Abstract
Recent evidence indicates that inactivation of the primate superior colliculus (SC) results in an increase in saccade target selection errors. The pattern of errors suggests that a winner-take-all competition selects the saccade goal, and that SC inactivation perturbs this process by biasing the competition against stimuli in the inactivated field. To investigate this idea, the difficulty of target selection was manipulated in a color-oddity search task by varying the number of homogenous distractors in the search array. Previous studies have shown that target selection is easier when a greater number of homogenous distractors is present, due to perceptual grouping of the distractors. These results were replicated when testing with the SC intact. Surprisingly, during SC inactivation, this normal trend was reversed: target selection performance declined significantly with more distractors, resulting in a greater proportion of errant saccades to distractors. Examination of the saccade endpoints indicates that after SC inactivation, many errant saccades were directed to distractors adjacent to the target. This pattern of results suggests that the salience signal used by the SC for target selection is relatively broad in spatial scope. As a result, when the area of the SC representing the target location is inactivated, distractors near the target are at a competitive advantage relative to more distant distractors, and consequently, are selected more often as the saccade goal. This contributes to the trend of worse performance with more distractors due to the greater proximity of distractors to the target.
INTRODUCTION
The primate superior colliculus (SC) plays a central role in the generation of saccadic eye movements (e.g., Sparks and Hartwich-Young 1989), and recent evidence indicates that it is also involved in the process of target selection when a saccade goal must be chosen from multiple stimuli (e.g., Glimcher and Sparks 1992; Basso and Wurtz 1998; Krauzlis and Dill 2002; McPeek and Keller 2002). Indeed, a causal relationship between SC activity and saccade target selection has been demonstrated using inactivation (McPeek and Keller 2004) and microstimulation (Carello and Krauzlis 2004) techniques.
In a visual search task, McPeek and Keller (2004) varied the strength of the sensory cues distinguishing the target from distractors. They found that in intact monkeys, target selection was slightly worse with a low-salience target. However, during SC inactivation, performance became dramatically worse when a low-salience target appeared in the inactivated field. The deficit was not a simple motor impairment, because the monkeys were consistently able to make saccades into the inactivated field when distractors were absent. Nor was it due to a simple visual impairment, because contrast sensitivity was unaffected by SC inactivation in monkeys (McPeek and Keller 2004).
Instead, they interpreted the results in terms of a salience map and winner-take-all selection of the saccade goal (e.g., Godijn and Theeuwes 2002; McPeek and Keller 2004; Krauzlis et al. 2004; Fecteau and Munoz 2006; Wilimzig et al. 2006). Under this idea, activity in the SC represents the salience and behavioral relevance of potential saccade goals. When a saccade is imminent, there is a competition across the SC, and the goal with the greatest activity in the SC wins the competition and suppresses other goals. If the activity of neurons representing a potential goal is perturbed by inactivation (or microstimulation), this competition is biased against the goal in the inactivated field (or in favor of the goal at the stimulated site). This bias would be exaggerated with a low-salience target, because there would be a smaller difference in activity between the target and distractor goals in the putative SC salience map. As a result, the competition would be more susceptible to disruption by SC inactivation, resulting in much worse performance when a low-salience target is presented in the inactivated field, as observed by McPeek and Keller (2004).
In the current study, I further examined the characteristics of this putative salience map by varying the number of distractors in the search array to manipulate the difficulty of target selection. Previous studies of saccade target selection (as well as studies of attention and of hand reaching) have found that target selection in color-oddity search is easier when a greater number of homogenous distractors is present (Bravo and Nakayama 1992; Maljkovic and Nakayama 1994; McPeek et al. 1999; McSorley and Findlay 2003; Arai et al. 2004; Song and Nakayama 2006; Song et al. 2008). This result may seem surprising at first, but, in fact, is consistent with bottom-up models of pop-out, which predict that an odd-colored target is more salient when it is embedded in a dense array of homogenous distractors rather than a sparse array (e.g., Koch and Ullman 1985; Julesz 1986). Thus, I predicted that the target selection deficit seen during SC inactivation would decrease when the target was made more salient by embedding it in a denser array of homogenous distractors. Unexpectedly, I found the opposite result: target selection performance after SC inactivation became markedly worse with more distractors. An examination of the endpoints of the error saccades suggests that this seemingly paradoxical finding is due, at least in part, to spatial interactions between target and distractor goals when distractors are located close to the target location.
METHODS
Physiological methods
Two male rhesus monkeys (Macaca mulatta, 4-7 kg) participated in this study. All experimental protocols were approved by the Institutional Animal Care and Use Committee at the California Pacific Medical Center and complied with the guidelines of the US Public Health Service policy on Humane Care and Use of Laboratory Animals. Recording, microstimulation, and microinjection procedures (McPeek and Keller 2004) and surgical procedures (McPeek et al. 2003) have been described in detail previously. Eye movements were recorded using implanted magnetic search coils (Fuchs and Robinson 1966; Judge et al. 1980), with the head fixed. In each experiment, a microelectrode attached to a metal cannula (33-gauge) was lowered into the SC. Injections of 1-1.25μl of 2% lidocaine or 0.5μl of muscimol (0.5μg/μl concentration) were delivered at a rate of 0.5μl/min at sites at which saccade-related activity was recorded and saccades were reliably elicited with low-current stimulation (less than 30μA at 400 Hz). The location within the SC motor map was estimated by evoking saccades using currents of twice the threshold current. Stimulation train duration was varied to obtain the site-specific maximal amplitude at each site (Paré et al., 1994; Stanford et al. 1996). The region of the visual field corresponding to the injection site was then estimated as the median endpoint of the saccades evoked using these current and duration parameters. Testing in behavioral tasks was conducted after placement of the cannula in the SC, before injection, after injection, and after recovery from the injection.
Behavioral testing
Detailed descriptions of the testing procedures are given in McPeek and Keller (2004) and Arai et al. (2004). Briefly, visual stimuli were presented on a video monitor. At the beginning of each trial, a central fixation spot appeared and monkeys fixed for 450-650 ms. The fixation spot was removed and a target appeared. The target was a disc, randomly colored red or green. In each trial, the target randomly appeared in one of four locations at 90° intervals around an imaginary circle. Target locations were selected for each injection site such that one location was at the center of the inactivated part of the visual field. The diameter of the target was scaled according to the cortical magnification factor in order to keep its salience constant across different eccentricities (Rovamo and Virsu 1979). At an eccentricity of 15°, the target subtended 2° of visual angle. Simultaneously with the target, 0, 2, 3, or 7 distractors appeared. The distractors were placed at the same eccentricity as the target, and were evenly spaced from the target and each other around the imaginary circle (see Fig. 1). The distractors were identical to each other, and differed from the target only in color: when the target was red, the distractors were green, and vice-versa. Trials with different numbers of distractors were randomly interleaved in a ratio of 1:2:2:2 for the 0, 2, 3, and 7 distractor conditions, respectively. Monkeys were given liquid rewards for making a single saccade to the target location within 50-750 ms after the onset of the target and distractors. To be rewarded, the saccade had to be directed within +/− 22.5° of the target direction, and had to have an amplitude at least 66% of the target eccentricity from initial fixation. Prior to the experiments, the animals were trained for several weeks until they became proficient in the task.
