Abstract
NOD2, a NOD-like receptor (NLR), is an intracellular sensor of bacterial muramyl dipeptide (MDP) that was suggested to promote secretion of the proinflammatory cytokine IL-1β. Yet, the molecular mechanism by which NOD2 can stimulate IL-1β secretion, and its biological significance were heretofore unknown. We found that NOD2 through its N-terminal caspase recruitment domain directly binds and activates caspase-1 to trigger IL-1β processing and secretion in MDP-stimulated macrophages, whereas the C-terminal leucine-rich repeats of NOD2 prevent caspase-1 activation in nonstimulated cells. MDP challenge induces the association of NOD2 with another NLR protein, NALP1, and gel filtration analysis revealed the formation of a complex consisting of NOD2, NALP1, and caspase-1. Importantly, Bacillus anthracis infection induces IL-1β secretion in a manner that depended on caspase-1 and NOD2. In vitro, Anthrax lethal toxin strongly potentiated IL-1β secretion, and that response was NOD2 and caspase-1-dependent. Thus, NOD2 plays a key role in the B. anthracis-induced inflammatory response by being a critical mediator of IL-1β secretion.
Keywords: inflammasome, lethal toxin, LPS
Secretion of IL-1β, −18, and −33 by activated macrophages depends on the protease caspase-1 that converts their precursors to the mature and biologically active cytokines (1, 2). IL-1β and −18 are potent mediators of inflammation, being responsible for a variety of effects associated with host responses to microbial invasion and tissue damage (3, 4). IL-33 mediates production of T helper type 2-associated cytokines (5). The mechanism by which caspase-1 is activated to induce processing of pro-IL-1β and related cytokines is not well understood. It was proposed that caspase-1 is activated within a large protein complex called the “inflammasome” (6). To date, several inflammasomes were identified and are defined by the NOD-like receptor (NLR) proteins that they contain, such as the NALP3 (7) and IPAF inflammasomes (8). NLR proteins are essential for activation of caspase-1 by various bacteria, such as Salmonella typhimurium, Staphylococcus aureus, and Listeria monocytogenes and are involved in sensing of bacterial cell wall products (8, 9).
Another member of the NLR family characterized by a N-terminal protein interaction motif, followed by a nucleotide-binding domain (NBD) and leucine-rich repeats (LRR) is NOD2 (2, 10). Mutations in the human NOD2 gene are linked to increased susceptibility to the chronic inflammatory disorders Crohn's disease (CD), psoriatic arthritis, and Blau syndrome (10). NOD2 was proposed to serve as an intracellular sensor for muramyl dipeptide (MDP), a fragment of peptidoglycan (PGN) from bacterial cell walls, and initiate activation of NF-κB and MAPK (11). Macrophages from mice carrying a frameshift mutation at position 2939 of Nod2, which corresponds to a common CD-associated human mutation, −3020insC-, secrete more IL-1β than WT macrophages upon MDP stimulation (12). However, human monocytes obtained from CD patients homozygous for the same mutation are MDP-nonresponsive (13). The basis for this discrepancy is not known (10). Based on its similarity to other NLRs that are involved in inflammasome assembly and activation of caspase-1 (6, 14), we postulated that NOD2 may have a similar function in addition to its previously documented involvement in NF-κB and MAPK activation (11). Indeed, we found that NOD2 can associate with NALP1 to form a complex that activates caspase-1 in response to MDP. Importantly, NOD2 is a critical mediator of IL-1β secretion during Bacillus anthracis infection and thus may be an important contributor to the severe inflammation associated with Anthrax (15).
Results
NOD2 Is Required for MDP-Induced IL-1β Secretion.
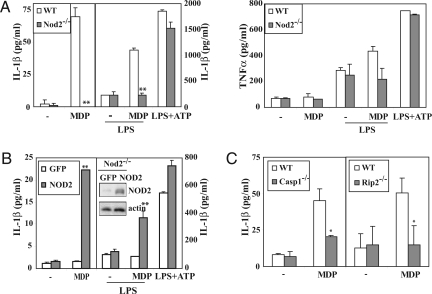
NLR-containing inflammasome complexes responsible for pro-IL-1β processing and IL-1β secretion have been identified (1). Given that mice carrying the Nod22939ic mutation produce more IL-1β during colonic inflammation (12), we examined whether NOD2 is also involved in caspase-1 activation and pro-IL-1β processing. We used peritoneal macrophages from WT and Nod2−/− mice (16) and to facilitate the uptake of poorly permeable MDP by the cell, we used microparticles (0.2-μm diameter) of dietary grade titanium dioxide (TiO2), which is a common food additive and pharmacological excipient that is readily taken up by Peyer's patches (17, 18). Incubation of macrophages with TiO2 microparticles alone did not induce cytokine secretion (no treatment in Fig. 1A). Stimulation of WT macrophages with MDP complexed with TiO2 induced efficient IL-1β secretion, but the response was markedly attenuated in Nod2−/− macrophages (Fig. 1A). By contrast, no difference in TNF-α production were noted between the genotypes. Because MDP is a modest NF-κB activator (12) whose activity is needed for pro-IL-1β mRNA production (19), we pretreated macrophages with a low concentration of LPS (0.5 ng/ml), a potent activator of NF-κB, for 6 h to induce pro-IL-1β accumulation and then stimulated these cells with TiO2 microparticles with or without MDP. Preincubation with LPS strongly enhanced MDP-stimulated IL-1β secretion in WT macrophages, but even under these conditions, Nod2−/− macrophages did not show a response to MDP (Fig. 1A). Incubation of macrophages with LPS+ATP resulted in IL-1β secretion that depended on the NALP3 inflammasome (9). The Nod2 status had no effect on this response (Fig. 1A). As expected, the LPS-mediated enhancement of IL-1β production was abrogated in Tlr4−/− and Myd88−/− macrophages (data not shown). Reintroduction of NOD2 into Nod2−/− macrophages restored MDP-stimulated IL-1β secretion without or with LPS preincubation (Fig. 1B). These data confirm that NOD2 is required for MDP-dependent IL-1β production as initially suggested by indirect in vivo experiments (12) and more recent in vitro experiments (20).
