Abstract
OBJECTIVE
To determine the association between in utero exposure to acute inflammation and long-term major neurodevelopmental disability at age 6 years among children born prior to 32 weeks’ gestation.
STUDY DESIGN
This was a follow-up investigation of a cohort of maternal-infant dyads delivered between 23 and <32 weeks’ gestation. Surviving infants (and their mothers or caregivers) underwent a battery of psychological and neurodevelopmental tests between 5 and 8 years of age. Pregnancy and neonatal data were analyzed among children with versus those without major neurodevelopmental disability (including IQ <70 [n=41], cerebral palsy [CP, n=11] and a composite major disability [n=52]).
RESULTS
A total of 261 (70%) of the 375 maternal-infant dyads with surviving children were successfully recruited and evaluated at 6.8 ± 0.7 years. Mean delivery gestational age (GA) and birthweight were 28.8 ± 2.2 weeks’ and 1163 ± 382 grams, respectively. Neither surrogate indicators for nor direct markers of in utero exposure to acute inflammation were significantly associated with severe adverse outcomes. Delivery GA was significantly associated with outcome. Logistic regression indicated that each increasing gestational week was associated with a significantly decreased risk of an IQ <70 (OR 0.75, 95% C.I. 0.6 – 0.9). An average 1.9 point increase in IQ at 6 years of age was observed per gestational week gained (23 to 32 weeks’). Periventricular leukomalacia was associated with a 9.6 point mean deficit in IQ. The perceptive vocabulary scores (IQ proxy) of primary caregivers were significantly lower among children with an IQ <70 vs. ≥70 (87.5 ± 11.5 vs. 92.1 ± 11.2, P=.016).
CONCLUSIONS
Among children born between 23 and 32 weeks’ gestation, neonatal complications, GA at delivery, and caregiver IQ, but not in utero exposure to acute inflammation was associated with increased risk of severe adverse neurodevelopmental outcomes.
Keywords: Cerebral palsy, Chorioamnionitis, Infection, Inflammation, Neurodevelopmental disability, Preterm birth
INTRODUCTION
Preterm delivery complicates 12.7% of all births in the United States and accounts for a major proportion of perinatal morbidity and mortality.1,2 Extremely low birthweight survivors have been reported to have significantly increased risk of adverse neurodevelopmental disability including cerebral palsy (CP), low intelligence quotient (IQ), vision impairment, poor motor skills, diminished academic skills, and poor adaptive functioning leading to significant long-term health and educational needs.3–6 A bountiful literature has identified in utero microbial infection and/or inflammation as a strong risk factor for preterm birth, particularly early spontaneous preterm birth as opposed to preterm delivery effected for specific indications.7,8
Published literature indicates in utero exposure to bacterial infection and/or inflammation as a risk factor for CP.9–14 Elevated proinflammatory cytokines in amniotic fluid and in fetal cord blood have been associated with brain white matter lesions such as periventricular leukomalacia (PVL), a strong risk marker for subsequent development of CP.15–17 In a case-control study including 31 predominantly term-born children with CP, Nelson et al.18 reported that numerous cytokines, chemokines, and colony stimulating factors were significantly elevated among children with CP compared to normal controls. In a longitudinal study of 123 preterm infants born prior to 36 weeks’ gestation and followed until age 3 years or older, Yoon et al.19 reported that elevations of interleukins 6 and 8 in amniotic fluid obtained by amniocentesis prior to delivery were associated with increased risk of CP.
Based on available data, conditions associated with a high likelihood of intrauterine microbial infection/inflammation (such as early preterm labor or preterm premature rupture of membranes, PPROM) that expose the fetus to a potentially “hostile environment” may increase neurological injury out of proportion to that associated with gestational age at delivery. However, the literature supporting this concern lacks longitudinal data from consecutively-delivered preterm infants with detailed information regarding characteristics of pregnancy, intrauterine environment, and short-term neonatal outcomes linked to long-term neurodevelopmental data. Also, most of the existing literature is focused on CP as the primary neurodevelopmental outcome of interest. We previously conducted a cohort study of maternal infant dyads with extensive pregnancy and neonatal outcome data.20–26 This cohort (described below) offered an excellent opportunity for detailed long-term neurodevelopmental follow-up that could be expanded to include CP as well as other neurodevelopmental outcomes of interest. For purposes of the current analysis, our hypothesis was that in utero exposure to acute inflammation would be associated with major neurodevelopmental disability at 6 years of age among children born very preterm.
MATERIALS AND METHODS
An earlier study conducted at the University of Alabama at Birmingham (UAB; HD33927) included a cohort of 424 consecutive single pregnancies delivered between 23 and <32 weeks during the interval from December 5, 1996 to December 31, 1999. Details of the study design, definitions of maternal and neonatal outcome variables, and results have been previously reported.20–26 Briefly, extensive pregnancy and neonatal (birth to discharge or death) outcome data were collected from these maternal-infant dyads by trained research nurses. Spontaneous preterm birth was defined as delivery due to the spontaneous onset of labor or PPROM. Indicated preterm birth was defined as delivery effected for maternal or fetal indications. Systemic inflammatory response syndrome was defined as the presence of negative cerebrospinal fluid and blood cultures plus clinically suspected sepsis or a band: band + polymorphonuclear cell ratio ≥0.15. Umbilical cord blood Mycoplasma hominis and Ureaplasma urealyticum cultures were performed27 as were placental cultures for these and other aerobic and anaerobic bacteria as previously described.28 Interleukin-6 (IL-6) concentrations were determined as previously described.20 Values of IL-6 greater than 34.5 pg/mL, the 95th percentile of women who had an indicated preterm birth in this population, were considered elevated. Placental histopathologic evaluation was performed by a single pathologist (OF-P), using a protocol26 adapted from Bendon et al.29
Research personnel contacted (by telephone and mail) mothers and/or caregivers of the children in the original cohort for participation in a follow-up evaluation of both the mother/caregivers and their children between 5 and 8 years of age. In cases where the mother was not available, the child’s primary caregiver/guardian was recruited to participate in the study with the child. Mothers and caregivers who initially agreed to participate presented with their children to the CWRH where the study was explained in detail and written informed consent for evaluation was obtained (from the parent or legal guardian). The study was approved by the UAB Institutional Review Board.
