Abstract
Root hairs show highly localized cell expansion focused to their growing tips. This growth pattern is accomplished through restriction of secretion to the elongating apex and modulation of cell wall properties, with the wall just behind the tip becoming rigidified to resist the lateral expansive forces of turgor. In this report we show that root hairs exhibit oscillating growth that is associated with oscillating increases in extracellular pH and reactive oxygen species (ROS), which lag growth by ≈7 s. Consistent with a role for these changes in growth control, artificially increasing extracellular pH arrested root hair elongation, whereas decreasing pH elicited bursting at the tip. Similarly, application of exogenous ROS arrested elongation, whereas scavenging of ROS led to root hair bursting. Roots hairs of the root hair-defective rhd2-1 mutant, which lack a functional version of the NADPH oxidase ATRBOH C, burst at the transition to tip growth. This phenotype could be rescued by elevating the pH of the growth medium to ≥6.0. Such rescued root hairs showed reduced cytoplasmic ROS levels and a lack of the oscillatory production of ROS at the tip. However, they exhibited apparently normal tip growth, including generation of the tip-focused Ca2+ gradient thought to drive apical growth, indicating that ATRBOH C is not absolutely required to sustain tip growth. These observations indicate that root hair elongation is coupled to spatially distinct regulation of extracellular pH and ROS production that likely affect wall properties associated with the polarized expansion of the cell.
Keywords: NADPH oxidase, AtRBOH C, rhd2, proton flux
Apically growing cells, such as root hairs, provide an important model with which to study the dynamic regulation of growth. Root hair elongation is driven by turgor pressure and maintained by the activity of the exocytotic machinery that delivers new material to the expanding point of the root hair tip (1, 2). A tube-like growth habit is then maintained by the subapical wall resisting the expansive forces of turgor.
In the apical cytoplasm, it is well established that Ca2+, the cytoskeleton, monomeric G proteins, and a host of other cytoplasmic factors play an important role in regulating the activity of the secretory apparatus that sustains growth (3–6). However, much less is known about how the spatial patterning in wall structure contributes to restricting growth to the tip (e.g., ref. 7). Reactive oxygen species (ROS) produced by the plasma membrane NADPH oxidase ATRBOH C are thought to be indispensable for tip growth because they activate Ca2+-permeable channels required to generate the tip-focused Ca2+ gradient that drives apical growth (8). However, such extracellular ROS production may also directly affect cell wall structure (e.g., refs. 9–11).
Changes in cell wall pH have also been reported to affect both diffuse (12) and localized cell expansion (13). Indeed, there is a large, tip-localized oscillatory influx of H+ accompanying the growth of lily pollen tubes (14), whereas small, steady, inward- or outward-directed H+ fluxes have been reported around growing root hairs (15, 16). Despite such evidence that H+ fluxes are associated with tip growth, their biological function remains to be defined.
We report here that, during root hair growth, oscillatory increases in extracellular pH and ROS work together to likely modulate wall properties to support tip growth. Analysis of root hair growth of the rhd2-1 mutant indicates that the NADPH oxidase AtRBOH C is required for localized, oscillatory ROS production. However, the lesion in tip growth in this mutant can be compensated for by increased extracellular pH, indicating that extracellular pH and ROS may be acting in a coordinated and complementary manner to restrict expansion of the growing hair.
Results
Root Hair Growth Is Associated with Oscillating H+ Fluxes.
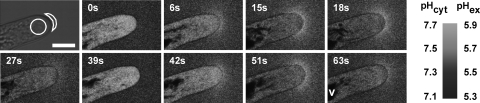
We developed an approach to visualize pH changes along the surface of growing root hairs based on supplementing the growth medium with the pH-sensitive fluorescent dye fluorescein (conjugated to a 10-kDa dextran). Confocal ratio imaging was then used to generate two-dimensional maps of pH around the root hairs [Fig. 1, supporting information (SI) Materials and Methods, and SI Movie 1]. This approach revealed that root hair tip growth was tightly associated with oscillatory changes in surface pH (Fig. 1 and SI Fig. 8). Although the amplitude of the extracellular pH oscillations varied considerably between different root hairs (0.1–1.2 pH units), their frequency was relatively constant at 2–4 oscillations per minute (Fig. 1 and SI Fig. 8). The pH oscillations were restricted to the apical 5–10 μm of the root hair tip, with the largest changes focused around the extreme apex of the growing hair (Fig. 1). No changes in extracellular pH were detected along the root hair flanks (Fig. 1) or base or around nongrowing root hairs (SI Fig. 9A). Simultaneous measurement of cytosolic pH using plants expressing a pH sensitive variant of GFP (17) revealed that a transient cytosolic acidification accompanied each extracellular alkalinization (Fig. 1, SI Fig. 8, and SI Movie 1), suggesting oscillatory H+ fluxes across the plasma membrane. Measurement of these fluxes by using a self-referencing H+-selective microelectrode showed peak influx densities of up to 90 pmol cm−2 s−1 (SI Fig. 9B).
Fig. 1.
Two-dimensional map of surface pH around a growing Arabidopsis root hair. Bright-field (leftmost) and fluorescence images of a root hair expressing cytosolic pH-sensitive GFP and immersed in fluorescein dextran, allowing measurement of cytosolic and surface pH, respectively. Numbers represent time of observation in seconds. The regions of interest (ROI) outlined in the bright-field image mark the areas selected for quantitative analysis of fluorescence intensity shown in SI Fig. 8. V, vacuole. Representative of n > 20 root hairs. (Scale bar, 10 μm.)