Fig. 1.
Caspase-1 and RIP2 are essential for MDP-induced IL-1β production in macrophages. (A) IL-1β and TNF-α secretion by WT and Nod2−/− macrophages. Macrophages were left untreated or primed with LPS (0.5 ng/ml) for 6 h before treatment with TiO2 microparticles without (−) or with MDP (10 μg/ml). For NOD2-independent inflammasome activation, macrophages were primed with high levels of LPS (500 ng/ml) followed by incubation with ATP (5 mM). Supernatants were collected, and secreted cytokines were measured by ELISA. This and all similar experiments were repeated at least three times, and one representative experiment done in triplicates is shown. Results are expressed as the mean ± SD, and the statistical analysis was performed by using a two-sided unpaired Student's t test. Significant differences, **, P < 0.01; *, P < 0.05. (B) Reconstitution of Nod2-deficient macrophages with NOD2 restores MDP-induced IL-1β release. Bone marrow of Nod2−/− mice was transduced with retroviruses encoding NOD2 or GFP, differentiated into macrophages and stimulated as in A. IL-1β levels in supernatants were determined by ELISA and NOD2 protein amount was monitored by immunobot. (C) Caspase-1 and RIP2 dependence of MDP-induced IL-1β secretion. Peritoneal macrophages from the indicated strains were treated as above and IL-1β release was measured.
Caspase-1 and RIP2 Are Required for MDP-Induced IL-1β Secretion but They Act Through Different Mechanisms.
Caspase-1 is a caspase recruitment domain (CARD)-containing protease required for processing of pro-IL-1β in macrophages (21). Another CARD-containing protein, RIP2, is used by NOD2 to drive MAPK and NF-κB activation (22). We examined the requirement for caspase-1 and RIP2 in MDP-stimulated IL-1β secretion. Caspase-1- (21) and Rip2- (23) null macrophages exhibited diminished MDP responsivenesss measured by IL-1β secretion (Fig. 1C), but only Rip2−/−macrophages exhibited defective MDP-induced TNF-α secretion [supporting information (SI) Fig. S1]. When preincubated with LPS, caspase-1−/− macrophages continued to exhibit normal MDP-enhanced TNF-α release but little IL-1β secretion, whereas Rip2−/− macrophages exhibited the opposite pattern: MDP-enhanced IL-1β secretion, but no MDP-enhanced TNF-α release. (Fig. S2). Mirroring its effect on TNF-α release, ablation of RIP2, but not caspase-1, prevented MDP-induced NF-κB activation (Fig. S3 A and C). Accordingly, RIP2 ablation blocked MDP induction of NF-κB target genes, including Il-1β and Tnf-α (Fig. S3B). RIP2 was also required for MDP-enhanced, but not for LPS-induced, expression of pro-IL-1β protein and mRNA, whereas caspase-1 was not required for pro-IL-1β mRNA accumulation in response to either stimulus (Fig. S4). Like RIP2, the absence of NOD2 abrogated the MDP-enhancement of LPS-induced pro-IL-1β mRNA expression.
NOD2 Directly Interacts with and Activates Caspase-1 Through Its N-Terminal CARD.
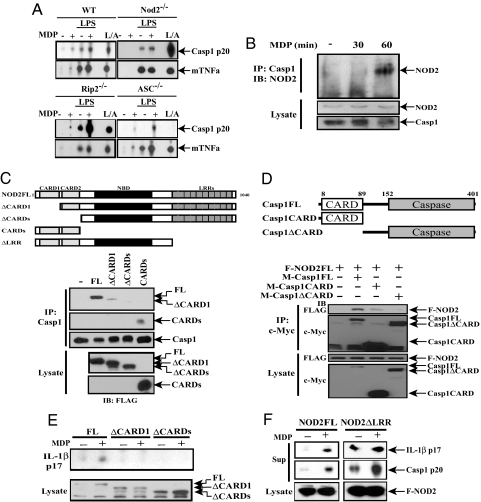
NOD2 is required for MDP-induced caspase-1 activation but has no role in the response to LPS+ATP (Fig. 2A). Although by itself MDP was a weak caspase-1 activator, it enhanced the release of activated p20 caspase-1 from LPS-primed WT macrophages, and this response was also abrogated in the absence of NOD2 (Fig. 2A). Rip2−/− macrophages, however, behaved like WT cells with respect to MDP- and LPS+MDP-induced caspase-1 activation (Fig. 2A). Thus, although RIP2 is important for MDP-stimulated NF-κB activation, it makes little contribution, if any, to MDP-enhanced caspase-1 activation. Consistent with a previous publication (8), Asc−/− macrophages were no longer responsive to LPS but retained almost normal responsiveness to MDP with respect to caspase-1 activation (Fig. 2A) and IL-1β secretion (Fig. S5).
