Abstract
High-pressure methods are increasingly used to produce new dense materials with unusual properties. Increasing efforts to understand the reaction mechanisms at the microscopic level, to set up and optimize synthetic approaches, are currently directed at carbon-based solids. A fundamental, but still unsolved, question concerns how the electronic excited states are involved in the high-pressure reactivity of molecular systems. Technical difficulties in such experiments include small sample dimensions and possible damage to the sample as a result of the absorption of intense laser fields. These experimental challenges make the direct characterization of the electronic properties as a function of pressure by linear and nonlinear optical spectroscopies up to several GPa a hard task. We report here the measurement of two-photon excitation spectra in a molecular crystal under pressure, up to 12 GPa in benzene, the archetypal aromatic system. Comparison between the pressure shift of the exciton line and the monomer fluorescence provides evidence for different compressibilities of the ground and first excited states. The formation of structural excimers occurs with increasing pressure involving molecules on equivalent crystal sites that are favorably arranged in a parallel configuration. These species represent the nucleation sites for the transformation of benzene into amorphous hydrogenated carbon. The present results provide a unified picture of the chemical reactivity of benzene at high pressure.
Keywords: benzene crystal, electronic transitions, high-pressure chemistry, two-photon spectroscopy
Both technological and fundamental research interests highlighted in recent years a remarkable growing focus on high-pressure studies. The discovery of new transformations, including insulator-to-metal transitions, and the search for superhard materials have stimulated great interest in the high-pressure behavior of molecules consisting of light elements, which has subsequently resulted in the merging of different disciplines such as chemistry, physics, geoscience, and material science. The formation of extended covalently bonded solids has been observed through pressurizing simple molecular systems (1). High-quality polymeric materials have been recovered at ambient conditions by high-pressure processing of small hydrocarbon molecules (2–5), demonstrating the potential of this approach for synthesis. In addition, attractive amorphous materials have been obtained by compressing model compounds. Because these processes are often reversible, a fundamental question concerns the way to quench at ambient conditions products existing in a metastable form that will allow them to be used, for example, as energetic materials in the case of nitrogen (6), or as an ultrahard glass for carbon dioxide (7).
The design and optimization of solid-state chemical reactions require knowledge of transformation mechanisms at the microscopic level. A comprehensive picture of these mechanisms is still lacking; studies as a function of pressure are a powerful tool to complement the concepts developed so far (8–12). Pressure is indeed the most efficient tool to reduce the intermolecular distances, allowing precise and continuous tuning of the corresponding interactions. The response of the system to density increases is a complex interplay between relative molecular arrangements and internal structural changes, and the energy excess generated can give access to new minima on the potential surfaces. An important step in understanding the mechanisms that regulate the solid-state reactivity is represented by recent high-pressure studies, which have revealed the role of lattice phonons in the activation and propagation of the chemical reaction that transforms crystalline benzene into a completely different material, specifically an amorphous hydrogenated carbon, which is recoverable at ambient conditions (13). During this process the long-range order that characterizes a crystalline structure is lost, and we observe a complete reconstruction of the molecular bonds, giving rise to a complex network made by both sp2 (having a planar trigonal bonding geometry) and sp3 (tetrahedrally coordinated) carbon atoms.
Another fundamental issue that remains far from being fully understood concerns the role of electronic excited states in high-pressure reactivity (14–16). Increasing overlap of molecular orbitals of adjacent molecules, leading to electronic delocalization, is produced by compression. In terms of electronic states, this generally corresponds to a reduction of the energy gap between the ground and excited electronic states (vertical contribution). These observations directly apply to systems containing conjugated π bonds. π orbitals are indeed more pressure sensitive than σ orbitals because of the smaller overlap occurring at atmospheric conditions. As a direct consequence of this, the π → π* transitions, generally the lowest in energy, are strongly affected by compression. However, it is not easy to quantify the extent to which the energy gap reduces with increasing pressure, because the energy surfaces of the different states also undergo various displacements along suitable configuration coordinates because of their different compressibility (horizontal contribution) (14). The combination of the two contributions in this regard can result in a significant lowering of the thermal barrier, which gives rise to a mixing of the ground and excited states. This mixing, tuned by pressure, has been invoked to explain the reaction occurring among neighboring molecules in some large aromatic hydrocarbon crystals (15), but it has also been suggested that the same process can be activated or increased at milder density through the optical excitation of the molecule to a suitable electronic state, as successfully demonstrated for several unsaturated hydrocarbons (16). In addition, a remarkably high selectivity in reaction products can also be achieved in the photoinduced high-pressure polymerization of butadiene (2), ethylene (4), and isoprene (17).