High-resolution growth analysis (14) indicated that, under our growth conditions, Arabidopsis root hairs consistently grew in an oscillating manner, with an average period of 18.2 ± 0.6 s (n = 10). Each large peak in growth was associated with a strong surface alkalinization localized to the growing apex; smaller growth peaks were coupled to less-pronounced pH increases (Fig. 2A). Cross-correlation analysis revealed that the oscillatory pH increases most likely lag growth oscillations by 7.0 ± 0.3 s (Fig. 2B).
Fig. 2.
Root hair surface pH oscillations lag growth oscillations. (A) Simultaneous measurement of pH-dependent fluorescence and tip growth showed that surface pH at the root hair tip and growth rate oscillate with the same periodicity but out of phase. Fluorescence intensity (F.I.) was measured in an ROI close to the apex of the growing root hair (see Fig. 1). Note that peaks in alkalinization follow peaks in growth. Data are representative of n = 10 root hairs. (B) Cross-correlation analysis showed that pH oscillations lag growth oscillations by 7.0 ± 0.3 s (means ± SD of n = 10 root hairs).
To investigate a potential functional role of these extracellular pH changes, we manipulated the extracellular pH by buffering the medium. When the medium was alkalinized to pH 8 and above, root hair growth was rapidly inhibited, whereas decreasing extracellular pH to 4.5 or below caused all growing root hairs to burst at their tips within 10 min (Table 1). Nongrowing root hairs did not burst under these same low pH conditions.
Table 1.
Effect of manipulation of extracellular pH and ROS concentration on Arabidopsis root hair growth
| Treatment | Growing, % | Stopping, % | Bursting, % |
|---|---|---|---|
| pH 4.5 (228) | 0 | 0 | 100 |
| pH 8 (54) | 4 | 96 | 0 |
| 1 μM H2O2 (24) | 0 | 100 | 0 |
| 100 μM ascorbate (104) | 16 | 0 | 84 |
| 10 μM MCLA (100) | 2 | 0 | 98 |
| 25 μM DPI (40) | 0 | 17 | 83 |
To determine the effect of extracellular pH and ROS on tip growth, root hairs were selected that exhibited stable growth rates of 1–2 μm min−1 in unbuffered minimal medium. After monitoring root hair growth for ≈10 min, treatment was started by adding compounds/buffers (10 mM DMGA for pH 4.5, 10 mM Hepes for pH 8) listed to the minimal growth medium. Numbers of root hairs are in parentheses.
Oscillating Changes in Extracellular ROS Production Are Associated with Root Hair Apical Growth.
Oxidative cross-linking and severing of wall polymers have been proposed to regulate plant growth by modulating the yield threshold of the cell wall (e.g., refs. 9–11). We therefore asked whether the polarized growth of root hairs is associated with localized changes in the oxidative environment of the apoplast. To visualize extracellular ROS with high spatial and temporal resolution, we developed an imaging approach based on OxyBURST green H2HFF, a nonfluorescent reagent that becomes fluorescent upon oxidation. OxyBURST was applied as a conjugate with BSA, making the ROS sensor cell impermeable. Importantly, the intensity of OxyBURST fluorescence does not vary with environmental factors such as pH or ionic strength of the medium (ref. 18 and data not shown)
In growing root hairs, there was a clear gradient in OxyBURST wall labeling with weaker fluorescence at the apex and much stronger fluorescence in subapical regions (Fig. 3A). In contrast, nongrowing root hairs showed strong homogenous fluorescence in the cell wall along the entire root hair length (Fig. 3B). The intense labeling of the apoplast by OxyBURST was not due to nonspecific adsorption of the OxyBURST-BSA conjugate because preoxidized OxyBURST-BSA was not accumulated in the wall (SI Fig. 10A). Increasing the imaging contrast revealed that tip growth was invariably accompanied by periodic bursts of increased OxyBURST fluorescence that occurred at a frequency of 2–4 peaks min−1 (Fig. 3C, SI Fig. 11, and SI Movie 2). The strongest fluctuations were detected along the subapical flanks of the root hair (SI Movie 2). The sensitivity of root hair growth to laser irradiation coupled to the need to perform OxyBURST imaging in liquid media imposed limitations on the resolution of our fluorescence and growth measurements (see SI Materials and Methods for detailed explanation). However, we were able to perform cross-correlation of OxyBURST fluorescence with growth, although with poorer resolution than for the pH measurements. Such analysis of the growth/ROS phase relationship indicated that the peaks of OxyBURST fluorescence most likely lag peaks in growth rate by 8.0 ± 1.4 s or precede the minima in growth rates by 7.5 ± 1.5 s (n = 6; Fig. 3D). Both timings are consistent with a model in which ROS production acts to help restrict cell expansion after a peak of elongation. Consistent with OxyBURST reporting ROS production to the apoplast of the root hair, the formation of these patterns of fluorescence was abolished by preincubating the root with the general ROS scavenger ascorbic acid (SI Fig. 10B).
Fig. 3.