Fig. 2.
NOD2 binds to and activates caspase-1. (A) Caspase-1 activation by MDP is NOD2-dependent. Peritoneal macrophages from the indicated strains were treated as described in Fig. 1A, and supernatants were analyzed by immunoblotting for the presence of activated caspase-1 (p20) and TNF-α. L/A = macrophages were primed with LPS + ATP as in Fig. 1A. (B) NOD2 interacts with caspase-1 upon MDP stimulation. TDM were treated with MDP (10 μg/ml). At the indicated time points, cells were lysed, caspase-1 was immunoprecipitated (IP), and the presence of NOD2 in immunoprecipitates and original lysates was examined by immunoblotting (IB). (C) The NOD2 CARD motif binds caspase-1. Myc-tagged caspase-1 was coexpressed in HEK293T cells with indicated FLAG-tagged NOD2 constructs. After 36 h, cells were lysed and caspase-1 was immunoprecipitated. The presence of FLAG-tagged NOD2 and caspase-1 in the immunoprecipitates was examined by immunoblotting. (D) Caspase-1 binds NOD2 via its CARD motif. FLAG-tagged NOD2 was coexpressed in HEK293T cells with the indicated Myc-tagged caspase-1 constructs. After 36 h, cell lysates were immunoprecipitated with Myc antibody and analyzed as above. (E) The NOD2-CARD motifs are required for MDP-induced IL-1β secretion. FLAG-tagged NOD2 expression vectors were cotransfected into HEK293T cells along with caspase-1 and pro-IL-1β expression vectors as above. After 36 h, culture supernatants and cell lysates were examined for mature IL-1β and the different NOD2 proteins, respectively. (F) The NOD2-LRR prevents caspase-1 activation in the absence of MDP. The different NOD2 constructs were coexpressed in HEK293T cells as shown. Secretion of mature IL-1β and activated caspase-1 was examined as above.
In transient expression experiments in HEK293T cells, coexpression of NOD2, caspase-1, and pro-IL-1β expression vectors allowed MDP-induced pro-IL-1β processing (data not shown). Mouse and human NOD2 proteins, but not RIP2, coprecipitated with caspase-1 when expressed in HEK293T cells (Fig. S6 A and B). Furthermore, NOD2 interacted only with inflammatory caspases: caspase-1 and −4, under these transient expression conditions (Fig. S7). In addition, MDP without the use of TiO2 microparticles, which were not required in THP-1-derived macrophages (TDM), induced the association of endogenous NOD2 with caspase-1 (Fig. 2B). Incubation of TDM with MDP activated caspase-1 and induced IL-1β processing (data not shown).
A series of NOD2 truncation mutants were examined for caspase-1 interaction (Fig. 2C). Deletion of the CARD domains abolished caspase-1 binding, whereas a fragment containing only the CARD domains did bind caspase-1. Removal of the CARD domain from caspase-1 abolished NOD2 binding, and the CARD domain alone was able to bind NOD2, albeit weakly (Fig. 2D). Thus, NOD2 associates with caspase-1 through CARD–CARD interactions. Removal of either the first or both CARD domains from NOD2 abolished its ability to stimulate pro-IL-1β processing (Fig. 2E).
The LRR of NOD2 was proposed to engage in protein–protein interactions and ligand binding (10) and inhibition of NOD2 function in the absence of a ligand (24). To examine whether the LRR negatively regulates NOD2 function, as shown for plant resistance (R) proteins, IPAF and NALP1 (6, 25, 26), we analyzed different NOD2 derivatives. Removal of the LRR rendered NOD2 a constitutive activator of pro-IL-1β processing and caspase-1 (Fig. 2F). Furthermore, the LRR interacted with the N-terminal half of NOD2 (CARDs + NOD domain), and this interaction was abolished by high MDP concentrations (Fig. S8). These data strongly suggest that, upon MDP presentation, the LRR of NOD2 may no longer engage in inhibitory intramolecular interactions, freeing the CARD domains to interact with caspase-1 and activate it.
MDP Triggers NOD2:NALP1 Complex Formation.