As mentioned above, the high-pressure transformation of benzene to an amorphous hydrogenated carbon is a case study of particular relevance; it has been studied in some considerable detail through a wide range of temperatures and pressures (18–20). The threshold pressure for activating the reaction is considerably lowered when the crystal is irradiated by laser light of suitable energy (21), which suggests the active role of excited states in the reaction. One open and intriguing question is how these findings are linked to recent results showing the existence of a critical intermolecular C–C distance used to induce the reaction in crystalline benzene (13), which implies that the microscopic mechanism of pressure-induced reactivity can be modeled only once the role of the excited states is fully understood. A characterization of the properties of electronic excited states as a function of pressure is not an easy task, and it should be noted that only the one-photon (OP) absorption, or fluorescence, spectra of a few model aromatic compounds have been studied so far, with pressures not exceeding 10 GPa.
We report here a detailed study of the pressure evolution of the lowest excited state of benzene. Two-photon (TP) excitation spectra were measured in the crystal phase as a function of pressure, allowing the characterization of the S0 → S1 transition up to 12 GPa. Fluorescence spectra were also obtained, and the formation of excimers was revealed. We suggest that these species, the same formed by pressure alone under more drastic conditions, drive the transformation of benzene into an amorphous hydrogenated carbon, thus providing a unique reaction mechanism for laser-assisted and purely pressure-induced reactions.
Results
TP absorption spectroscopy has advantages over conventional OP absorption spectroscopy in high-pressure experiments. The lowest electronic transitions of small hydrocarbons, which are model systems for high-pressure reactivity, lie at around 200 nm. Observation of these transitions is partly or completely precluded in OP experiments by the diamond absorption edge occurring (for type-IIa diamonds) at ≈220 nm at ambient conditions and shifting to the red with increasing pressure. In contrast, TP spectroscopy provides access to these excited states through the simultaneous absorption of two visible photons. In addition, the small cross section (10−50 cm4·sec per photon per molecule) of TP transitions minimizes the production of excited species that are able to trigger the reactivity of the system.
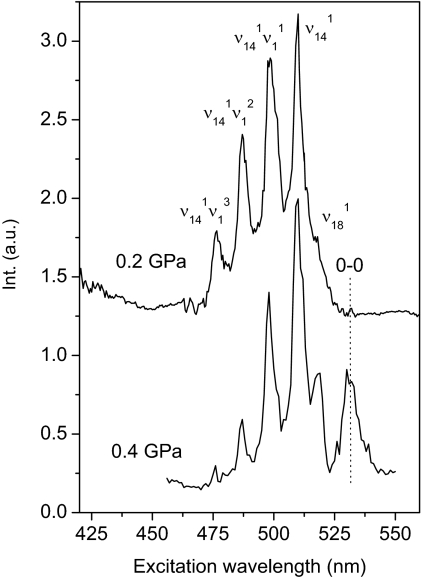
The TP excitation spectra that we measured in the liquid phase, at both ambient and low pressure, perfectly coincide with data given in the literature (22). The structure of the spectrum is also very similar in the crystal phases, and it can be directly compared to those reported in refs. 23 and 24 once the effect of temperature is taken into account. TP excitation spectra of the S0 → S1 transition recorded on two different samples in the crystal phase I (where benzene crystallizes once the liquid is compressed at room temperature), are shown in Fig. 1. The two spectra are very similar except if one takes into account the intensities of the symmetry-forbidden exciton line (0–0) and the weakly allowed ν18 vibronic origin (24). This difference can be attributed to different levels of crystal defects (e.g., vacancies, dislocations, and grain boundaries), which relax the selection rules by breaking the translational symmetry. The vibronic structure is reproducible in the two spectra once the small pressure shift is taken into account. The main peaks are readily assigned based on literature data for ambient pressure (24), despite observations of a systematic red shift of 2–3 nm likely because of the different pressure and temperature conditions. An unstructured absorption tail below 450 nm indicates the edge of the higher energy allowed transitions.
Fig. 1.