After obtaining consent, the mother/caregiver and child were placed in separate rooms. Primary caregivers were asked to complete a battery of tests (requiring approximately 2½ hours to complete) that included a broad array of psychometric measures. The current analysis includes the results of the Peabody Picture Vocabulary Test 3rd edition (PPVT-III)30 that was administered early in the battery and used as a proxy measure of the IQ of the mother/caregiver.
Each child was given a battery of tests assessing a wide range of psychometric measures (requiring approximately 3 hours to complete) including the Wechsler Intelligence Scale for Children-IV (WISC-IV)31 or the Differential Ability Scales (DAS, for children who were not yet six-years-old or were unable to complete the WISC-IV)32 used to assess IQ. The testing sequence was varied to overcome possible effects of fatigue. A 15 minute break was given at the midpoint of testing during which the child was given a snack. Psychometric testing was administered by psycometrists under the supervision of a licensed child psychologist (FB). The children also underwent a complete physical and neurological examination including assessment of gross and finemotor function, hearing and vision screening evaluations performed by a certified nurse practitioner under the supervision of a developmental pediatrician (AMP-C).
The primary outcome for this analysis was the presence of severe adverse neurodevelopment in the children at age 5 to 8 years. The IQ derived from the score on the WISC-IV or DAS was analyzed as a continuous and dichotomous (IQ <70 vs. ≥70) variable. Cerebral palsy was defined as an abnormal muscle tone in at least one extremity and abnormal control of movement and posture. Major neurodevelopmental disability was defined using a composite that included one or more of the following: IQ <70, CP, blindness, deafness, or other severe neurological motor deficit such as abnormal balance, impaired coordination, dystonia, or a seizure disorder that affected function.
Statistical analyses were performed using SAS, version 9.1. Categorical variables were compared between groups using chi-square or Fisher’s exact test where appropriate. Risk ratios and 95% confidence intervals were calculated for significant predictors of adverse outcome. Mean intelligence quotients were compared using student’s t-test. Logistic regression models were constructed to determine the adjusted odds ratio for adverse outcome for the two dichotomous outcome variables, and analysis of covariance models were developed for the continuous variable IQ. All factors determined to be significant in bivariate analyses were included in initial modeling and were adjusted for gestational age, ethnicity, and socioeconomic status. Final models retained those factors with p < 0.1, adjusting for gestational age andethnicity. Otherwise, statistical significance was considered at an alpha of .05. Models for IQ and major disability were also adjusted for the mother/caregiver’s Peabody score.
RESULTS
During the initial study interval, a cohort of 424 maternal-infant dyads was enrolled including 381 infants who were alive at discharge from the nursery. Six infants died after discharge leaving 375 survivors potentially available for follow-up. We failed to locate 63 (16.8%) of these, while 47(12.5%) declined to participate, and 4 (1%) were repetitive no-shows. Thus, we successfully enrolled 261 (70%) of the surviving dyads in the follow-up study. In 232 cases (90%), the caregiver was the biological mother. Among the other 28, the mother had a limited role if any as a caregiver for the child (n=15) or the family was complex with varying participation of the mother as a caregiver (n=13). In these 28 cases, the individual who accompanied the child to the clinic underwent the adult testing battery. These included the father (n=5), the grandmother (n=9), grandfather (n=1), an adopting mother (n=7) or father (n=1), aunt (n=1), other female (n=2), and unspecified (n=2). Mean maternal age at delivery, birthweight, and gestational age at delivery, respectively, were 24.9 ± 6.2 years, 1163 ± 382 grams, and 28.8 ± 2.2 weeks’. The mean mother/caregiver Peabody standard score (IQ proxy) was 91.4 ± 11.3 and the mean IQ of the children was 83.4 ± 14.9. The children were evaluated at 6.8 ± 0.7 years of age.
The association between demographic and other characteristics of the study cohort and IQ <70, CP, and major disability are depicted in Table 1. No statistically significant differences were observed in association with IQ <70, although this outcome tended to be more frequent among African-American children and those born to single mothers (Table 1). Cerebral palsy was significantly less common among African-American vs. non-African American children (1.3% vs. 9.0%, P=.004) and those born to single mothers (1.3%vs. 8.7%, P=.008). While no characteristic was associated with a significantly increased risk of major disability (Table 1), this outcome was more frequent among children born to mothers with ≤12 years of education (24.1% vs. 14.9%, p=.066).
Table 1.
Frequency of the neurodevelopmental outcomes according to demographic and other characteristics of the study cohort (n=261).