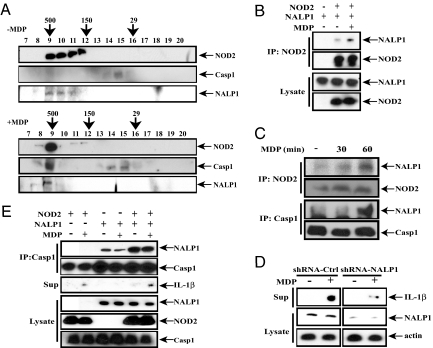
Although our results demonstrate the dependence of MDP-stimulated caspase-1 activation and IL-1β secretion on NOD2, it was also suggested that these responses depend on NALP1 (27). One possible explanation for this apparent discrepancy is formation of a NOD2:NALP1 inflammasome complex, in which both NLRs contribute to MDP-induced caspase-1 activation. Indeed, gel filtration analysis of TDM extracts revealed that MDP stimulation affected the elution profiles of NOD2, NALP1, and caspase-1, but not NALP3, leading to their coelution in a single high-molecular-weight fraction (Fig. 3A and data not shown). Coimmunoprecipitation experiments demonstrated that MDP enhanced the association of NALP1 with NOD2 in transiently transfected HEK293T cells (Fig. 3B), whereas in TDM, MDP induced association of endogenous NOD2 and NALP1 (Fig. 3C). The time course of NALP1 binding to NOD2 was similar to its association with caspase-1. Consistent with these data, NALP3 did not bind NOD2 under these conditions (data not shown). We used RNAi to knockdown NALP1 in TDM and found decreased MDP-stimulated IL-1β secretion (Fig. 3D). Accordingly, NALP1 enhanced the ability of NOD2 to stimulate IL-1β secretion in response to MDP in 293T cells and NOD2 enhanced the binding of NALP1 to caspase-1 (Fig. 3E). These results suggest that a complex (inflammasome) consisting of NOD2 and NALP1 regulates caspase-1 activation and IL-1β secretion in response to MDP.
Fig. 3.
NALP1 binds NOD2 and enhances MDP-induced IL-1β release. (A) NOD2 and NALP1 coelute with caspase-1 after MDP stimulation. TDM were left untreated or treated with MDP (10 μg/ml) for 2 h. Cell lysates were collected and separated on a Superdex 200 size exclusion column. Fractions were immunoblotted with antibodies against NOD2 (Genentech), caspase-1, and NALP1 (Abcam). Fraction number and molecular mass markers (in kDa) are shown above each image. (B) MDP enhances association of NOD2 with NALP1. HEK293T cells were transfected with NALP1 or NOD2 vectors in the absence or presence of MDP (2 μg/ml). After 36 h, cells were lysed, and NOD2 was immunoprecipitated. Presence of NALP1 in the immunoprecipitates and original lysates was examined by immunoblotting. (C) MDP induces binding of NALP1 to endogenous NOD2, and caspase-1. TDM were stimulated with MDP (10 μg/ml). NOD2 and caspase-1 were separately immunoprecipitated, and the presence of NALP1 in the immunoprecipitates was examined as above. (D) NALP1 is required for MDP-induced IL-1β release in TDM. TDM were infected with lentiviruses expressing either scrambled or NALP1-specific shRNA and cultured for 72 h before stimulation with 10 μg/ml MDP for 12 h. Secretion of mature IL-1β and expression of NALP1 were examined by immunoblotting. (E) NALP1 potentiates NOD2-induced IL-1β secretion by MDP. NOD2 and NALP1 were coexpressed as indicated with caspase-1 and pro-IL-1β in HEK293T cells with or without MDP. After 36 h, culture supernatants were examined for mature IL-1β. Caspase-1 was immunoprecipitated from cell lysates, and the presence of coprecipitated NALP1 was examined by immunoblotting. NOD2, NALP1, and caspase-1 expression was analyzed as above.
B. anthracis-Induced IL-1β Secretion Requires NOD2 and Caspase-1.
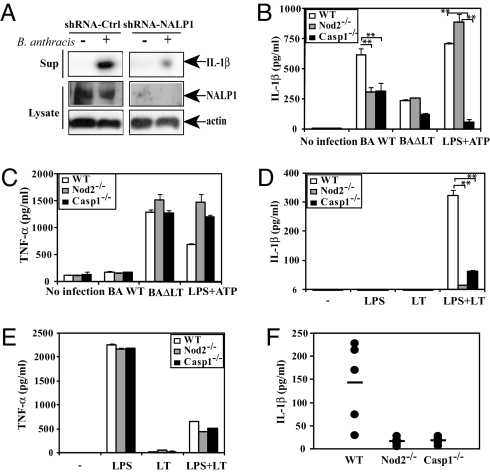
B. anthracis lethal toxin (LT) was reported to be a major cause of Anthrax-related death as well as being responsible for caspase-1 activation and IL-1β induction (28). NALP1, which is encoded by a polymorphic complex in mouse, was recently suggested to control macrophage death and IL-1β secretion in response to Anthrax LT (28). However, the role of NALP1 in responsiveness to intact B. anthracis was not examined. Because the ability of B. anthracis to induce severe inflammation was suggested to depend on IL-1β secretion (15), we examined the role of NALP1 and NOD2 in the host response. The knockdown of NALP1 in TDM, however, decreased IL-1β secretion induced by B. anthracis infection (Fig. 4A). Unfortunately, the polymorphic nature of the mouse Nalp1 locus precluded similar experiments in mouse macrophages. Nonetheless, mouse macrophages deficient in either NOD2 or caspase-1 exhibited a marked decrease in IL-1β release upon B. anthracis infection compared to WT macrophages (Fig. 4B). B. anthracis-induced IL-1β secretion was LT-dependent, as evident by the attenuated response to a LT-deficient mutant (Fig. 4B) or a B. anthracis strain lacking the virulence plasmid (pX01) (Fig. S9A). B. anthracis-induced TNF-α release, however, was similar in WT, caspase-1−/−, and Nod2−/− macrophages and was strongly augmented by the absence of LT (Fig. 4C and Fig. S9B), an effect consistent with the ability of LT to inhibit activation of p38α, a protein kinase required for TNF-α production (29). Defective Anthrax-induced IL-1β secretion in Nod2−/− or caspase-1−/− macrophages was not due to decreased pro-IL-1β expression (SI Text and Fig. S9C). Our data suggest that B. anthracis-triggered IL-1β secretion is mainly caspase 1-dependent; however, a caspase1-independent pathway is also likely to operate, because IL-1β secretion is still found in infected caspase-1 knockout macrophages.
