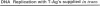Abstract
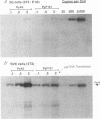
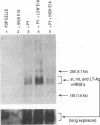
Polyomavirus (Py) DNA replication may be regulated to a low-level replication state in specific target cells in mice as well as in certain undifferentiated murine cell lines, such as embryocarcinoma (EC) cells. To investigate possible mechanisms by which such control may occur, we have examined the effects of E1A on Py DNA replication. Adenovirus E1A proteins repress transcriptional activation of various enhancers, including those of Py, and can stimulate DNA replication in quiescent cells, but E1A effects on Py DNA replication were unknown. We found that constitutive E1A expression in NIH 3T3 cells depressed Py DNA replication very strongly. Two F9 EC cell-selected Py enhancer variants, PyF441 and PyF101, were also examined because undifferentiated EC cells are hypothesized to have an E1A-like activity responsible for the Py restriction, and these variants activate Py DNA replication in cis in undifferentiated F9 cells. Both variants were repressed by E1A, indicating that E1A activity in 3T3 cells is not equivalent to undifferentiated F9 cell E1A-like activity. We also examined transient inducible E1A expression in cells supplying Py large tumor antigen (T-Ag). Py DNA replication was again repressed, and the inhibition increased with E1A induction. Analysis of T-Ag mRNA levels indicated that E1A repression of Py DNA replication was not an indirect result of depression of T-Ag transcription. This suggests that E1A may repress Py DNA replication by a more direct mechanism, possibly by blocking enhancer activation of DNA replication in a manner uncoupled with enhancer transcriptional control.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Berk A. J. Functions of adenovirus E1A. Cancer Surv. 1986;5(2):367–387. [PubMed] [Google Scholar]
- Borrelli E., Hen R., Chambon P. Adenovirus-2 E1A products repress enhancer-induced stimulation of transcription. Nature. 1984 Dec 13;312(5995):608–612. doi: 10.1038/312608a0. [DOI] [PubMed] [Google Scholar]
- Braithwaite A. W., Sturzbecher H. W., Addison C., Palmer C., Rudge K., Jenkins J. R. Mouse p53 inhibits SV40 origin-dependent DNA replication. Nature. 1987 Oct 1;329(6138):458–460. doi: 10.1038/329458a0. [DOI] [PubMed] [Google Scholar]
- Campbell B. A., Villarreal L. P. Host species specificity of polyomavirus DNA replication is not altered by simian virus 40 72-base-pair repeats. Mol Cell Biol. 1985 Jun;5(6):1534–1537. doi: 10.1128/mcb.5.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell B. A., Villarreal L. P. Lymphoid and other tissue-specific phenotypes of polyomavirus enhancer recombinants: positive and negative combinational effects on enhancer specificity and activity. Mol Cell Biol. 1986 Jun;6(6):2068–2079. doi: 10.1128/mcb.6.6.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos R., Villarreal L. P. An SV40 deletion mutant accumulates late transcripts in a paranuclear extract. Virology. 1982 May;119(1):1–11. doi: 10.1016/0042-6822(82)90059-9. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J. Animal virus DNA replication. Annu Rev Biochem. 1989;58:671–717. doi: 10.1146/annurev.bi.58.070189.003323. [DOI] [PubMed] [Google Scholar]
- Cheng L., Kelly T. J. Transcriptional activator nuclear factor I stimulates the replication of SV40 minichromosomes in vivo and in vitro. Cell. 1989 Nov 3;59(3):541–551. doi: 10.1016/0092-8674(89)90037-8. [DOI] [PubMed] [Google Scholar]
- Dandolo L., Aghion J., Blangy D. T-antigen-independent replication of polyomavirus DNA in murine embryonal carcinoma cells. Mol Cell Biol. 1984 Feb;4(2):317–323. doi: 10.1128/mcb.4.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone V., Amati P. Replicative cis-advantage of polyomavirus regulatory region mutants in different murine cell lines. J Virol. 1987 May;61(5):1615–1620. doi: 10.1128/jvi.61.5.1615-1620.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone V., La Mantia G., Lania L., Amati P. Polyomavirus mutation that confers a cell-specific cis advantage for viral DNA replication. Mol Cell Biol. 1985 Aug;5(8):2142–2146. doi: 10.1128/mcb.5.8.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L. Transcriptional elements as components of eukaryotic origins of DNA replication. Cell. 1988 Mar 11;52(5):635–638. doi: 10.1016/0092-8674(88)90398-4. [DOI] [PubMed] [Google Scholar]