Extracellular ROS around growing Arabidopsis root hair. (A and B) Distribution of OxyBURST fluorescence along the cell wall of a growing (n > 30, A) and nongrowing (n = 20, B) root hair. The image was acquired shortly after addition of OxyBURST. (C) Time course of growth and extracellular ROS production of a growing root hair. Note that extracellular ROS and growth rate oscillate with the same periodicity but out of phase. Fluorescence intensity (F.I.) was measured in an ROI close to the apex of the root hair (see A). Data are representative of n = 6 root hairs. (D) Cross-correlation analysis showed that ROS oscillations lag behind growth oscillations by 8.0 ± 1.4 s or precede the minima in growth by 7.5 ± 1.5 s (means ± SE of n = 6 root hairs). (E) Pretreatment with MCLA prevents oxidation of OxyBURST around a nongrowing root hair. The root was incubated in 20 μM MCLA for 45 s before addition of OxyBURST; n = 8. Fluorescence images in A, B, and E were collected and processed by using the same microscope settings. (Scale bars, 10 μm.)
OxyBURST is documented to react with only certain forms of ROS, showing great sensitivity for superoxide and being oxidized by H2O2 only in the presence of peroxidases (19). Therefore, to determine which endogenous ROS species was responsible for OxyBURST fluorescence around the root hair, we preincubated the root with the superoxide scavenger MCLA (methoxy cypridina luciferin analog; 20), which completely inhibited the development of OxyBURST fluorescence (Fig. 3E), confirming that the OxyBURST signal most likely depended on root hair production of superoxide.
To investigate a potential functional role of extracellular ROS, we elevated the oxidative activity in the cell wall by applying 1 μM H2O2, which caused immediate cessation of root hair apical growth (Table 1). Cytoplasmic streaming continued in these H2O2-treated hairs, which also continued to label with the vital stain fluorescein diacetate (data not shown), indicating that the ROS-induced cessation of growth was unlikely to be due to a nonspecific cytotoxic action of the treatment. Similarly, application of hydroxyl radicals using the Fenton reaction (10 μM H2O2, 10 μM ascorbic acid, 10 μM CuCl2) led to growth arrest (SI Fig. 12). Conversely, scavenging endogenously produced ROS by adding 100 μM ascorbic acid or 10 μM less-membrane-permeant MCLA to the growth medium induced rapid (within 5 min) bursting of growing, but never of nongrowing root hair apices (Table 1). The DMSO solvent control for MCLA indicated no detectable effect on growth or development of OxyBURST fluorescence (n = 7, data not shown). These results are consistent with the hypothesis that increasing extracellular ROS concentration leads to cell wall rigidification, whereas a decrease in ROS concentration enhances cell wall extensibility.
The NADPH Oxidase ATRBOH C Is Important for Maintaining Root Hair Cell Wall Integrity.
ATRBOH C, a superoxide-producing NADPH oxidase expressed in the root epidermis and root hairs of Arabidopsis, has been shown to play an important role in root hair growth (8). The lack of a functional ATRBOH C in the root hair-defective Arabidopsis mutant rhd2-1 does not impede root hair initiation but was reported to cause root hair development to arrest before the onset of tip growth (8, 21). On closer inspection, we observed that, in this mutant, initiating root hairs ruptured at the tip upon switching to apical growth (SI Fig. 13, SI Movie 3, and ref. 22); consistent with this observation, treatment with 25 μM DPI (diphenyleneiodonium), a broad-range inhibitor of flavoproteins (such as NADPH oxidases; 23) also induced bursting of most tip growing root hairs (Table 1). These findings support the idea that decreased extracellular oxidative activity in the mutant weakens the cell wall and renders it less resistant to turgor pressure.
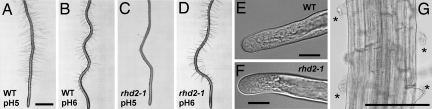
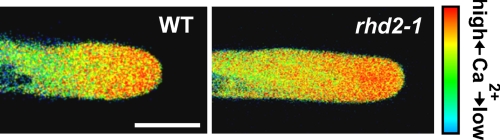
The data presented above indicate that elevated apoplastic pH and ROS both contribute to stabilize the root hair cell wall at the growing apex. To test the hypothesis that these elements act in concert to support root hair elongation, we investigated the effect of increased extracellular pH on root hair development in rhd2-1. When grown on the surface of 1/4-strength Murashige and Skoog medium buffered to pH 5, WT roots produced an abundance of long, regularly shaped root hairs (Fig. 4A). Under identical conditions, the root hairs of rhd2-1 ruptured just after their initiation (Fig. 4 C and G). This growth pattern changed dramatically, however, when the pH of the medium was increased to ≥6. At this less acidic pH, rhd2-1 root hairs developed apparently normally, showing no obvious disruption of the tip growth process and differing neither in morphology, density, nor length from the root hairs of WT plants grown under identical conditions (Fig. 4 B and D–F and Table 2). The same pH-dependent rescue of the root hair-defective phenotype was observed in the Salk_071801 T-DNA insertional mutant of AtrbohC (Table 2). The ROS produced by rhd2-1 have been proposed to regulate the apical Ca2+ gradient thought to drive tip growth (8). However, the Ca2+ gradient observed in WT root hairs was also seen in rhd2-1 pH rescued tip growing root hairs (Fig. 5).