Fig. 4.
B. anthracis-induced IL-1β secretion depends on NOD2, NALP1, and caspase-1. (A) NALP1 is required for IL-1β release in B. anthracis-infected TDM. TDM were infected with scrambled or NALP1 shRNA lentivirus as above and cultured for 72 h before B. anthracis infection. Supernatants were collected 6 h postinfection, and secretion of mature IL-1β and expression of NALP1 were examined by immunoblotting. (B and C) NOD2 is necessary for IL-1β, but not TNF-α, secretion in B. anthracis-infected mouse macrophages. Peritoneal macrophages from WT, Nod2−/− and caspase1−/− mice were infected or not with the indicated B. anthracis Sterne strains (BaWT or BaΔLT) at a multiplicity of infection of 2. Macrophages were also pretreated with LPS and then pulsed with ATP as a positive control. Supernatants were collected 6 h postinfection, and secreted IL-1β (B) and TNF-α (C) were measured. (D and E) NOD2 is required for IL-1β, but not TNF-α, release by LT-treated macrophages. Macrophages were left untreated or treated with LT (200 ng/ml LF + 2.5 μg/ml PA63) in the absence or presence LPS (100 ng/ml) for 18 h. Supernatants were analyzed for secreted IL-1β (D) and TNF-α (E). Significant differences, **, P < 0.01; *, P < 0.05. (F) IL-1β secretion in B. anthracis infected mice requires caspase-1 and NOD2. Mice (n = 5) were injected i.p. with 107 cfu of early log-phase B. anthracis BaWT. IL-1β in plasma was measured 17 h after infection.
To further confirm the role of LT in NOD2-dependent IL-1β processing, we measured IL-1β secretion by macrophages treated with recombinant LT. Because both Nod2−/− and caspase-1−/− mice were of the C57BL/6 genetic background, we used C57BL/6 macrophages as controls despite their poor LT responsiveness (28). As expected, C57BL/6 mouse macrophages produced less IL-1β when incubated with LT relative to 129S1/SvlmJ macrophages (data not shown). Nonetheless, the absence of NOD2 or caspase-1 in C57BL/6 macrophages abolished LT-induced IL-1β secretion, which required coincubation with LPS (Fig. 4D), a condition shown to strongly enhance the killing of C57BL/6 macrophages by LT (29). TNF-α secretion in response to LT treatment was also potentiated by LPS costimulation, but unlike IL-1β secretion, did not depend on NOD2 or caspase-1 (Fig. 4E). Consistent with the IL-1β secretion results, Nod2−/− and caspase-1−/− macrophages were relatively resistant to induction of cell death in response to LT+LPS (data not shown).
We next examined the response to B. anthracis infection in vivo. After i.p. challenge with live B. anthracis, serum IL-1β was elevated in WT C57BL/6 mice but not in Nod2−/− and caspase-1−/− counterparts (Fig. 4F). The caspase-1 or NOD2 deficiencies did not exert a major effect on TNF-α secretion (Fig. S9D). These results confirm that NOD2 is an important mediator of Anthrax-induced IL-1β production ex vivo and in vivo. Because this response is LT-dependent, it may also depend on NALP1, which was shown to be responsible for strain-specific variation in the response to recombinant LT (28).
Discussion
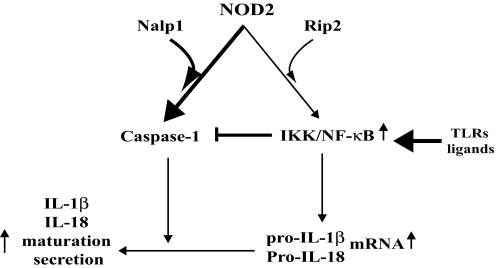
At least two distinct events are required for production of mature IL-1β, −18, and −33 (2). The first event is enhanced precursor synthesis that is mainly regulated transcriptionally by NF-κB and other transcription factors (2). The second event is precursor processing, which in macrophages chiefly depends on caspase-1 activation (2, 19). Our results strongly suggest that, although NOD2 can signal to NF-κB (11), its more critical function in the context of an actual bacterial infection is activation of caspase-1. This conclusion is consistent with a recent report that demonstrates that NOD2 plays dual roles in pro-IL-1β synthesis and caspase-1-dependent IL-1β maturation in human monocytes (30). Indeed, the absence of NOD2 prevents B. anthracis-induced IL-1β secretion but has little effect on pro-IL-1β synthesis in infected macrophages. Like other Gram-positive bacteria, B. anthracis is likely to activate TLR2, but its anthrolysin protein is a potent TLR4 activator (31). Although NOD2 can contribute to NF-κB and MAPK activation, it should be noted that Toll-like receptors (TLRs) are much more effective in triggering these responses (12), and therefore IL-1β transcription during a “real” bacterial infection is most likely TLR- rather than NLR-mediated. We thus propose that a key function of NOD2 is formation of an MDP-responsive “inflammasome” together with NALP1, and this is responsible for pro-IL-1β processing and secretion of the mature cytokine (Fig. 5).