- Delli Bovi P., De Simone V., Giordano R., Amati P. Polyomavirus growth and persistence in Friend erythroleukemic cells. J Virol. 1984 Feb;49(2):566–571. doi: 10.1128/jvi.49.2.566-571.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley T. P., Miranda M., Jones N. C., DePamphilis M. L. Transactivation of the adenovirus EIIa promoter in the absence of adenovirus E1A protein is restricted to mouse oocytes and preimplantation embryos. Development. 1989 Dec;107(4):945–956. doi: 10.1242/dev.107.4.945. [DOI] [PubMed] [Google Scholar]
- Dubensky T. W., Murphy F. A., Villarreal L. P. Detection of DNA and RNA virus genomes in organ systems of whole mice: patterns of mouse organ infection by polyomavirus. J Virol. 1984 Jun;50(3):779–783. doi: 10.1128/jvi.50.3.779-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubensky T. W., Villarreal L. P. The primary site of replication alters the eventual site of persistent infection by polyomavirus in mice. J Virol. 1984 May;50(2):541–546. doi: 10.1128/jvi.50.2.541-546.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura F. K., Deininger P. L., Friedmann T., Linney E. Mutation near the polyoma DNA replication origin permits productive infection of F9 embryonal carcinoma cells. Cell. 1981 Mar;23(3):809–814. doi: 10.1016/0092-8674(81)90445-1. [DOI] [PubMed] [Google Scholar]
- Fujimura F. K., Linney E. Polyoma mutants that productively infect F9 embryonal carcinoma cells do not rescue wild-type polyoma in F9 cells. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1479–1483. doi: 10.1073/pnas.79.5.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon J. V., Lane D. P. p53 and DNA polymerase alpha compete for binding to SV40 T antigen. Nature. 1987 Oct 1;329(6138):456–458. doi: 10.1038/329456a0. [DOI] [PubMed] [Google Scholar]
- Gerard R. D., Gluzman Y. New host cell system for regulated simian virus 40 DNA replication. Mol Cell Biol. 1985 Nov;5(11):3231–3240. doi: 10.1128/mcb.5.11.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass D. S., Read D., Lewis E. D., Manley J. L. Cell- and promoter-specific activation of transcription by DNA replication. Genes Dev. 1987 Dec;1(10):1065–1074. doi: 10.1101/gad.1.10.1065. [DOI] [PubMed] [Google Scholar]
- Hen R., Borrelli E., Fromental C., Sassone-Corsi P., Chambon P. A mutated polyoma virus enhancer which is active in undifferentiated embryonal carcinoma cells is not repressed by adenovirus-2 E1A products. Nature. 1986 May 15;321(6067):249–251. doi: 10.1038/321249a0. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Imperiale M. J., Hart R. P., Nevins J. R. An enhancer-like element in the adenovirus E2 promoter contains sequences essential for uninduced and E1A-induced transcription. Proc Natl Acad Sci U S A. 1985 Jan;82(2):381–385. doi: 10.1073/pnas.82.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperiale M. J., Kao H. T., Feldman L. T., Nevins J. R., Strickland S. Common control of the heat shock gene and early adenovirus genes: evidence for a cellular E1A-like activity. Mol Cell Biol. 1984 May;4(5):867–874. doi: 10.1128/mcb.4.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. C., Rigby P. W., Ziff E. B. Trans-acting protein factors and the regulation of eukaryotic transcription: lessons from studies on DNA tumor viruses. Genes Dev. 1988 Mar;2(3):267–281. doi: 10.1101/gad.2.3.267. [DOI] [PubMed] [Google Scholar]
- Katinka M., Vasseur M., Montreau N., Yaniv M., Blangy D. Polyoma DNA sequences involved in control of viral gene expression in murine embryonal carcinoma cells. Nature. 1981 Apr 23;290(5808):720–722. doi: 10.1038/290720a0. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Rosenfeld P. J., Wides R. J., O'Neill E. A., Li J. J., Wold M. S. Replication of adenovirus and SV40 chromosomes in vitro. Philos Trans R Soc Lond B Biol Sci. 1987 Dec 15;317(1187):429–438. doi: 10.1098/rstb.1987.0070. [DOI] [PubMed] [Google Scholar]
