. 2 and 4. As shown in this diagram, brain slices were prepared on day 4 for all groups except naïve controls. To avoid possible changes in synaptic properties caused by the test stimuli, one set of rats was killed to prepare brain slices, and another set was used to monitor conditioned freezing. White and gray tones in the rectangles represent context A and B, respectively. (Lower) Pooled behavioral results. Context controls represent context B-exposed groups without tone presentation in days 2 and 3. Note that freezing in context controls was not significantly different from that in conditioned groups, whereas freezing in extinction was significantly reduced (F3,102 = 328.5, P < 0.01; P > 0.05 for context controls-conditioned groups; P < 0.01 for all of the other pairs, Newman–Keuls posttest). (C) Input–output curves for EPSCs in naïve controls (n = 18), unpaired (n = 17), conditioned (n = 22), and extinction (n = 17) groups. Representative current traces are an average of five consecutive responses with input stimulations of 35 μA. (Scale bars: 50 ms and 150 pA.) Experiments were initially done nonblindly, but were validated in a blind fashion later on, so all data were pooled (for the blind portion of the experiments, naïve, 5.47 ± 0.99 pA/μA; unpaired, 3.80 ± 0.93 pA/μA; conditioned, 15.16 ± 3.59 pA/μA; extinction, 6.35 ± 1.66 pA pA/μA; F3,18 = 6.531, P < 0.005; P < 0.01 for conditioned group-the other three groups, P > 0.05 for extinction group-naïve group or for extinction group-unpair group, Newman–Keuls posttest). (D) Input–output curves for EPSCs in conditioned rats (n = 8) and context controls (n = 7). The series resistance of conditioned groups was not different from that of extinction-context controls (14.95 ± 0.66 and 14.06 ± 0.53 MΩ for conditioned and extinction-context groups, respectively; P > 0.3, unpaired t test). Representative current traces are averages of four consecutive responses with input stimulations of 35 μA. (Scale bars: 50 ms and 150 pA.) Experiments were done nonblindly.
Ex Vivo Depotentiation as a Mechanism for the Extinction-Induced Reversal of Conditioning-Induced Potentiation.
To identify cellular mechanisms underlying the extinction-induced reversal of conditioning-induced potentiation at T-LA synapses, we first searched for ex vivo depotentiation, that is, where depotentiation stimuli produced reversal of in vivo synaptic potentiation preserved in amygdala slices. Ex vivo depotentiation needs to satisfy two criteria for it to be a viable mechanism underlying the extinction-induced reversal: (i) depotentiating stimuli should produce synaptic depression in amygdala slices prepared only from fear-conditioned rats, but not from naïve and unpaired controls, and (ii) the stimulation-induced depression should be lower in extinction-group amygdala slices than in conditioned-group slices, so as to ensure that extinction occludes ex vivo depotentiation. Successful occlusion would indicate that the above two processes involve the same (or similar) mechanisms, and no effect in the unpaired group would show that ex vivo depotentiation is specific to the associative cued learning-induced changes.
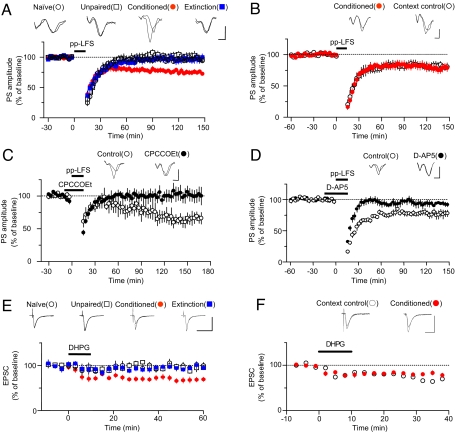
Two representative paradigms exist for induction of depotentiation [or long-term depression (LTD)]: (i) prolonged single-pulse (1 or 5 Hz) stimulation and (ii) paired-pulse (1 Hz) low-frequency stimulation (14, 15). Among various stimulation paradigms tested, paired-pulse low-frequency stimulation (pp-LFS; 50-ms interstimulus interval) at 1 Hz for 15 min was found to meet all of the criteria (Fig. 2A). ANOVA indicated a main effect of group (F3,28 = 4.537, P < 0.02), with post hoc tests confirming that synaptic responses after ex vivo depotentiation induction in the conditioned group was significantly depressed compared with the other three groups (P < 0.01 for naïve-conditioned, P < 0.05 for all other designated pairs, Newman–Keuls