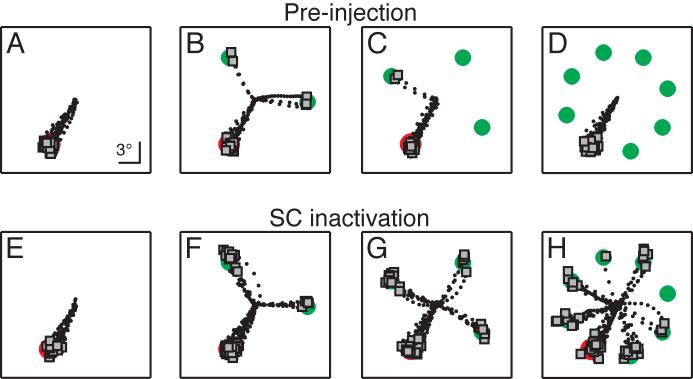
Figure 1.
Effects of SC inactivation in a search task with variable numbers of distractors. Panels show spatial plots of eye position when the target appeared in the part of the field affected by the injection, pre-injection (A-D) and during inactivation (E-H). Trials began with fixation at the center. Black dots indicate eye position samples and gray squares indicate saccade endpoints, superimposed on a cartoon of the stimulus. The task was to make a saccade to the odd-colored target. Pre-injection, search performance improved when a greater number of homogenous distractors was present (B-D). After inactivation, saccades to a target without distractors could still be made (E), but performance declined as more distractors were presented (F-H), a reversal of the pre-injection trend. Notably, more errors are directed to stimuli flanking the target (H). The conditions with different numbers of distractors were randomly intermixed. The sizes of the target and distractor stimuli are not shown to scale.
For each lidocaine injection site, the number of pre-injection trials collected for each target location in each distractor condition (2, 3, or 7 distractors) ranged from 24-38 (mean = 32), the corresponding number of post-injection trials ranged from 42-72 (mean = 58), and the number of recovery trials ranged from 26-36 (mean = 30). For the randomly-interleaved 0 distractor condition, half the number of trials was collected. Pre-injection, post-injection, and recovery data were collected in the same experimental session. Collection of post-injection trials began approximately one minute after the injection was made, and continued for 15 minutes. Recovery data were collected beginning at least 40 minutes after the injection. Only a single lidocaine injection was made on each day, and lidocaine experiments occurred 1-5 days apart. For each muscimol site, the number of pre-injection trials collected for each target location in each distractor condition (2, 3, or 7 distractors) ranged from 32-62 (mean = 47), the corresponding number of post-injection trials ranged from 50-70 (mean = 62), and the corresponding number of recovery trials ranged from 80-120 (mean = 98). Half this number of trials was collected in the 0 distractor condition. For muscimol injections, the pre-injection and post-injection data were collected on the same day, and the recovery data were collected on the following day. The time interval between muscimol injections in the same animal was 7 days.
RESULTS
Target-selection performance was measured in color-oddity search for 11 lidocaine inactivation sites (6 in Monkey A, 5 in Monkey B; see Table 1). The number of distractors (0, 2, 3, and 7 distractors) was randomly varied on a trial-by-trial basis. Results from a typical site are shown in Figure 1. Each panel shows a spatial plot of the saccades made in one of the distractor conditions when the target was presented in the inactivated field. Fig. 1A-D shows pre-injection performance. In the absence of distractors, saccades were made to the target location in every trial (Fig. 1A). When two distractors were added to the display, the proportion of correct saccades to the target decreased to 78%, with the remainder of the saccades going to distractors (Fig. 1B). However, performance progressively improved as more distractors were added (Fig. 1C-D, 3 distractors: 92% correct; 7 distractors: 100%). The statistical significance of this improvement with more distractors was verified by a linear contrast (P < 0.05; Rosenthal et al. 2000). Thus, before SC inactivation, the expected pattern of results is seen for this site: saccade target selection is facilitated when a greater number of homogenous distractors is present.
Table 1.
Superior colliculus injection sites
| Injection | Monkey | Amplitude (deg) |
Direction (deg) |
Agent, Concentration | Volume (μl) |
|---|---|---|---|---|---|
| 1 | A | 12 | 10 | lidocaine, 2% | 1.25 |
| 2 | B | 25 | 138 | lidocaine, 2% | 1.25 |
| 3 | B | 9 | 355 | lidocaine, 2% | 1 |
| 4 | A | 7 | 240 | lidocaine, 2% | 1 |
| 5 | A | 12 | 70 | lidocaine, 2% | 1 |
| 6 | A | 11 | 140 | lidocaine, 2% | 1 |
| 7 | A | 15 | 345 | lidocaine, 2% | 1 |
| 8 | B | 20 | 350 | lidocaine, 2% | 1 |
| 9 | A | 18 | 160 | lidocaine, 2% | 1 |
| 10 | B | 8 | 40 | lidocaine, 2% | 1 |
| 11 | B | 14 | 170 | lidocaine, 2% | 1 |
| 12 | B | 13 | 320 | muscimol, 0.5μg/μl | 0.5 |
| 13 | A | 10 | 170 | muscimol, 0.5μg/μl | 0.5 |
| 14 | B | 16 | 220 | muscimol, 0.5μg/μl | 0.5 |
| 15 | A | 8 | 40 | saline | 1 |
| 16 | B | 14 | 150 | saline | 1 |
After injection of a small amount of lidocaine, saccades to the inactivated region of the visual field showed relatively modest motor deficits, including lower peak velocities, longer latencies, lower amplitude gain, and an increase in endpoint error, similar to what has been reported previously (e.g., Hikosaka and Wurtz 1986; McPeek and Keller 2004). Importantly, correct saccades to the target were still be made in every trial when distractors were absent (Fig. 1E). However, when two distractors were presented along with the target, the proportion of saccades directed to the target declined from its pre-injection level (Fig. 1F; 65% correct) and more saccades were made to distractors, consistent with an impairment in target selection (McPeek and Keller 2004). I expected the overall pattern of better target-selection performance with more distractors to persist during SC inactivation, but, surprisingly, a very different pattern emerged (Fig. 1F-H): as the number of distractors increased, target selection performance became even worse (Fig. 1G, H; 3 distractors: 48% correct; 7 distractors: 34% correct), a reversal of the normal trend (linear contrast, P < 0.01).