Fig. 4.
pH-dependent rescue of rhd2-1 root hair phenotype. (A–D) Seeds of WT Arabidopsis (A and B) and rhd2-1 (C and D) were germinated for 4 d in continuous light on agar plates containing 1/4-strength Murashige and Skoog (M&S) basal salts, 1% (wt/vol) sucrose and 5 mM Mes, titrated to pH 5 (A and C) or pH 6 (B and D). Roots were imaged by using a Leica Wild M420 stereo microscope. Representative of n > 15 WT and rhd2-1 roots, respectively. (E and F) WT and rescued rhd2-1 root hair at higher magnification. (Scale bar in A, 1 mm for A–D; scale bar in E, 10 μm for E and F. (G) Higher magnification of the bursting phenotype seen in rhd2-1 grown in 1/4-strength M&S medium, pH 5. * indicates burst root hairs. (Scale bar, 100 μm.)
Table 2.
pH-dependent rescue of root hair growth in Arabidopsis knockout mutants of Atrboh C
| Genotype, treatment* | Root hair density (root hairs per mm of root length) | Root hair length, μm |
|---|---|---|
| WT, pH5 | 25 ± 7 (11)† | 492 ± 67 (15) |
| WT, pH6 | 25 ± 5 (12) | 343 ± 97 (24) |
| rhd2-1, pH5 | 0.6 ± 0.6 (16) | na |
| rhd2-1, pH6 | 21 ± 4 (20) | 357 ± 71 (25) |
| Salk_071801, pH5 | 0.2 ± 0.2 (9) | na |
| Salk_071801, pH6 | 25 ± 8 (8) | 334 ± 64 (42) |
na, not applicable.
*Seedlings were grown on 1% (wt/vol) agar plates containing 1/4-strength Murashige and Skoog basal salts, 1% (wt/vol) sucrose, buffered to indicated pH with 5 mM MES/NaOH.
†Mean values ± SD (n).
Fig. 5.
Tip-focused Ca2+ gradients measured in growing cameleon YC3.6-expressing Arabidopsis root hairs. (A) WT. (B) “pH-rescued” rhd2-1. Cytosolic Ca2+ levels have been pseudocolor-coded according to the scale at the right. (Scale bar, 10 μm.)
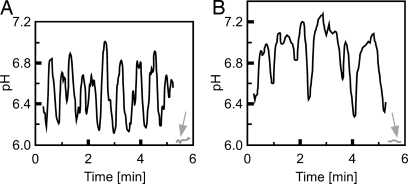
The observation that experimentally alkalinizing the growth medium helped rescue the burst root hair phenotype of rhd2-1 led us to investigate whether the endogenous tip growth-associated pH regulatory system was altered in the rhd2-1 background. Although rescued rhd2-1 root hairs showed fluctuating changes in surface pH, the degree of alkalinization was higher than in WT, regularly reaching pH values ≥7 (Fig. 6). The duration of these alkalinizations was also significantly increased (Fig. 6 and SI Fig. 14).
Fig. 6.
Surface pH dynamics at apex of growing Arabidopsis root hairs measured with a H+-selective microelectrode. Surface pH of WT (A) and rescued rhd2-1 (B) root hair. The gray arrows indicate the pH measured in the medium at 30-μm distance from the root hair apices. Representative of n > 20 WT and rhd2-1 root hairs, respectively.
ATRBOH C Is Required for Oscillatory Changes in ROS Production During Root Hair Tip Growth.
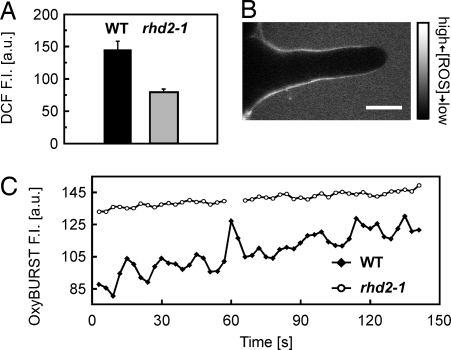
It has been reported (8) that initiating root hair bulges of rhd2-1 show reduced accumulation of cytoplasmic ROS compared with WT. To verify that pH-rescued root hairs of rhd2-1 are also deficient in the production of ROS, we first investigated the intracellular redox status using the ROS sensor dichlorofluorecin (DCF). ROS-dependent fluorescence of DCF was significantly greater in WT root hairs than in root hairs of rhd2-1 (Fig. 7A), whereas no such difference in fluorescence intensity was observed in control experiments with the structurally similar, but ROS-independent, fluorescent dye fluorescein diacetate (SI Fig. 15). No tip-focused accumulation of ROS was detectable in either WT or pH-rescued rhd2-1 tip growing root hairs (SI Fig. 16).
Fig. 7.
Intra- and extracellular ROS of pH rescued rhd2-1 root hair. (A) Intracellular redox status of WT and rhd2-1 root hairs monitored with DCF. Higher DCF fluorescence intensity indicates higher cytoplasmic oxidative activity. Values are means ± SE of n = 16 (WT) and 23 (rhd2-1) root hairs. Means are significantly different (P < 0.001, Student t test). Fluorescence intensity was measured in a region of interest (ROI) in the apical cytoplasm (see Fig. 1). (B) ROS-dependent OxyBURST fluorescence along a pH-rescued growing rhd2-1 root hair. The image was acquired by using the same settings as for Fig. 3 A, B, and E. (Scale bar, 10 μm.) (C) Time course of extracellular ROS production of WT and rhd2-1 root hairs. OxyBURST fluorescence intensity (F.I.) was measured in an ROI behind the extreme apex. Data are representative of n = 14 experiments for WT and rhd2-1, respectively.