posttest; Fig. 2A). Also, pp-LFS failed to produce significant depression in naïve, unpaired controls, and extinction groups (naïve, 97.0 ± 2.9%, n = 9, P > 0.3; unpaired, 97.3 ± 5.6%, n = 8, P > 0.9; extinction, 100.1 ± 9.4%, n = 10, P > 0.5; paired t test vs. baseline). To rule out the possibility that the occlusion effect by extinction was caused merely by exposure to the extinction chambers, the magnitude of ex vivo depotentiation was compared between fear-conditioned groups and extinction-context controls. We found no significant difference between the two groups (context controls, 81.6 ± 5.4%, n = 7; conditioned, 78.5 ± 5.7%, n = 14; P > 0.6, unpaired t test; Fig. 2B), showing that the observed occlusion was specific to the extinguishing tone stimuli. pp-LFS-induced ex vivo depotentiation was blocked by either CPCCOEt, an antagonist for group I metabotropic GluRs (mGluRs) (control, 65.7 ± 7.7%, n = 6; CPCCOEt, 100.9 ± 10.7%, n = 6; P < 0.02, unpaired t test; Fig. 2C) or D-AP5, a NMDA receptor (NMDAR) antagonist (control, 76.9 ± 5.3%, n = 5; D-AP5, 93.1 ± 2.9%, n = 5; P < 0.05, unpaired t test; Fig. 2D). Thus, pp-LFS-induced ex vivo depotentiation appears to depend on coactivation of both group I mGluRs and NMDARs, a case similar to LTD in the perirhinal cortex (16, 17). It has been proposed that cooperativity between group I mGluRs and NMDARs may be required to increase calcium levels beyond a threshold for induction of LTD or depotentiation (17).
Fig. 2.
pp-LFS-induced ex vivo depotentiation of conditioning-induced potentiation as a mechanism of the extinction-induced reversal. (A) pp-LFS produced significant depression only in conditioned groups (ex vivo depotentiation) and the ex vivo depotentiation was occluded by extinction. Population spike (PS) amplitudes were plotted as a function of the recording time in four experimental groups (naïve, n = 9; unpaired, n = 8; conditioned, n = 14; extinction, n = 10). (B) The occlusive effect of extinction on pp-LFS-induced ex vivo depotentiation was not observed when rats were exposed to extinction context without tone stimuli (context control). (C) The mGluR1 antagonist CPCCOEt blocked ex vivo depotentiation (control, n = 6; CPCCOEt, n = 6). Representative paired traces are averages of five traces before and after pp-LFS, respectively. (Scale bars: 2 ms and 0.2 mV.) (D) The NMDAR antagonist D-AP5 blocked ex vivo depotentiation (control, n = 5; CPCCOEt, n = 5). Representative paired traces are averages of five traces before and after pp-LFS. (Scale bars: 2 ms and 0.2 mV.) (E) DHPG-induced ex vivo depotentiation at T-LA synapses. Brief application of DHPG produced significant depression only in conditioned groups, and ex vivo DHPG-induced depotentiation was occluded by extinction. EPSC amplitudes were plotted as a function of the recording time in four experimental groups (naïve, n = 5; unpaired, n = 6; conditioned, n = 12; extinction, n = 8). Representative paired traces are averages of five consecutive traces 5 min before and 60 min after DHPG application. (Scale bars: 20 ms and 100 pA.) (F) The occlusive effect of extinction on DHPG-induced ex vivo depotentiation was not observed when rats were exposed merely to an extinction context without tone stimuli (context control). (Scale bars: 20 ms and 100 pA.) Experiments shown in A were initially done nonblindly, but were validated in a blind fashion later on, so all data were pooled. For the blind portion of the experiments, the difference between conditioned and extinction groups was verified (n = 6 and n = 4 for conditioned and extinction groups, respectively; P < 0.05, unpaired t test). Experiments shown in E were nonblinded. Subsequent blinded repetitions confirmed the difference between conditioned (n = 7) and extinction (n = 3) groups (P < 0.05, unpaired t test; see SI Fig. 6). In all experiments, brain slices were prepared on day 4 for all groups except naïve controls (see Fig. 1B).
