Abstract
Hematopoietic prostaglandin D2 synthase (hPGD2S) metabolizes cyclooxygenase (COX)-derived PGH2 to PGD2 and 15-deoxyΔ12–14 PGJ2 (15d-PGJ2). Unlike COX, the role of hPGD2S in host defense is ambiguous. PGD2 can be either pro- or antiinflammatory depending on disease etiology, whereas the existence of 15d-PGJ2 and its relevance to pathophysiology remain controversial. Herein, studies on hPGD2S KO mice reveal that 15d-PGJ2 is synthesized in a self-resolving peritonitis, detected by using liquid chromatography–tandem MS. Together with PGD2 working on its DP1 receptor, 15d-PGJ2 controls the balance of pro- vs. antiinflammatory cytokines that regulate leukocyte influx and monocyte-derived macrophage efflux from the inflamed peritoneal cavity to draining lymph nodes leading to resolution. Specifically, inflammation in hPGD2S KOs is more severe during the onset phase arising from a substantial cytokine imbalance resulting in enhanced polymorphonuclear leukocyte and monocyte trafficking. Moreover, resolution is impaired, characterized by macrophage and surprisingly lymphocyte accumulation. Data from this work place hPGD2S at the center of controlling the onset and the resolution of acute inflammation where it acts as a crucial checkpoint controller of cytokine/chemokine synthesis as well as leukocyte influx and efflux. Here, we provide definitive proof that 15d-PGJ2 is synthesized during mammalian inflammatory responses, and we highlight DP1 receptor activation as a potential antiinflammatory strategy.
Keywords: antiinflammatory, cyclooxygnease, drug development, eicosanoids, innate immunity
Cyclooxygenase (COX) metabolizes phospholipase A2-derived arachidonic acid to prostaglandin (PG)H2, which is further metabolized by a series of downsteam synthases to the prostanoids. Indeed, the expression of the particular downstream enzyme and its coupling either to constitutively expressed COX1 or to inducible COX2 will determine the profile and levels of arachidonic metabolites released by cells. Thus, targeting COX will diminish most if not all prostanoids, which, in the case of new-generation COX2 inhibitors, resulted in prostacyclin abatement, enhanced risk of cardiovascular side effects, and the eventual withdrawal of selective COX2 inhibitors from clinical usage. In this event, attention has now shifted to understanding the role of COX downstream synthases in inflammation and the cardiovascular system in the hope of adding more selectivity with fewer side effects.
In an attempt to understand the role of COX-related downstream synthase in host defense, we found that the COX2/hematopoietic PGD2 synthase pathway resolves both acute innate (1, 2) and adaptive immune responses (3). Hematopoietic PGD2 synthase (hPGD2S) metabolizes COX-derived PGH2 to PGD2 (4), which may activate two G protein-coupled receptors, DP1 and DP2. DP1 regulates dendritic cell function (5), and DP2 promotes allergic inflammation (6–8). Although controversial, it is believed that PGD2 is initially converted to PGJ2 and 15-deoxy-PGD2 (15d-PGD2) in an albumin-independent manner. Thereafter, the cyclopentenone PGs (cyPGs) 15-deoxy-Δ12, 14-PGJ2 (15d-PGJ2) and Δ12-PGJ2 are generated from PGJ2 by albumin-independent and -dependent reactions, respectively (9). Although 15d-PGJ2 is a putative ligand for peroxisome proliferator-activated receptor (PPAR)γ (10), the skepticism with cyPGs lies in whether 15d-PGJ2 is formed in pathophysiological settings at sufficient levels to exert meaningful biological effects in mammalian systems.
Investigating the role of hPGD2S in acute inflammation, we provide definitive proof using liquid chromatography–tandem MS (LC-MS/MS) that 15d-PGJ2 is synthesized in vivo. In a series of mechanistic studies, we show that along with PGD2 acting on its DP1 receptor, 15d-PGJ2 controls the balance of cytokines and chemokines that regulate leukocyte trafficking during acute inflammation as well as the efflux of macrophage to draining lymphatics leading to its resolution such that in hPGD2S-deficient mice, inflammation is grossly exaggerated and fails to resolve. These data not only prove that cyPGs exist in mammalian systems and exert potent antiinflammatory and proresolution properties, but data from this work also highlight the potential of activating DP1 receptors as a future antiinflammatory strategy.
Results
hPGD2S-Derived Lipid Mediators in Acute Inflammation.