We then visualized extracellular ROS at the surface of rescued rhd2-1 root hairs using OxyBURST-BSA. Similar to WT, these growing rhd2-1 root hairs showed strong OxyBURST fluorescence in the lateral walls with weak fluorescence at the apex (Fig. 7B). However, in none of the monitored root hairs was growth ever accompanied by detectable oscillatory changes in ROS-dependent fluorescence (Fig. 7C), indicating that the oscillating production of ROS seen in WT root hairs depends on the presence of a functional ATRBOH C.
Discussion
Extracellular pH as a Regulator of Expansion at the Growing Root Hair Apex.
In this study, we explored the role of ROS and pH in maintaining the highly defined growth pattern associated with root hair elongation. Cell wall pH is thought to play an important role in regulating wall yielding and, therefore, the rate at which a cell can expand (24). We found that tip growth of an Arabidopsis root hair is associated with cyclic changes in H+ influx around the extreme apex of the cell (Fig. 1 and SI Figs. 8 and 9) that correlated with the periodicity of growth (Fig. 2). Similar growth oscillations have been documented for the more rapidly tip-growing pollen tubes that are also characterized by oscillating changes in H+ fluxes (14, 25). In pollen tubes, periods of intense and slower growth are preceded by periods of low and high H+ influx density, respectively (14). Cross-correlation analysis of growth and surface pH demonstrated a similar relationship in Arabidopsis root hairs (Fig. 2), indicating that growth accelerates after apoplastic acidification and slows upon alkalinization. This is consistent with our observation that suddenly imposing a stable alkaline environment of pH 8 on root hairs arrested tip growth, whereas acidifying the medium to pH 4.5 resulted in bursting, i.e., uncontrolled expansion, of all growing root hairs (Table 1).
Rapid changes in apoplastic pH are likely to modulate the activity of a number of regulatory elements such as cell wall proteins, e.g., the expansins (26) and pectin methylesterases (27–30). In addition, plasma membrane proteins such as ion transporters may also be affected, either directly, such as the pH-sensitive potassium channels (31, 32) or indirectly by alterations in the steepness of the proton gradient across the membrane. The concomitant large swings in cytoplasmic pH (Fig. 1, SI Fig. 8) may also affect a myriad of cytosolic protein activities, providing a global integrator of cellular growth-related activities.
Extracellular ROS and Tip Growth.
Apoplastic ROS have recently emerged as an important element in the regulation of plant cell expansion (9, 10, 33–36). Although nitro blue tetrazolium (NBT) has been used to monitor extracellular ROS, in our hands, this reagent has proven to be cytotoxic (SI Fig. 17 and SI Movie 4). We therefore developed an assay using OxyBURST green H2HFF-BSA, a nonfluorescent reagent that becomes highly fluorescent upon oxidation (Figs. 3 and 7 and SI Fig. 12) and which proved to be nontoxic to roots and root hair growth. Our measurements revealed a characteristic pattern of fluorescence in the cell wall of the growing root hair with high signal intensity along root hair flanks and less fluorescence at the apex (Fig. 3). This observation indicates that lower levels of extracellular ROS are associated with the rapidly expanding region of the root hair tip, whereas higher ROS concentrations are present along the root hair flanks where wall yielding is reduced (2). Interestingly, ROS levels also strongly increased at the extreme apex when a root hair ceased to grow (Fig. 3), and low concentrations of exogenous ROS (1 μM H2O2 or 10 μM H2O2 driving hydroxyl radical production from the Fenton reaction) immediately inhibited root hair expansion (Table 1, SI Fig. 12). Conversely, ROS scavengers such as ascorbic acid and MCLA caused root hair bursting (Table 1). All of these findings are consistent with the idea that growing cells generate extracellular ROS to promote the rigidification of the cell wall (33, 37) and that the localization and intensity of ROS production is tightly regulated to control where such rigidification will occur.
ROS have been proposed to modulate root hair tip growth by activating a Ca2+ channel required to generate the tip-focused Ca2+-gradient, with the production of these regulatory cytosolic ROS mediated by the NADPH oxidase ATRBOH C (8). However, NADPH oxidases generate extracellular ROS by transferring electrons from cytosolic NADPH across the plasma membrane to reduce molecular oxygen (38). Thus, NADPH oxidases are likely to also directly affect wall ROS status. Root hairs of Arabidopsis rhd2-1 mutants, which lack a functional ATRBOH C (8), showed normal root hair initiation but then burst (SI Fig. 13, SI Movie 3, and ref. 22), indicating that, in addition to a possible role for this enzyme in modulating cytosolic ROS and subsequent signaling (8), it may also be involved in wall rigidification during tip growth. Significantly, this phenotype was rescued by increasing extracellular pH. Thus, when grown at pH ≥6, rhd2-1 root hairs developed normally with regard to morphology, length, and density (Fig. 4, Table 2). In addition, although these rescued root hairs showed reduced cytosolic ROS levels, they formed a tip-focused Ca2+ gradient (Fig. 5). This observation indicates that ATRBOH C is not absolutely essential for root hair development or gating of the Ca2+ channels needed to generate the tip focused gradient and that some of its function (possibly a role in initial wall rigidification) can be compensated by more-alkaline pH. However, technical limitations on the analysis of wall composition at the subcellular level have so far prevented us from directly monitoring this likely alteration in the structure of the wall at the root hair initiation site in rhd2-1 plants.