Next, we tested whether the group I mGluR agonist, 3,5-dihydroxyphenylglycine (DHPG), could induce ex vivo depotentiation. In whole-cell recordings, a 10-min application of DHPG (100 μM) did not produce any significant changes in EPSC amplitudes in naïve, unpaired, and extinction groups (P > 0.05 for naïve, P > 0.4 for unpaired, P > 0.05 for extinction, paired t test vs. baseline), whereas it induced long-lasting depression in conditioned groups (68.4 ± 5.5%, n = 14) compared with the other three groups (F3,24 = 10.50, P < 0.0002; P < 0.01 for all designated pairs, Newman–Keuls posttest), thereby satisfying all of the criteria for ex vivo depotentiation (Fig. 2E). We also found no significant difference between fear-conditioned groups and context controls (context controls, 68.1 ± 5.8%, n = 4; conditioned, 80.9 ± 3.1%, n = 6; P > 0.05, unpaired t test; Fig. 2F), showing that the observed occlusion was specific to the extinguishing tone stimuli. Together, these results show that prolonged overstimulation of group I mGluRs by a synthetic agonist, DHPG, are sufficient for induction of ex vivo depotentiation, whereas both NMDARs and group I mGluRs are necessary for induction of ex vivo depotentiation elicited by synaptically released glutamate.
Expression of Surface AMPARs in LA Synaptosomes.
Recent findings indicate that encoding of auditory fear memories in the LA is mediated, at least in part, by delivery of AMPARs to the surface of LA excitatory synapses (6, 8, 10). Thus, one of the most plausible mechanisms for ex vivo depotentiation is removal of AMPARs from the synapse surface. To examine surface expression of synaptic AMPARs, we isolated surface membranes of LA synaptosomes by using a biochemical surface biotinylation technique developed in hippocampal cultures and slices (18–20). The use of synaptosomes to study surface receptors is an improvement over previous studies that routinely assessed the surface fraction of total proteins.
As in the hippocampus, most AMPARs in excitatory neurons in the LA are composed of GluR1/GluR2 or GluR2/GluR3 subunits, whereas AMPARs in inhibitory interneurons are devoid of GluR2 subunits (21–25). Therefore, observing GluR2 expression levels has the merit of ruling out changes in surface AMPARs on inhibitory interneuron synapses [see supporting information (SI) Text for additional details].
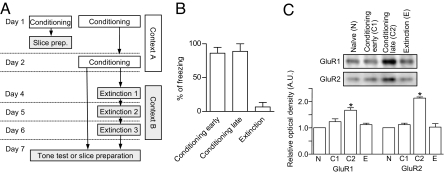
As shown in Fig. 3A, stronger conditioning and extinction protocols were adopted to improve the signal-to-noise ratio (see also SI Text). We compared the surface expression of GluR1 and GluR2 subunits among four groups of rats [naïve, conditioning-early (rats were conditioned with three tone-shock pairing and the samples were prepared 20 min after the last shock), conditioning-late (rats were conditioned with 2-day scheduled, six tone-shock pairing and the samples were prepared 5 days after the last shock), and extinction; n = 3 for each treatment group, with each sample comprising proteins prepared from five to six rats for a total of 68 rats; Fig. 3A].
Fig. 3.
Expression of surface AMPAR subunits of LA synaptosomes for behavior-trained rats. (A) The behavioral procedure for the experiments shown in B and C. As shown in this diagram, brain slices were prepared on day 7 for all groups except naïve controls. (B) Pooled behavioral results (conditioning-early, 86.3 ± 8.6%, n = 6; conditioning-late, 89.0 ± 11.0%, n = 6; extinction, 6.7 ± 6.7%, n = 6). (C) Relative optical band densities of GluR1 and GluR2 immunoreactivity expressed as mean ± SE (arbitrary unit). *, P < 0.05 vs. naïve controls (paired t test). (Inset) Representative immunoblots showing relative optical band densities of GluR1 and GluR2.
The surface expression of both GluR1 and GluR2 was enhanced in the conditioning-late group (P < 0.05, paired t test), but not in the conditioning-early group (P > 0.1, paired t test) relative to naïve controls. The subunit levels were reversed in the extinction groups (P > 0.1 vs. naïve controls, paired t test) (Fig. 3C). ANOVA indicated a main effect of group (GluR1, F3,8 = 14.12, P = 0.0015; GluR2, F3,8 = 52.26, P < 0.0001), with post hoc tests confirming that the expression levels of both GluR1 and GluR2 were significantly higher in the conditioned-late group than in the other three groups (P < 0.01 for all designated pairs, Newman–Keuls posttest), and that the expression levels of the three other groups did not differ significantly (P > 0.05 for all designated pairs, Newman–Keuls posttest).
Attenuation of both Ex Vivo Depotentiation and Extinction by a GluR2-Derived Peptide, a Blocker for Regulated AMPAR Endocytosis.