| Characteristics | IQ<70* (%) | P value | CP (%) | P Value | Major* ND (%) | P Value |
|---|---|---|---|---|---|---|
| Race | ||||||
| AA | 19.0 | 0.082 | 1.3 | 0.004 | 20.3 | 0.930 |
| Non-AA | 10.9 | 9.0 | 19.8 | |||
| Maternal Age (years) | ||||||
| <20 | 12.7 | 0.184 | 3.2 | 1.000 | 19.1 | 0.202 |
| 20–30 | 19.2 | 4.7 | 23.2 | |||
| >30 | 8.9 | 4.4 | 11.1 | |||
| Maternal Education (years) | ||||||
| ≤12 | 19.3 | 0.084 | 4.2 | 1.000 | 24.1 | 0.066 |
| >12 | 11.4 | 4.4 | 14.9 | |||
| Income | ||||||
| <$1600/month | 15.9 | 0.914 | 1.6 | 0.060 | 20.6 | 0.779 |
| >$1600/month | 15.4 | 7.0 | 19.2 | |||
| Maternal smoking/pregnancy | ||||||
| Yes | 9.1 | 0.365 | 10.0 | 0.207 | 18.2 | 1.000 |
| No | 16.5 | 3.8 | 20.3 | |||
| Marital status at delivery | ||||||
| Married | 10.6 | 0.066 | 8.7 | 0.008 | 17.3 | 0.386 |
| Single | 19.1 | 1.3 | 21.7 | |||
| Maternal BMI | ||||||
| <19.8 | 16.7 | 0.924 | 16.7 | 0.121 | 16.7 | 0.896 |
| 19.8–<26 | 13.2 | 2.6 | 17.1 | |||
| 26 – <29 | 16.7 | 8.8 | 22.2 | |||
| ≥29 | 16.6 | 2.9 | 20.9 | |||
| Child Gender | ||||||
| Male | 18.3 | 0.334 | 5.3 | 0.545 | 23.5 | 0.222 |
| Female | 13.9 | 3.5 | 17.4 | |||
Two children were not evaluated
IQ = Intelligence Quotient
CP = Cerebral Palsy
ND = Neurodevelopmental disability
AA = African American
Two children were not evaluated for IQ and 4 did not have a physical examination for CP. A total of 41 (15.8%) had an IQ < 70, 11 (4.3%) had CP (5 mild, 5 moderate, and 1 severe), and 52 (20.1%) had a major disability. It should be noted that 41 of the 52 children with a major disability (78.9%) had an IQ <70; therefore, low IQ was the predominant component of the major disability composite outcome. Compared to children with an IQ ≥70, those with an IQ <70 had a significantly lower mean gestational age at delivery (27.4 ± 2.3 vs. 29.0 ± 2.1 weeks, P<.0001), lower birthweight (912 ± 331 vs. 1211 ± 374 grams, p<.0001), and their mother/caregivers had a lower score on the Peabody receptive vocabulary test (87.5 ± 11.5 vs. 92.1 ± 11.2, P=.016). Children with CP and those with a major disability also had a significantly lower mean gestational age at delivery (CP, 26.7 ± 1.9 vs. 28.8 ± 2.2 weeks’, P=.002; major disability, 27.6 ± 2.2 vs. 29.1 ± 2.1 weeks’, P<.0001), and lower birthweight (CP, 831 ± 340 vs. 1177 ± 379 grams, P=.003; major disability,968 ± 375 vs. 1213 ± 370 weeks, P<.0001) compared to those without these conditions. Mother/caregiver receptive vocabulary was not significantly different among children with CP (95.9 ± 10.1 vs. 91.3 ± 11.4, P=.188) or major disability (89.0 ± 11.3 vs. 92.0 ± 11.3, P=.089) compared to mother/caregivers of children without these conditions.
Obstetrical characteristics of the cohort according to neurodevelopmental outcome are depicted in Table 2. There was a strong, statistically significant association between early gestational age at birth and all three adverse neurodevelopmental outcomes (Table 2). Surprisingly, conditions that might be anticipated to be associated with an increased risk of in utero exposure to bacterial infection and/or mediators of acute inflammation (labor, spontaneous preterm delivery, and clinical chorioamnionitis) were not associated with an increased frequency of severe adverse neurodevelopmental outcomes (Table 2). Neither was in utero exposure to magnesium sulfate or antenatal steroids protective against such outcomes (Table 2). Interestingly, birth specifically due to PPROM was associated with a significantly lower frequency of IQ <70 (P=.017), and major disability (P=.016), but not CP (P=.688; Table 2).
Table 2.
Frequency of the neurodevelopmental outcomes according to obstetrical characteristics of the study cohort (n=261).
| Characteristics | IQ<70* (%) | P value | CP (%) | P Value | Major* ND (%) | P Value |
|---|---|---|---|---|---|---|
| Parity | ||||||
| Para 0 | 15.0 | 0.687 | 3.9 | .778 | 19.7 | 0.853 |
| Para 1+ | 16.8 | 4.7 | 20.6 | |||
| Delivery GA (weeks) | ||||||
| 23–26 | 31.7 | 0.0006 | 13.2 | 0.0009 | 36.7 | 0.0003 |
| 27–28 | 16.4 | 3.3 | 24.6 | |||
| 29–30 | 10.7 | 0.0 | 11.9 | |||
| 31–32 | 5.6 | 1.9 | 9.3 | |||
| SGA | ||||||
| Yes | 23.8 | 0.345 | 0.0 | 0.608 | 23.8 | 0.583 |
| No | 15.1 | 4.7 | 19.8 | |||
| PTB Type | ||||||
| Spontaneous | 14.7 | 0.525 | 3.7 | 0.537 | 19.6 | 0.816 |
| Indicated** | 17.7 | 5.3 | 20.8 | |||
| PTB classification | ||||||
| Spontaneous labor | 22.4 | 0.017 | 4.7 | 0.688 | 28.2 | 0.016 |
| PPROM | 6.4 | 2.6 | 10.3 | |||
| Indicated** | 17.7 | 5.3 | 20.8 | |||
| C-section | ||||||
| Yes | 18.8 | 0.213 | 6.4 | 0.105 | 23.4 | 0.192 |
| No | 13.1 | 2.3 | 16.9 | |||
| Any labor | ||||||