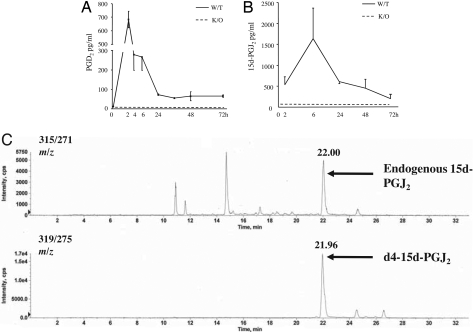
In the first instance, levels of PGD2 and 15d-PGJ2 were quantified throughout the time course of resolving murine peritonitis. PGD2 was maximal 2 h after zymosan injection, with levels waning by 24 h (Fig. 1A). Contrary to common belief, 15d-PGJ2 was detectable in cell-free inflammatory exudates of wild-type mice by LC-MS/MS (Fig. 1 B and C). It was elevated at 2 h, maximum between 6 and 24 h, and had declined by 48 h/72 h, with average levels of between 0.5 and 5 ng/ml. PGD2 and 15d-PGJ2 were absent from experimental WT controls (0 h). Given its instability (9), we controlled for potential ex vivo degradation of PGD2 to 15d-PGJ2 during sample processing by spiking inflammatory fluids in situ with deuterated PGD2 and found that the detected 15d-PGJ2 was native and not deuterated 15d-PGJ2. These data, coupled with the finding that neither PGD2 nor 15d-PGJ2 was detectable in the exudates of hPGD2S KOs (Fig. 1 A and B), confirmed that 15d-PGJ2 is a bona fide PGD2 eicosanoid metabolite formed in vivo during resolving inflammation.
Fig. 1.
hPGD2S synthesizes 15d-PGJ2 during zymosan-induced resolving peritonitis. (A and B) hPGD2S-derived PGD2 (A) and 15d-PGJ2 (B) were extracted from the cell-free inflammatory exudates and quantified by EIA and electrospray triple quadruple LC-MS/MS, respectively. Ex vivo degradation of PGD2 to 15d-PGJ2 during sample processing was controlled for by spiking inflammatory fluids in situ with deuterated PGD2. (C) LC-MS/MS spectra of detected 15d-PGJ2, which was found to be native and not deuterated. For eicosanoid analysis n = 6–8 animals were present in each group with the exception of 15d-PGJ2, which had 2–6 samples per group. Data are represented as the mean ± SEM.
hPGD2S Tempers the Severity of Acute Inflammation Through DP1 and 15d-PGJ2.
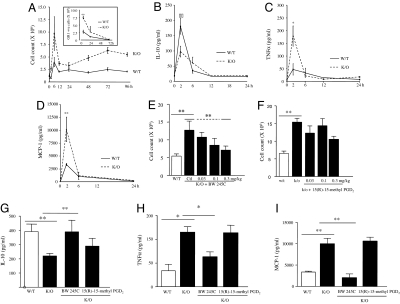
We sought to determine the role that PGD2 and 15d-PGJ2 play in inflammation. hPGD2S−/− mice show a more aggressive early (4–6 h) response to zymosan, characterized by a 2-fold increase in polymorphonuclear leukocyte (PMN) influx compared with WT (Fig. 2A). By 48 h, PMN numbers in KOs were reduced to levels similar to that found in WT (Fig. 2A, Inset), indicating that although hPGD2S controls early PMN influx it does not influence their survival or clearance from inflammatory sites. This was confirmed by detecting little difference in PMN apoptosis by annexin V/propidium iodide (PI) labeling as well as total leukocyte apoptosis by the same technique and by caspase-3 activity [supporting information (SI) Fig. 5]. Increased inflammation in hPGD2S−/− mice at onset was associated with a decrease in antiinflammatory IL-10 (Fig. 2B) and an increase in proinflammatory TNFα (Fig. 2C) and monocyte chemoattractant protein 1 (MCP-1) (Fig. 2D). This hyperinflammatory phenotype in KOs was rescued to that of WT at 6 h by a selective DP1 (BW245C, Fig. 2E) but not a DP2 receptor agonist [15(R)-15-methyl PGD2] (Fig. 2F), independently of apoptosis. Treatment of hPGD2S−/− mice with BW245C also restored the imbalance in TNFα and MCP-1 vs. IL-10 to levels found in WT (Fig. 2 G–I). Thus, by signaling through the DP1 receptor, hPGD2S-derived PGD2 controls the onset phase of acute inflammation and its balance of pro- and antiinflammatory cytokines and chemokines. A range of other cytokines and chemokines were measured, and their relative levels in wild types and hPGD2S KOs are presented in SI Table 1. 15d-PGJ2 also reduced cell numbers in the peritoneal cavity of mice but by increasing leukocyte apoptosis as determined by both annexin V/PI labeling and caspase activity, which resulted in a concomitant elevation in IL-10 and TGFβ1, cytokines typically released by macrophage upon recognition of apoptotic leukocytes (SI Fig. 6 A–F). We believe that these data do not represent the true role of 15d-PGJ2 in vivo because there is little difference in total cell apoptosis between WT and KOs at onset (5.05 ± 0.6% of controls expressing phosphatidylserine vs. 3.9 ± 0.64% for KOs). These potentially misleading data may arise from injecting a bolus dose of exogenous and highly reactive electrophilic 15d-PGJ2 vs. its controlled endogenous release by intracellular hPGD2S. To understand how endogenous 15d-PGJ2 works in situ and to bypass the apoptosis-inducing effects caused by a single bolus injection, we examined its effects on peritoneal leukocytes ex vivo below.
Fig. 2.