Previous studies have shown that short C-terminal sequences of GluR2 subunits are critical for regulated AMPAR endocytosis, which allows removal of surface AMPARs during hippocampal LTD (26). A synthetic peptide derived from the GluR2 carboxyl tail (GluR23Y; 869YKEGYNVYG877) has been shown to block regulated, but not constitutive, AMPAR endocytosis. This peptide appears to inhibit CA1 and nucleus accumbens LTD, but not hippocampal LTP (27). Furthermore, GluR23Y has been successfully introduced into neurons by fusing GluR23Y to the cell membrane transduction domain of the HIV-1 Tat protein (Tat-GluR2-derived peptide; see ref. 27). Tat-GluR23Y has also been shown to block NMDA-induced AMPAR endocytosis in cultured neurons, but had no discernible effects on constitutive endocytosis (27). Microinjection of Tat-GluR23Y into the nucleus accumbens, in which GluR23Y blocks LTD, appears to attenuate behavioral sensitization, indicating that the Tat-GluR2-derived peptide can be used to test the role of regulated AMPAR endocytosis in vivo (27). In sum, the GluR2-derived peptide appears to act as a selective antagonist that can block regulated AMPAR endocytosis in vitro and in vivo.
As shown in Fig. 4A, inclusion of the GluR23Y peptide (100 μg/ml) in the internal recording solution abolished the expression of ex vivo depotentiation (98.8% of control, n = 11, P > 0.8 vs. baseline, paired t test). In contrast, a control peptide in which the three tyrosine residues critical for the effectiveness of GluR23Y were replaced by alanine (GluR23A; AKEGANVAG) failed to alter the expression of ex vivo depotentiation (70.32% of control, n = 7, P < 0.01 vs. baseline, paired t test), supporting the specificity of GluR23Y in blocking ex vivo depotentiation. No GluR2-derived peptides altered basal synaptic transmission at T-LA synapses in amygdala slices prepared from conditioned rats, suggesting that the GluR2-derived peptides do not affect constitutive AMPAR endocytosis (Fig. 4B). These results are consistent with the notion that the GluR23Y peptide specifically blocks regulated AMPAR endocytosis, and thus blocks the expression of ex vivo depotentiation.
Fig. 4.
The GluR2-derived peptide, a blocker for regulated AMPAR endocytosis, inhibited ex vivo depotentiation. (A) Ex vivo depotentiation was blocked by the dialysis of GluR23Y into a postsynaptic neuron (100 μg/ml), but not by the dialysis of the control GluR23A (100 μg/ml). Conditioned rats were used, and brain slices were prepared on day 4 (see Fig. 1B). Postsynaptic neurons were dialyzed with the peptides for 28.6 ± 1.5 min (GluR23Y) or 28.1 ± 1.1 min (GluR23A) before the DHPG application. Representative paired traces are averages of four consecutive traces −2 to 0 min before and 38–40 min after DHPG application. (Scale bars: 20 ms and 50 pA.) (B) The dialysis of the GluR2-derived peptides had no significant effects on basal transmission. Conditioned rats were used. In the experiments shown, data acquisition was initiated 19.3 ± 1.2 min after the start of whole-cell recordings. Experimenters were blinded to the peptides.