- Kovesdi I., Satake M., Furukawa K., Reichel R., Ito Y., Nevins J. R. A factor discriminating between the wild-type and a mutant polyomavirus enhancer. Nature. 1987 Jul 2;328(6125):87–89. doi: 10.1038/328087a0. [DOI] [PubMed] [Google Scholar]
- Kryszke M. H., Piette J., Yaniv M. Induction of a factor that binds to the polyoma virus A enhancer on differentiation of embryonal carcinoma cells. Nature. 1987 Jul 16;328(6127):254–256. doi: 10.1038/328254a0. [DOI] [PubMed] [Google Scholar]
- La Thangue N. B., Rigby P. W. An adenovirus E1A-like transcription factor is regulated during the differentiation of murine embryonal carcinoma stem cells. Cell. 1987 May 22;49(4):507–513. doi: 10.1016/0092-8674(87)90453-3. [DOI] [PubMed] [Google Scholar]
- Lebkowski J. S., Clancy S., Calos M. P. Simian virus 40 replication in adenovirus-transformed human cells antagonizes gene expression. Nature. 1985 Sep 12;317(6033):169–171. doi: 10.1038/317169a0. [DOI] [PubMed] [Google Scholar]
- Levine A. J. The nature of the host range restriction of SV40 and polyoma viruses in embryonal carcinoma cells. Curr Top Microbiol Immunol. 1982;101:1–30. doi: 10.1007/978-3-642-68654-2_1. [DOI] [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione R., Passananti C., De Simone V., Delli-Bovi P., Augusti-Tocco G., Amati P. Selection of mouse neuroblastoma cell-specific polyoma virus mutants with stage differentiative advantages of replication. EMBO J. 1985 Dec 1;4(12):3215–3221. doi: 10.1002/j.1460-2075.1985.tb04068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. E., Piette J., Yaniv M., Tang W. J., Folk W. R. Activation of the polyomavirus enhancer by a murine activator protein 1 (AP1) homolog and two contiguous proteins. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5839–5843. doi: 10.1073/pnas.85.16.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran E., Mathews M. B. Multiple functional domains in the adenovirus E1A gene. Cell. 1987 Jan 30;48(2):177–178. doi: 10.1016/0092-8674(87)90418-1. [DOI] [PubMed] [Google Scholar]
- Muller W. J., Dufort D., Hassell J. A. Multiple subelements within the polyomavirus enhancer function synergistically to activate DNA replication. Mol Cell Biol. 1988 Nov;8(11):5000–5015. doi: 10.1128/mcb.8.11.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei R., Berk A. J. Multiple transcription factor binding sites mediate adenovirus E1A transactivation. J Virol. 1989 Aug;63(8):3499–3506. doi: 10.1128/jvi.63.8.3499-3506.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J., Yaniv M. Two different factors bind to the alpha-domain of the polyoma virus enhancer, one of which also interacts with the SV40 and c-fos enhancers. EMBO J. 1987 May;6(5):1331–1337. doi: 10.1002/j.1460-2075.1987.tb02372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C., Murakami Y., Kern F. G., Folk W., Basilico C., Hurwitz J. DNA sequence requirements for replication of polyomavirus DNA in vivo and in vitro. Mol Cell Biol. 1987 Oct;7(10):3694–3704. doi: 10.1128/mcb.7.10.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel R., Kovesdi I., Nevins J. R. Developmental control of a promoter-specific factor that is also regulated by the E1A gene product. Cell. 1987 Feb 13;48(3):501–506. doi: 10.1016/0092-8674(87)90200-5. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Miller J. S., Kimelman D., Cepko C. L., Lemischka I. R., Mulligan R. C. Individual adenovirus type 5 early region 1A gene products elicit distinct alterations of cellular morphology and gene expression. J Virol. 1985 Nov;56(2):404–413. doi: 10.1128/jvi.56.2.404-413.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. M., Weintraub H. Negative control of DNA replication in composite SV40-bovine papilloma virus plasmids. Cell. 1986 Aug 29;46(5):741–752. doi: 10.1016/0092-8674(86)90350-8. [DOI] [PubMed] [Google Scholar]
- Rochette-Egly C., Fromental C., Chambon P. General repression of enhanson activity by the adenovirus-2 E1A proteins. Genes Dev. 1990 Jan;4(1):137–150. doi: 10.1101/gad.4.1.137. [DOI] [PubMed] [Google Scholar]