PGD2 acting by the DP1 receptor controls the balance of pro- vs. antiinflammatory mediators and leukocyte trafficking during acute inflammation. (A) Intraperitoneal zymosan resulted in a self-limiting inflammatory response that peaked at 6–12 h. Inflammation in hPGD2S−/− mice (K/O) was 2-fold greater than in WT at 6 h and failed to resolve, with the early-onset phase characterized by predominantly GR1-positive PMNs with higher numbers of monocytes/macrophages compared with WT (see Fig. 4A). (Inset) Appropriate isotype control antibodies. (B–D) Cell-free exudate levels of IL-10 (B) were lower in hPGD2S−/− mice whereas TNFα (C) and MCP-1 (D) were higher. (E–I) The hyperinflammation in hPGD2S−/− mice was redressed by the DP1 receptor agonist BW245C (E) but not by the DP2 agonist 15(R)-15-methyl PGD2 (F), resulting in a corresponding (G–I) equilibration of inflammatory cytokines and chemokines to levels similar to controls. n = 6–8 animals per group; *, P ≤ 0.05; **, P ≤ 0.01, as determined by ANOVA followed by Bonferroni t test, with data expressed as mean ± SEM.
hPGD2S Exerts Its Protective Effects on Resident Macrophages and Lymphocytes.
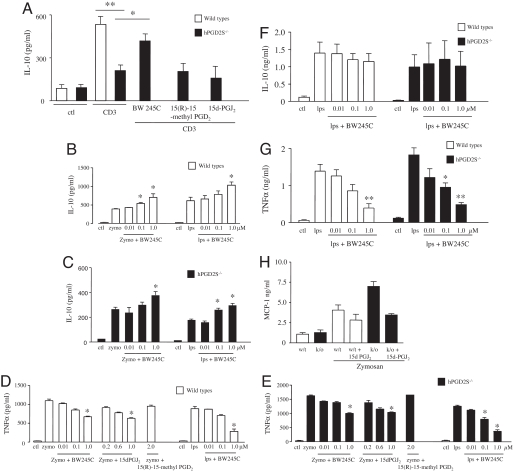
In the naïve or uninflamed murine peritoneum, T and B lymphocytes constitute ≈40% of the total cell population (≈1.5 × 106), with the remaining being resident macrophages, determined by using CD3/CD19, B220, CD5, and F4/80 labeling quantified by FACS. In the case of the peritoneal cavity, resident macrophages dictate the magnitude of the ensuing response with peritoneal lymphocytes possessing innate immune regulatory effects (11–13). In this event, we next determined on which cell types within the peritoneum PGD2 and 15d-PGJ2 exert their antiinflammatory effects. Separated peritoneal T and B cells as well as macrophages were stimulated with either LPS or zymosan (B cells and macrophages) or anti-CD3 antibody (T cells). Cytokines were measured 24 h later in response to BW245C (DP1 receptor agonist), 15(R)-15-methyl PGD2 (DP2 receptor agonist), or 15d-PGJ2 at concentrations that do not increase apoptosis (SI Fig. 7). IL-10 from hPGD2S-deficient peritoneal T cells was significantly lower than WT but was reversed by BW245C (DP1 agonist); neither 15(R)-15-methyl PGD2 (DP2 receptor agonist) nor 15d-PGJ2 had any effect (Fig. 3A). Similarly, IL-10 secretion from zymosan or LPS-stimulated hPGD2S-deficient peritoneal B cells (265 ± 20 and 178 ± 12 pg/ml, respectively) was lower compared with WT [387 ± 25 (P ≤ 0.05) and 625 ± 35 (P ≤ 0.01) pg/ml, respectively] and was rescued by BW245C only (Fig. 3 B and C). Conversely, synthesis of TNFα from these zymosan- or LPS-stimulated hPGD2S-deficient B cells (1,625 ± 175 and 1,260 ± 152 pg/ml, respectively) was higher compared with WT [1,160 ± 105 (P ≤ 0.05) and 810 ± 65 pg/ml (P ≤ 0.05), respectively] but were reduced by BW245C and 15d-PGJ2 but not with 15(R)-15-methyl PGD2 (Fig. 3 D and E). In contrast to T and B lymphocytes, IL-10 from peritoneal macrophages was not elevated by BW245C (Fig. 3F), DP2 receptor activation, or by 15d-PGJ2 (data not shown). However, these cells did show a reduction in TNFα and MCP-1 after treatment with BW245C and 15d-PGJ2, respectively (Fig. 3 G and H). Similar results were obtained by using bone marrow-derived macrophages from these animals. These data show that hPGD2S-derived lipid mediators exert differentially protective effects on peritoneal-resident macrophages as well as lymphocytes, thereby controlling the balance of cytokines and chemokines that orchestrate innate inflammatory responses.