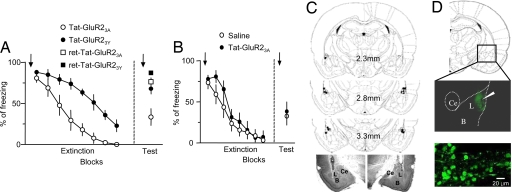
Accordingly, we used Tat-GluR23Y to determine whether ex vivo depotentiation plays a critical role in the extinction of fear memory. We performed intracranial microinfusion of Tat-GluR2-derived peptides or saline (15 pmol, 60 min before the first tone of both extinction and testing sessions) into the LA. We infused peptides for testing sessions and extinction training: during testing sessions, neurons in the LA may be exposed to a condition similar to extinction training because the LA is thought to be signaled from other brain areas that store extinction experience (28). As predicted, microinfusion of Tat-GluR23Y into the LA attenuated fear extinction compared with Tat-GluR23A-injected groups (Fig. 5A). The attenuating effect of the Tat-GluR23Y was evident on both short-term and long-term extinction [short-term extinction, for drug, F(1,91) = 25.46, P = 0.0002, for drug × trial interaction, F(7,91) = 2.542, P = 0.0195]. Tat-GluR23Y did not appear to impair retention of consolidated fear memory, as shown in experiments using retention controls to which the same behavioral and injection procedures as the extinction groups were applied except that a tone presentation during extinction sessions was omitted. One-way ANOVA indicated a main effect of group [ANOVA: F(3,22) = 9.585, P < 0.01; Tat-GluR23Y, n = 8; Tat-Glur23A, n = 7; ret-Tat-GluR23Y, n = 4; ret-Tat-GluR23A, n = 7] with post hoc tests confirming that freezing in the Tat-GluR23A-injected group differed significantly from that in the Tat-GluR23Y-injected group, Tat-GluR23Y-injected retention controls and Tat-GluR23A-injected retention controls (P < 0.01), and that freezing did not differ between Tat-GluR23Y-injected retention controls and Tat-GluR23A-injected retention controls (P > 0.05). Microinjection of Tat-GluR23A into the LA failed to affect fear extinction compared with the saline-injected group [short-term extinction, for drug, F(1,98) = 0.826, P = 0.3788, for drug × trial interaction, F(7,98) = 1.152, P = 0.3375; long-term extinction, unpaired t test, P = 0.7278; Fig. 5B]. Thus, these findings provide strong evidence that removal of GluR2-containing AMPARs at LA excitatory synapses contributes to fear extinction, which is consistent with the idea of extinction-induced reversal of the LA fear memory trace.
Fig. 5.
The GluR2-derived peptide, a blocker for regulated AMPAR endocytosis, attenuated extinction. (A) Microinjection of Tat-GluR23Y attenuated short-term and long-term extinction compared with injection of Tat-GluR23A, but it did not alter maintenance of consolidated fear memory as shown in the retention controls (see more details in Results). (B) Microinjection of Tat-GluR23A failed to alter fear extinction compared with injection of saline. (A and B) Extinction training was performed 48 h after fear conditioning, and the tone test was performed 24 h after completion of extinction training. The data were analyzed in blocks of two trials. The arrows indicate infusion and the error bars indicate SEM. (C) Location of cannula tips in the LA (L) of GluR23A and GluR23Y-injected groups, which received extinction training in A. (Upper) Schematic representation of the LA at three different rostrocaudal planes. The numbers represent the posterior coordinate from bregma. Injector placements in the LA are represented by the symbols (○, GluR23A injected; ●, GluR23Y injected). (Lower) Photomicrographs of representative cannula placements in the LA. Histology drawings were adapted from Paxinos and Watson (52). L, lateral nucleus; B, basal nucleus; CE, central nucleus. (D) (Upper) Diffusion of the fluorescent dansyl-Tat-GluR33Y peptide (1.5 nmol) within 1 h after the microinjection, as visualized with a multiphoton microscope (the flattened image of 10 optical sections, Δz = 10 μm). The white arrow indicates the end of injector cannula. (Lower) Peptide transduction in individual LA neurons at high magnification. Conditioned freezing was quantified by trained observers that were blind to the experimental groups.
Discussion
We have shown that fear extinction results in the reversal of conditioning-induced potentiation that has been consolidated at T-LA synapses. This reversal is mediated by a novel form of depotentiation that depends on activation of NMDARs and mGluRs. Accordingly, extinction results in reversal of the conditioning-enhanced expression of surface GluR1 and GluR2 in LA synaptosomal preparations. A GluR2-derived peptide that blocks regulated AMPAR endocytosis attenuated both depotentiation and extinction, supporting a link between these two events. The results described here are in line with previous findings. Neural activity in the LA has been shown to decrease after extinction in the rat and human (29–31) (but see also ref. 32). The NMDAR dependency of pp-LFS-induced ex vivo depotentiation fits nicely with a large body of evidence that fear extinction depends on amygdala NMDARs (33, 34). Similarly, blockade of group I mGluRs in the LA has recently been shown to attenuate fear extinction (35).