TP excitation spectra of crystalline benzene measured in two different samples in the orthorhombic phase I. Assignment of the main peaks is in accordance with ref. 24. Int. (a.u.), intensity in arbitrary units.
The pressure dependence of the TP excitation spectra is shown in Fig. 2. The effects of compression are negligible, as far as the intensity and bandshape of the S0 → S1 transitions are concerned, up to ≈7 GPa. Above this pressure, a weakening and broadening in this part of the spectrum takes place, accompanied by a change of the relative intensity of the vibronic structure. The spectral quality further worsens above 11.8 GPa, and as a result, the S1 vibronic structure becomes barely distinguishable from the background. It should also be mentioned that a consistent contribution to the background derives from the overlap with the absorption because of transitions occurring to higher electronic excited states that exhibit a pronounced red pressure shift.
Fig. 2.
Pressure evolution of the TP excitation spectra of crystalline benzene measured in the monoclinic phase II.
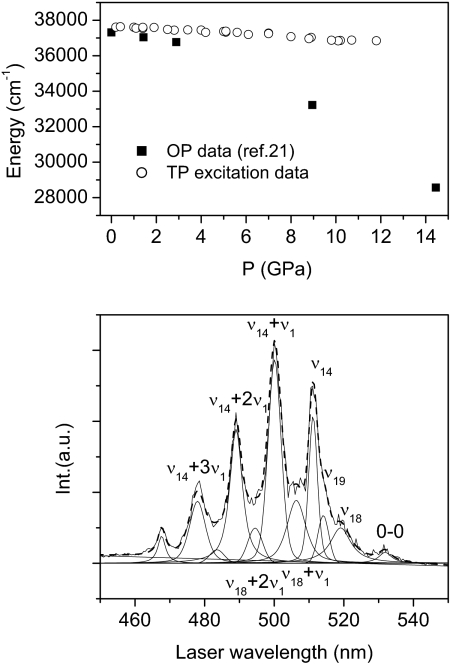
The spectra were reproduced by using the minimum number of peaks having a pseudo-Voigt profile. The electronic origin, vibronic origins that occur due to ν18 (C–H bending e1u), and ν14 (C–C stretching b2u) modes, and the progression observed as a result of the breathing mode (ν1) built on this latter vibronic origin are readily identified in these experiments (see Fig. 3). In addition, the vibronic origin formed as a result of ν19 (C–C stretching e1u) and progression occurring through the breathing mode (ν1) built on ν18 vibronic origin, are obtained from the fit. It should be noted that a crystal field splitting is not observed due to the temperature and pressure broadening, and the relative intensities of all these peaks coincide with those reported in ref. 24. The pressure shift of the exciton band is shown in Fig. 3 Upper, together with the data relative to the S0 → S1 transition from the OP absorption spectra (21). The discrepancy between the two sets of data is large (≈1 eV at 14 GPa) and is attributed to the method used to estimate the energy gap in the saturated OP absorption spectra. It is evident that in this case, determination of the peak energy was heavily influenced by the intensification and broadening of the absorption line. The precise determination of the 0–0 transition reveals a moderate red shift (approximately −60 cm−1/GPa), which is 1/10 as large as previously reported (21).
Fig. 3.
Analysis of TP spectra. (Lower) Deconvolution of a TP excitation spectrum measured in phase II crystalline benzene at 2.4 GPa; assignment is derived from ref. 24. (Upper) Pressure evolution of the 0–0 exciton transition energy. The open circles represent the values obtained in this study through the TP excitation profile, and filled squares represent the data gathered from ref. 21 extracted by saturated OP absorption spectra.