Fig. 3.
hPGD2S controls the inflammatory phenotype of peritoneal leukocytes. (A–C) IL-10 release from stimulated hPGD2S-deficient T cells (A) as well as B cells (B and C) was not only lower than that from wild types but was rescued by DP1 activation (BW245C). DP2 receptor activation with 15(R)-15-methyl PGD2 was without effect in these experiments. (D and E) Conversely, TNFα release from hPGD2S-deficient B cells was greater than that from wild types and was reversed with BW245C and 15d-PGJ2 but not 15(R)-15-methyl PGD2. (F–H) In macrophages, whereas hPGD2S had little effect on IL-10 (F), BW245C reduced TNFα (G) whereas 15d-PGJ2 also ameliorated levels of MCP-1 secretion (H). These data show the differential regulatory effects of hPGD2S-derived lipid mediators on inflammatory leukocyte phenotype. Cells were isolated from n = 3 animals, and all experiments done in triplicate on two separate occasions to confirm original findings. *, P ≤ 0.05; **, P ≤ 0.01, as determined by ANOVA, followed by Bonferroni t test, with data expressed as mean ± SEM.
hPGD2S Deficiency Severely Compromises Resolution.
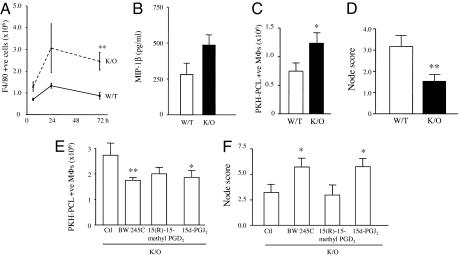
Finally, one striking finding of these studies was impaired resolution in hPGD2S−/− mice characterized by macrophage (Fig. 4A) as well as lymphocyte accumulation (0.24 ± 0.04 × 106 T and B cells in WT vs. 0.8 ± 0.1 × 106 in KOs; P ≤ 0.01). SI Fig. 8 shows a breakdown of lymphocyte subtypes in these animals. The implication of lymphocyte accumulation during resolution in hPGD2S−/− mice is unknown at this stage but is reminiscent of results published showing the persistence of lymphocytes in the inflamed paws of COX2 KO mice (14) and raises questions regarding the long-term impact of COX inhibitors on the progression of chronic inflammatory diseases. Although increased macrophage numbers during resolution in hPGD2S−/− mice may have arisen from elevated MCP-1 (Fig. 2D) and macrophage inflammatory protein 1β (MIP-1β levels) (Fig. 4B), a failure to clear to the draining lymphatics (15, 16) may also provide an explanation. To investigate this hypothesis, the selective macrophage label PKH26-PCL (15, 16) was injected i.p. at the peak of macrophage accumulation (24 h), revealing increased numbers of PKH26-PCL-labeled macrophages in the peritoneal cavities of hPGD2S−/− mice (Fig. 4C) and a corresponding reduction in the parathymic load of these cells compared with WT at 72 h (Fig. 4D). Adding BW245C or 15d-PGJ2 to hPGD2S−/− mice alleviated peritoneal macrophage accumulation (Fig. 4E) and increased numbers of PKH26-PCL macrophages in the parathymic lymph nodes (Fig. 4F). The DP2 receptor agonist was without effect. Notably, BW245C can be added 6 h, 12 h, or 24 h after inflammation and will exert a macrophage clearance effect at all of these time points, attributing a robust proresolution property to DP1 (data not shown). Thus, hPGD2S-derived PGD2 and 15d-PGJ2 play a role in clearing inflammatory macrophages from the inflamed peritoneum to local draining lymph nodes.
Fig. 4.
hPGD2S deficiency compromises inflammatory resolution. (A and B) Although PMNs dominate the inflamed peritoneal cavity at the early-onset phase in hPGD2S−/− (K/O) mice (Fig. 2A, Inset) F4/80 positive macrophages were the predominant cell types during resolution (A) WT, wild type. Increased macrophage numbers in hPGD2S−/− mice could arise from enhanced MIP-1β (B) as well as MCP-1 (Fig. 2D) synthesis and/or a failure to exit the peritoneum to the parathymic lymph nodes (15, 16). To determine the latter, mice were injected i.p. with the macrophage label PKH-PCL26 at either 24 h or 48 h. (C) A significantly greater number of PKH26-PCL-positive macrophages (double labeling with FITC-labeled F4/80) were recorded in the cavity of hPGD2S−/− mice compared with WT at 72 h. (D) Correspondingly fewer labeled macrophages were found by histology in the parathymic lymph nodes of the KOs. (E and F) Adding BW245C (DP1 receptor agonist) or 15d-PGJ2 to the inflamed cavity of hPGD2S−/− mice reversed the accumulation of macrophages in the peritoneum (E) and resulted in increased numbers of labeled macrophages in parathymic lymph nodes (F). n = 10 animals per group; *, P ≤ 0.05; **, P ≤ 0.01, as determined by ANOVA, followed by Bonferroni t test, with data expressed as mean ± SEM.