Our findings apparently contradict the prevailing theory of fear extinction. It is generally accepted that, after consolidation of fear memory, extinction of auditory fear memory does not erase the original fear memory (but see also refs. 36–41) but generates a new memory that inhibits the persistent original memory (28, 38, 42). There is strong evidence, mainly from behavioral studies, challenging the erasure or unlearning mechanism by showing that extinguished fear memory can relapse in specific retrieval conditions (28, 38, 42). However, this evidence does not rule out the possibility that multiple mechanisms underlie extinction of consolidated memory (38). That is, some fear memory traces are erased during extinction, but other traces may be spared and inhibited, allowing for relapse upon disinhibition. Our electrophysiological observations were restricted to a small subregion within the dorsolateral division of the LA (see Fig. 1A and SI Text for additional details), so neurons in other subregions may behave differently. In fact, Repa et al. (32) have shown that there are at least two different populations of neurons in the LA based on their responsiveness to fear conditioning. Memory traces encoded in cortical inputs into the LA (43–46) may also be resistant to extinction. In support of the inhibition theory, numerous behavioral and electrophysiological studies have provided evidence that intercalated inhibitory neurons in the medial side of the basolateral amygdala (BLA) receive excitatory inputs from the BLA and the prefrontal cortex and that LTP in these pathways (or at synapses in the prefrontal cortex) is responsible for encoding the inhibitory memory (47–49). Thus, the degree to which the depotentiation mechanism proposed here contributes to extinction of consolidated fear memory compared with the prevailing inhibitory mechanism remains to be determined.
Our studies do not exclude the involvement of presynaptic mechanisms in maintaining fear memory traces. McKernan and Shinnick-Gallagher (3) have suggested that an enhancement in presynaptic functions underlies conditioning-induced potentiation of synaptic efficacy. In addition, two other studies (50, 51) have demonstrated that activation of presynaptic group II mGluRs depresses synaptic transmission at both thalamic and cortical input synapses onto the LA through presynaptic mechanisms and reduces conditioned fear (only before consolidation). These previous findings suggest the presence of conditioning-induced potentiation of presynaptic functions that may be depotentiated during fear extinction.
In a previous study (37), where the surface expression of GluR1 in the whole neuron preparation was examined, extinction failed to reverse conditioning-induced enhancement in the surface expression of GluR1 after consolidation of fear memory, contradicting our results. There may be several explanations for this apparent discrepancy. First, we used a synaptosomal preparation rather than the whole neuronal preparation. Thus, we were able to eliminate extrasynaptic receptors that could interfere with detection of changes in surface receptors. Second, we adopted an extinction protocol that completely eliminated conditioned responses, whereas the authors of the previous study used a protocol that partially reduced them. This difference would be very critical if the relationship between synaptic weights and behavioral outputs were nonlinear. In fact, those authors observed a significant reversal of the GluR1 surface expression when conditioned rats were extinguished more strongly with the aid of d-cycloserine, a coagonist for NMDARs, although the same result could be caused by the recruitment of completely different extinction mechanisms induced by d-cycloserine as alluded to by those authors.
Collectively, our findings provide strong evidence that regulated endocytosis of AMPARs at excitatory synapses in LA neurons underlies extinction. In addition, our findings are consistent with the idea that the fear memory traces encoded in the LA are weakened during extinction. Understanding of the cellular mechanisms underlying memory extinction would help in designing new drugs and strategies for treating emotional malfunctioning.
Methods
Behavioral Procedures.
All procedures were approved by the Institute of Laboratory Animal Resources of Seoul National University. Male Sprague–Dawley rats (4–5 weeks old, except for experiments in Fig. 5, which used 10-week-old animals) were given free access to food and water and housed under an inverted 12/12-h light/dark cycle.
Cannula Implantation.
When fully anesthetized, rats were mounted on a stereotaxic apparatus (Kopf Instruments) and bilaterally implanted with 26-gauge stainless-steel cannulas (model C315G; Plastic Products) into the LA.
Slice Preparation and Electrophysiological Recordings.
Sprague–Dawley rats (4–5 weeks old) were anesthetized with halothane and decapitated. The isolated whole brains were placed in an ice-cold modified artificial cerebrospinal fluid (aCSF) solution. Coronal slices (300 or 400 μm) including the LA were cut and incubated in normal aCSF. A submersion-type recording chamber was continuously superfused with aCSF (33.0–34.5°C for field recordings; 31.0–33.0°C for whole-cell recordings). Extracellular field-potential recordings were made by using a parylene-insulated microelectrode (573210; A-M Systems) in 400-μm-thick slices. Whole-cell recordings were made by using an Axopatch 200A amplifier (Molecular Devices) in 300-μm-thick slices. Recordings were obtained by using pipettes with series resistances of 2.5–3.5 Mohm when filled with the following solution: 100 mM Cs-gluconate, 0.6 mM EGTA, 10 mM Hepes, 5 mM NaCl, 20 mM tetraethylammonium, 4 mM Mg-ATP, 0.3 mM Na-GTP, and 3 mM QX314, with the pH adjusted to 7.2 (SI Fig. 7).