Although indistinguishable in appearance from WT, rescued rhd2-1 root hairs differed in their pattern of extracellular ROS. Wall ROS were present in both WT and rhd2-1 root hairs (Figs. 3 and 7) and presumably reflected the activity of multiple sources for extracellular ROS. These likely include the range of superoxide-producing NADPH oxidases (39), although wall-bound peroxidases (40) and apoplastic amine oxidases (41) may also contribute. However, the cyclic ROS production seen at the tip of WT root hairs was not detected in the rhd2-1 mutant (Figs. 3 and 7). This observation indicates that ATRBOH C contributes to the oscillatory generation of ROS associated with root hair tip growth.
ATRBOH C activity also affects the intracellular redox state of the root hair (Fig. 7), likely through consumption of cytosolic NADPH (42) and/or generation of apoplastic ROS that then diffuse across the plasma membrane into the cytoplasm. Thus, in addition to growth control through modulating wall properties, ROS generated at the apex and well behind the growing root hair tip (Fig. 3A) are likely involved in many other physiological responses known to be modulated by ROS (43). Interestingly, the loss of ATRBOH C-dependent ROS production in rescued rhd2-1 root hairs was accompanied by altered pH oscillations with very strong alkalinizations that lasted significantly longer than in WT (Fig. 6), suggesting that feedback between these two systems is required to support normal tip growth.
Although ATRBOH C appears to be a key element in modulating growth-related ROS production, the molecular mechanisms responsible for the highly dynamic H+ fluxes we have described remain to be identified. It seems likely that pH changes involve the oscillatory activation/deactivation of H+ transporters such as H+-ATPases and cation- (H+) or anion- (OH−) permeable channels. Identifying these H+ transporters and the signaling elements involved in the concerted regulation of H+ fluxes and ATRBOH C activity is the challenge for future research.
Materials and Methods
Plant Material.
Seeds of Arabidopsis thaliana (ecotype Columbia) were surface sterilized and germinated aseptically on Murashige and Skoog medium (Sigma) supplemented with 1% (wt/vol) sucrose and 1% (wt/vol) agar at 21°C under continuous light conditions. Four- to 5-day-old seedlings were chosen for experiments. Seeds of rhd2-1 were kindly provided by J. Lynch (Pennsylvania State University, State College, PA) and plants expressing YC3.6 by Jeff Harper (University of Nevada, Reno, NV). Seeds of Salk_071801 were obtained from the European Arabidopsis Stock Center.
Imaging of Intra- and Extracellular pH.
Arabidopsis seedlings expressing the pH-sensitive GFP variant H148D were transferred to purpose-built cuvettes (44) and covered with 2% (wt/vol) agarose (type VII; Sigma) in minimal medium (0.1 mM KCl, 0.1 mM CaCl2, 1 mM NaCl, 1% (wt/vol) sucrose, ≈pH 6). After 3 h, the agarose was carefully removed from near the root tip, the exposed surface covered with minimal medium, and the root allowed to grow out of the cut surface for an additional 3 h. To measure extracellular pH around the exposed root hairs, the medium was supplemented with 30 μg ml−1 of the pH-sensitive fluorescent dye fluorescein, conjugated to 10-kDa dextran (Sigma), and ratio imaged (18) with the Zeiss LSM 510 laser scanning confocal microscope (Carl Zeiss Inc.) using a ×40 water-immersion, 1.2 numerical aperture, C-Apochromat objective. Excitation was alternated between 458 and 488 nm (switching between wavelengths after each scanned line at ≪1 s), emission collected by using a 488-nm dichroic mirror and 505-nm long-pass filter. Bright-field images were acquired simultaneously by using the transmission detector of the confocal microscope. For time-lapse analysis, images were collected every 3 s, with each individual image scan lasting 2.2 s. For extracellular pH, calibration was performed in 100 mM Mes buffer at pH 5.0, 5.25, 5.5, 6.0, and 6.5 with identical imaging parameters. Endpoint calibration of cytosolic pH was performed with 100 mM NH4Cl, pH 9.3, and 100 mM KHCO3, pH 6.2. The pH was calculated by using the formula [H+]cyt = Kd (Rmax − R)/(R − Rmin), with a Kd of GFP H148D of ≈10−7.8M (45). Data were analyzed by using the Zeiss LSM software.
Measurement of Root Hair Growth.
Bright-field images were collected every 2 s simultaneously with fluorescence images using single wavelength (488 nm) excitation. High-resolution growth measurements were made by using the computer vision tracking software as described (14).
Measurement of Intra- and Extracellular Oxidative Activity.
Arabidopsis seedlings were mounted as described for extracellular pH measurements (see above), incubated for 20 min with 20 μM 2′,7′-dichlorofluorescin diacetate (HDCF-DA; Sigma, 20 mM stock in DMSO), and imaged for no more than 5 min. Root hairs were imaged with a Zeiss LSM 510 confocal microscope by using the ×40 lens described above. The fluorescent dye was excited with the 488-nm line of the argon laser, and emission was collected by using a 488 dichroic mirror and 505-nm long-pass filter.
