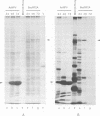
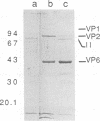
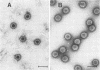
Abstract
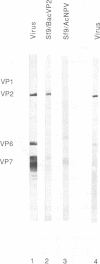
The complete VP2 gene of bovine rotavirus strain RF has been inserted into the baculovirus transfer vector pVL941 under the control of the polyhedrin promoter. Cotransfection of Spodoptera frugiperda 9 cells with wild-type baculovirus DNA and transfer vector DNA led to the formation of recombinant baculoviruses which contain bovine rotavirus gene 2. Infection of S. frugiperda cells with this recombinant virus resulted in the production of a protein similar in size and antigenic properties to the authentic rotavirus VP2. The protein binds double-stranded RNA and DNA in an overlay protein blot assay. Expressed VP2 assembles in the cytoplasm of infected cells in corelike particles 45 nm in diameter. These corelike particles were purified by sucrose gradient centrifugation and found to be devoid of nucleic acid. Coexpression of VP2 and VP6 from heterologous rotavirus strains (bovine and simian) resulted in the formation of single-shelled particles. These results definitively show the existence of an innermost protein shell in rotavirus which is formed independently of other rotavirus proteins. These results have implications for schemes of rotavirus morphogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bican P., Cohen J., Charpilienne A., Scherrer R. Purification and characterization of bovine rotavirus cores. J Virol. 1982 Sep;43(3):1113–1117. doi: 10.1128/jvi.43.3.1113-1117.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. F., Holmes K. V. RNA-binding proteins of bovine rotavirus. J Virol. 1986 May;58(2):561–568. doi: 10.1128/jvi.58.2.561-568.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüssow H., Bruttin A., Marc-Martin S. Polypeptide composition of rotavirus empty capsids and their possible use as a subunit vaccine. J Virol. 1990 Aug;64(8):3635–3642. doi: 10.1128/jvi.64.8.3635-3642.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Charpilienne A., Chilmonczyk S., Estes M. K. Nucleotide sequence of bovine rotavirus gene 1 and expression of the gene product in baculovirus. Virology. 1989 Jul;171(1):131–140. doi: 10.1016/0042-6822(89)90519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Laporte J., Charpilienne A., Scherrer R. Activation of rotavirus RNA polymerase by calcium chelation. Arch Virol. 1979;60(3-4):177–186. doi: 10.1007/BF01317489. [DOI] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Ernst H., Duhl J. A. Nucleotide sequence of genomic segment 2 of the human rotavirus Wa. Nucleic Acids Res. 1989 Jun 12;17(11):4382–4382. doi: 10.1093/nar/17.11.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Crawford S. E., Penaranda M. E., Petrie B. L., Burns J. W., Chan W. K., Ericson B., Smith G. E., Summers M. D. Synthesis and immunogenicity of the rotavirus major capsid antigen using a baculovirus expression system. J Virol. 1987 May;61(5):1488–1494. doi: 10.1128/jvi.61.5.1488-1494.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Mason B. B. Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. J Virol. 1981 Sep;39(3):879–888. doi: 10.1128/jvi.39.3.879-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- French T. J., Roy P. Synthesis of bluetongue virus (BTV) corelike particles by a recombinant baculovirus expressing the two major structural core proteins of BTV. J Virol. 1990 Apr;64(4):1530–1536. doi: 10.1128/jvi.64.4.1530-1536.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gottlieb P., Strassman J., Qiao X. Y., Frucht A., Mindich L. In vitro replication, packaging, and transcription of the segmented double-stranded RNA genome of bacteriophage phi 6: studies with procapsids assembled from plasmid-encoded proteins. J Bacteriol. 1990 Oct;172(10):5774–5782. doi: 10.1128/jb.172.10.5774-5782.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray W. L., Yalamanchili R., Raengsakulrach B., Baumann R. P., Staczek J., O'Callaghan D. J. Viral transcripts in cells infected with defective interfering particles of equine herpesvirus type 1. Virology. 1989 Sep;172(1):1–10. doi: 10.1016/0042-6822(89)90101-3. [DOI] [PubMed] [Google Scholar]
- Kapahnke R., Rappold W., Desselberger U., Riesner D. The stiffness of dsRNA: hydrodynamic studies on fluorescence-labelled RNA segments of bovine rotavirus. Nucleic Acids Res. 1986 Apr 25;14(8):3215–3228. doi: 10.1093/nar/14.8.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Charpilienne A., Cohen J. Nucleotide sequence of the gene encoding for the RNA binding protein (VP2) of RF bovine rotavirus. Nucleic Acids Res. 1989 Mar 11;17(5):2126–2126. doi: 10.1093/nar/17.5.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Haridon R., Scherrer R. Culture in vitro du rotavirus associé aux diarrhées néonatales du veau. Ann Rech Vet. 1976;7(4):373–381. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu M., Estes M. K. Nucleotide sequence of the simian rotavirus SA11 genome segment 3. Nucleic Acids Res. 1989 Oct 11;17(19):7991–7991. doi: 10.1093/nar/17.19.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow V. A., Summers M. D. High level expression of nonfused foreign genes with Autographa californica nuclear polyhedrosis virus expression vectors. Virology. 1989 May;170(1):31–39. doi: 10.1016/0042-6822(89)90348-6. [DOI] [PubMed] [Google Scholar]
- Mitchell D. B., Both G. W. Completion of the genomic sequence of the simian rotavirus SA11: nucleotide sequences of segments 1, 2, and 3. Virology. 1990 Jul;177(1):324–331. doi: 10.1016/0042-6822(90)90487-c. [DOI] [PubMed] [Google Scholar]
- Nakata S., Petrie B. L., Calomeni E. P., Estes M. K. Electron microscopy procedure influences detection of rotaviruses. J Clin Microbiol. 1987 Oct;25(10):1902–1906. doi: 10.1128/jcm.25.10.1902-1906.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B. V., Wang G. J., Clerx J. P., Chiu W. Three-dimensional structure of rotavirus. J Mol Biol. 1988 Jan 20;199(2):269–275. doi: 10.1016/0022-2836(88)90313-0. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Chen G. M., Hung T., Beards G. M., Brown D. W., Flewett T. H. Effects of two negative staining methods on the Chinese atypical rotavirus. Arch Virol. 1987;94(3-4):305–308. doi: 10.1007/BF01310723. [DOI] [PubMed] [Google Scholar]
- Urakawa T., Roy P. Bluetongue virus tubules made in insect cells by recombinant baculoviruses: expression of the NS1 gene of bluetongue virus serotype 10. J Virol. 1988 Nov;62(11):3919–3927. doi: 10.1128/jvi.62.11.3919-3927.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager M., Dryden K. A., Olson N. H., Greenberg H. B., Baker T. S. Three-dimensional structure of rhesus rotavirus by cryoelectron microscopy and image reconstruction. J Cell Biol. 1990 Jun;110(6):2133–2144. doi: 10.1083/jcb.110.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]