This pattern of results was confirmed in the summary data, pooled across all 11 injection sites. Figure 2 shows percent correct saccades, as a function of the number of distractors, for each of the four target locations tested. To combine data across sites, I categorized each target location according to its position relative to the inactivated field (Fig. 2E): the “inject” location corresponds to the center of the inactivated field; the “ipsi” location is 90° in direction from the inject location in the same hemifield; the “contra” location is 90° from the inject location in the opposite hemifield; and the “opposite” location is diametrically opposite the inject location.
Figure 2.
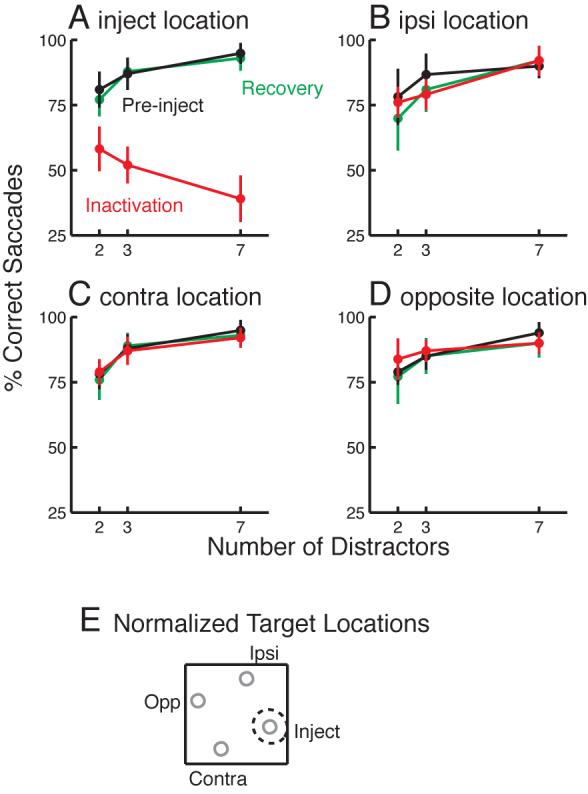
Effects of SC inactivation on target selection performance across 11 lidocaine injection sites. Each panel shows performance for one of the four target locations, pre-injection, during SC inactivation, and after recovery from inactivation. The relative positions of the four target locations are shown in E: the “inject” location (A) was near the estimated center of the inactivated field (dashed circle); “ipsi” (B) was 90° in direction from inject in the same hemifield; “contra” (C) was 90° away in the opposite hemifield; and “opposite” (D) was 180° in direction from inject. Pre-injection and after recovery, performance improved when a greater number of homogenous distractors was present in the display, for all four target locations (A-D). During SC inactivation, performance at the inject location declined with more distractors (A), while performance at the other locations was similar to pre-injection performance (B-D). Error bars show +/− standard deviation across sites.
Pre-injection and after recovery from the inactivation, percent correct performance across sites improved significantly with more distractors at all four target locations (black and green lines; Fig. 2A-D), as verified using linear contrasts (all P < 0.05). After SC inactivation, saccades showed modest motor deficits consisting of decreased peak velocities (mean decrease = 19%; P = 0.01), increased latency (mean increase = 76 ms; P = 0.001); decreased amplitude gain (pre gain: 0.93; post gain: 0.82; P = 0.014), and increased endpoint error (pre mean error = 1.25°; post mean error = 2.33°; P = 0.003) when the target appeared at the inject location, but not at the other locations (all P > 0.18). Importantly, in the absence of distractors, saccades were still made to the target in every trial. When the target appeared with distractors, target selection performance at the ipsi, contra, and opposite locations was unaffected (red lines; Fig. 2B-D). On the other hand, at the inject location, overall target selection performance dropped after SC inactivation. Furthermore, performance became significantly worse as the number of distractors increased (linear contrast: P < 0.01), a clear reversal of the pre-injection trend.
Another interesting feature of the data is suggested by Figure 1H: in the 7 distractor condition, many of the error saccades after SC inactivation are directed to distractors near the correct target location. To examine this across sites, in Figure 3 I plotted histograms of saccade endpoint direction before (Fig. 3A-C) and during (Fig. 3D-F) SC inactivation when the target was presented at the inject location. The data were normalized, such that the target location was always at a direction of 0°, the ipsi location at 90°, and so on (see Fig. 3 insets). With two distractors, errant saccades during SC inactivation appear to be randomly distributed between the two distractor locations (Fig. 3D), and a chi-squared test did not show any significant difference between the number of saccades directed to the two distractors (P = 0.96). With three distractors, significantly more errors are directed to the ipsi and contra locations than to the opposite location (Fig. 3E; chi-squared test: P < 0.05). In the seven distractor condition (Fig. 3F), the error endpoints are clearly not uniformly spread among the distractors (chi-squared test: P < 0.01): many more saccades are directed to the two nearest distractors flanking the target than to the other distractors. This suggests that the increase in errors with more distractors after SC inactivation results, at least in part, from an increase in the attractiveness (or salience) of distractors near the target location. This increase in salience appears to be present for distractors within approximately 90° in direction from the target, and even stronger at the closer separation of 45°.
Figure 3.
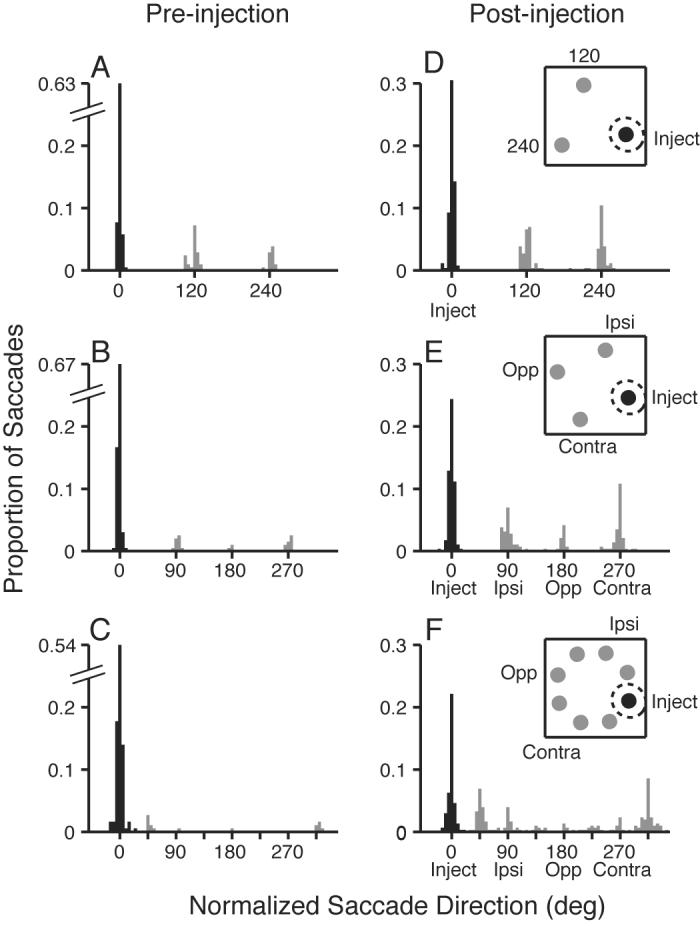
Histograms of saccade endpoint direction for the different distractor conditions before (A-C) and during (D-F) SC inactivation, when the target was presented at the center of the inactivated field. Black bars show correct saccades to the target; gray bars show error saccades. A, D show endpoints in the 2 distractor condition before and during inactivation, respectively. B, E show endpoints in the 3 distractor condition. C, F show endpoints in the seven distractor condition. Data are pooled across 11 lidocaine inactivation sites. Inset cartoons show stimulus conditions, and dashed circle indicates estimated center of the inactivated region.