Extracellular release of ROS was determined by using the fluorogenic reagent OxyBURST green H2HFF (dihydro-2′,4,5,6,7,7′-hexafluorofluorescein)-BSA (Invitrogen). Roots were incubated with 100 μg ml−1 OxyBURST, and fluorescence was recorded with the Zeiss LSM 510 microscope with the same imaging parameters as described above for DCF.
Neither HDCF nor OxyBURST had any detectable effect on normal root hair growth or development (data not shown).
Imaging of Cytosolic Ca2+ Levels.
Arabidopsis seedlings expressing the FRET-based Ca2+ sensor cameleon YC3.6 (46) were mounted as described above and ratio imaged with the Zeiss LSM 510 microscope using the ×40 objective described above. The sensor was excited with the 458-nm line of the argon laser. The CFP (473–505 nm) and FRET-dependent (536–546 nm) emission were collected by using a 458-nm primary dichroic mirror and the Meta detector of the microscope.
Electrophysiology.
Extracellular pH and H+ fluxes at the surface of Arabidopsis root hairs were measured with H+-selective microelectrodes. The microelectrode and reference electrode were fabricated and calibrated as described (47), see also SI Materials and Methods for detailed protocols.
To prepare plants for measurement, seedlings were transferred onto holders coated with 2% (wt/vol) agar in minimal medium and fixed in place by covering the roots with 1% (wt/vol) agarose. The holders were then placed into cuvettes containing minimal medium. After several hours, the growing root tip had emerged from the agar, and the developing root hairs and epidermal cells were accessible to H+-selective microelectrodes.
To measure extracellular pH, the microelectrode was moved to within 1 μm of the cell surface and the voltage recorded in 1-s intervals. To measure H+ fluxes, the microelectrode was vibrated close (1–2 μm) to the surface of the root hair apex. One measurement cycle consisted of waiting for 1.5 s, measuring for 0.3 s, and then stepping the electrode 10 μm away from the previous position (perpendicular to the surface), waiting for 1.5 s, and again measuring for 0.3 s, followed by the next cycle. Each pair of measurements was used to calculate the H+ concentration gradient and the corresponding H+ flux according to Fick's first law of diffusion.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Prof. M. H. Weisenseel for his generous support; Dr. Adam Bertl (Technische Universität, Darmstadt, Germany), Dr. Andreas Meyer (University of Heidelberg, Heidelberg), and Phillip Day (Pennsylvania State University) for many helpful discussions; and Dr. Sarah Swanson for critical reading of the manuscript. This research was supported by National Aeronautics and Space Agency Grant NAG-1594 (to S.G.), by National Science Foundation Grants MCB 02-12099, MCB 0641288, IBN 03-36738, and DBI 03-01460 (to S.G.) and MCB 0641288 (to G.B.M.), and by National Institutes of Health Grant NCRR P41 RR001395 (to M.A.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 20649.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708586104/DC1.
References
- 1.Hepler PK, Vidali L, Cheung AY. Annu Rev Cell Dev Biol. 2001;17:159–187. doi: 10.1146/annurev.cellbio.17.1.159. [DOI] [PubMed] [Google Scholar]
- 2.Shaw SL, Dumais J, Long SR. Plant Physiol. 2000;124:959–970. doi: 10.1104/pp.124.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussey PJ, Ketelaar T, Deeks MJ. Annu Rev Plant Biol. 2006;57:109–125. doi: 10.1146/annurev.arplant.57.032905.105206. [DOI] [PubMed] [Google Scholar]
- 4.Sieberer BJ, Ketelaar T, Esseling JJ, Emons AMC. New Phytol. 2005;167:711–719. doi: 10.1111/j.1469-8137.2005.01506.x. [DOI] [PubMed] [Google Scholar]
- 5.Brownlee C, Goddard H, Hetherington AM, Peake LA. J Exp Bot. 1999;50:1001–1011. [Google Scholar]
- 6.Fu Y, Yang Z. Trends Plants Sci. 2001;6:545–547. doi: 10.1016/s1360-1385(01)02130-6. [DOI] [PubMed] [Google Scholar]
- 7.Emons AM, Mulder BM. Trends Plants Sci. 2000;5:35–40. doi: 10.1016/s1360-1385(99)01507-1. [DOI] [PubMed] [Google Scholar]
- 8.Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, et al. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 9.Schopfer P. Planta. 1996;199:43–49. [Google Scholar]
- 10.Pedreira J, Sanz N, Pena MJ, Sanchez M, Queijeiro E, Revilla G, Zarra I. Plant Cell Physiol. 2004;45:530–534. doi: 10.1093/pcp/pch059. [DOI] [PubMed] [Google Scholar]
- 11.Schopfer P. Plant J. 2001;28:679–688. doi: 10.1046/j.1365-313x.2001.01187.x. [DOI] [PubMed] [Google Scholar]
- 12.Cosgrove DJ. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:391–417. doi: 10.1146/annurev.arplant.50.1.391. [DOI] [PubMed] [Google Scholar]