Fig. 5.
A model summarizing the control of IL-1β secretion by NOD2. We propose that in the context of a bacterial infection, TLR engagement provides the major input leading to activation of NF-κB and induction of cytokine precursors. The unique function of NOD2 in this context is to activate caspase-1 in response to bacterial MDP or intact B. anthracis. This function depends on the association of NOD2 with NALP1. Activated NF-κB inhibits caspase-1 activation through synthesis of PAI-2.
In addition to B. anthracis infection, NOD2 is required together with NALP1 for caspase-1 activation in response to MDP. In the experiments performed on mouse macrophages, we incubated MDP with TiO2 microparticles, a common food additive readily taken up by these cells (17). TiO2 was shown to facilitate MDP entry into mouse macrophages, because MDP itself is poorly cell-permeable (32), but fine TiO2 microparticles are actively phagocytosed (17). The negatively charged surface of the TiO2 microparticle favors adsorption of cations (Ca+2 in our case), which may further potentiate caspase-1 activation (17). Although by themselves TiO2 microparticles do not induce cytokine release or macrophage death (ref. 33 and Fig. 1A), we note that they also facilitate LPS-induced IL-1β release and thus mimic the action of ATP, which may act through induction of K+ efflux (17, 34) or inhibition of NF-κB, a negative regulator of caspase-1 activation (19). Importantly, even in the presence of TiO2, the response to MDP strongly depended on both NOD2 and caspase-1, and similar dependence was still seen when the macrophages were preincubated with LPS to induce pro-IL-1β synthesis via a RIP2-independent pathway. However, if LPS preincubation was omitted, pro-IL-1β synthesis in MDP-stimulated cells fully depended on RIP2, shown to be required for MDP-induced NF-κB activation (22). Thus, other than enhancing the responses to MDP or LPS, TiO2 does not alter the dependence of IL-1β secretion on caspase-1 or RIP2.
Although RIP2 was also shown to interact with caspase-1 in an LPS-inducible manner (35), we could not detect MDP-induced interaction between the two molecules (Fig. S6B). Consistent with these findings, Rip2−/− macrophages did not exhibit defective caspase-1 activation after MDP stimulation (Fig. 2A). Nonetheless, RIP2 is required for pro-IL-1β precursor synthesis through its effect on NF-κB, and therefore Rip2−/− macrophages exhibit defective MDP-induced IL-1β secretion, as reported (20).
Another NLR family member, NALP3, is required for LPS+ATP- (9) or LPS+MDP- (20) induced IL-1β release, whereas NALP1 is responsible for MDP-induced caspase-1 activation and IL-1β secretion in response to in vitro incubation with MDP (27). To reconcile these and our results, we examined whether NOD2 interacts with either NALP1 or NALP3. We found MDP-induced interaction between NOD2 and NALP1 under a variety of experimental settings (Fig. 3), but under no circumstances could we detect an interaction between NOD2 and NALP3 (data not shown). Furthermore, in our hands, Nalp3−/− mouse macrophages were still MDP-responsive (Fig. S5). Based on these results, we suggest that NALP3 may be important for MDP responsiveness under different conditions from those that trigger NOD2-dependent IL-1β secretion, which also requires NALP1.
ASC is a CARD-containing protein, often used by NLRs as an adaptor molecule for caspase-1 binding and activation (2). Our results suggest that NOD2 binds and activates caspase-1 independently of ASC. Our observations are consistent with a recent report, which demonstrates that LPS but not MDP can induce ASC oligomerization leading to formation of a “pyroptosome” complex that activates caspase-1 (36).
Based on structural considerations and our deletion analysis, we propose that NOD2 binds caspase-1 directly through its N-terminal CARD domains, and that the role of NALP1 in MDP-induced IL-1β processing depends on its association with NOD2. However, it is unclear whether MDP is directly sensed by either NALP1 or NOD2 or both proteins, but it should be noted that NALP1-mediated caspase-1 activation is MDP-responsive in a minimal in vitro system (27). Thus, NALP1 may be an actual MDP receptor. Undoubtedly, further work is needed to elucidate the exact biochemical functions carried out by NOD2 and NALP1 within the inflammasome complex. Based on our results, each protein must have a unique and nonredundant function.