Estimated extent of inactivation
When considering spatial proximity effects during SC inactivation, it is worthwhile to consider the portion of the colliculus inactivated, since this will determine whether a given distractor lies inside or outside of the inactivated zone. Due to the topography of the collicular map of visual space, similar-sized injections in different parts of the colliculus could potentially affect different-sized regions of the visual field. In particular, the angular spread of the lidocaine effect across the visual field would be expected to depend on the rostro-caudal location of the injection site. The lidocaine injections in these experiments were confined to a fairly narrow rostro-caudal region of the colliculus representing visual eccentricities of 7-25° (see Table 1). To examine this in more detail, the location on the colliculus of each injection site was estimated using the endpoints of stimulation-evoked saccades (see Methods), and mapping from visual to collicular coordinates using an established function (Ottes et al. 1986). Using these collicular injection coordinates, I then estimated the region of the colliculus inactivated for each lidocaine injection using estimates of lidocaine spread (Tehovnik and Sommer 1997) and the actual volume injected at each site. In order to compute the expected directional spread of the lidocaine effect across the visual field for each site, I transformed the estimated inactivated collicular region back into a region of the visual field using an established function for mapping collicular space into visual space (Ottes et al., 1986). Across sites, the estimated directional spread of the inactivation effect did not differ dramatically, ranging from a minimum of approximately +/− 22° to a maximum of +/− 28°. Thus, in all cases, the spread was significantly less than the minimum angular distance between stimuli (45°), making it unlikely that the proportion of error saccades to the nearest distractors was influenced by rostro-caudal injection location. To confirm this, for each site I calculated the proportion of saccades in the 7 distractor condition to distractors neighboring the target in the same hemifield, when the target was at the inject location. I then tested for a significant correlation between this proportion of error saccades and the estimated angular spread of the lidocaine inactivation. I found no significant correlation (P = 0.71), most likely due to the restricted range of angular spreads tested in these experiments.
Muscimol Injections
One disadvantage of using lidocaine as an inactivating agent is that it affects axons as well as cell bodies. In contrast, muscimol inactivation leaves axons intact. McPeek and Keller (2004) demonstrated that target selection deficits are seen for injections of either agent. In the current study, I preferentially used lidocaine because the effects of muscimol appear to have a much wider spatial spread across the SC than lidocaine (McPeek and Keller 2004; Quaia et al. 1998; Hikosaka and Wurtz 1985). However, the use of lidocaine raises the possibility that the effects observed here could be due to inactivation of fibers.
To address this issue, I performed muscimol inactivations at three additional sites (one in monkey A, two in monkey B), and pooled the data across sites. In the pre-injection condition, I observed a significant improvement in performance as the number of distractors increased, for each of the four target locations (linear contrasts, all P < 0.01), as expected. After muscimol injection, this trend remained intact for the contra and opposite locations (linear contrasts: all P < 0.02). Performance at all four target locations (inject, ipsi, contra, and opposite) is shown in Figure 4.
Figure 4.
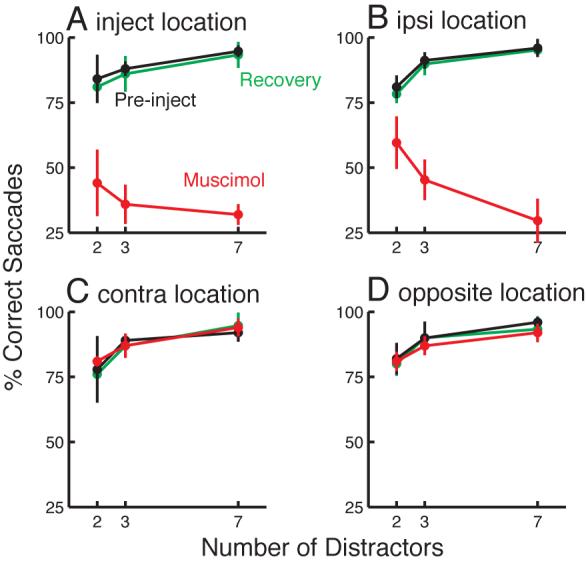
Effects of SC muscimol inactivation on target selection performance. Each panel shows performance for one of the four target locations, immediately pre-injection, during muscimol inactivation, and after recovery from inactivation (on the following day). Data are pooled across 3 injection sites, and target positions are normalized across sites as in Fig. 2. Pre-injection and after recovery, at all four target locations performance improved when a greater number of homogenous distractors was present in the display. A: At the inject location, performance during muscimol inactivation was worse overall, and declined slightly with more distractors, although the trend was not significant. B: At the ipsi location, performance also dropped after inactivation, and there was a highly significant decline with more distractors. C, D: At the other locations, performance during muscimol inactivation was similar to pre-injection performance. Error bars show +/− standard deviation across sites.
Prior work indicates that muscimol injections of the size used here should strongly affect performance at the inject location, and should also moderately affect the ipsi location due to spreading of the muscimol (e.g., McPeek and Keller 2004; Hikosaka and Wurtz 1985). In agreement with this, I found that during muscimol inactivation, performance at the inject location was dramatically worse overall (Figure 4A). However, perhaps due to spread of the muscimol effect to the nearby distractor locations, there was no significant trend in error rate as the number of distractors increased (P = 0.08), and examination of the endpoints of the error saccades in the 7 distractor condition showed that few of the error saccades were directed to distractors flanking the target. Thus, it seems that the wide area affected by muscimol undermined analysis of error saccade endpoints for the inject location.