| Yes | 15.4 | 0.738 | 3.0 | 0.070 | 19.4 | 0.614 |
| No | 17.2 | 8.8 | 22.4 | |||
| Clinical chorioamnionitis | ||||||
| Yes | 16.1 | 1.000 | 3.2 | 1.000 | 19.4 | 0.915 |
| No | 15.8 | 4.4 | 20.2 | |||
| Steroids | ||||||
| Yes | 16.0 | 0.957 | 4.5 | 1.000 | 20.0 | 0.805 |
| No | 15.6 | 3.1 | 21.9 | |||
| Preeclampsia | ||||||
| Yes | 17.4 | 0.616 | 4.8 | 0.753 | 20.9 | 0.809 |
| No | 15.0 | 4.1 | 19.7 | |||
| Magnesium sulfate | ||||||
| Yes | 16.7 | 0.823 | 3.7 | 1.000 | 19.1 | 0.893 |
| No | 15.6 | 4.2 | 19.8 | |||
= Two children were not evaluated, IQ = Intelligent Quotient, CP = Cerebral Palsy, ND = Neurodevelopmental disability, GA = gestational age, PPROM = Preterm Premature Rupture of Membranes, PTB = Preterm birth, SGA = Small for gestational age
Indications included preeclamsia (60%), non-reassuring fetal heart rate tracing (16%), clinical chorioamnionitis (without PPROM or labor; 9.6%), abruption placenta (3.2%), uterine bleeding (2.4%), intrauterine growth restriction (1.6%), and other (7.2%)
The association between markers of in utero exposure to acute inflammation and severe neurodevelopmental disability at age 6 years is depicted in Table 3. A positive placental culture for any microorganism, histologic chorioamnionitis, acute inflammation of the membranes or fetal plate of the placenta, elevated umbilical cord IL-6, and a positive umbilical cord blood culture for Ureaplasma urealyticum or Mycoplasma hominis were not associated with an increased likelihood of IQ <70, CP, or major disability. The frequency of an IQ <70 was lower in the presence of funisitis (10.1% vs. 19.6%, P=.061; RR 0.5, 95% C.I. 0.2 – 1.1) but this difference was not statistically significant.
Table 3.
Frequency of the neurodevelopmental outcomes according to markers of acute inflammation in the placenta and umbilical cord among the cohort (n=261).
| Characteristics | IQ<70 (%) | P value | CP (%) | P Value | Major* ND (%) | P Value |
|---|---|---|---|---|---|---|
| Placenta: | ||||||
| Histologic chorioamnionitis | ||||||
| Yes | 13.5 | 0.251 | 4.1 | 0.842 | 19.3 | 0.628 |
| No | 18.8 | 4.7 | 21.8 | |||
| Acute inflammation - Membranes | ||||||
| Yes | 12.8 | 0.229 | 4.5 | 0.978 | 19.3 | 0.670 |
| No | 18.5 | 4.6 | 21.5 | |||
| Acute inflammation – Fetal Plate | ||||||
| Yes | 12.6 | 0.221 | 4.1 | 1.000 | 17.9 | 0.384 |
| No | 18.5 | 4.8 | 22.5 | |||
| Funisitis | ||||||
| Yes | 10.1 | 0.061 | 4.9 | 0.756 | 16.5 | 0.224 |
| No | 19.6 | 4.3 | 23.2 | |||
| Culture positive (any microbe) | ||||||
| Yes | 15.7 | 0.761 | 3.8 | 0.588 | 18.7 | 0.389 |
| No | 17.1 | 5.2 | 23.1 | |||
| Cord Blood: | ||||||
| Elevated IL-6 | ||||||
| Yes | 9.6 | 0.275 | 3.9 | 0.633 | 13.5 | 0.314 |
| No | 15.9 | 2.4 | 19.8 | |||
| UU/Mycoplasma positive | ||||||
| Yes | 15.7 | 0.713 | 0.0 | 0.342 | 17.7 | 0.909 |
| No | 13.6 | 4.1 | 18.4 | |||
Two children were not evaluated
IQ = Intelligence Quotient
CP = Cerebral Palsy
ND = Neurodevelopmental disability
UU = Ureaplasma Urealyticum
The association between neonatal characteristics and conditions and subsequent development of severe neurodevelopmental disability at age 6 years is depicted in Table 4. Periventricular leukomalacia (PVL) was a strong and significant risk factor for subsequent IQ <70 (RR 3.4, 95% C.I. 1.7 – 6.7), CP (RR 13.8, 95% C.I. 4.8 – 39.5) and major disability (RR 2.6, 95% C.I. 1.3 – 5.1; Table 4). Intraventricular hemorrhage (IVH, grades 3 and 4) was also associated with increased risk of these three outcomes, particularly CP (RR 17.6, 95% C.I. 6.0 – 51.6), and major disability (RR 2.7, 95% C.I. 1.5 – 4.7; Table 4). Necrotizing enterocolitis (NEC) was associated with a significantly higher risk of CP (RR 4.7, 95% C.I. 1.5 – 14.5), but not IQ <70 or major disability (Table 4). There were only 4 cases of confirmed neonatal sepsis in the cohort (defined as a positive neonatal blood or cerebrospinal fluid culture), but none of these had an adverse neurodevelopmental outcome (Table 4). An IQ <70, CP, and major disability were all significantly more common, respectively, among children with a history of seizures (RR 3.7, 95% C.I. 2.1 – 6.6; RR 8.4, C.I. 2.8 – 26.4; RR 2.8, 95% C.I. 1.6 – 4.8), bronchopulmonary dysplasia (BPD; RR 2.7, 95% C.I. 1.5 – 4.6; RR 5.4, C.I. 1.7 – 16.8; RR 2.5, 95% C.I. 1.4 – 3.6) or chronic lung disease (CLD; BPD; RR 3.2, 95% C.I. 1.8 –5.7; RR 4.2, C.I. 1.2 – 14.7; RR 3.0, 95% C.I. 1.9 – 5.0). As depicted in Table 4, respiratory distress syndrome (RDS) was associated with an increased risk of IQ <70 (RR 2.1, 95% C.I. 1.0 – 4.3), major disability (RR 2.1, 95% C.I. 1.1 –4.0), and CP (a RR could not be calculated because all children with CP had RDS).