- Rochford R., Campbell B. A., Villarreal L. P. A pancreas specificity results from the combination of polyomavirus and Moloney murine leukemia virus enhancer. Proc Natl Acad Sci U S A. 1987 Jan;84(2):449–453. doi: 10.1073/pnas.84.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochford R., Davis C. T., Yoshimoto K. K., Villarreal L. P. Minimal subenhancer requirements for high-level polyomavirus DNA replication: a cell-specific synergy of PEA3 and PEA1 sites. Mol Cell Biol. 1990 Sep;10(9):4996–5001. doi: 10.1128/mcb.10.9.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekikawa K., Levine A. J. Isolation and characterization of polyoma host range mutants that replicate in nullipotential embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1100–1104. doi: 10.1073/pnas.78.2.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler K. R., Eng C. Y., Berk A. J. An adenovirus early region 1A protein is required for maximal viral DNA replication in growth-arrested human cells. J Virol. 1985 Mar;53(3):742–750. doi: 10.1128/jvi.53.3.742-750.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R. W., Whelan J. Insulin gene enhancer activity is inhibited by adenovirus 5 E1a gene products. Mol Cell Biol. 1989 Oct;9(10):4531–4534. doi: 10.1128/mcb.9.10.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R. W., Ziff E. B. Repression of insulin gene expression by adenovirus type 5 E1a proteins. Mol Cell Biol. 1987 Mar;7(3):1164–1170. doi: 10.1128/mcb.7.3.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. Initiation of eukaryotic DNA replication in vitro. Annu Rev Cell Biol. 1989;5:197–245. doi: 10.1146/annurev.cb.05.110189.001213. [DOI] [PubMed] [Google Scholar]
- Suemori H., Hashimoto S., Nakatsuji N. Presence of the adenovirus E1A-like activity in preimplantation stage mouse embryos. Mol Cell Biol. 1988 Aug;8(8):3553–3555. doi: 10.1128/mcb.8.8.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers H. T., van Dam H., Pronk G. J., Bos J. L., Van der Eb A. J. Adenovirus E1A represses transcription of the cellular JE gene. J Virol. 1989 Mar;63(3):1470–1473. doi: 10.1128/jvi.63.3.1470-1473.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velcich A., Kern F. G., Basilico C., Ziff E. B. Adenovirus E1a proteins repress expression from polyomavirus early and late promoters. Mol Cell Biol. 1986 Nov;6(11):4019–4025. doi: 10.1128/mcb.6.11.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velcich A., Ziff E. Adenovirus E1a proteins repress transcription from the SV40 early promoter. Cell. 1985 Mar;40(3):705–716. doi: 10.1016/0092-8674(85)90219-3. [DOI] [PubMed] [Google Scholar]
- Veldman G. M., Lupton S., Kamen R. Polyomavirus enhancer contains multiple redundant sequence elements that activate both DNA replication and gene expression. Mol Cell Biol. 1985 Apr;5(4):649–658. doi: 10.1128/mcb.5.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Imler J. L., Chatton B., Schatz C., Wasylyk C. Negative and positive factors determine the activity of the polyoma virus enhancer alpha domain in undifferentiated and differentiated cell types. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7952–7956. doi: 10.1073/pnas.85.21.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster K. A., Muscat G. E., Kedes L. Adenovirus E1A products suppress myogenic differentiation and inhibit transcription from muscle-specific promoters. Nature. 1988 Apr 7;332(6164):553–557. doi: 10.1038/332553a0. [DOI] [PubMed] [Google Scholar]
- White R. J., Stott D., Rigby P. W. Regulation of RNA polymerase III transcription in response to F9 embryonal carcinoma stem cell differentiation. Cell. 1989 Dec 22;59(6):1081–1092. doi: 10.1016/0092-8674(89)90764-2. [DOI] [PubMed] [Google Scholar]
- Young K. S., Weigel R., Hiebert S., Nevins J. R. Adenovirus E1A-mediated negative control of genes activated during F9 differentiation. Mol Cell Biol. 1989 Jul;9(7):3109–3113. doi: 10.1128/mcb.9.7.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers J., Schaffner W., Tyndall C., Lupton S., Kamen R. Polyoma virus DNA replication requires an enhancer. Nature. 1984 Nov 15;312(5991):242–246. doi: 10.1038/312242a0. [DOI] [PubMed] [Google Scholar]
- van Dam H., Offringa R., Smits A. M., Bos J. L., Jones N. C., van der Eb A. J. The repression of the growth factor-inducible genes JE, c-myc and stromelysin by adenovirus E1A is mediated by conserved region 1. Oncogene. 1989 Oct;4(10):1207–1212. [PubMed] [Google Scholar]