To circumvent this problem, I focused analysis on the more-moderately affected ipsi target location, instead of the inject location. When the target appeared at the ipsi location, performance during muscimol inactivation was worse than pre-injection, and I observed a clear trend of worse performance with more distractors (Fig. 4B; P < 0.01). To determine whether this reversal of the normal distractor effect was associated with an increase in saccades to distractors near the target, I plotted the distribution of saccade endpoint directions during muscimol inactivation when the target appeared at the ipsi location (Fig. 5; 90° position).
Figure 5.
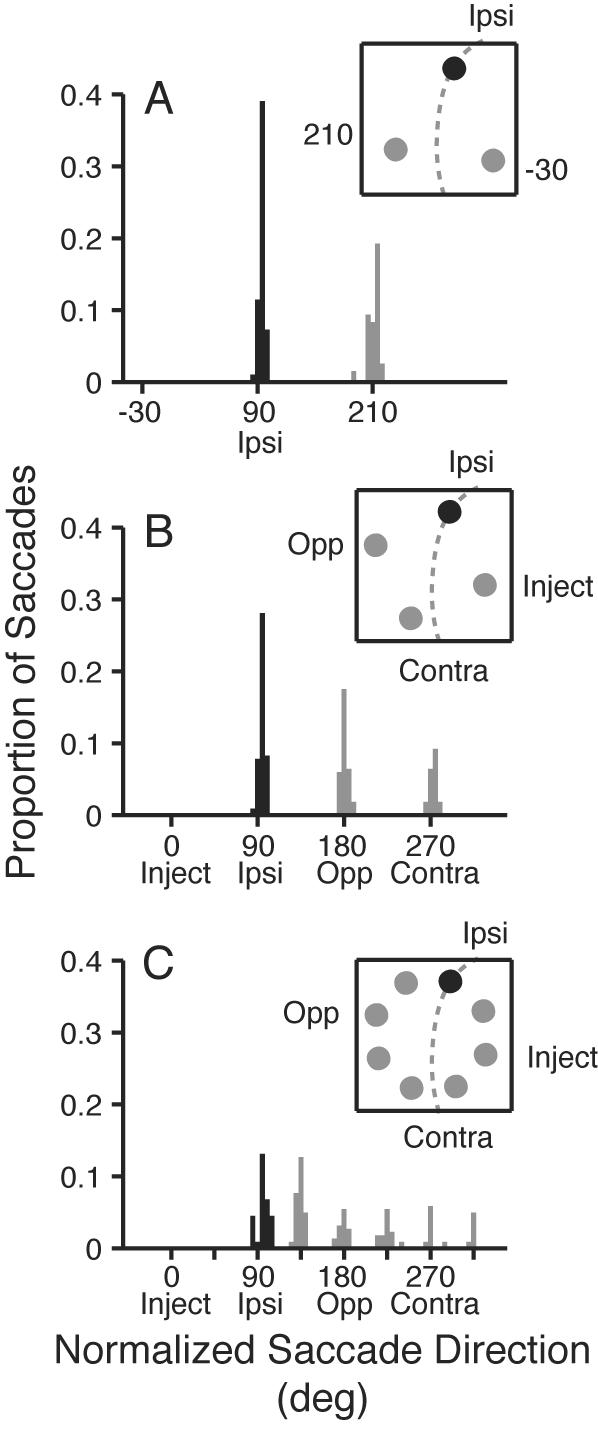
Histograms of saccade endpoint direction during SC muscimol inactivation when the target was presented at the ipsi target location. Black bars show correct saccades to the target; gray bars show error saccades. A: endpoints in the 2 distractor condition. One of the distractors (−30° location) lies inside the inactivated field; the other lies outside. B: endpoints in the 3 distractor condition. More error saccades are directed to the opposite location, which is adjacent to the target, than to the contra location. C: endpoints in the seven distractor condition. Here, many error saccades are directed to the distractor flanking the target location (135° position) which is further from the inactivated field. No saccades are directed to the 0° and 45° locations because they lie near the center of the inactivated region. Data are pooled across 3 muscimol inactivation sites. Inset cartoons show stimulus conditions, and dashed circle approximates the inactivated region.
In the 2 distractor condition (Fig. 5A), one of the distractors lay within the inactivated field (−30° position), and thus, all the errors were directed to the distractor at the 120° position. The 3 distractor condition (Fig. 5B) provides a more informative case. Here, two distractors lie outside the inactivated field: the 180° position, which is near the ipsi target location, and the 270° position, which is far from the ipsi target location. A chi-squared test revealed that significantly more error saccades were directed to the distractor near the ipsi target location than to the far distractor (P = 0.04), similar to what was seen for the lidocaine inactivations.
The most informative condition is the 7 distractor case. Of the two distractors adjacent to the ipsi target location, no errors were made to the distractor at 45°, which lies between the ipsi target and the inject location, presumably because SC neurons coding this location were affected by the muscimol injection at the 0° position. However, a disproportionate number of error saccades were made to the other flanking distractor on the side of the ipsi target away from the inactivated field (135° distractor). A chi-squared test on the errors to this position and to the other distractor positions lying outside the inactivated colliculus (180°, 225°, and 270° positions) indicated that the distribution of errors was significantly non-uniform (chi-squared test: P < 0.01). These results confirm that the increased attractiveness of nearby distractors is not dependent upon the inactivation of fibers, since it is clearly seen with muscimol.
Interestingly, a significant decline in performance with more distractors is seen only at the ipsi location, and not at the inject location (Fig. 4). Presumably, this is because at the inject location, both flanking distractors are near the center of the inactivated region, while at the ipsi location, one of the flanking distractors lies further from the inactivated field. This is consistent with the idea that saccades to flanking distractors contribute to the decline in performance with more distractors during SC inactivation.
Saline injections
As a final control, I examined the effects of injecting saline, rather than lidocaine or muscimol, at two sites in the SC (one in each animal). If the effects seen here are due to SC inactivation, rather than to the injection procedure, they should be absent for saline injections. I tested performance in the search task with 0, 2, 3, and 7 distractors intermixed, as before. As expected, saline injections did not reverse the normal effect of fewer errors with more homogenous distractors, which was observed in the pooled data for all four target locations, both pre- and post-injection (linear contrasts: all P < 0.05).