Discussion
We describe a central role for hPGD2S in controlling the onset and resolution of innate immune-mediated inflammation. First, we show that the protective nature of hPGD2S is mediated by DP1 and 15d-PGJ2, which control the balance of pro- and antiinflammatory cytokine synthesis. These data contrast with the pathogenic role PGD2 plays in allergic inflammation through DP2 (6–8). However, as with the entire field of eicosanoid biology, lipid mediators can be either protective or pathogenic depending on disease etiology, cell types present, and eicosanoid receptor expression (17). For instance, signaling through DP2 causes bronchoconstriction as well as Th2 and eosinophil cell accumulation (6–8). In contrast, PGD2 activation of DP1 may also suppress asthmatic symptoms by targeting lung dendritic cells, resulting in increased Treg cells that dampen inflammation in an IL-10-dependent manner (18). We found no difference in the numbers of CD4+/CD25+ cells between hPGD2S−/− and WT in experimental peritonitis (SI Fig. 9), and we propose that in acute inflammation at least, the protective effects of PGD2 are directed toward modulating resident leukocyte inflammatory phenotypes and are not reliant on recruitment of Treg cells. On this theme, PGD2 along with PGE2 can also induce PMN 15-lipoxygenase expression in a partly cAMP-dependent manner, causing the subsequent release of antiinflammatory and proresolution lipoxin A4 (19). It is conceivable, therefore, that some of the protective and cytokine regulatory effects of PGD2 in acute inflammation may also arise from its induction of other families of protective lipid mediators. Thus, from the current data and that published by others (5, 18), we suggest that DP1 receptor activation may be an attractive antiinflammatory target capable of modulating both innate and adaptive immune responses of different etiologies. In particular, we found that DP1 activation exerts its antiinflammatory effects at many stages throughout the inflammatory response, i.e., when administered either prophylactically or therapeutically, thereby demonstrating greater pharmacological flexibility than, for example NSAIDs, which although effective during acute stages of inflammation, subvert proresolution pathways (1, 19).
Controversy exists over whether 15d-PGJ2 is synthesized in vivo and whether it possesses relevant patho/physiological functions. This debate arose, in part, when we originally reported that COX2-derived PGD2 and 15-dPGJ2 brought about acute inflammatory resolution (1). Controversy also arose not so much because we suggested that COX2 was protective at a time when the emphasis was on developing COX2 inhibitors, but because we used an ELISA to quantify 15d-PGJ2. In particular, the reactive nature of 15d-PGJ2 raised questions regarding the accuracy of using an antibody-based measuring system. This result, coupled with subsequent reports using physical methods to show only negligible levels of 15d-PGJ2 in various biological systems (20), questioned the importance of 15d-PGJ2 in biology. In this work we show, by using LC-MS/MS on samples obtained from a resolving inflammation, that 15d-PGJ2 does exist in vivo at levels up to 5 ng/ml. We controlled for degradation of unstable PGD2 to 15d-PGJ2 during sample processing by spiking inflammatory fluids in situ with deuterated PGD2, and we found that the detected 15d-PGJ2 was native and not deuterated 15d-PGJ2. These data, coupled with the finding that neither PGD2 nor 15d-PGJ2 was detectable in the exudates of hPGD2S KOs (Fig. 1 A and B) and that the hyperproliferative phenotype of hPGD2S−/− T cells from a delayed-type hypersensitivity reaction was reversed with 15d-PGJ2 (3), confirm that 15d-PGJ2 is an endogenously generated protective PGD2 metabolite formed in vivo during resolving inflammatory reactions. The importance of this finding cannot be underestimated. cyPGs, derived from PGs of the A or D series possess antiinflammatory (21), antiviral (22), and anticancer (23) properties by activating either nuclear membrane-bound PPARs (24) or by forming covalent adducts with thiols through the unsaturated carbonyl group in the cyclopentenone moiety (25, 26). Importantly, protein modification by cyPGs does not occur randomly with cyPGs targeting defined cysteine residues within certain proteins in an apparently pH-dependent manner (27). Moreover, structural determinants of either the protein or the cyPG may be important for the specificity of protein modification such that cyPGs with diverse structures could selectively modify distinct proteins in cells. Thus, levels of cyPGs in the extracellular environment may only represent the tip of the iceberg, and their true level in biological systems may be much higher. The lack of detectable cyPGs in biological fluids does not necessarily exclude their existence within cells and their potential to exert meaningful biological effects. Indeed, in a series of experiments we estimate that of 15d-PGJ2 added exogenously to biological systems, >50% binds BSA. Moreover, in cardiomyocyte cell cultures, >80% binds to culture medium supplemented with 10% FCS with ≈16% binding to or being metabolized by cardiomyocytes, leaving <4% detectable by LC-MS/MS (SI Fig. 10). It is for this reason that we need to add quantitatively more 15d-PGJ2 back to inflammatory models to mimic the biological effects of endogenously produced 15d-PGJ2. This requirement is illustrated in SI Fig. 6, where more 15d-PGJ2 is injected i.p. than is detectable in peritoneal fluids (Fig. 1B). We suggest that the role of 15d-PGJ2 in self-limiting inflammatory responses is manifold and does not necessarily overlap with that of PGD2, controlling chemokine and cytokine synthesis as well as intracellular signaling and leukocyte apoptosis. We found that exogenous 15d-PGJ2 exerts proresolution effects if used pharmacologically by enhancing leukocyte apoptosis, the net outcome being similar to that found with the cyclin-dependent kinase inhibitor roscovitine, which enhanced PMN apoptosis and hastened resolution (28).