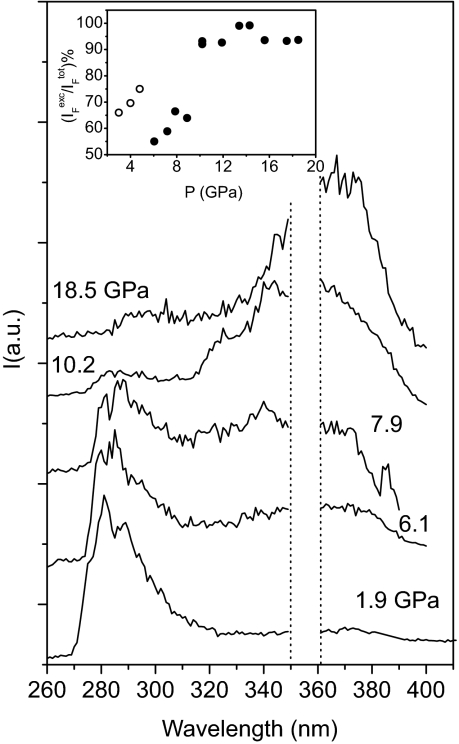
TP fluorescence spectra were recorded at each pressure through the use of excitation wavelengths corresponding to the peak maxima of the ν14 vibronic origin. There were no differences observed when excitation wavelengths gave access to either different regions of the S0 → S1 transition or when higher energy states were excited. In Fig. 4, selected spectra collected in compression cycles are reported. At a pressure below 5 GPa, the fluorescence spectrum is dominated by the monomer emission (270–310 nm) although a broad weak band ascribable to excimer emission can be detected at lower energies. The excimer formation at high pressure has been reported for several polycyclic aromatic hydrocarbons (25–28), particularly anthracene, which is the most studied (28). In all cases, the spectroscopic evidence consists of a broad band, intensifying with pressure, and red shifted by ≈5,000 cm−1 from the monomer fluorescence band. Here, we also observed a broad unstructured band growing ≈5,500 cm−1 below the monomer fluorescence suddenly intensified above 5 GPa.
Fig. 4.
Pressure evolution of the TP fluorescence spectra. The high-frequency (280–300 nm) and broader low-frequency (320–400 nm) bands are due to the monomer and excimer emissions, respectively. The region between the dotted lines has been cut because of the presence of spurious signals resulting from the 355-nm laser line. In the Inset the pressure evolution of the intensity ratio between the excimer and total detected fluorescence is reported: filled circles represent spectra measured on compression, and open circles refer to spectra collected on releasing pressure on a sample previously compressed up to 13 GPa, and then decompressed down to 5 GPa, avoiding irradiation.
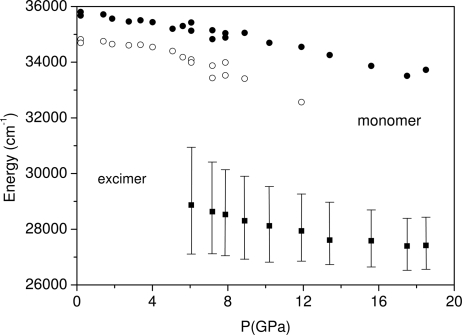
The ratio between the excimer and the total fluorescence intensity is reported as a function of pressure in the Inset of Fig. 4. The monomer and excimer fluorescence intensities are comparable at 6 GPa; however, the latter intensifies dramatically with rising pressure, and at 11 GPa constitutes 90–95% of the total emission intensity. A further pressure increase does not appreciably modify this ratio up to 20 GPa. The pressure shift of the monomer and excimer bands is shown in Fig. 5. The frequencies of the two main bands obtained from the deconvolution process are reported for the monomer. As regards the excimer, and because of the unstructured and asymmetric bandshape, we report the centroid of the band (defined as the frequency value dividing the emission band area into two equivalent portions). The dispersion of the entire emission pattern is also indicated. The pressure shifts of the monomer and excimer bands are similar (−125 and −135 cm−1/GPa, respectively). The excimer fluorescence in anthracene (28) and anthraquinone (27) undergoes a pressure shift that is 25–30% smaller than the monomer fluorescence. By contrast, we observed in benzene a slightly larger pressure shift for the excimer than for the monomer. This result indicates an increased stabilization of the excimer with pressure, thus suggesting its active role in the high-pressure reaction. The pressure shift of the monomer fluorescence band (−125 cm−1/GPa) is 2 times larger than that of the exciton line as determined by the TP absorption spectra (−60 cm−1/GPa).
Fig. 5.
Pressure evolution of the monomer and excimer fluorescence. For the excimer, the data points refer to the centroid of the broad emission band, and the bars account for its energy dispersion and indicate the frequency region ranging between 20% and 80% of the total integrated intensity.