Table 4.
Frequency of the neurodevelopmental outcomes according to neonatal and childhood characteristics of the cohort (n=261).
| Characteristics | IQ<70* (%) | P value | CP (%) | P Value | Major* ND (%) | P Value |
|---|---|---|---|---|---|---|
| Neonatal Factors: | ||||||
| PVL | ||||||
| Yes | 50.0 | 0.012 | 40.0 | <.0001 | 50.0 | 0.034 |
| No | 14.8 | 2.9 | 19.3 | |||
| IVH (3 or 4) | ||||||
| Yes | 31.3 | 0.150 | 37.5 | <.0001 | 50.0 | 0.006 |
| No | 15.2 | 2.1 | 18.6 | |||
| SIRS | ||||||
| Yes | 20.0 | 0.271 | 4.0 | 1.000 | 25.3 | 0.206 |
| No | 14.4 | 4.5 | 18.3 | |||
| NEC | ||||||
| Yes | 18.0 | 0.694 | 12.8 | 0.015 | 25.6 | 0.347 |
| No | 15.5 | 2.8 | 19.1 | |||
| RDS | ||||||
| Yes | 19.2 | 0.038 | 6.4 | 0.018 | 24.4 | 0.014 |
| No | 9.2 | 0.0 | 11.5 | |||
| BPD | ||||||
| Yes | 32.6 | 0.0006 | 12.8 | 0.006 | 37.0 | 0.002 |
| No | 12.2 | 2.4 | 16.4 | |||
| CLD | ||||||
| Yes | 42.9 | 0.0004 | 14.3 | 0.051 | 52.4 | .0005 |
| No | 13.5 | 3.4 | 17.2 | |||
| Confirmed Sepsis | ||||||
| Yes | 0.0 | 1.000 | 0.0 | 1.000 | 0.0 | 0.586 |
| No | 16.1 | 4.4 | 20.4 | |||
| Surfactant Use | ||||||
| Yes | 23.9 | 0.002 | 8.0 | 0.010 | 29.2 | 0.001 |
| No | 9.6 | 1.4 | 13.1 | |||
| History of SZ’s | ||||||
| Yes | 50.0 | 0.001 | 25.0 | <.003 | 50.0 | .006 |
| No | 13.6 | 2.9 | 18.1 | |||
Two children were not evaluated, IQ = Intelligence Quotient, CP = Cerebral Palsy, ND = Neurodevelopmental disability, IVH = Intraventricular Hemorrhage (grade 3 or 4), PVL = Periventricular Leukomalacia, SIRS = Systemic Inflammatory Response System, NEC – Necrotizing Enterocolitis, RDS = Respiratory Distress Syndrome, BPD = Brochopulmonary Dysplasia, CLD = Chronic Lung Disease, SZ’s = Seizures in the child
In logistic regression models adjusting for potential confounders, each increasing gestational week was associated with a significantly decreased risk of an IQ <70 and a major disability (Both: OR 0.75, 95% C.I. 0.6 – 0.9). A history of seizures remained significantly associated with an IQ <70 (OR 4.2, 95% C.I. 1.1 – 15.1) and a major disability (OR 4.2, 95% C.I. 1.1 – 15.2). Although not statistically significant, PVL (n=10) was associated with an increased risk for an IQ <70 and a major disability (Both: OR 4.9, 95% C.I. 0.9 – 26.0). A history of PPROM remained associated with a lower risk of IQ <70 (OR 0.2, 95% C.I. 0.1 – 0.7) and a major disability (Both: OR 0.2, 95% C.I. 0.1 –0.7). Risk factors for CP included IVH (grade 3 or 4; OR 25.6, 95% C.I. 3.8 – 172.2), a history of seizures (OR 11.2, 95% C.I. 1.5 – 82.1) and NEC (OR 5.7, 95% C.I. 0.9 –34.1). African American ethnicity was associated with a lower risk of CP (OR 0.1, 95% C.I. 0.01 – 0.6).
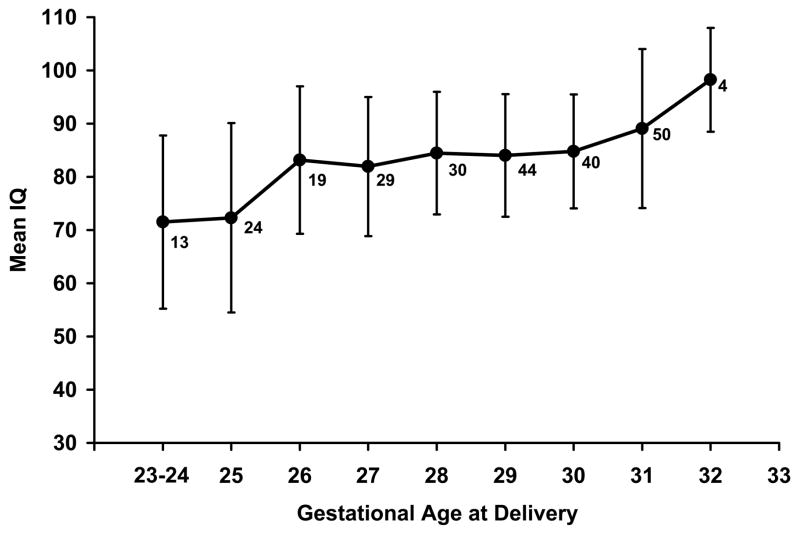
An analysis of co-variance was performed to analyze IQ as a continuous variable. In the model adjusting for mother/caregiver’s receptive vocabulary score and race, the following remained in the model with P<0.1 (expressed as the mean difference in IQ followed by the range and associated P-value): gestational age at delivery 1.9 (1.0, 2.7; P<.0001), PVL −9.6 (−18.8, −0.04; P=.041), PPROM 3.6 (−0.2, 7.4; P=.055), and a history of seizures −6.6 (−4.2, 1.0; P=.079). These results imply that an average 1.9 point increase in IQ occurs for every gestational week increase between 23 and 32 weeks’ (graphically depicted in Figure 1) and that PVL is associated with a 9.6 point mean decrease in IQ at age 6 years.