In addition to tissue resident histiocytes, there is the influx of monocytes during inflammation, which differentiate into large granular macrophages designed to phagocytose effete leukocytes. The fate of Reiter cells (macrophages containing dead cells) during resolution is poorly understood, with evidence that macrophages can undergo programmed cell death (29) and/or clearance from the peritoneum to the parathymic lymph nodes in a very late antigen (VLA)-4- and VLA-5-dependent manner (15, 16). Although the control of phagocyte clearance is poorly understood, we found an excess of macrophages in the inflamed peritoneum of hPGD2S−/− mice and a corresponding deficit of these cells in the draining lymph nodes compared with controls. This result could have arisen from accelerated monocyte influx, mediated by elevated MCP-1 and MIP-1β (Figs. 2D and 3B) and/or failed lymphatic efflux. Using a phagocytosable fluorescent cell tracker, the peritoneal pool of accumulated macrophages in hPGD2S−/− mice at resolution was found to be reduced by BW245C or 15d-PGJ2 and was associated with an increase in macrophages in the parathymic lymph node. We therefore suggest that DP1 is a robust pharmacological target that, in addition to stemming PMN trafficking, could be used to disseminate macrophages from sites of chronic inflammation where macrophages play a pathogenic role. A similar result was found recently with ω-3 polyunsaturated fatty acid-derived resolvin E1 and protectin D1, which facilitated leukocyte trafficking to lymph nodes and spleen, collectively underscoring the proresolution properties of lipid mediators in acute inflammation (30).
Lymphocytes were also found to accumulate in hPGD2S−/− mice at resolution, which we suspect resulted from enhanced influx in response to elevated chemokines in these animals rather than increased local lymphocyte proliferation because there was no significant difference in thymidine incorporation in hPGD2S−/− lymphocytes compared with controls (data not shown). Indeed, there was a trend toward a reduction in lymphocytes from KOs in these experiments, in contrast to results obtained with hPGD2S-deficient lymphocytes from an antigen-induced arthritis model (3), which showed elevated proliferation in hPGD2S KO mice. These differences are most likely the result of a differential role for both hPGD2S-derived lipid mediators and lymphocyte subsets and activation states in inflammatory disease of dissimilar etiologies.
In summary, we provide proof that hPGD2S synthesizes 15d-PGJ2 during mammalian defense responses and together with PGD2, acting through the DP1 receptor, plays a central role in controlling the onset of acute inflammation and its resolution by controlling the balance of pro- vs. antiinflammatory cytokines as well as macrophage clearance through draining lymphatics. Here, we have highlighted the potentially antiinflammatory and proresolution properties of cyPGs as well as DP1 receptors.
Materials and Methods
Animal Maintenance and Induction of Inflammation.
hPGD2S KO mice were generated as described (3). Animals were bred under standard conditions and maintained in a 12 h/12 h light/dark cycle at 22 ± 1°C and given food and tap water ad libitum in accordance with United Kingdom Home Office regulations. Peritonitis was induced by the i.p. injection of 1 mg of type A zymosan (Sigma) in 0.5 ml of sterile PBS. Cells were obtained by using 2 ml of sterile PBS to wash out the inflamed peritoneal cavity, and they were enumerated by hemocytometer at the time points stated in Results.
Eicosanoid Analysis.
Samples stored at −20°C were thawed at room temperature and spiked with 4 ng of the internal standard d4–15d-PGJ2 and acidified to pH 3. Solid-phase extraction was performed with Varian 3M Empore high-performance extraction disk cartridges, and the columns were washed with 1 ml of H2O and 1 ml of heptane and eluted with 1 ml of ethyl acetate. The eluate was dried under nitrogen and analyzed by electrospray triple/quadruple LC-MS/MS (Sciex API 3000; PerkinElmer). The conditions for the LC-MS/MS were: C18 columns (100 × 0.2 mm) with elution volume 200 μl/min consisting of a mobile phase 0–1 min of distilled H2O (pH 3), MeCN 75:25%, followed by a gradient mobile phase to 100% MeCN. 15d-PGJ2 was detected and quantified in negative ion mode, and the electrospray potential was maintained at −4 to 4.5 kV and heated to 500°C. For MS-MS analysis, 15d-PGJ2/internal standards were subjected to collision-induced fragmentation. Tetradeuterated (d4) 15d-PGJ2, PGD2, and 15d-PGJ2 were purchased from Cayman Chemicals. Varian 3M Empore high-performance extraction disk cartridges were purchased from JVA Analytical, Ltd. All other common laboratory chemicals were purchased from Sigma. For PGD2, samples were extracted C18 columns, treated with methoxylamine hydrochloride (MOX HCl), and the resulting stable PGD2-MOX was measured by EIA (Cayman Chemicals).
Cytokine/Chemokine, FACS, and Caspase Activity Analysis.