Discussion
The different pressure shifts measured for absorption and emission processes can be explained on the basis of a different compressibility of the ground and of the lowest excited states. The pressure shift of the exciton line determined by the TP absorption spectra yields a precise measurement (within the Born–Oppenheimer approximation) of the vertical shift of the two energy surfaces. The red shift measured in excess of the monomer fluorescence spectra should be entirely related to a different horizontal displacement of the two energy surfaces involved in the transition. This horizontal displacement of electronic potential energy surfaces was suggested and modeled by Drickamer et al. (14, 15) to quantify the difference between optical and thermal electronic transitions in organic solids as a function of pressure. This displacement is actually measured here. The direction of this horizontal displacement can be understood through the pressure evolution of the TP excitation profile. The intensity distribution of the Franck–Condon progression that is built on the ν14 vibronic origin (see Fig. 2) changes continuously on rising pressure as the maximum transfers over from the 0 + ν14 + ν1 to the ν14 vibronic origin. This process corresponds to an increased similarity of structure between the ground and lowest excited states as a result of increasing pressure; i.e., there is increased superposition of the minima between the two potential surfaces that is driven by greater compressibility of molecules in the excited state.
The formation and stabilization of excimer species at high pressure has been studied in only a few molecular systems. Irradiation (26) and crystal defects (28) have been identified as sources of the excimer emission occurring in the anthracene crystal. In general, once the excimer is formed, the original fluorescence spectrum can be recovered only by thermal annealing of the sample at low pressure (26). In the present case, there were no differences observed between the fluorescence spectra recorded above 6 GPa along pressurization cycles on well annealed samples in which irradiation was completely avoided during the compression and on those samples irradiated after applying very low pressure to record absorption and fluorescence spectra. Furthermore, we measured fluorescence spectra on samples compressed above 13 GPa and then decompressed down to 5 GPa while avoiding any irradiation of the sample. Also in this case, the excimer emission was observed and, apart from a small hysteresis (see Fig. 4 Inset), the process appears to have been fully reversible. In fact, the original low-pressure emission spectra were almost recovered at 2.5 GPa.
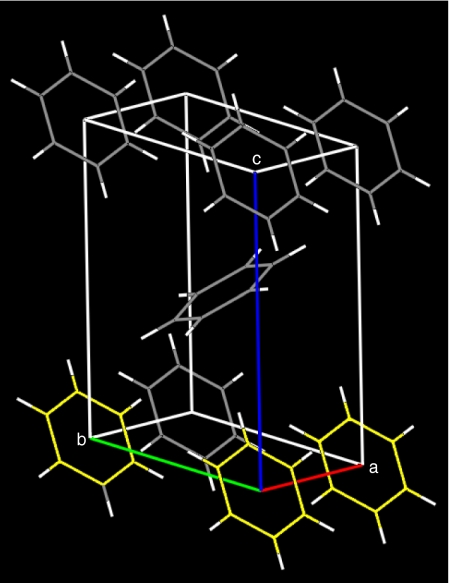
The above results imply that there is a structural origin for excimer emission, as suggested in ref. 25 for crystals in which the intermolecular contact allows for pair formation, particularly when the molecular planes of nearest neighbors remain parallel. In the case of anthracene, nearest neighbor rings are rather far from this condition as a result of being ordered through the so-called “herringbone structure,” where the angle between the normal vector perpendicular to the molecular planes is ≈130° (29). This arrangement prevents an overlap of the π densities and supports the proposed excimer formation through crystal defects (28). In phase II benzene, the aromatic rings along the a and b axes are arranged in a slipped-parallel configuration, i.e., the molecules lie on parallel planes whereas their centers are displaced (see Fig. 6), which could allow for excimer formation (20). The eclipsed configuration (D6h) was calculated through using time-dependent density functional theory as the most energetically stable dimer of benzene; however, the slipped-parallel configuration was found to be only slightly higher in energy (30).
Fig. 6.
Crystal structure of benzene phase II. The molecules aligned along the a and b axes (three of them are shown by yellow coloration) are arranged in parallel configuration and exhibit the shortest C–C contacts.