Figure 1.
Mean intelligence quotient (IQ) score of children at age 6 years versus gestational age at delivery. The numbers represent the n for each gestational age and the vertical lines represent the standard deviation.
Because 30% of the surviving cohort were not available for long-term neurodevelopmental assessment, it is important to note that there were no significant differences between those who did and did not undergo neurodevelopmental testing regarding delivery gestational age, spontaneous preterm delivery, SIRS, IVH (grade 3 and 4), PVL, NEC, BPD, CLD, RDS, histologic chorioamnionitis, positive placental cultures, and elevated cord blood IL-6 (data not shown, P-value range from .159 to .843). Among those with vs. without neurodevelopmental follow-up, there were 7.1% more African Americans and 6.3% fewer of Latino/other ethnicity (P=.001).
COMMENT
This longitudinal study of a cohort of consecutively-delivered maternal-infant dyads between 23 and 32 weeks’ gestation offers a unique opportunity to evaluate the potential impact of in utero exposure to acute inflammation on long-term neurodevelopment. Strengths of the study include the longitudinal design and the extensive pregnancy and short-term neonatal outcome data collected by trained research nurses. Other strengths include the comprehensive psychological and neurodevelopmental outcome data derived from testing using standardized instruments and overseen by a certified child psychologist and a developmental pediatrician, and the evaluation of the mother/caregivers including an assessment of their receptive vocabulary as an IQ proxy. The available information allowed for assessment of potential confounding variables such as neonatal conditions and mother/caregiver language ability.
Although this is one of the largest longitudinal cohorts of its kind, a potential weakness of this study is that the sample size was not initially designed for the long-term follow-up assessment at an average of 6 years. Thus, some apparent associations that were not statistically significant may represent type II errors. For example, although the frequency of an IQ <70 was only half as common among those with versus those without funisitis (Table 3), this difference was not statistically significant (p=0.061). Likewise, the frequency of an IQ <70 was twice as common among those who had IVH (grade 3 or 4) compared to those who did not (Table 4), but this also was notstatistically significant (p=0.150). Perhaps future studies with a larger sample size could establish whether or not such associations may be valid observations or type II errors.
Our a priori hypothesis was that in utero exposure to acute inflammation would be associated with an increased risk for severe adverse neurodevelopmental outcomes at 6 years of age including an IQ <70, cerebral palsy, and/or a composite major disability variable. This hypothesis was developed based on published literature associating such exposure with an increased risk of cerebral palsy.9–14,18,19 Interestingly, we observed no direct relationship between in utero exposure to acute inflammation and subsequent severe adverse neurodevelopmental outcome in this study of children born very preterm. Neither surrogate indicators of fetal exposure to acute inflammation, such as the presence of clinical chorioamnionitis or spontaneous preterm birth, nor direct evidence of such exposure such as histologic chorioamnionitis, funisitis, a positive chorioamnion microbial culture, or an elevated umbilical cord IL-6 value was associated with a severe adverse neurodevelopmental outcome. Indeed, PPROM may even be protective against such outcomes. The mechanism underlying this apparent protective effect of PPROM remains uncertain but is not explained by use of antibiotics, antenatal steroids, or an increased frequency of death among infants delivered after PPROM (data not shown).
The presence of certain neonatal or childhood conditions such as IVH, PVL, RDS, BPD, CLD, and a history of seizures were associated with increased risk for severe adverse neurodevelopmental outcome. Indeed, an analysis of covariance indicated that the presence of PVL at birth was associated with a mean decrease in IQ of nearly 10 points at 6 years of age.
An expected observation was the consistent association between delivery gestational age and adverse neurodevelopment. Children with an IQ <70, cerebral palsy, and a major disability at age 6 years had significantly lower mean delivery gestational ages and birthweights. Logistic regression models indicated that as the gestational age at birth increased by one week between 23 and 32 weeks’, there was a 25% lower odds of an IQ <70 and major disability at 6 years of age per gestational week. Similarly, an analysis of covariance indicated that for each gestational week gained between 23 and 32 weeks’, there was an approximate 2-point increase in IQ at 6 years of age.
These results indicate that the in utero exposure to inflammation and inflammatory mediators may not have a strong direct effect on risk for severe adverse neurodevelopmental outcomes. Alternatively, such exposure may have an indirect effect via a pathway that is mediated through the association of such exposure to increased risk of certain neonatal conditions (i.e. IVH, PVL, and lung disease) that, in turn, result in increased risk for a severe adverse neurodevelopmental outcome. We have previously reported in this cohort that acute histologic chorioamnionitis is more common at earlier delivery gestational ages, and is associated with increased risk for development of SIRS and NEC.21 Such chorioamnionitis is also associated with elevated umbilical cord blood levels of IL-6 that, in turn is associated with an increased risk for SIRS, PVL, and NEC.20 Thus, if in utero exposure to acute inflammation does increase the risk of severe adverse long-term neurodevelopment among infants delivered before 32 weeks’ gestation, this effect may be mediated through a pathway that involves development of certain neonatal complications. Alternatively, earlier gestational age at delivery (which is associated with increased risk of neonatal morbidities) may be associated with increased risk of long-term severe adverse neurodevelopment via mechanisms unrelated to fetal exposure to acute inflammation other than that such inflammation may have precipitated the preterm birth.