Cytokines and chemokines were measured initially by multiplex cytokine array analysis (Bio-Rad) using the manufacturer's protocols. Specific mediators of interest [IL-10, TNFα (eBiosciences), and MCP-1 (Becton Dickinson)] were further quantified by ELISA according to manufacturer's instructions. FACS was carried out on Becton Dickinson FACSCalibur with data analyzed by Cellquest. Leukocytes were incubated with antibodies to CD3 (Serotec), B cells (Ly220; Serotec), natural killer and γ/δ T cells (gift from T. Hussell, Kennedy Institute, U.K.), GR1 (BD PharMingen), or F4/80 (Caltag Laboratories) using isotype antibodies (Serotec) and compensated as appropriate for dual labeling. For apoptosis, cells were incubated with annexin V/PI (Becton Dickinson). For caspase 3 activity, frozen cell pellets were lysed in ice-cold RIPA buffer [20 nM Tris (pH 7.4), 150 mM NaCl, 1% Nonidet P-40, 0.5% (wt/vol) sodium deoxycholate, 0.1% SDS Promega)] for 10 min. After centrifugation at 16,000 × g for 15 min at 4°C, supernatants were aspirated, and protein was quantified by the Bradford reaction. Samples were incubated with 50 μM substrate in caspase assay buffer [213.5 mM Hepes (pH 7.5), 31.25% (wt/vol) sucrose, and 0.3125% CHAPS] for 1 h, and fluorescence was measured on a microplate reader (Fluostar Galaxy; BMG Laboratory Technologies) with excitation at 380 nm and emission set at 460 nm. For each sample, four replicates were assayed, with two replicates containing 50 μM the caspase-3 inhibitor (Ac-DEVD-CHO) and the remaining pair containing vehicle. Fluorescence readings from wells containing inhibitor were subtracted from total fluorescence, and results were calculated as nmol of aminomethyl couramin (AMC) per mg of protein per min.
Leukocyte Separation and in Vitro Culturing.
Bone marrow-derived or peritoneal macrophages were isolated by adherence to the bottom of 6-well tissue culture plates after incubation for 90 min at 37°C in a humid incubator. Nonadherent cells were removed and used to isolate T and B lymphocytes. The adherent cells were eluted with Versene, washed with 2% FCS in HBSS, and resuspended at 107 cells per ml. These cells were further depleted of contaminant T and B cells by using magnetic beads coated with rat monoclonal antibodies to mouse CD3 or B220 (Dynal Biotech, Ltd.). T and B lymphocytes were isolated by using the Dynal mouse B cell (or T cell) negative isolation kit according to the manufacturer's instructions. In brief, a mixture of rat monoclonal antibodies with specificity to all mouse non-B cells (or non-T cells when isolating T cells) was added to cell suspensions and incubated for 20 min at 4°C. Cells coated with the added monoclonal antibodies were then removed with magnetic beads coated with sheep polyclonal antibody to rat Ig. The purity of the cells was regularly >95%. B lymphocytes and macrophages were counted by hemocytometer and cultured in RPMI medium 1640 with 10% (vol/vol) FCS and antibiotics [100 units/ml benzylpencillin, 10 μg/ml streptomycin, 2.5 μg/ml amphotericin (all from Sigma–Aldrich)] in sterile 24-well culture plates (VWR) and incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2. Cells were incubated with the selective DP1 agonist (BWC245; Cayman), DP2 agonist [15(R)-15 methyl PGD2; Cayman), or 15d-PGJ2 (Cayman) with medium as control and then stimulated with 100 μg/ml zymosan type A (Sigma), 1 μg/ml LPS, or vehicle. The experiment was terminated 24 h later, and supernatant was stored at −80°C until further use. The protocol for T cells was similar apart from stimulation with anti-CD3 antibody.
Macrophage Clearance Assays.
Two milliliters of 500 nM macrophage-specific stain PKH26-PCL (Sigma) was injected into the inflamed peritoneal cavity with cells and parathymic lymph nodes isolated at time points stated in results section. Cells staining positive for PKH26-PCL (FL2 channel) were identified with FITC-labeled F4/80 (FL1 channel). Staining was found almost exclusively within macrophages. Parathymic lymph nodes were extracted and snap frozen in OCT1, and serial sections (minimum 15 sections per node) were examined for the presence of fluorescent PKH26-PCL-labeled macrophages. All sections were scored blindly by two independent observers by using an Olympus Axioscop microscope and our validated analog scale (16).
Supplementary Material
ACKNOWLEDGMENTS.
We acknowledge the technical assistance of Steven Bottoms, Rita Jones, and Julius Kieswich, and we thank Dr. Tracy Hussell, Kennedy Institute for Rheumatology, U.K., for supplying antibodies to natural killer and γ/δ T cells along with Dr. Y. Urade of the Osaka Bioscience Institute Japan for supplying hPGD2S KO mice. D.W.G. is a Wellcome Trust-funded Career Development Fellow, and R.R. is a Kidney Research U.K.-funded Clinical Fellow.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.S. is a guest editor invited by the Editorial Board.
See Commentary on page 20647.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707394104/DC1.