Most importantly, NOD2 is required for IL-1β secretion in vivo, during a pathophysiologically relevant condition, B. anthracis infection. Interestingly, B. anthracis can kill murine macrophages by necrosis depending on their genetic background in a manner depending on its LT (28). An important mediator of LT-induced macrophage necrosis is NALP1b, and due to polymorphisms at the Nalp1 locus, C57BL/6 macrophages are resistant to LT-induced necrosis (28). Nonetheless, C57BL/6 macrophages are susceptible to LT-induced apoptosis, especially when TLR4 is activated either by LPS or by B. anthracis-produced anthrolysin (29, 31, 37). Our results suggest that both NOD2 and NALP1, possibly acting together within an inflammasome complex, are required for IL-1β secretion in response to B. anthracis infection of C57BL/6 mice and macrophages (Fig. 4 A and B). NOD2-dependent IL-1β secretion in response to B. anthracis infection required expression of LT, but it is rather unlikely that LT is a direct NOD2:NALP1 agonist. Notably, LT induces macrophage death through its proteolytic activity that disrupts activation of p38α, whose activity is required for macrophage survival (29). The survival function of p38α is mediated in part through induction of plasminogen activator inhibitor-2 (PAI-2) (38), which we recently found to serve as an indirect inhibitor of caspase-1 activation acting downstream of NF-κB (19). Thus, caspase-1 is subject to both positive and negative regulation, and a pathogen such as B. anthracis, uses more than one mechanism to induce IL-1β secretion. Interestingly, although being a positive regulator of caspase-1-dependent IL-1β secretion, LT inhibits TNF-α production, which depends on p38α activation (39). These findings support our earlier proposal that inflammasome activation provides an alternative way to trigger innate immunity in response to microbes that are capable of inhibiting critical effectors such as p38α and IKKβ (19).
NOD2 and yet-to-be-identified enteric bacteria have been implicated in the pathogenesis of CD (10). The effect of CD-associated NOD2 mutations on IL-1β production is controversial (10) and differs between human (40) and mouse (12) macrophages. The present results suggest that the actual effect of NOD2 mutations may depend on the interaction of NOD2 with NALP1 and the presence of bacteria that can produce factors that further modulate caspase-1 activation through effects on macrophage survival and pathways that negatively control caspase-1 activation.
Experimental Procedures
Mice, Plasmids, and Reagents.
Nod2−/−, caspase-1−/−, Asc−/−, CIAS1/Nalp3−/−, and Rip2−/− mice were described (8, 16, 21, 23, 41) and were all in the C57BL/6 background.
Macrophage Isolation and Stimulation.
Macrophages were pretreated with or without 0.5 ng/ml LPS for 6 h and then washed with PBS and incubated for 16 additional hours with Ca2+-complexed TiO2 (dietary grade, Tioxide U.K.) microparticles preloaded or not with 10 μg/ml MDP as described in ref. 18.
Bacterial Infections.
Early log-phase WT B. anthracis Sterne (BaWT) or mutants lacking the entire virulence plasmid pX01 (BaΔpX01) or just lethal factor (BaΔLT) (42) were added to mouse peritoneal macrophages at a multiplicity of infection of 2. After 1 h, gentamicin (50 μg/ml) was added to kill extracellular bacteria, and culture supernatants were analyzed for cytokines 6 h after infection. WT, Nod2−/−, or caspase1−/− mice were injected i.p. with 107 cfu of early log-phase BaWT. Mice were killed, and blood was collected 17 h after injection to measure cytokines. Further experimental details can be found in SI Text.
Supplementary Material
Acknowledgments.
We thank Drs. R. A. Flavell (Yale University, New Haven, CT) and G. Cheng (University of California, Los Angeles) for various knockout mice, J. Yuan (Harvard University, Boston) and J. Tschopp (Lausanne University, Lausanne, Switzerland) for gifts of plasmids and antibodies, and D. G. Guiney (University of California, San Diego) for B. anthracis strains. Macrophages from Cryopyrin/NALP3 and ASC knockout mice were provided by H. M. Hoffman (University of California, San Diego), J. Bertin, E. P. Grant, A. J. Coyle, and Millennium Pharmaceuticals. This work was supported by grants from the Crohn's and Colitis Foundation of America and the National Institutes of Health. L.-C.H., S.R.A., and S.McGillavray were supported by career development award from the Crohn's and Colitis Foundation of America, postdoctoral fellowship from the Philip Morris Foundation, and a University of California, San Diego/San Diego State University Institutional Research and Academic Career Development Award, respectively. M.K. is an American Cancer Society Research Professor.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802726105/DCSupplemental.