DISCUSSION
These results reveal that after focal SC inactivation, there is a reversal of the usual distractor effect in a color-oddity target selection task. In the intact monkey and human, target selection for saccades, hand reaches, and shifts of attention is facilitated when a greater number of homogenous distractors is present (Bravo and Nakayama 1992; Maljkovic and Nakayama 1994; McPeek et al. 1999; McSorley and Findlay 2003; Arai et al. 2004; Song and Nakayama 2006; Song et al. 2008). This finding is consistent with models of target selection, which predict that a greater density of distractors will make the target's odd color more conspicuous; in other words, there is greater “feature contrast” between the target and distractors when more distractors are present (Koch and Ullman 1985; Julesz 1986).
I replicated this effect in the pre-injection data, and predicted that after SC inactivation, overall performance would worsen, but the general trend of better performance with more distractors would remain intact. Indeed, an earlier study found that when the perceptual salience of a search target was reduced by making the distractors more similar to the target, the normal trend of worse performance with more difficult target selection persisted, and was exaggerated in magnitude after SC inactivation (McPeek and Keller 2004).
Surprisingly, a very different pattern of results emerged: rather than exaggerating the normal trend, focal lidocaine SC inactivation led to its reversal: monkeys made more errant saccades to distractors as the number of distractors increased. Moreover, a disproportionate share of the error saccades were directed to stimuli near the correct target location. Since the SC is non-selective for color (Ottes et al. 1987; McPeek and Keller 2002), SC inactivation presumably does not impair identification of the odd-colored target in cortical visual areas. Thus, I assume that even after SC inactivation, the odd target continues to perceptually pop-out more strongly with a greater number of homogenous distractors. Why, then, did the previous study (McPeek and Keller 2004) show an exaggeration of the normal effects of target selection difficulty on performance, while the current results show a reversal in the normal trend after SC inactivation? A key difference is that here, varying the number of distractors in the display changes not only the difficulty of target selection, but also the spatial separation between the target and distractors. Judging from the distribution of error saccade endpoints, it seems that when the target is presented in the inactivated field, distractors outside the inactivated region become more attractive the closer they are to the target. This effect is stronger when a dense array of distractors is present, due to the greater proximity of distractors to the target.
Possible mechanisms underlying the pattern of target selection errors
One mechanism which could explain the pattern of target selection errors observed here involves lateral inhibitory interactions among nearby competing saccade goals. Several models of collicular function have posited that competition among saccade goals in the colliculus is mediated by lateral inhibitory interactions (e.g., Van Opstal and Van Gisbergen 1989; Arai et al. 1999; Trappenberg et al. 2001), and physiological studies have reported evidence for such interactions (Meredith and Ramoa 1998; Munoz and Istvan 1998). Other studies have suggested that if lateral inhibitory interactions do occur among collicular stimuli, these interactions arise from circuitry outside the colliculus (Özen et al. 2004; Watanabe et al. 2005; Lee et al. 2006). Nonetheless, the idea of lateral competitive interactions in the SC is plausible, and recent models have proposed that these interactions have a limited spatial extent across the SC.
To explain the current data, I posited that each target and distractor goal exerts an inhibitory influence on competing goals within a restricted spatial extent (approximately +/− 90° in direction), and that this inhibition is stronger for closer separations (i.e., +/− 45°). Thus, in the normal animal, each stimulus in the 7 distractor condition would inhibit other nearby stimuli. Due to the perceptual salience (pop-out) of the target, the level of activity at the target location would be higher than at the distractor locations, leading to selection of the target as the saccade goal. However, after SC inactivation, activity at the target location would be lower than normal. This would produce an increase in target selection errors, as the strength of the target representation relative to the distractors would be reduced across all 3 distractor conditions. Lower target activity would also produce a decrease in lateral inhibition for distractors located near the target. This would lead to a net increase in attractiveness for nearby distractors, most strongly for the nearest distractors (+/− 45° positions) and to a lesser extent for distractors in the +/− 90° positions. This increase in attractiveness for a subset of the distractors would further degrade target selection performance, as these nearby distractors would be more likely to be selected as the saccade goal. Similar to the 7 distractor case, in the 3 distractor condition, this scheme would predict that inactivation would decrease the attractiveness of the target, and would additionally lead to a greater number of error saccades to the distractors in the +/− 90° positions, due to the decrease in target-related lateral inhibition. However, the absence of distractors at the +/− 45° positions would make the effect on saccade target selection less pronounced than in the 7 distractor condition. Finally, in the 2 distractor condition, inactivation of the target location would again lead to an increase in target selection errors. However, the error rate would not be as great as in the other conditions because the larger distance between the distractors and the target would mean that inactivation of the target site would lead to little or no disinhibition of distractors, because the distractors would lie outside the range of the lateral inhibitory connections from the target.
An alternative proposal that could account for the pattern of errors seen here is that the salience signal indicating the odd-colored target (which is received by the SC from visual areas) is fairly broad in spatial scope. In this scheme, the strongest salience signal would be in the region of the SC coding the target, but SC neurons coding nearby stimuli could also be facilitated depending on their distance from the target. Under normal conditions, this would result in most of the saccades being directed to the correct target. However, when the region of the SC representing the target is inactivated, the target would be less likely to win the competition in all the distractor conditions. Moreover, the broad zone of facilitation within the SC around the target location would make distractors near the target particularly attractive. As a result, the decline in target selection performance due to weakening of activity at the target site would be exacerbated by the increase in attractiveness of distractors when they are located near the target. This second factor would most strongly reduce performance in the 7 distractor condition, and to a lesser extent in the 3 distractor condition, contributing to an overall pattern of worse performance with more distractors.
This second proposal is compatible with the finding that the spatial distribution of SC activity for saccades extends fairly broadly, spreading across approximately 25-28% of the structure (Munoz and Wurtz 1995; Anderson et al. 1998; Ottes et al. 1986). Similarly, Basso and Wurtz (1998) found that facilitation associated with selection of a saccade target extends to locations approximately 45-90° in direction from the saccade goal, in agreement with the scope of the flanking-distractor facilitation seen here. Interestingly, this is in contrast to the frontal eye fields, where it has been shown that distractors 45° in direction from the target tend to be inhibited more strongly than more distant distractors (Schall et al. 1995). A broad facilitation of the SC around the target location could be mediated by the substantia nigra pars reticulata, as proposed in a model by Arai and Keller (2005). Nigral neurons have large response fields, and pause around the time of saccade onset to disinhibit a region of the SC (Hikosaka and Wurtz 1983; Karabalis and Moschovakis 1985; Handel and Glimcher 1999; Basso and Wurtz 2002; Bayer et al. 2004).
Interestingly, it has been reported by several groups that there is some tendency for more error saccades to be directed to distractors near the correct target location even in normal, intact human and monkey subjects (Findlay 1997; Bichot and Schall 1999; Gilchrist et al. 1999; McPeek and Keller 2001; Dorris et al. 2007). Indeed, this trend is evident in the pre-injection data presented here (Fig. 3B,C). This tendency is compatible with the second proposed mechanism, namely that the signal in the SC for saccade target selection is broadly-tuned spatially. On the other hand, it appears to be at odds with the lateral inhibition explanation, because lateral inhibition would predict that in the normal animal, distractors near the target would be more strongly suppressed than far distractors, the opposite of what is observed.