The features of the high-pressure room temperature reactivity of benzene do not change when the reaction is purely pressure induced (19) or when it is activated at lower pressure by light absorption (21). In both processes most of the monomer reacts only during decompression, and the recovered amorphous hydrogenated carbons obtained in the two syntheses present the same characteristics. This result indicates that the same reaction mechanism occurs in both cases, which is rather peculiar behavior in comparison with other small unsaturated hydrocarbons, where the structural changes determined by the optical excitation open new reactive paths, as observed in isoprene (17), or select specific ones, as in butadiene (2). In benzene, the molecular geometry is not significantly altered by excitation to the S1 state; only a moderate lengthening of the C–C bonds (0.03 Å) takes place. The resulting reduction of the intermolecular distances is much smaller than that due to the thermal oscillations, which have recently been shown to explain the purely pressure-induced reactivity (13). The arrangements of benzene molecules in the lattice are nearly parallel, allowing charge-transfer processes to become predominant at high pressure, giving rise to transient dimers (structural excimers) that represent the seeds of the chemical reaction. The stabilization of benzene dimers at high pressure is demonstrated by the present experiment, in agreement with first-principle simulations (13). This suggests that dimerization is the first stage of benzene amorphization. The formation of these species is driven by lattice motions, and their reproducibility over a wide pressure–temperature range (13) indicates that the process occurs not only at specific points (defects) but also in the bulk crystal. When the crystal is optically excited, the excimers form at lower pressure because of the dipolar interaction between the excited molecule and the nearest neighbor benzene molecule favorably arranged in the slipped parallel configuration. Therefore, the same mechanism characterizes the two reactions, as they mainly occur on releasing pressure and lead to the same product.
Conclusions
Increased gigapascal pressure has been used to induce chemical transformations in the simplest molecular systems. Notwithstanding the synthetic interest, high-pressure studies represent a powerful tool for understanding the mechanisms driving solid-state reactivity. In this study, we investigated how the electronic excited states participate to the pressure-induced benzene amorphization. We measured TP excitation spectra and obtained a precise characterization of the evolution with pressure of the first electronic excited state applied up to 12 GPa, close to the onset of the photoinduced high-pressure reaction. The pressure shift of the S0 → S1 exciton transition is remarkably different, about 1/10 as large as that previously estimated from OP absorption spectra. TP fluorescence spectra present a complex structure changing with pressure because of simultaneous emission of monomeric and excimeric species. The pressure shift of the monomer fluorescence, compared with the frequency and intensity evolution with pressure observed through the absorption data, reveals a different compressibility for the ground and first electronic excited state. The formation of the excimeric species, the amount of which depends on pressure and steeply increases above 5 GPa, also occurs when irradiation of the sample is avoided, thus demonstrating its structural origin. The parallel arrangement of the molecules along the a and b crystal axes allows for dimer formation. These species are identified as the nuclei of the chemical reaction; i.e., they are the same in the purely pressure-induced and photo-assisted reaction. This behavior, which is unusual for the reactivity of hydrocarbons under pressure, can be explained through the emergence of a progressive stabilization of the dimer with rising pressure. Finally, the simultaneous observation of a moderate pressure shift of the lowest electronic transition and the formation of structural dimers is of interest. In general, the progressive coloration observed when compressing several molecular crystals is attributed to a decrease in the energy gap between the monomer fundamental and excited states. In this analysis, we provide evidence for a more complex phenomenon in which stable excimers are present in the sample and whose formation not only is related to the presence of crystal defects or the absorption of radiation through a suitable wavelength but is intrinsically dependent on the crystal structure. The formation of these structural dimers is, in principle, always possible in π-bonded systems for molecules related by translational symmetry through the assistance of zone boundary acoustic modes. The stability and concentration of these species can strongly modify optical properties and represent a preferential site for chemical transformation.
Methods
Liquid benzene (Merck; purity ≥ 99.9%) was loaded in a membrane diamond anvil cell (MDAC) equipped with IIa diamonds. The sample dimensions were typically 40–50 μm thick and 150 μm in diameter. Phase I crystals were produced by slowly compressing the liquid. After the transition to phase II, which occurs at room temperature at ≈1.5 GPa, the sample was annealed to 450–500 K to completely remove phase I remnants (20). At each pressure both the TP fluorescence spectrum and the corresponding excitation profile were measured. Before this and immediately after these measurements, IR absorption spectra were measured in the mid-infrared region to identify whether any chemical change took place in the sample and determine its pressure. In fact, we did not use the standard local pressure calibration through R1 ruby fluorescence band shifting to avoid any possible damage of the sample because of absorption of visible light by the ruby and as a result of local heating. To achieve internal calibration, the pressure shift of the ν15 IR active vibrational mode at ≈1,150 cm−1 (ambient pressure) was used. The frequency of this mode was carefully calibrated in a separate experiment performed up to 20 GPa on a perfectly annealed phase II crystal by measuring the pressure by the ruby fluorescence band shift. The apparatus used to perform infrared experiments under pressure, including the optical beam condenser, has been described elsewhere (31, 32). A Fourier-transform infrared (FTIR) spectrometer (Bruker IFS-120 HR) was used to measure the IR absorption spectra with an instrumental resolution better than 1 cm−1. For fluorescence measurements we used an optical parametric generator (OPG) as excitation source, pumped by the third harmonic (355 nm) of a mode-locked Nd-YAG laser (from EKSPLA). Tunable light in the 420- to 680-nm spectral range was generated by a lithium triborate crystal. The pulse duration from the OPG was ≈25 psec and the repetition rate was 10 Hz. Pulse energies not exceeding 0.5 μJ were applied during the experiment to avoid damage to the diamonds and to reduce the possibility of activating any chemical reaction. The fluorescence from the sample was collected in a 180° geometry, filtered by a single stage monochromator (2,400 grooves per mm), revealed by a photomultiplier (ET-9235QB) and both averaged and integrated by an oscilloscope (LeCroy LC584A). A quadratic dependence of the fluorescence signal on the excitation energy was found in all of the ranges investigated.