For an extensive description, see SI Text.
Supplementary Material
ACKNOWLEDGMENTS.
This work was supported by Korea Research Foundation Grants KRF-2002-041-C00266, 2003-041-C20266, 2004-041-C00360, and 2005-041-C00422 funded by the Korean Ministry of Education and Human Resource Development, Korea Science and Engineering Foundation Grant R01-2004-000-10613-0 funded by the Korean Ministry of Science and Technology, and Brain Research Center of the 21st Century Frontier Research Program Grant M103KV010027-07K2201-02710 funded by the Korean Ministry of Science and Technology. S.L., J.K., K.P., H.P., B.S., and I.H. were supported by Brain Korea 21 Research Fellowships from the Korean Ministry of Education.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710548105/DC1.
References
- 1.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 2.Bliss TV, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 3.McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- 4.Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 7.Schafe GE, Doyere V, LeDoux JE. Tracking the fear engram: The lateral amygdala is an essential locus of fear memory storage. J Neurosci. 2005;25:10010–10014. doi: 10.1523/JNEUROSCI.3307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Doyere V, Debiec J, Monfils MH, Schafe GE, LeDoux JE. Synapse-specific reconsolidation of distinct fear memories in the lateral amygdala. Nat Neurosci. 2007;10:414–416. doi: 10.1038/nn1871. [DOI] [PubMed] [Google Scholar]
- 10.Yeh SH, Mao SC, Lin HC, Gean PW. Synaptic expression of glutamate receptor after encoding of fear memory in the rat amygdala. Mol Pharmacol. 2006;69:299–308. doi: 10.1124/mol.105.017194. [DOI] [PubMed] [Google Scholar]
- 11.Schafe GE, LeDoux JE. Memory consolidation of auditory Pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gale GD, et al. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiltgen BJ, Brown RA, Talton LE, Silva AJ. New circuits for old memories: The role of the neocortex in consolidation. Neuron. 2004;44:101–108. doi: 10.1016/j.neuron.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci USA. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thiels E, Barrionuevo G, Berger T. Excitatory stimulation during postsynaptic inhibition induces long-term depression in hippocampus in vivo. J Neurophysiol. 1994;72:3009–3116. doi: 10.1152/jn.1994.72.6.3009. [DOI] [PubMed] [Google Scholar]
- 16.Cho K, et al. A new form of long-term depression in the perirhinal cortex. Nat Neurosci. 2000;3:150–156. doi: 10.1038/72093. [DOI] [PubMed] [Google Scholar]
- 17.Cho K, Bashir ZI. Cooperation between mglu receptors: a depressing mechanism? Trends Neurosci. 2002;25:405–411. doi: 10.1016/s0166-2236(02)02228-2. [DOI] [PubMed] [Google Scholar]
- 18.Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunah AW, Standaert DG. Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J Neurosci. 2001;21:5546–5558. doi: 10.1523/JNEUROSCI.21-15-05546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Son GH, et al. Maternal stress produces learning deficits associated with impairment of NMDA receptor-mediated synaptic plasticity. J Neurosci. 2006;26:3309–3318. doi: 10.1523/JNEUROSCI.3850-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- 22.Farb CR, Aoki C, Ledoux JE. Differential localization of NMDA and AMPA receptor subunits in the lateral and basal nuclei of the amygdala: A light and electron microscopic study. J Comp Neurol. 1995;362:86–108. doi: 10.1002/cne.903620106. [DOI] [PubMed] [Google Scholar]
- 23.McDonald AJ. Localization of AMPA glutamate receptor subunits in subpopulations of non-pyramidal neurons in the rat basolateral amygdala. Neurosci Lett. 1996;208:175–178. doi: 10.1016/0304-3940(96)12585-4. [DOI] [PubMed] [Google Scholar]
- 24.Washburn MS, Numberger M, Zhang S, Dingledine R. Differential dependence on GluR2 expression of three characteristic features of AMPA receptors. J Neurosci. 1997;17:9393–9406. doi: 10.1523/JNEUROSCI.17-24-09393.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radley JJ, et al. Distribution of NMDA and AMPA receptor subunits at thalamo-amygdaloid dendritic spines. Brain Res. 2007;1134:87–94. doi: 10.1016/j.brainres.2006.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmadian G, et al. Tyrosine phosphorylation of GluR2 is required for insulin-stimulated AMPA receptor endocytosis and LTD. EMBO J. 2004;23:1040–1050. doi: 10.1038/sj.emboj.7600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brebner K, et al. Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science. 2005;310:1340–1343. doi: 10.1126/science.1116894. [DOI] [PubMed] [Google Scholar]