In conclusion, the results support the idea that the SC plays an essential role in saccade target selection. The spatial pattern of saccade errors can be explained by at least two different mechanisms, but overall, the data tend to favor the idea that the signals underlying target selection in the SC have a broad spatial tuning, such that selection of a target can increase the salience for the oculomotor system of nearby distractor stimuli. Of course, the presence of the broadly-tuned selection signal hypothesized here does not necessarily preclude the existence of an additional mechanism involving lateral inhibitory interactions among more-distant saccade goals. Indeed, shorter-range excitatory interactions and longer-range inhibitory interactions among saccade goals has been a feature of several models of SC function (e.g., Van Opstal and Van Gisbergen 1989; Arai et al. 1999; Trappenberg et al. 2001; Arai and Keller 2005). Such a scheme is also supported by a recent study by Dorris et al. (2007), which examined spatial interactions between a target and distractor using behavioral, recording, and microstimulation techniques in the SC. Our results additionally suggest that distractors near the saccade target continue to receive excitation even when SC neurons at the target site have been inactivated. This suggests that the proposed short-range excitation is mediated, at least in part, by extracollicular mechanisms, as proposed in a recent neural model (Arai and Keller 2005).
ACKNOWLEDGMENTS
I would like to thank J.-H. Song for helpful comments on the manuscript and N. Takahashi for expert technical assistance.
GRANTS
This work was supported by National Eye Institute Grant EY014885 to R. M. McPeek.
REFERENCES
- Anderson RW, Keller EL, Gandhi NJ, Das S. Two-dimensional saccade-related population activity in superior colliculus in monkey. J Neurophysiol. 1998;80:798–817. doi: 10.1152/jn.1998.80.2.798. [DOI] [PubMed] [Google Scholar]
- Arai K, Das S, Keller EL, Aiyoshi E. A distributed model of the saccade system: simulations of temporally perturbed saccades using position and velocity feedback. Neural Netw. 1999;12:1359–1375. doi: 10.1016/s0893-6080(99)00077-5. [DOI] [PubMed] [Google Scholar]
- Arai K, Keller EL. A model of the saccade-generating system that accounts for trajectory variations produced by competing visual stimuli. Biol Cybern. 2005;92:21–37. doi: 10.1007/s00422-004-0526-y. [DOI] [PubMed] [Google Scholar]
- Arai K, McPeek RM, Keller EL. Properties of saccadic responses in monkey when multiple competing visual stimuli are present. J Neurophysiol. 2004;91:890–900. doi: 10.1152/jn.00818.2003. [DOI] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Modulation of neuronal activity in superior colliculus by changes in target probability. J Neurosci. 1998;18:7519–7534. doi: 10.1523/JNEUROSCI.18-18-07519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Neuronal activity in substantia nigra pars reticulata during target selection. J Neurosci. 2002;22:1883–1894. doi: 10.1523/JNEUROSCI.22-05-01883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer HM, Handel A, Glimcher PW. Eye position and memory saccade related responses in substantia nigra pars reticulata. Exp Brain Res. 2004;154:428–441. doi: 10.1007/s00221-003-1735-7. [DOI] [PubMed] [Google Scholar]
- Bichot NP, Schall JD. Saccade target selection in macaque during feature and conjunction visual search. Vis Neurosci. 1999;16:81–89. doi: 10.1017/s0952523899161042. [DOI] [PubMed] [Google Scholar]
- Bravo MJ, Nakayama K. The role of attention in different visual search tasks. Percept Psychophys. 1992;51:465–472. doi: 10.3758/bf03211642. [DOI] [PubMed] [Google Scholar]
- Carello CD, Krauzlis RJ. Manipulating intent: evidence for a causal role of the superior colliculus in target selection. Neuron. 2004;43:575–583. doi: 10.1016/j.neuron.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Olivier E, Munoz DP. Competitive integration of visual and preparatory signals in the superior colliculus during saccadic programming. J Neurosci. 2007;27:5053–5062. doi: 10.1523/JNEUROSCI.4212-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau JH, Munoz DP. Salience, relevance, and firing: a priority map for target selection. Trends Cogn Sci. 2006;10:382–90. doi: 10.1016/j.tics.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Findlay JM. Saccade target selection during visual search. Vision Res. 1997;37:617–631. doi: 10.1016/s0042-6989(96)00218-0. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966;21:1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Gilchrist ID, Heywood CA, Findlay JM. Saccade selection in visual search: evidence for spatial frequency specific between-item interactions. Vision Res. 1999;39:1373–1383. doi: 10.1016/s0042-6989(98)00242-9. [DOI] [PubMed] [Google Scholar]
- Glimcher PW, Sparks DL. Movement selection in advance of action in the superior colliculus. Nature. 1992;355:542–545. doi: 10.1038/355542a0. [DOI] [PubMed] [Google Scholar]
- Godijn R, Theeuwes J. Programming of endogenous and exogenous saccades: evidence for a competitive integration model. J Exp Psychol Hum Percept Perform. 2002;28:1039–1054. doi: 10.1037//0096-1523.28.5.1039. [DOI] [PubMed] [Google Scholar]
- Handel A, Glimcher PW. Quantitative analysis of substantia nigra pars reticulata activity during a visually guided saccade task. J Neurophysiol. 1999;82:3458–3475. doi: 10.1152/jn.1999.82.6.3458. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J Neurophysiol. 1983;49:1285–1301. doi: 10.1152/jn.1983.49.5.1285. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J Neurophysiol. 1985;53:266–291. doi: 10.1152/jn.1985.53.1.266. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Saccadic eye movements following injection of lidocaine into the superior colliculus. Exp Brain Res. 1986;61:531–539. doi: 10.1007/BF00237578. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Julesz B. Texton gradients: the Texton theory revisited. Biol Cybern. 1986;54:245–251. doi: 10.1007/BF00318420. [DOI] [PubMed] [Google Scholar]
- Karabelas AB, Moschovakis AK. Nigral inhibitory termination on efferent neurons of the superior colliculus: an intracellular horseradish peroxidase study in the cat. J Comp Neurol. 1985;239:309–329. doi: 10.1002/cne.902390305. [DOI] [PubMed] [Google Scholar]
- Koch C, Ullman S. Shifts in selective visual attention: towards the underlying neural circuitry. Hum Neurobiol. 1985;4:219–227. [PubMed] [Google Scholar]