References
- 1.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 2.Gilroy DW, Newson J, Sawmynaden P, Willoughby DA, Croxtall JD. FASEB J. 2004;18:489–498. doi: 10.1096/fj.03-0837com. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi SG, Newson J, Rajakariar R, Jacques TS, Hannon R, Kanaoka Y, Eguchi N, Colville-Nash P, Gilroy DW. Proc Natl Acad Sci USA. 2006;103:5179–5184. doi: 10.1073/pnas.0507175103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urade Y, Hayaishi O. Vitam Horm. 2000;58:89–120. doi: 10.1016/s0083-6729(00)58022-4. [DOI] [PubMed] [Google Scholar]
- 5.Hammad H, de Heer HJ, Soullie T, Hoogsteden HC, Trottein F, Lambrecht BN. J Immunol. 2003;171:3936–3940. doi: 10.4049/jimmunol.171.8.3936. [DOI] [PubMed] [Google Scholar]
- 6.Mandal AK, Zhang Z, Ray R, Choi MS, Chowdhury B, Pattabiraman N, Mukherjee AB. J Exp Med. 2004;199:1317–1330. doi: 10.1084/jem.20031666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu MC, Bleecker ER, Lichtenstein LM, Kagey-Sobotka A, Niv Y, McLemore TL, Permutt S, Proud D, Hubbard WC. Am Rev Respir Dis. 1990;142:126–132. doi: 10.1164/ajrccm/142.1.126. [DOI] [PubMed] [Google Scholar]
- 8.Fujitani Y, Kanaoka Y, Aritake K, Uodome N, Okazaki-Hatake K, Urade Y. J Immunol. 2002;168:443–449. doi: 10.4049/jimmunol.168.1.443. [DOI] [PubMed] [Google Scholar]
- 9.Scher JU, Pillinger MH. Clin Immunol. 2005;114:100–109. doi: 10.1016/j.clim.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Nat Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 11.Ajuebor MN, Das AM, Virag L, Flower RJ, Szabo C, Perretti M. J Immunol. 1999;162:1685–1691. [PubMed] [Google Scholar]
- 12.Cailhier JF, Partolina M, Vuthoori S, Wu S, Ko K, Watson S, Savill J, Hughes J, Lang RA. J Immunol. 2005;174:2336–2342. doi: 10.4049/jimmunol.174.4.2336. [DOI] [PubMed] [Google Scholar]
- 13.Haas KM, Poe JC, Steeber DA, Tedder TF. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Wallace JL, Bak A, McKnight W, Asfaha S, Sharkey KA, MacNaughton WK. Gastroenterology. 1998;115:101–109. doi: 10.1016/s0016-5085(98)70370-1. [DOI] [PubMed] [Google Scholar]
- 15.Bellingan GJ, Caldwell H, Howie SE, Dransfield I, Haslett C. J Immunol. 1996;157:2577–2585. [PubMed] [Google Scholar]
- 16.Bellingan GJ, Xu P, Cooksley H, Cauldwell H, Shock A, Bottoms S, Haslett C, Mutsaers SE, Laurent GJ. J Exp Med. 2002;196:1515–1521. doi: 10.1084/jem.20011794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris T, Rajakariar R, Stables M, Gilroy DW. Trends Pharmacol Sci. 2006;27:609–611. doi: 10.1016/j.tips.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Hammad H, Kool M, Soullie T, Narumiya S, Trottein F, Hoogsteden HC, Lambrecht BN. J Exp Med. 2007;204:357–367. doi: 10.1084/jem.20061196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 20.Bell-Parikh LC, Ide T, Lawson JA, McNamara P, Reilly M, FitzGerald GA. J Clin Invest. 2003;112:945–955. doi: 10.1172/JCI18012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Straus DS, Glass CK. Med Res Rev. 2001;21:185–210. doi: 10.1002/med.1006. [DOI] [PubMed] [Google Scholar]
- 22.Santoro MG. Trends Microbiol. 1997;5:276–281. doi: 10.1016/S0966-842X(97)01066-4. [DOI] [PubMed] [Google Scholar]
- 23.Conti M. Anticancer Drugs. 2006;17:1017–1022. doi: 10.1097/01.cad.0000231471.54288.00. [DOI] [PubMed] [Google Scholar]
- 24.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 25.Cernuda-Morollon E, Pineda-Molina E, Canada FJ, Perez-Sala D. J Biol Chem. 2001;276:35530–35536. doi: 10.1074/jbc.M104518200. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Sala D, Cernuda-Morollon E, Canada FJ. J Biol Chem. 2003;278:51251–51260. doi: 10.1074/jbc.M309409200. [DOI] [PubMed] [Google Scholar]
- 27.Bickley JF, Ciucci A, Evans P, Roberts SM, Ross N, Santoro MG. Bioorg Med Chem. 2004;12:3221–3227. doi: 10.1016/j.bmc.2004.03.061. [DOI] [PubMed] [Google Scholar]
- 28.Rossi AG, Sawatzky DA, Walker A, Ward C, Sheldrake TA, Riley NA, Caldicott A, Martinez-Losa M, Walker TR, Duffin R, et al. Nat Med. 2006;12:1056–1064. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- 29.Gilroy DW, Colville-Nash PR, McMaster S, Sawatzky DA, Willoughby DA, Lawrence T. FASEB J. 2003;17:2269–2271. doi: 10.1096/fj.02-1162fje. [DOI] [PubMed] [Google Scholar]
- 30.Schwab JM, Chiang N, Arita M, Serhan CN. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.