Although the reported association between intrauterine exposure to infection/inflammation and increased risk for cerebral palsy is relatively consistent for children born at or near term, this association is less clear among children born very preterm and published studies have yielded conflicting results. In a recent study of children born prior to 32 weeks’ gestation, Grether et al. reported that neither clinical (such as clinical chorioamnionitis or maternal fever) nor placental histopathological indicators of intrauterine infection/inflammation were associated with CP.33 Additionally, Nelson et al. recently reported no relationship between cytokine concentrations in neonatal blood of infants born prior to 32 weeks’ gestation and a later diagnosis of CP.34 This observation34 was in contrast to an earlier report from this group indicating an impressive association between CP and elevated concentrations of selected proinflammatory cytokines and coagulation factors in predominantly term-born children.18 Our results confirm the above two studies33,34 and extend the results to include an analysis of severe neourodevelopmental outcomes other than CP (including IQ). Like most studies in this area, the studies of Grether et al. and Nelson et al. are retrospective case control studies. Our current study offers the advantage of a prospectively evaluated cohort that is extremely well characterized regarding pregnancy and neonatal factors as well as caregiver information (including an estimate of caregiver IQ).
In a longitudinal study of infants born prior to 36 weeks’ gestation, Yoon et al.19 reported that elevations of IL-6 and 8 in amniotic fluid were associated with increased risk of CP at age 3 years.19 We observed no association between elevated umbilical cord IL-6 and subsequent severe neurodevelopmental outcome. It is important to note that the mean birth gestational age in the study by Yoon et al.19 was higher than that in the current study as well as that of Grether et al.33 and Nelson et al.34 Thus, the results of Yoon et al.19 are consistent with other studies of children born closer to term9,11,18 and, as such, are not necessarily in conflict with the current study or that of Nelson et al.34
Because of the observed consistent and significant correlation between later delivery gestational age and improved long-term neurodevelopmental outcome, the results of this study do not support rescue of fetuses from a “hostile” uterine environment by earlier delivery between 23 and 32 weeks’ gestation. Rather, these results support current clinical practice employing efforts to delay delivery during this gestational age interval in the setting of preterm labor or PPROM, at least in the absence of overt intrauterine infection or apparent fetal compromise. Notably, The mother/caregivers of children with an IQ <70 at age 6 years had significantly lower receptive language scores (IQ proxy) representing a consistent risk factor for adverse neurodevelopment that maynot easily be altered. Additional research is warranted to unravel the mysterious and inconsistently observed link between in utero exposure to acute inflammation and adverse long-term outcomes.
Acknowledgments
The authors would like to acknowledge Nicole Burrell, Amy Grubbs and June Weston who located and recruited the maternal participants to join the study, Vicki Gordon, MSN, CRNP and Mickey Parks, MSN, CRNP who performed the physical assessments, Robin Steele, MPH, data manager, and Rachel Copper, MSN, CRNP who was the research coordinator for the project.
This study was funded by two grants from the National Institute of Child Health and Human Development (HD33927 and HD43949).
Footnotes
No reprints available
Condensation
Among births between 23 and 32 weeks’, in utero exposure to acute inflammation is not associated with severe adverse neurodevelopment at age 6 years.
References
- 1.Hamilton BE, Minino AM, Martin JA, Kochanek KD, Strobino DM, Guyer B. Annual summary of vital statistics: 2005. Pediatrics. 2007;119:345–60. doi: 10.1542/peds.2006-3226. [DOI] [PubMed] [Google Scholar]
- 2.Hack M, Fanaroff AA. Outcomes of children of extremely low birthweight and gestational age in the 1990s. Semin Neonatol. 2000;5:89–106. doi: 10.1053/siny.1999.0001. [DOI] [PubMed] [Google Scholar]
- 3.Hack M, Taylor HG, Drotar D, Schluchter M, Cartar L, Andreias L, et al. Chronic conditions, functional limitations, and special health care needs of school-aged children born with extremely low-birth-weight in the 1990’s. JAMA. 2005;294:318–25. doi: 10.1001/jama.294.3.318. [DOI] [PubMed] [Google Scholar]
- 4.Anderson P, Doyle LW the Victorian Infant Collaborative Study Group. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990’s. JAMA. 2003;289:3264–72. doi: 10.1001/jama.289.24.3264. [DOI] [PubMed] [Google Scholar]
- 5.Anderson PJ, Doyle LW the Victorian Infant Collaborative Study Group. Executive functioning in school-age children who were born very preterm or extremely low birth weight in the 1990’s. Pediatrics. 2004;114:50–57. doi: 10.1542/peds.114.1.50. [DOI] [PubMed] [Google Scholar]
- 6.Mikkola K, Ritari N, Tommiska V, Salokorpi T, Lehtonen Tammela O, et al. neurodevelopmental outcome at 5 years of age of a national cohort of extremely low birth weight infants who were born in 1196–1997. Pediatrics. 2005;116:1391–1400. doi: 10.1542/peds.2005-0171. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg RL, Hauth JC, Andrews WW. Mechanisms of disease: intrauterine infection and preterm birth. N Engl J Med. 2000;342:1500–7. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 8.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu YW, Escobar GJ, Grether JK, Coen LA, Greene JD, Newman TB. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA. 2003;290:2677–84. doi: 10.1001/jama.290.20.2677. [DOI] [PubMed] [Google Scholar]