- 13.Bibikova TN, Jacob T, Dahse I, Gilroy S. Development (Cambridge, UK) 1998;125:2925–2934. doi: 10.1242/dev.125.15.2925. [DOI] [PubMed] [Google Scholar]
- 14.Messerli MA, Danuser G, Robinson KP. J Cell Sci. 1999;112:1497–1509. doi: 10.1242/jcs.112.10.1497. [DOI] [PubMed] [Google Scholar]
- 15.Herrmann A, Felle HH. New Phytol. 1995;129:523–533. [Google Scholar]
- 16.Jones DL, Shaff JE, Kochian LV. Planta. 1995;197:672–680. [Google Scholar]
- 17.Fasano JM, Swanson SJ, Blancaflor EB, Dowd PE, Kao TH, Gilroy S. Plant Cell. 2001;13:907–921. doi: 10.1105/tpc.13.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen CS. BMC Cell Biol. 2002;3:21. doi: 10.1186/1471-2121-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haugland RP. Handbook of Fluorescent Probes and Research Chemicals. Eugene, OR: Molecular Probes; 2005. [Google Scholar]
- 20.Nakano M. Cell Mol Neurobiol. 1998;18:565–579. doi: 10.1023/A:1020621616389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schiefelbein JW, Somerville C. Plant Cell. 1990;2:235–243. doi: 10.1105/tpc.2.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones MA, Raymond MJ, Yang Z, Smirnoff N. J Exp Bot. 2007;58:1261–1270. doi: 10.1093/jxb/erl279. [DOI] [PubMed] [Google Scholar]
- 23.Mori IC, Pinontoan R, Kawano T, Muto S. Plant Cell Physiol. 2001;42:1383–1388. doi: 10.1093/pcp/pce176. [DOI] [PubMed] [Google Scholar]
- 24.Cosgrove DJ. Nature. 2000;407:321–326. doi: 10.1038/35030000. [DOI] [PubMed] [Google Scholar]
- 25.Feijo JA, Sainhas J, Hackett GR, Kunkel JG, Hepler PK. J Cell Biol. 1999;144:483–496. doi: 10.1083/jcb.144.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cosgrove DJ, Li LC, Cho HT, Hoffmann-Benning S, Moore RC, Blecker D. Plant Cell Physiol. 2002;43:1436–1444. doi: 10.1093/pcp/pcf180. [DOI] [PubMed] [Google Scholar]
- 27.Sherrier DJ, Vandenbosch KA. Plant J. 1994;5:185–195. doi: 10.1104/pp.104.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li YQ, Chen F, Linskens HF, Cresti M. Sex Plant Reprod. 1994;7:145–152. [Google Scholar]
- 29.Bosch M, Hepler PK. Plant Cell. 2005;17:3219–3226. doi: 10.1105/tpc.105.037473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Micheli F. Trends Plants Sci. 2001;6:414–419. doi: 10.1016/s1360-1385(01)02045-3. [DOI] [PubMed] [Google Scholar]
- 31.Ilan N, Schwartz A, Moran N. J Membr Biol. 1996;154:169–181. doi: 10.1007/s002329900142. [DOI] [PubMed] [Google Scholar]
- 32.Hartje S, Zimmermann S, Klonus D, Mueller-Roeber B. Planta. 2000;210:723–731. doi: 10.1007/s004250050673. [DOI] [PubMed] [Google Scholar]
- 33.Hohl M, Greiner H, Schopfer P. Physiol Plant. 1995;94:491–498. [Google Scholar]
- 34.Bradley DJ, Kjellbom P, Lamb CJ. Cell. 1992;70:21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]
- 35.Wojtaszek P. Biochem J. 1997;322:681–692. doi: 10.1042/bj3220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brisson LF, Tenhaken R, Lamb C. Plant Cell. 1994;6:1703–1712. doi: 10.1105/tpc.6.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Held MA, Tan L, Kamyab A, Hare M, Shpak E, Kieliszewski MJ. J Biol Chem. 2004;279:55474–55482. doi: 10.1074/jbc.M408396200. [DOI] [PubMed] [Google Scholar]
- 38.Sumimoto H, Miyano K, Takeya R. Biochem Biophys Res Commun. 2005;338:677–686. doi: 10.1016/j.bbrc.2005.08.210. [DOI] [PubMed] [Google Scholar]
- 39.Torres MA, Dangl JL. Curr Opin Plant Biol. 2005;8:397–403. doi: 10.1016/j.pbi.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 40.Passardi F, Penel C, Dunand C. Trends Plants Sci. 2004;9:534–540. doi: 10.1016/j.tplants.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Cona A, Rea G, Angelini R, Federico R, Tavladoraki P. Trends Plants Sci. 2006;11:80–88. doi: 10.1016/j.tplants.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 42.Cardenas L, McKenna ST, Kunkel JG, Hepler PK. Plant Physiol. 2006;142:1460–1468. doi: 10.1104/pp.106.087882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mori IC, Schroeder JI. Plant Physiol. 2004;135:702–708. doi: 10.1104/pp.104.042069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wymer CL, Bibikova TN, Gilroy S. Plant J. 1997;12:427–439. doi: 10.1046/j.1365-313x.1997.12020427.x. [DOI] [PubMed] [Google Scholar]
- 45.Elsliger MA, Wachter RM, Hanson GT, Kallio K, Remington SJ. Biochemistry. 1999;38:5296–5301. doi: 10.1021/bi9902182. [DOI] [PubMed] [Google Scholar]
- 46.Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A. Proc Natl Acad Sci USA. 2004;101:10554–10559. doi: 10.1073/pnas.0400417101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arend M, Monshausen G, Wind C, Weisenseel MH, Fromm J. Plant Cell Environ. 2004;27:1288–1296. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.