- Krauzlis RJ, Dill N. Neural correlates of target choice for pursuit and saccades in the primate superior colliculus. Neuron. 2002;35:355–363. doi: 10.1016/s0896-6273(02)00756-0. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Liston D, Carello CD. Target selection and the superior colliculus: goals, choices and hypotheses. Vision Res. 2004;44:1445–1451. doi: 10.1016/j.visres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Lee P, Hall WC. An in vitro study of horizontal connections in the intermediate layer of the superior colliculus. J Neurosci. 2006;26:4763–4768. doi: 10.1523/JNEUROSCI.0724-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maljkovic V, Nakayama K. Priming of popout: I. Role of features. Mem Cognit. 1994;22:657–672. doi: 10.3758/bf03209251. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Han JH, Keller EL. Competition between saccade goals in the superior colliculus produces saccade curvature. J Neurophysiol. 2003;89:2577–2590. doi: 10.1152/jn.00657.2002. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Short-term priming, concurrent processing, and saccade curvature during a target selection task in the monkey. Vision Res. 2001;41:785–800. doi: 10.1016/s0042-6989(00)00287-x. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Saccade target selection in the superior colliculus during a visual search task. J Neurophysiol. 2002;88:2019–2034. doi: 10.1152/jn.2002.88.4.2019. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci. 2004;7:757–763. doi: 10.1038/nn1269. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Maljkovic V, Nakayama K. Saccades require focal attention and are facilitated by a short-term memory system. Vision Res. 1999;39:1555–1566. doi: 10.1016/s0042-6989(98)00228-4. [DOI] [PubMed] [Google Scholar]
- McSorley E, Findlay JM. Saccade target selection in visual search: accuracy improves when more distractors are present. J Vis. 2003;3:877–892. doi: 10.1167/3.11.20. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Ramoa AS. Intrinsic circuitry of the superior colliculus: pharmacophysiological identification of horizontally oriented inhibitory interneurons. J Neurophysiol. 1998;79:1597–1602. doi: 10.1152/jn.1998.79.3.1597. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Istvan PJ. Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus. J Neurophysiol. 1998;79:1193–1209. doi: 10.1152/jn.1998.79.3.1193. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Saccade-related activity in monkey superior colliculus. II. Spread of activity during saccades. J Neurophysiol. 1995;73:2334–2348. doi: 10.1152/jn.1995.73.6.2334. [DOI] [PubMed] [Google Scholar]
- Ottes FP, Van Gisbergen JA, Eggermont JJ. Visuomotor fields of the superior colliculus: a quantitative model. Vision Res. 1986;26:857–873. doi: 10.1016/0042-6989(86)90144-6. [DOI] [PubMed] [Google Scholar]
- Ottes FP, Van Gisbergen JAM, Eggermont JJ. Collicular involvement in a saccadic colour discrimination task. Exp Brain Res. 1987;66:465–478. doi: 10.1007/BF00270679. [DOI] [PubMed] [Google Scholar]
- Özen G, Helms MC, Hall WC. The Intracollicular neuronal network. In: Hall WC, Moschovakis AK, editors. The Superior Colliculus: New Approaches for Studying Sensorimotor Integration. CRC Press; Boca Raton, FL: 2004. pp. 147–158. [Google Scholar]
- Quaia C, Aizawa H, Optican LM, Wurtz RH. Reversible inactivation of monkey superior colliculus. II. Maps of saccadic deficits. J Neurophysiol. 1998;79:2097–2110. doi: 10.1152/jn.1998.79.4.2097. [DOI] [PubMed] [Google Scholar]
- Paré M, Crommelinck M, Guitton D. Gaze shifts evoked by stimulation of the superior colliculus in the head-free cat conform to the motor map but also depend on stimulus strength and fixation activity. Exp Brain Res. 1994;101:123–139. doi: 10.1007/BF00243222. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Rosnow RL, Rubin DB. Contrasts and Effect Sizes in Behavioral Research. Cambridge University Press; New York: 2000. [Google Scholar]
- Rovamo J, Virsu V. An estimation and application of the human cortical magnification factor. Exp Brain Res. 1979;37:495–510. doi: 10.1007/BF00236819. [DOI] [PubMed] [Google Scholar]
- Schall JD, Hanes DP, Thompson KG, King DJ. Saccade target selection in frontal eye field of macaque. I. Visual and premovement activation. J Neurosci. 1995;15:6905–6918. doi: 10.1523/JNEUROSCI.15-10-06905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J-H, McPeek RM, Takahashi N. Target selection for visually-guided reaching in macaque. J Neurophysiol. 2008;99:14–24. doi: 10.1152/jn.01106.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J-H, Nakayama K. Role of focal attention on latencies and trajectories of visually guided manual pointing. J Vis. 2006;6:982–995. doi: 10.1167/6.9.11. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Hartwich-Young R. The deep layers of the superior colliculus. In: Wurtz RH, Goldberg ME, editors. The Neurobiology of Saccadic Eye Movements, Reviews of Oculomotor Research. III. Elsevier; Amsterdam: 1989. pp. 213–256. [PubMed] [Google Scholar]
- Stanford TR, Freedman EG, Sparks DL. Site and parameters of microstimulation: evidence for independent effects on the properties of saccades evoked from the primate superior colliculus. J Neurophysiol. 1996;76:3360–3381. doi: 10.1152/jn.1996.76.5.3360. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA. Effective spread and timecourse of neural inactivation caused by lidocaine injection in monkey cerebral cortex. J Neurosci Methods. 1997;74:17–26. doi: 10.1016/s0165-0270(97)02229-2. [DOI] [PubMed] [Google Scholar]
- Trappenberg TP, Dorris MC, Munoz DP, Klein RM. A model of saccade initiation based on the competitive integration of exogenous and endogenous signals in the superior colliculus. J Cogn Neurosci. 2001;13:256–271. doi: 10.1162/089892901564306. [DOI] [PubMed] [Google Scholar]
- Van Opstal AJ, Van Gisbergen JA. A nonlinear model for collicular spatial interactions underlying the metrical properties of electrically elicited saccades. Biol Cybern. 1989;60:171–183. doi: 10.1007/BF00207285. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Kobayashi Y, Inoue Y, Isa T. Effects of local nicotinic activation of the superior colliculus on saccades in monkeys. J Neurophysiol. 2005;93:519–534. doi: 10.1152/jn.00558.2004. [DOI] [PubMed] [Google Scholar]
- Wilimzig C, Schneider S, Schoner G. The time course of saccadic decision making: dynamic field theory. Neural Netw. 2006;19:1059–1074. doi: 10.1016/j.neunet.2006.03.003. [DOI] [PubMed] [Google Scholar]