- 10.Nelson KB, Ellenberg JH. Antecedents of cerebral palsy, I: univariate analysis of risks. ALDC. 1985;139:1031–38. doi: 10.1001/archpedi.1985.02140120077032. [DOI] [PubMed] [Google Scholar]
- 11.Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birthweight. JAMA. 1997;278:207–11. (published correction in JAMA 1998;279:118) [PubMed] [Google Scholar]
- 12.Nelson KB, Ellenberg JH. Antecedents of cerebral palsy; multivariate analysis of risk. N Engl J Med. 1986;315:81–86. doi: 10.1056/NEJM198607103150202. [DOI] [PubMed] [Google Scholar]
- 13.Redline RW, Wilson-Costello D, Borawski E, Fanaroff AA, Hack M. Placental lesions associated with neurologic impairment and cerebral palsy in very low-birth-weight infants. Arch Pathol Lab Med. 1998;122:1091–98. [PubMed] [Google Scholar]
- 14.Barshiri A, Burstein E, Moshe M. Cerebral palsy and the fetal inflammatory response syndrome: a review. J Perinat Med. 2006;34:5–12. doi: 10.1515/JPM.2006.001. [DOI] [PubMed] [Google Scholar]
- 15.Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, et al. Amniotic fluid cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 16.Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174:1433–40. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- 17.Yoon BH, Romero R, Kim CJ, Koo JN, Choe G, Chi JG. High expression of tumor-necrosis factor-alpha and interleukin-6 in periventricular leukomalacia. Am J Obstet Gynecol. 1997;177:406–11. doi: 10.1016/s0002-9378(97)70206-0. [DOI] [PubMed] [Google Scholar]
- 18.Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665–75. doi: 10.1002/ana.410440413. [DOI] [PubMed] [Google Scholar]
- 19.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, et al. Fetal exposure to intra-amniotic cytokines and development of cerebral palsy at age three years. Am J Obstet Gynecol. 2000;182:675–81. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 20.Goepfert A, Andrews WW, Carlo W, Ramsey P, Cliver SP, Goldenberg RL, et al. Umbilical cord plasma interleukin-6 concentrations in preterm infants and risk of neonatal morbidity. Am J Obstet Gynecol. 2004;191:1375–1381. doi: 10.1016/j.ajog.2004.06.086. [DOI] [PubMed] [Google Scholar]
- 21.Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver SP, Goepfert A, Hauth JC. The Alabama preterm birth study: Polymorphonuclear and mononuclear cell placental infiltration, other markers of inflammation and outcomes in preterm newborns. Am J Obstet Gynecol. 2006;195:803–8. doi: 10.1016/j.ajog.2006.06.083. [DOI] [PubMed] [Google Scholar]
- 22.Goldenberg RL, Andrews WW, Faye-Petersen O, Goepfert A, Cliver SP, Hauth JC. The Alabama preterm birth study: Intrauterine infection and placental histologic findings in male and female <32 week preterm births. Am J Obstet Gynecol. 2006;195:1533–37. doi: 10.1016/j.ajog.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 23.Goldenberg RL, Andrews WW, Faye-Petersen O, Cliver SP, Goepfert A, Hauth JC. The Alabama preterm birth study: Corticosteroids and newborn outcomes in 23–32 week newborns with various markers of placental infection. Am J Obstet Gynecol. 2006;195:1020–4. doi: 10.1016/j.ajog.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 24.Goldenberg RL, Andrews WW, Faye-Petersen O, Cliver SP, Goepfert A, Hauth JC. The Alabama preterm birth project: Placental histology in recurrent preterm birth. Am J Obstet Gynecol. 2006;195:792–6. doi: 10.1016/j.ajog.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 25.Goldenberg RL, Faye-Petersen O Andrews WW, Goepfert A, Cliver SP, Hauth JC. The Alabama preterm birth study: diffuse decidual leukocytoclastic necrosis of the decidua basalis, A placental lesion associated with preeclampsia, indicated preterm birth, and decreased fetal growth. J Mat Fet Med. 2006 doi: 10.1080/14767050701236365. (In press) [DOI] [PubMed] [Google Scholar]
- 26.Goldenberg RL, Andrews WW, Goepfert AR, Faye-Petersen O, Cliver SP, Carlo WA, Hauth JC. The Alabama preterm birth study: Umbilical cord blood ureaplasma urealyticum and mycoplasma hominis cultures in very preterm newborns. Am J Obstet Gynecol. doi: 10.1016/j.ajog.2007.07.033. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waites KB, Rikihisa Y, Taylor-Robinson D. Mycoplasma and Ureaplasma. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of Clinical Microbiology. 8. ASM Press; 2003. pp. 972–990. [Google Scholar]
- 28.Andrews WW, Hauth JC, Goldenberg RL, Gomez R, Romero R, Cassell GH. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol. 1995;173:606–12. doi: 10.1016/0002-9378(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 29.Bendon RW, Faye-Petersen O, Pavlova Z, Qureshi F, Elder N, Das A, et al. Histologic features of chorioamnion membrane rupture: Development of methodology. Pediatr Pathol Lab Med. 1997;17:27–42. [PubMed] [Google Scholar]
- 30.Williams KT, Wang JJ. Technical references to the Peabody Picture Vocabulary Test-Third Edition (PPVT-III) Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- 31.Wechsler D. WISC-IV administration and scoring manual. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 32.Eliot CD. Differential ability scales. San Antonio, TX: The Psychological Corporation; 1990. [Google Scholar]
- 33.Grether JK, Nelson KB, Walsh E, Willoughby RE, Redline RW. Intrauterine exposure to infection and risk of cerebral palsy in very preterm infants. Arch Pediatr Adolesc Med. 2003;157:26–32. doi: 10.1001/archpedi.157.1.26. [DOI] [PubMed] [Google Scholar]
- 34.Nelson KB, Grether JK, Dambrosia JM, Walsh E, Kohler S, Satyanarayana G, et al. Neonatal cytokines and cerebral palsy in very preterm infants. Pediatr Res. 2003;53:600–07. doi: 10.1203/01.PDR.0000056802.22454.AB. [DOI] [PubMed] [Google Scholar]