Acknowledgments.
We gratefully acknowledge M. De Pas for his valuable technical support. This work was supported by the European Union under Contract RII3-CT2003-506350, by the Italian Ministero dell'Università e della Ricerca Scientifica e Tecnologica (MURST) and by “Firenze Hydrolab” through a grant by Ente Cassa di Risparmio di Firenze.
Footnotes
The authors declare no conflict of interest.
References
- 1.Schettino V, Bini R, Ceppatelli M, Ciabini L, Citroni M. Chemical reactions at very high pressure. In: Rice SA, editor. Advances in Chemical Physics. Vol. 131. New York: Wiley; 2005. pp. 105–242. [Google Scholar]
- 2.Citroni M, Ceppatelli M, Bini R, Schettino V. Laser-induced selectivity for dimerization versus polymerization of butadiene under pressure. Science. 2002;295:2058–2060. doi: 10.1126/science.1068451. [DOI] [PubMed] [Google Scholar]
- 3.Citroni M, Ceppatelli M, Bini R, Schettino V. The high-pressure chemistry of butadiene crystal. J Chem Phys. 2003;118:1815–1820. [Google Scholar]
- 4.Chelazzi D, Ceppatelli M, Santoro M, Bini R, Schettino V. High-pressure synthesis of crystalline polyethylene using optical catalysis. Nat Mater. 2004;3:470–475. doi: 10.1038/nmat1147. [DOI] [PubMed] [Google Scholar]
- 5.Chelazzi D, Ceppatelli M, Santoro M, Bini R, Schettino V. Pressure-induced polymerization in solid ethylene. J Phys Chem B. 2005;109:21658–21663. doi: 10.1021/jp0536495. [DOI] [PubMed] [Google Scholar]
- 6.Eremets ML, Hemley RJ, Mao H, Gregoryanz E. Semiconducting non-molecular nitrogen up to 240 GPa and its low-pressure stability. Nature. 2001;411:170–174. doi: 10.1038/35075531. [DOI] [PubMed] [Google Scholar]
- 7.Santoro M, et al. Amorphous silica-like carbon dioxide. Nature. 2006;441:857–860. doi: 10.1038/nature04879. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MD, Schmidt GMJ. Topochemistry 1. Survey. J Chem Soc. 1964:1996–2000. [Google Scholar]
- 9.Luty T, Eckhardt CJ. General theoretical concepts for solid-state reactions—Quantitative formulation of the reaction cavity, steric compression and reaction-induced stress using an elastic multipole representation of chemical pressure. J Am Chem Soc. 1995;117:2441–2452. [Google Scholar]
- 10.Cohen MD. Photochemistry of organic solids. Angew Chem Int Ed Engl. 1975;14:386–393. [Google Scholar]
- 11.McBride JM, Segmuller BE, Hollingsworth MD, Mills DE, Weber BA. Mechanical stress and reactivity in organic solids. Science. 1986;234:830–835. doi: 10.1126/science.234.4778.830. [DOI] [PubMed] [Google Scholar]
- 12.Dwarakanath K, Prasad PN. Raman phonon spectroscopy of solid state reactions—thermal rearrangement of methyl para-dimethylaminobenzenesulfonate in solid-state. J Am Chem Soc. 1980;102:4254–4256. [Google Scholar]
- 13.Ciabini L, et al. Triggering dynamics of the high-pressure benzene amorphization. Nat Mater. 2007;6:39–43. doi: 10.1038/nmat1803. [DOI] [PubMed] [Google Scholar]
- 14.Drickamer HG, Frank CW, Slichter CP. Proc Natl Acad Sci USA. 1972;69:933–937. doi: 10.1073/pnas.69.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drickamer HG, Frank CW. Electronic Transitions and the High Pressure Chemistry and Physics of Solids. London: Chapman and Hall; 1973. [Google Scholar]