References
- 1.Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- 2.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 3.Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002;20:S1–13. [PubMed] [Google Scholar]
- 4.Dinarello CA. Infection, fever, and exogenous and endogenous pyrogens: some concepts have changed. J Endotoxin Res. 2004;10:201–222. doi: 10.1179/096805104225006129. [DOI] [PubMed] [Google Scholar]
- 5.Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 7.Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14:1929–1934. doi: 10.1016/j.cub.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 8.Mariathasan S, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 9.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 10.Eckmann L, Karin M. NOD2 and Crohn's disease: loss or gain of function? Immunity. 2005;22:661–667. doi: 10.1016/j.immuni.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Girardin SE, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 12.Maeda S, et al. Nod2 mutation in Crohn's disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;307:734–738. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 13.van Heel DA, et al. Muramyl dipeptide and toll-like receptor sensitivity in NOD2-associated Crohn's disease. Lancet. 2005;365:1794–1796. doi: 10.1016/S0140-6736(05)66582-8. [DOI] [PubMed] [Google Scholar]
- 14.Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Baillie L. The development of new vaccines against Bacillus anthracis. J Appl Microbiol. 2001;91:609–613. doi: 10.1046/j.1365-2672.2001.01498.x. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi KS, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 17.Ashwood P, Thompson RP, Powell JJ. Fine particles that adsorb lipopolysaccharide via bridging calcium cations may mimic bacterial pathogenicity towards cells. Exp Biol Med. 2007;232:107–117. [PubMed] [Google Scholar]
- 18.Evans SM, et al. The role of dietary microparticles and calcium in apoptosis and interleukin-1beta release of intestinal macrophages. Gastroenterology. 2002;123:1543–1553. doi: 10.1053/gast.2002.36554. [DOI] [PubMed] [Google Scholar]
- 19.Greten FR, et al. NF-kappaB Is a Negative Regulator of IL-1beta Secretion as Revealed by Genetic and Pharmacological Inhibition of IKKbeta. Cell. 2007;130:918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan Q, et al. MDP-induced interleukin-1beta processing requires Nod2 and CIAS1/NALP3. J Leukoc Biol. 2007;82:177–183. doi: 10.1189/jlb.1006627. [DOI] [PubMed] [Google Scholar]
- 21.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 22.Ogura Y, et al. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 23.Chin AI, et al. Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature. 2002;416:190–194. doi: 10.1038/416190a. [DOI] [PubMed] [Google Scholar]
- 24.Tanabe T, et al. Regulatory regions and critical residues of NOD2 involved in muramyl dipeptide recognition. EMBO J. 2004;23:1587–1597. doi: 10.1038/sj.emboj.7600175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moffett P, Farnham G, Peart J, Baulcombe DC. Interaction between domains of a plant NBS-LRR protein in disease resistance-related cell death. EMBO J. 2002;21:4511–4519. doi: 10.1093/emboj/cdf453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poyet JL, et al. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J Biol Chem. 2001;276:28309–28313. doi: 10.1074/jbc.C100250200. [DOI] [PubMed] [Google Scholar]
- 27.Faustin B, et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 28.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 29.Park JM, Greten FR, Li ZW, Karin M. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science. 2002;297:2048–2051. doi: 10.1126/science.1073163. [DOI] [PubMed] [Google Scholar]
- 30.Ferwerda G, et al. Engagement of NOD2 has a dual effect on proIL-1beta mRNA transcription and secretion of bioactive IL-1beta. Eur J Immunol. 2008;38:184–191. doi: 10.1002/eji.200737103. [DOI] [PubMed] [Google Scholar]
- 31.Park JM, Ng VH, Maeda S, Rest RF, Karin M. Anthrolysin O and other gram-positive cytolysins are toll-like receptor 4 agonists. J Exp Med. 2004;200:1647–1655. doi: 10.1084/jem.20041215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abbott DW, Wilkins A, Asara JM, Cantley LC. The Crohn's disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr Biol. 2004;14:2217–2227. doi: 10.1016/j.cub.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 33.Butler M, Boyle JJ, Powell JJ, Playford RJ, Ghosh S. Dietary microparticles implicated in Crohn's disease can impair macrophage phagocytic activity and act as adjuvants in the presence of bacterial stimuli. Inflamm Res. 2007;56:353–361. doi: 10.1007/s00011-007-7068-4. [DOI] [PubMed] [Google Scholar]
- 34.Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- 35.Humke EW, Shriver SK, Starovasnik MA, Fairbrother WJ, Dixit VM. ICEBERG: a novel inhibitor of interleukin-1beta generation. Cell. 2000;103:99–111. doi: 10.1016/s0092-8674(00)00108-2. [DOI] [PubMed] [Google Scholar]
- 36.Fernandes-Alnemri T, et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muehlbauer SM, et al. Anthrax lethal toxin kills macrophages in a strain-specific manner by apoptosis or caspase-1-mediated necrosis. Cell Cycle. 2007;6:758–766. doi: 10.4161/cc.6.6.3991. [DOI] [PubMed] [Google Scholar]
- 38.Park JM, et al. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis–CREB and NF-kappaB as key regulators. Immunity. 2005;23:319–329. doi: 10.1016/j.immuni.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Pellizzari R, Guidi-Rontani C, Vitale G, Mock M, Montecucco C. Anthrax lethal factor cleaves MKK3 in macrophages and inhibits the LPS/IFNgamma-induced release of NO and TNFalpha. FEBS Lett. 1999;462:199–204. doi: 10.1016/s0014-5793(99)01502-1. [DOI] [PubMed] [Google Scholar]
- 40.Netea MG, et al. NOD2 3020insC mutation and the pathogenesis of Crohn's disease: impaired IL-1beta production points to a loss-of-function phenotype. Neth J Med. 2005;63:305–308. [PubMed] [Google Scholar]
- 41.Sutterwala FS, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Janes BK, Stibitz S. Routine markerless gene replacement in Bacillus anthracis. Infect Immun. 2006;74:1949–1953. doi: 10.1128/IAI.74.3.1949-1953.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.