- 28.Maren S, Quirk GJ. Neuronal signaling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 29.Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: Parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 30.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 31.Hobin JA, Goosens KA, Maren S. Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J Neurosci. 2003;23:8410–8416. doi: 10.1523/JNEUROSCI.23-23-08410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat Neurosci. 2001;4:724–731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- 33.Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: Blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sotres-Bayon F, Bush DE, LeDoux JE. Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology. 2007;32:1929–1940. doi: 10.1038/sj.npp.1301316. [DOI] [PubMed] [Google Scholar]
- 35.Kim J, et al. Blockade of amygdala metabotropic glutamate receptor subtype 1 impairs fear extinction. Biochem Biophys Res Commun. 2007;355:188–193. doi: 10.1016/j.bbrc.2007.01.125. [DOI] [PubMed] [Google Scholar]
- 36.Medina JF, Nores WL, Mauk MD. Inhibition of climbing fibers is a signal for the extinction of conditioned eyelid responses. Nature. 2002;416:330–333. doi: 10.1038/416330a. [DOI] [PubMed] [Google Scholar]
- 37.Mao SC, Hsiao YH, Gean PW. Extinction training in conjunction with a partial agonist of the glycine site on the NMDA receptor erases memory trace. J Neurosci. 2006;26:8892–8899. doi: 10.1523/JNEUROSCI.0365-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myers KM, Ressler KJ, Davis M. Different mechanisms of fear extinction dependent on length of time since fear acquisition. Learn Mem. 2006;13:216–223. doi: 10.1101/lm.119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cain CK, Godsil BP, Jami S, Barad M. The L-type calcium channel blocker nifedipine impairs extinction, but not reduced contingency effects, in mice. Learn Mem. 2005;12:277–284. doi: 10.1101/lm.88805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rescorla RA, Wagner AR. A Theory of Pavlovian Conditioning: Variations in the Effectiveness of Reinforcement and Nonreinforcement. New York: Appleton-Century-Crofts; 1972. [Google Scholar]
- 41.Lin CH, et al. Identification of calcineurin as a key signal in the extinction of fear memory. J Neurosci. 2003;23:1574–1579. doi: 10.1523/JNEUROSCI.23-05-01574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- 43.Huang YY, Kandel ER. Postsynaptic induction and PKA-dependent expression of LTP in the lateral amygdala. Neuron. 1998;21:169–178. doi: 10.1016/s0896-6273(00)80524-3. [DOI] [PubMed] [Google Scholar]
- 44.Tsvetkov E, Carlezon WA, Benes FM, Kandel ER, Bolshakov VY. Fear conditioning occludes LTP-induced presynaptic enhancement of synaptic transmission in the cortical pathway to the lateral amygdala. Neuron. 2002;34:289–300. doi: 10.1016/s0896-6273(02)00645-1. [DOI] [PubMed] [Google Scholar]
- 45.Schroeder BW, Shinnick-Gallagher P. Fear learning induces persistent facilitation of amygdala synaptic transmission. Eur J Neurosci. 2005;22:1775–1783. doi: 10.1111/j.1460-9568.2005.04343.x. [DOI] [PubMed] [Google Scholar]
- 46.Boatman JA, Kim JJ. A thalamo-cortico-amygdala pathway mediates auditory fear conditioning in the intact brain. Eur J Neurosci. 2006;24:894–900. doi: 10.1111/j.1460-9568.2006.04965.x. [DOI] [PubMed] [Google Scholar]
- 47.Royer S, Pare D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience. 2002;115:455–462. doi: 10.1016/s0306-4522(02)00455-4. [DOI] [PubMed] [Google Scholar]
- 48.Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- 49.Likhtik E, Pelletier JG, Paz R, Pare D. Prefrontal control of the amygdala. J Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin CH, Lee CC, Huang YC, Wang SJ, Gean PW. Activation of group II metabotropic glutamate receptors induces depotentiation in amygdala slices and reduces fear-potentiated startle in rats. Learn Mem. 2005;12:130–137. doi: 10.1101/lm.85304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heinbockel T, Pape HC. Input-specific long-term depression in the lateral amygdala evoked by theta frequency stimulation. J Neurosci. 2000;20:RC68. doi: 10.1523/JNEUROSCI.20-07-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.