- 16.Bini R. Laser-assisted high-pressure chemical reactions. Acc Chem Res. 2004;37:95–101. doi: 10.1021/ar030015c. [DOI] [PubMed] [Google Scholar]
- 17.Citroni M, Ceppatelli M, Bini R, Schettino V. Dimerization and polymerization of isoprene at high pressures. J Phys Chem B. 2007;111:3910–3917. doi: 10.1021/jp0701993. [DOI] [PubMed] [Google Scholar]
- 18.Cansell F, Fabre D, Petitet JP. Phase-transitions of benzene up to 550°C and 30 GPa. J Chem Phys. 1993;99:7300–7304. [Google Scholar]
- 19.Ciabini L, Santoro M, Bini R, Schettino V. High pressure reactivity of solid benzene probed by infrared spectroscopy. J Chem Phys. 2002;116:2928–2935. [Google Scholar]
- 20.Ciabini L, et al. High-pressure and high-temperature equation of state and phase diagram of solid benzene. Phys Rev B. 2005;72 094108. [Google Scholar]
- 21.Ciabini L, Santoro M, Bini R, Schettino V. High pressure photoinduced ring opening of benzene. Phys Rev Lett. 2002;88 doi: 10.1103/PhysRevLett.88.085505. 085505. [DOI] [PubMed] [Google Scholar]
- 22.Scott TW, Callis PR, Albrecht AC. Alternant hydrocarbon selection rules in the two-photon spectroscopy of perturbed benzene. Chem Phys Lett. 1982;93:111–114. [Google Scholar]
- 23.Hochstrasser RM, Sung HN, Wessel JE. Moderate resolution study of the two-photon spectrum of the 1B2u state of benzene-h6 and benzene-d6. Chem Phys Lett. 1974;24:7–10. [Google Scholar]
- 24.Hochstrasser RM, Climcak CM, Meredith GR. Vibronic spectra of the benzene crystal at 4.2 K using two-photon fluorescence excitation. J Chem Phys. 1979;70:870–880. [Google Scholar]
- 25.Offen HW. Fluorescence spectra of several aromatic crystals under high pressures. J Chem Phys. 1966;44:699–703. [Google Scholar]
- 26.Jones PF, Nicol M. Excimer emission of naphthalene, anthracene, and phenanthrene crystals produced by very high pressures. J Chem Phys. 1968;48:5440–5447. [Google Scholar]
- 27.Brillante A, Della Valle RG, Farina R, Venuti E. Pressure-induced phase-transitions in 9,10-anthracene derivatives—anthraquinone. Chem Phys. 1995;191:177–184. [Google Scholar]
- 28.Dreger ZA, Lucas H, Gupta YM. High-pressure effects on fluorescence of anthracene crystals. J Phys Chem B. 2003;107:9268–9274. [Google Scholar]
- 29.Oehzelt M, Resel R, Nakayama A. High-pressure structural properties of anthracene up to 10 GPa. Phys Rev B. 2002;66:174104. [Google Scholar]
- 30.Amicangelo JC. Theoretical study of the benzene excimer using time-dependent density functional theory. J Phys Chem A. 2005;109:9174–9182. doi: 10.1021/jp053445o. [DOI] [PubMed] [Google Scholar]
- 31.Bini R, Ballerini R, Pratesi G, Jodl HJ. Experimental setup for Fourier transform infrared spectroscopy studies in condensed matter at high pressure and low temperatures. Rev Sci Instrum. 1997;68:3154–3160. [Google Scholar]
- 32.Gorelli FA, Santoro M, Ulivi L, Bini R. The epsilon phase of solid oxygen: Evidence of an O4 molecule lattice. Phys Rev Lett. 1999;83:4093–4096. [Google Scholar]